Abstract
Objective: To evaluate the impact of sequential blood purification therapy on myocardial injury and serum inflammatory cytokine levels in patients with organophosphorus poisoning. Methods: The clinical data of 67 patients hospitalized for organophosphorus poisoning from January 2020 to January 2021 in Anxi County Hospital were retrospectively analyzed. The patients were divided into a control group (n = 30, received conventional treatment plus hemoperfusion) and a research group (n = 37, received conventional treatment plus sequential blood purification therapy [hemoperfusion plus continuous veno-venous hemofiltration (CVVH)]). The clinical efficacy, myocardial injury indicators, inflammatory cytokine levels, liver and kidney function, incidence of complications, and mortality rate were compared between the two groups. Results: The research group showed a significantly higher total effective rate of 94.59% compared to 73.33% in the control group (P < 0.05). Fewer doses of penehyclidine were administered in the research group, compared to the control group (P < 0.05). In addition, the duration of penehyclidinezation, wakefulness, recovery of CHE activity, mechanical ventilation and hospitalization in the research group was significantly shorter than those in the control group (all P < 0.05). After treatment, both groups exhibited decreased levels of serum NSE, cTnI, CK-MB, LDH, Myo, WBC, CRP, PCT and IL-6, with lower levels in the research group (P < 0.05). Conversely, both groups showed elevated levels of serum CHE, BUN, Scr, urine protein, AST, TBIL and ALT, with higher levels in the control group (P < 0.05). On the 1st and 2nd days after treatment, the serum NSE levels in the observation group were lower than those in the control group, while the serum CHE levels were higher than those in the control group (all P < 0.05). On the 3rd day after treatment, serum NSE and CHE levels were not significantly different between the two groups. The incidence of rebound in the research group was significantly lower (8.11% vs. 30.00%, P < 0.05) than that in the control group. The mortality rates did not show statistically significant differences between the two groups (10.00% vs. 2.70%, P > 0.05). Conclusion: Sequential blood purification therapy can effectively mitigate myocardial injury, reduce the expressions of inflammatory cytokines, and protect liver and kidney function in patients with organophosphorus poisoning, with a favorable safety profile.
Keywords: Organophosphorus poisoning, sequential blood purification therapy, myocardial injury, inflammatory cytokines, liver and kidney function
Introduction
Organophosphate poisoning is a common clinical critical emergency, with patients typically presenting with respiratory failure, coma, and pulmonary edema, posing a serious threat to their life and health [1]. It can cause myocardial damage, liver and kidney dysfunction, and systemic inflammatory responses. Without prompt treatment, this condition can even progress to multiple organ dysfunction [2]. Currently, the treatment of organophosphate poisoning in clinical practice typically focuses on thorough gastric lavage, prompt catharsis, employment of acetylcholinesterase reactivators and antidotes such as anticholinergic receptor drugs. However, the overall mortality rate from organophosphate poisoning still exceeds 10%, with unsatisfactory prognosis in some patients [3,4]. Therefore, how to effectively treat organophosphate poisoning remains a hot research topic in major medical institutions.
In recent years, with the continuous development of medical technology, blood purification therapies have been widely employed in clinical treatment for patients with organophosphate poisoning, which has effectively reduced the mortality rates and improved the quality of life [5]. Even though there are many blood purification therapies with varying efficacy, consensus on treatment plans for organophosphate poisoning hasn’t been reached yet in clinical settings. Sequential blood purification therapy is a treatment approach that combines hemoperfusion (HP) with continuous veno-venous hemofiltration (CVVH), which can effectively remove inflammatory mediators, toxic substances, etc. This therapy has demonstrated ideal effects in clinical treatment of conditions, such as multiple organ dysfunction caused by mushroom poisoning, sepsis complicated with acute kidney injury and so on [6,7]. However, there are few reports on the use of sequential blood purification therapy in patients with organophosphate poisoning in clinical practice. Therefore, this study investigated the effects of sequential blood purification therapy and its impact on myocardial injury and serum inflammatory cytokine levels in patients suffering from organophosphate poisoning, with aims to establish a theoretical foundation for the treatment of the disease.
Materials and methods
General data
A retrospective analysis was conducted on the clinical data of 67 patients with organophosphate poisoning admitted to Anxi County Hospital from January 2020 to January 2021. The general data of these patients were as follows: gender distribution: male/female = 39/28; age: 23-68 years old, with an average age of (38.21±5.12) years; poisoning duration: 1-8 hours, with an average of (4.17±1.57) hours; body mass index (BMI): 16-32 kg/m2, with an average of (23.16±2.01) kg/m2; types of toxins: dichlorvos/methamidophos/others = 35/19/13. The patients were divided into the control group (n = 30) and the research group (n = 37) in accordance with treatment plans, with the control group receiving conventional treatment plus HP and the research group being treated with conventional treatment plus sequential blood purification therapy (HP in addition to CVVH). This research has gained approval from the Ethics Committee of Anxi County Hospital. The general information of the two groups was comparable (P > 0.05).
Inclusion and exclusion criteria
Inclusion criteria: Patients who met the diagnostic criteria for organophosphate poisoning and were accompanied by symptoms such as pulmonary edema, delirium, and vomiting [8]; with complete clinical data; aged between 20 and 70 years; less than 8-h interval from poisoning to receiving treatment. Exclusion criteria: Patients who were unable to comply with the treatment plan; pregnant and lactating patients; combined with other drug poisonings; complicated with other severe heart, liver, or kidney conditions.
Treatment plans
All patients immediately underwent conventional treatments including catharsis, gastric lavage, mechanical ventilation, anti-infection management, and nutritional support after admission. On the basis of the above care, patients in the control group were treated with HP using an HA230 perfusion apparatus (purchased from Lijie Medical Biomaterials Co., Ltd.), with low molecular weight heparin calcium for anticoagulation, blood flow rate set at 150-250 ml/min, duration of 3-4 hours per session, once a day for 3 days. In addition to conventional treatments, patients in the research group underwent additional sequential blood purification therapy, namely the HP plus CVVH. This treatment plan involved connecting a filter in series with the HA230 perfusion apparatus. After treatment for 3 hours, the perfusion apparatus was disconnected from the filter and replaced with CVVH therapy. The replacement liquid was prepared in accordance with the bicarbonate formula, with a flow rate of 3000 ml/h, including pre-replacement at 2500 ml/h and post-replacement at 500 ml/h. The blood flow rate was maintained at 150-200 ml/min, with low molecular weight heparin calcium used for anticoagulation. The perfusion apparatus and filter were replaced daily, and the treatment continued for 3 days.
Outcome measures
(1) Clinical efficacy. Markedly effective: Symptoms significantly improved or disappeared, cardiac troponin I (cTnI) returned to normal levels, and cholinesterase (CHE) activity significantly increased. Effective: Symptoms improved, cTnI levels improved but did not yet reached normal level, and CHE activity increased. Ineffective: No significant or effective improvements were observed. The total effective rate is the sum of the significant improvement rate and the effective rate. (2) Clinical indicators. The dosage of penehyclidine as well as the duration of penehyclidinezation, wakefulness, recovery of CHE activity, mechanical ventilation and hospitalization were compared between the two groups. (3) Myocardial injury indicators. Three milliliters of fasting venous blood were collected from patients in both groups before and after treatment. After centrifugation at 3000 rpm for 10 minutes (radius: 6 cm), the upper serum layer was transferred to a 1.5 ml EP tube. The levels of cTnI, myoglobin (Myo), creatine kinase myocardial isoenzyme (CK-MB), and lactate dehydrogenase isoenzyme (LDH) were measured using enzyme-linked immunosorbent assay (ELISA) kits, with reagents provided by Wenzhou Kemiao Biotechnology Co., Ltd. (Batch No.: 0722, 1009, 1212). (4) Inflammatory cytokine levels. White blood cell count (WBC) before and after treatment were measured using a blood analyzer. The levels of C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) before and after treatment were detected using ELISA kits. The CRP, PCT and IL-6 ELISA kits were purchased from Wenzhou Kemiao Biotechnology Co., Ltd. (Batch No.: 0920, 1208, 1130). (5) Serum levels of cholinesterase (CHE) and neuron-specific enolase (NSE). Blood samples were collected from peripheral veins of all patients before treatment and on the 1st, 2nd and 3rd day after treatment. Serum levels of NSE and CHE were measured by ELISA. The CHE and NSE ELISA kits were purchased from Wenzhou Kemiao Biotechnology Co., Ltd. (Batch No.: 1120 and 1011). All tests were carried out by trained professionals in the same laboratory using the same batch of kits, operating in strict accordance with the kit instructions. (6) Liver and kidney function. Blood urea nitrogen (BUN), serum creatinine (Scr), aspartate aminotransferase (AST), total bilirubin (TBIL), alanine aminotransferase (ALT), and urine protein levels before and after treatment were measured using a fully automated biochemical analyzer. (7) Safety. The incidence of complications was compared between the two groups. (8) Mortality rate. The mortality rates were compared between the two groups.
Statistical analysis
SPSS 23.0 software was used to perform statistical analysis on data concerning clinical efficacy, safety, mortality rate and so on, which were expressed as n (%) and χ2. The sample size calculation was based on the primary outcome measures of myocardial injury and inflammatory factors. PASS software was used to calculate the sample size with α = 0.05 and 1-β = 0.80. Based on pre-trial data, considering the differences in the primary outcome measures between the two groups, 67 patients were ultimately included in this study. Comparison of clinical indicators, myocardial injury indicators, inflammatory cytokine levels, and hepatic and renal function indicators between the two groups was conducted by t test and expressed as (x̅ ± S). P < 0.05 was considered statistically significant.
Results
Baseline data
The comparison of gender, average poisoning duration, BMI and toxin types between the two groups showed no statistically significant difference (P > 0.05). See Table 1.
Table 1.
Comparison of baseline data between the two groups
| Groups | Gender (male/female, cases) | Average age (years) | Average poisoning duration (h) | BMI (kg/m2) | Toxin types (dichlorvos/methamidophos/others, cases) |
|---|---|---|---|---|---|
| Control group (n = 30) | 18/12 | 38.95±12.38 | 4.75±1.65 | 23.11±2.02 | 15/8/7 |
| Research group (n = 37) | 21/16 | 35.47±10.09 | 4.28±3.53 | 24.20±2.99 | 20/11/6 |
| χ2/t | 0.072 | 1.232 | 0.672 | 1.705 | 0.539 |
| P | 0.789 | 0.223 | 0.504 | 0.093 | 0.764 |
Clinical efficacy
The clinical efficacy was 94.59% in the research group, which was significantly higher in comparison to 73.33% in the control group (P < 0.05). See Table 2.
Table 2.
Comparison of clinical efficacy between the two groups [n (%)]
| Groups | Markedly effective | Effective | Ineffective | Total effectiveness |
|---|---|---|---|---|
| Control group (n = 30) | 13 (43.33) | 9 (30.00) | 8 (26.67) | 22 (73.33) |
| Research group (n = 37) | 24 (64.86) | 11 (29.73) | 2 (5.41) | 35 (94.59) |
| χ2 | 4.342 | |||
| P | 0.037 |
Clinical indicators
Patients in the research group had lower doses of long-acting nifedipine compared to those in the control group. Besides, the research group demonstrated shorter time in administering long-acting nifedipine, to regain consciousness, for CHE activity recovery as well as shorter duration of mechanical ventilation and hospitalization in comparison to the control group (P < 0.05). See Table 3.
Table 3.
Comparison of clinically indicators between the two groups (x̅ ± SD)
| Groups | Dosage of long-acting nifedipine (mg) | Time of administering long-acting nifedipine (h) | Conscious time (h) | CHE recovery time (h) | Mechanical ventilation time (d) | Length of hospitalization (d) |
|---|---|---|---|---|---|---|
| Control group (n = 30) | 308.51±45.18 | 5.33±1.19 | 18.81±2.37 | 12.34±1.91 | 5.38±0.85 | 10.85±2.25 |
| Research group (n = 37) | 245.39±25.98 | 3.91±1.05 | 14.51±2.12 | 9.02±1.55 | 4.12±0.77 | 8.74±1.78 |
| t | 7.168 | 5.185 | 7.831 | 7.857 | 6.358 | 4.287 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Note: CHE: Cholinesterase.
Myocardial injury indicators
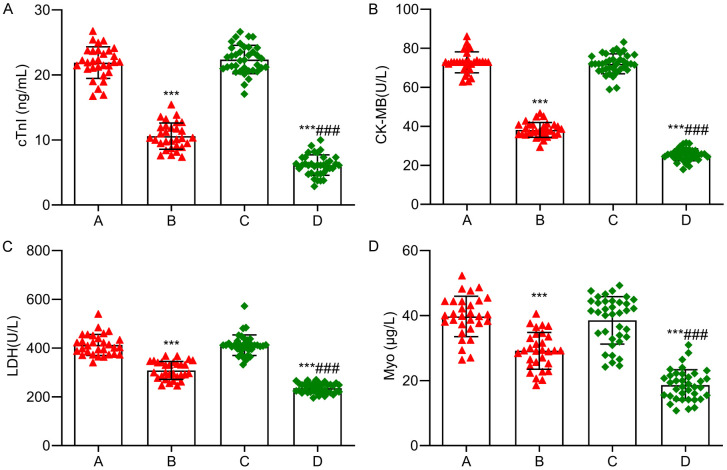
No significant differences were observed in myocardial injury indicators between the two groups before treatment (P > 0.05). After treatment, the cTnI, CK-MB, LDH and Myo levels significantly decreased in both groups, with lower levels observed in the research group than those in the control group (P < 0.05). See Figure 1.
Figure 1.
Comparison of myocardial injury indexes between the two groups. A: cTnI; B: CK-MB; C: LDH; D: Myo. Note: Compared with before treatment, ***P < 0.001; Compared with the control group, ###P < 0.001. A: Control group before treatment; B: Control group after treatment; C: Research group before treatment; D: Research group after treatment. cTnI: Troponin I; CK-MB: Creatine kinase myocardial isoenzyme; LDH: Lactate dehydrogenase isoenzyme; Myo: myoglobin.
Inflammatory cytokine levels
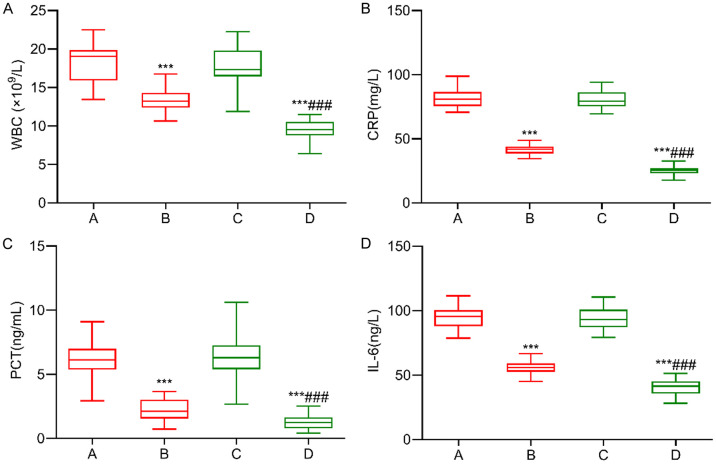
There were no significant differences in inflammatory cytokine indicators between the two groups before treatment (P > 0.05). After treatment, the WBC, CRP, PCT, and IL-6 levels significantly decreased in both groups, with lower levels observed in the research group than those in the control group (P < 0.05). See Figure 2.
Figure 2.
Comparison of levels of inflammatory factors between the two groups. A: WBC; B: CRP; C: PCT; D: IL-6. Note: Compared with before treatment, ***P < 0.001; Compared with the control group, ###P < 0.001. A: Control group before treatment; B: Control group after treatment; C: Research group before treatment; D: Research group after treatment. WBC: White blood cell count; CRP: C-reactive protein; PCT: Procalcitonin; IL-6: Interleukin-6.
Comparison of serum NSE and CHE levels
After treatment, the levels of serum NSE were significantly decreased, while the levels of serum CHE were significantly increased (all P < 0.05) in both groups. On the 1st and 2nd day after treatment, the serum NSE levels in the observation group were significantly lower than those in the control group, while the serum CHE levels were significantly higher than those in the control group (all P < 0.05). However, there was no significant difference in serum NSE and CHE levels between the two groups on day 3 after treatment. See Table 4.
Table 4.
Comparison of serum NSE and CHE levels between the two groups (x̅ ± SD)
| Groups | NSE (μg/L) | CHE (×1000 UL) | |
|---|---|---|---|
| Control group (n = 30) | Before treatment | 57.83±7.22 | 1.72±1.33 |
| 1 day after treatment | 43.35±6.74* | 2.89±1.55* | |
| 2 days after treatment | 29.24±6.17* | 4.17±1.31* | |
| 3 days after treatment | 15.22±5.59* | 5.58±1.46* | |
| Research group (n = 37) | Before treatment | 58.22±8.17 | 1.81±1.14 |
| 1 day after treatment | 32.93±7.17*,# | 3.91±1.22*,# | |
| 2 days after treatment | 15.61±6.63*,# | 4.85±1.37*,# | |
| 3 days after treatment | 14.73±4.55* | 5.61±1.29* |
Note: NSE: neuron-specific enolase; CHE: Cholinesterase.
Compared with before treatment;
P < 0.05.
Compared with the control group at the same time;
P < 0.05.
Liver and kidney function indicators
After treatment, all liver and kidney function indicators in the control group significantly increased and were all higher than those in the research group (P < 0.05). See Table 4.
Safety
The rebound rate was 8.11% in the research group, which was markedly lower than 30.00% in the control group (P < 0.05). No statistically significant differences were found in the incidence of complications between the two groups (P > 0.05). See Table 5.
Table 5.
Comparison of liver and kidney function indexes between the two groups (x̅ ± SD)
| Groups | BUN (mmol/L) | Scr (μmol/L) | Urinary protein (mg) | |||
|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
|
| ||||||
| Control group (n = 30) | 4.15±1.39 | 6.88±1.83* | 88.25±6.95 | 108.95±14.34* | 78.33±6.37 | 98.47±9.24* |
| Research group (n = 37) | 4.04±1.51 | 3.78±1.46 | 88.73±7.19 | 90.01±12.05 | 77.92±6.82 | 78.91±7.02 |
| t | 0.307 | 7.715 | 0.276 | 5.875 | 0.252 | 9.846 |
| P | 0.760 | < 0.001 | 0.784 | < 0.001 | 0.802 | < 0.001 |
|
| ||||||
| Groups | AST (U/L) | TBIL (μmol/L) | ALT (U/L) | |||
|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
|
| ||||||
| Control group (n = 30) | 37.81±3.27 | 50.28±5.37* | 13.58±2.54 | 24.05±3.05* | 28.95±2.84 | 45.89±5.08* |
| Research group (n = 37) | 38.12±3.41 | 37.65±3.15 | 13.79±2.39 | 14.21±2.73 | 29.31±3.02 | 28.66±2.98* |
| t | 0.377 | 11.997 | 0.348 | 13.921 | 0.498 | 17.301 |
| P | 0.708 | < 0.001 | 0.729 | < 0.001 | 0.620 | < 0.001 |
Note: BUN: Urea nitrogen; Scr: Serum creatinine; AST: Aspartate aminotransferase; TBIL: Total bilirubin; ALT: Alanine aminotransferase.
Compared with the group before treatment;
P < 0.05.
Mortality rate
In the study, the control group had 3 deaths and the research group had only 1 death, with mortality rates of 10.00% and 2.70%, respectively. There was no significant difference in the mortality rates between the two groups (χ2 = 0.540, P = 0.462). See Table 6.
Table 6.
Comparison of the incidence of complications between the two groups n (%)
| Groups | Rebound | Intermediate syndrome | Multiple organ dysfunction | Pulmonary infection | Gastrointestinal hemorrhage |
|---|---|---|---|---|---|
| Control group (n = 30) | 9 (30.00) | 2 (6.67) | 4 (13.33) | 3 (10.00) | 2 (6.67) |
| Research group (n = 37) | 3 (8.11) | 1 (2.70) | 2 (5.41) | 1 (2.70) | 1 (2.70) |
| χ2 | 5.400 | 0.035 | 0.490 | 0.540 | 0.035 |
| P | 0.020 | 0.852 | 0.484 | 0.462 | 0.852 |
Discussion
Organophosphate poisoning is a common but serious emergent condition with a high mortality rate. Currently, clinical treatments for organophosphate poisoning mainly involves gastric lavage, catharsis, and the use of antidotes. Although these treatments have presented certain clinical efficacy, organophosphates are still be rapidly absorbed into the body and accumulate in the kidney and liver, making the toxins difficult to be removed from the blood [9,10]. Over recent years, blood purification therapies have been gradually introduced for the treatment of different types of poisoning, showing promising results in reducing mortality rates [11,12]. In this study, it was demonstrated that the clinical efficacy of the treatment plan employed in the research group was 94.59%, significantly higher compared to 73.33% in the control group. Additionally, patients in the research group administered lower doses of long-acting nifedipine than those in the control group. They also had long-acting nifedipine for a shorter period, needed shorter time to regain consciousness, realized cholinesterase activity recovery faster, and relied on shorter duration of mechanical ventilation and hospitalization compared to those in the control group. Therefore, sequential blood purification therapy has shown significant treatment effects with the ability to promote a fast recovery for patients from organophosphate poisoning. The underlying mechanism is that HP, through perfusion with adsorbents such as activated charcoal or resin, removes metabolic products and exogenous toxins from the body, thereby alleviating poisoning symptoms and saving peoples’ lives [13]. However, the clearance of urea- and water-soluble toxins, as well as other small molecular substances in the body by HP is not complete [14]. Thus comes CVVH. It simulates the reabsorption and filtration functions of renal tubules and glomeruli, utilizes adsorption and convection principles to remove excessive solutes, water, and inflammatory mediators from the body, while regulating acid-base and electrolyte balance to maintain hemodynamic stability [15,16].
Organophosphate poisoning inhibits the activity of cholinesterase (CHE), which leads to a decrease in serum CHE levels. Therefore, the serum CHE level can be used as an important index for the diagnosis and severity classification of acute organophosphorus pesticide poisoning (AOPP). After treatment, the serum CHE levels in both groups were significantly increased (P < 0.05), and on the 1st and 2nd day after treatment, the serum CHE levels in the observation group were significantly higher than those in the control group (P < 0.05). These results suggested that blood purification therapy can rapidly restore serum CHE activity and promote the recovery of physiological function in patients with AOPP. HP can adsorb free organophosphorus in blood, shorten the recovery time of CHE, improve the recovery rate, shorten the course of disease, reduce the mortality and improve the curative effect. Serum CHE activity correlates with the severity of organophosphate poisoning and is a specific marker for the diagnosis of AOPP. Organophosphorus can lead to the inactivation of CHE by phosphorylation, the accumulation of acetylcholine, resulting in nervous system excitement, causing systemic symptoms and nervous system syndrome. The rapid recovery of CHE activity is helpful to the recovery of patients.
Neuron-specific enolase (NSE) is an enolase isoenzyme involved in glycolysis, which is a specific and sensitive index of neuronal injury. Serum NSE level can reflect brain injury and central nervous system injury, and it has important significance for judging the severity of disease, evaluating and guiding prognosis. After neuronal injury in AOPP patients, NSE escapes from the neuronal cytoplasm and enters the blood through the blood-brain barrier, resulting in an increase in serum NSE levels. Therefore, serum NSE level can be used to judge the condition and evaluate the prognosis of patients with AOPP. In this study, the serum NSE level of the two groups was significantly decreased after treatment (all P < 0.05), and the serum NSE level of the observation group was significantly lower than that of the control group at each time point (all P < 0.05). These results suggest that sequential blood purification therapy can slow down nerve damage in AOPP patients and promote their recovery.
Clinical observations have shown that organophosphate poisoning can lead to damage in various organs, especially in patients with severe poisoning, resulting in myocardial injury [17]. When organophosphates enter the body, they combine with CHE to form phosphorylated cholinesterase, thereby sabotaging CHE activity. This sabotage leads to the accumulation of acetylcholine and intense stimulation of myocardial cells, resulting in the release of large amounts of myocardial enzymes. The released enzymes cause abnormalities in calcium ion channels, reduced myocardial contractility, atrioventricular conduction block, and ultimately, myocardial cell degeneration and necrosis, resulting in myocardial injury [18]. Furthermore, toxins from organophosphate poisoning stimulate the body to secrete large amounts of inflammatory mediators, triggering a cascade-like inflammatory response, leading to inflammatory damage to myocardial cells. cTnI, CK-MB, and LDH are all typical markers of myocardial injury, the levels of which would markedly increase when myocardial injury occurs. Awasthi P et al. found that the treatment and prognosis of organophosphate poisoning were closely related to changes in inflammatory cytokine levels [19]. Acute poisoning triggers a strong stress response in the body, leading to the release of factors from the hypothalamus, increased levels of β-endorphins in the anterior pituitary, and the generation of a large number of free radicals, which damage cells and endothelial function. This activates the mononuclear macrophage system, leading to the release of large amounts of inflammatory cytokines, causing a systemic inflammatory response and multiple organ dysfunction. The results of this study showed that after treatment, the levels of cTnI, CK-MB, LDH, WBC, CRP, PCT, and IL-6 all significantly decreased in both groups, with lower levels observed in the research group than those in the control group. Zhang Qing et al. found that the average CHE activity recovery time, average coma time, the dosage of long-acting tacrine administered per patient, and average hospitalization duration in the sequential the blood purification group were significantly shorter than those in the hemoperfusion group [20]. In addition, the cTnI of patients on days 2, 3, 5 and 7 of hospitalization and the left ventricular ejection fraction within 24 hours after blood purification therapy were significantly lower than those following HP treatment. These results were similar to those of this study. However, the underlying mechanisms of the treatment were not explored in their study. The results of this study indicated that sequential blood purification therapy effectively mitigated myocardial injury, decreased the expression of inflammatory cytokines, and inhibited inflammatory damage. The reason might be related to the effective removal of toxins and inflammatory mediators by sequential blood purification therapy from the body [21].
In addition to damaging the heart, organophosphate poisoning also affects liver and kidney functions. This is primarily because most organophosphates are excreted through the kidneys, and the ischemia-hypoxia caused by organophosphate poisoning can lead to changes in renal hemodynamics and damage renal tubules. Furthermore, organophosphates can damage red blood cells, which are metabolized and eliminated by the liver, potentially causing liver cell damage [22,23]. In this study, the results showed that after treatment, the levels of BUN, Scr, urine protein, AST, TBIL, and ALT in the control group all significantly increased and were all higher than those in the research group, which indicated that sequential blood purification therapy effectively protected the liver and kidney function from the sabotage of organophosphate poisoning. Rebound is one of the common complications in patients with organophosphate poisoning, referring to the sudden recurrence of poisoning symptoms after significant improvement of cholinergic crisis symptoms following treatment. This occurs without obvious warning signs and has a rapid onset and progression, even after the condition appears to be controlled. Research by Kaiser J et al. demonstrated that rebound was one of the main reasons for treatment failure in patients with organophosphate poisoning and was closely related to poor prognosis [24]. In this study, it was indicated that the incidence of rebound was 8.11% in the research group, which was significantly lower than 30.00% in the control group. The comparison of mortality rates between the two groups (10.00% vs. 2.70%) showed no significant difference. These results suggested that sequential blood purification therapy was safe in treating patients suffering from organophosphate poisoning, with advantages to effectively reduce complications, thereby improving the prognosis of patients. However, this is a retrospective study with a relatively small number of cases, thus the severity of myocardial injury in patients wasn’t categorized. What was focused on as outcome measures in the study were improvements on myocardial injury in patients before and after treatment. In the subsequent research, we would categorize the severity of myocardial injury in patients into various groups and treat each group differently.
Patients enrolled in this study all received treatment within 8 hours after hospitalization. Thanks to in-time treatment, the mortality of patients is relatively low (only 3 cases). However, due to the small case number, we were unable to perform COX multivariate regression analysis for comparing factors that influence patients’ prognoses, which is a limitation of this study. What we found in the study was that influencing factors like HP, thorough gastric lavage, prompt catharsis, and reasonable and sufficient doses of reactivator and long-acting tacrine were critical to the success of preventing patients from dying. In addition, Tian Fei et al. found that the dosage of toxins, the time from administering toxins to gastric lavage, the employment of CRP, ChE, and APACHE II were all independent factors affecting the prognosis of patients with acute severe organophosphorus poisoning [25]. In future research, we will expand the number of cases, conduct prospective studies, so as to further explore the independent factors affecting the prognosis of patients with organophosphorus poisoning.
In summary, sequential blood purification therapy has significant clinical efficacy in alleviating myocardial damage and reducing the expression of inflammatory cytokines in patients with organophosphate poisoning. This thereby helps protect liver and kidney function, ensuring optimal safety for patients.
Disclosure of conflict of interest
None.
References
- 1.John H, Thiermann H. Poisoning by organophosphorus nerve agents and pesticides: an overview of the principle strategies and current progress of mass spectrometry-based procedures for verification. J Mass Spectrom Adv Clin Lab. 2021;19:20–31. doi: 10.1016/j.jmsacl.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramadori GP. Organophosphorus poisoning: acute respiratory distress syndrome (ARDS) and cardiac failure as cause of death in hospitalized patients. Int J Mol Sci. 2023;24:6658. doi: 10.3390/ijms24076658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey S, Shrestha N. Organophosphorus poisoning among acute poisoning cases presenting to the emergency department of a secondary care centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2022;60:435–438. doi: 10.31729/jnma.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhusal S, Bhandari R, Dahal S, Niroula A, Basnet K, Chaudhary A, Pant S. Organophosphorus poisoning among patients admitted to the intensive care unit of the department of internal medicine in a tertiary care centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2022;60:766–769. doi: 10.31729/jnma.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faiz Norrrahim MN, Idayu Abdul Razak MA, Ahmad Shah NA, Kasim H, Wan Yusoff WY, Halim NA, Mohd Nor SA, Jamal SH, Ong KK, Zin Wan Yunus WM, Knight VF, Mohd Kasim NA. Recent developments on oximes to improve the blood brain barrier penetration for the treatment of organophosphorus poisoning: a review. RSC Adv. 2020;10:4465–4489. doi: 10.1039/c9ra08599h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Zhang W, Zhao S, Tian X, Fu G, Wang B. Hemoperfusion in combination with hemofiltration for acute severe organophosphorus pesticide poisoning: a systematic review and meta-analysis. J Res Med Sci. 2022;27:33. doi: 10.4103/jrms.JRMS_822_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang SZ, Ma BE, Liu C, Wang R. Clinical efficacy of intravenous infusion of atropine with micropump in combination with hemoperfusion on organophosphorus poisoning. Saudi J Biol Sci. 2019;26:2018–2021. doi: 10.1016/j.sjbs.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emergency Medical Branch of Chinese Medical Doctor Association. Clinical guideline for the diagnosis and treatment of acute organophosphorus pesticide poisoning (2016) Chin J Crit Care Med. 2017;36:1057–1065. [Google Scholar]
- 9.Allard JL, Shields KA, Munro TP, Lua LHL. Strategies for developing a recombinant butyrylcholinesterase medical countermeasure for Organophosphorus poisoning. Chem Biol Interact. 2022;363:109996. doi: 10.1016/j.cbi.2022.109996. [DOI] [PubMed] [Google Scholar]
- 10.Patil A, Kumar S, Inamdar A, Acharya S, Wanjari A, Bawankule S, Agrawal S, Sontakke T. Impact of serum amylase level in the outcome of acute organophosphorus poisoning: 2-year cross-sectional study at rural teaching hospital. J Lab Physicians. 2021;14:1–5. doi: 10.1055/s-0041-1734015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam A, Chowdhury D, Palit PK, Sohel M, Mozibullah M, Islam MJ, Al Mamun A, Datta J, Dev A, Nath PK, Chowdhury MFF, Nath SK, Mujib ASM. Serum creatinine phosphokinase: a potential prognostic marker in assessing clinical severity with organophosphorus poisoning. J Clin Lab Anal. 2023;37:e24980. doi: 10.1002/jcla.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mousavi SR, Moshiri M, Darchini-Maragheh E, Ghasempouri SK, Dadpour B, Sardar Antighechi F, Balali-Mood M. Therapeutic effects of HESA-A (a herbal-marine compound) in acute organophosphorus pesticide poisoning. Avicenna J Phytomed. 2020;10:235–242. [PMC free article] [PubMed] [Google Scholar]
- 13.Ke J, Wei Y, Chen B. Application of hemoperfusion in the treatment of acute poisoning. Blood Purif. 2024;53:49–60. doi: 10.1159/000532050. [DOI] [PubMed] [Google Scholar]
- 14.Kocoğlu Barlas Ü, Akçay N, Sofuoğlu Aİ, Şevketoğlu E. Charcoal hemoperfusion in calcium channel antagonist poisoning. Turk Arch Pediatr. 2023;58:112–114. doi: 10.5152/TurkArchPediatr.2022.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Z, Wang P, Fu Q, Song Q, Wang W, Liu A, Zhang P. Efficacy and safety of plasma exchange combined with hemoperfusion in the treatment of organophosphorus poisoning: a meta-analysis. Blood Purif. 2023;52:578–590. doi: 10.1159/000530117. [DOI] [PubMed] [Google Scholar]
- 16.Chen AB, Li F, Di EM, Zhang X, Zhao QY, Wen J. Influence of strengthened hemoperfusion combined with continuous venovenous hemofiltration on prognosis of patients with acute paraquat poisoning: SHP + CVVH improve prognosis of acute PQ patients. BMC Pharmacol Toxicol. 2020;21:49. doi: 10.1186/s40360-020-00428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar HM, Pannu AK, Kumar S, Sharma N, Bhalla A. Magnesium sulfate in organophosphorus compound poisoning: a prospective open-label clinician-initiated intervention trial with historical controls. Int J Crit Illn Inj Sci. 2022;12:33–37. doi: 10.4103/ijciis.ijciis_67_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu G, Li Y, Jian T, Shi L, Cui S, Zhao L, Jian X, Kan B. Clinical analysis of acute organophosphorus pesticide poisoning and successful cardiopulmonary resuscitation: a case series. Front Public Health. 2022;10:866376. doi: 10.3389/fpubh.2022.866376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi P, Jindal A, Sharma Y, Williams V, Ravikumar N, Nallasamy K, Angurana SK. Continuous venovenous hemofiltration as a rescue therapy for severe acetaminophen toxicity in a toddler. J Pediatr Intensive Care. 2021;10:159–161. doi: 10.1055/s-0040-1712158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Li C. Comparison of the efficacy of two blood purification methods in treating myocardial injury in patients with severe acute organophosphorus poisoning. Chin J Emerg Med. 2016;25:4. [Google Scholar]
- 21.Lee KH, Ou SM, Tsai MT, Tseng WC, Yang CY, Lin YP, Tarng DC. AN69 filter membranes with high ultrafiltration rates during continuous venovenous hemofiltration reduce mortality in patients with sepsis-induced multiorgan dysfunction syndrome. Membranes (Basel) 2021;11:837. doi: 10.3390/membranes11110837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaviya NB, Parikh R, Pancholi K, Belim OB. Assessment of the peradeniya organophosphorus poisoning scale as a severity and prognostic marker in patients with acute organophosphorus poisoning presenting to an emergency medicine department. Cureus. 2023;15:e40277. doi: 10.7759/cureus.40277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia JD, Wang H, Hua LW, Xu M, Zheng X, Zhang K. Comparative analysis of organophosphorus versus carbamate pesticide poisoning: a case study. Arh Hig Rada Toksikol. 2024;75:81–84. doi: 10.2478/aiht-2024-75-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser J, Gertzen CGW, Bernauer T, Höfner G, Niessen KV, Seeger T, Paintner FF, Wanner KT, Worek F, Thiermann H, Gohlke H. A novel binding site in the nicotinic acetylcholine receptor for MB327 can explain its allosteric modulation relevant for organophosphorus-poisoning treatment. Toxicol Lett. 2023;373:160–171. doi: 10.1016/j.toxlet.2022.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Tian F, Xi ZY, Li CX, Wan FZ, Gui SM, Peng ZK, Liu M, Zhang R, Qv XG, Zhang CH. Analysis of clinical efficacy and prognostic factors of gastric lavage in patients with acute severe organophosphorus poisoning. Bachu Med J. 2023;6:35–41. [Google Scholar]