Abstract
Objective: Frailty status is closely related to cerebral infarction, but there is a lack of objective biomarkers to determine frailty status in cerebral infarction patients. This study explores frailty status and frailty-related serum markers in patients with acute cerebral infarction and determines their diagnostic value for frailty. Methods: A total of 146 patients with acute cerebral infarction admitted to Hangzhou Third people’s Hospital from January 2021 to December 2023 were enrolled prospectively. The Edmonton scale was used to evaluate the patients in the frailty and non-frailty groups. The clinical frailty scale (CFS) was used to divide frailty patients into mild, moderate, and severe frailty groups, comparing clinical data and levels of serum markers among different groups, and analyzing the risk factors for frailty in cerebral infarction. Results: Among the 146 patients, 70 cases (47.9%) were in the frailty group, and 76 cases (52.1%) in the non-frailty group. Compared with patients in the non-frailty group, patients in the frailty group had significantly lower levels of hemoglobin, triglycerides, low-density lipoprotein, and albumin (P<0.05 or 0.01), while levels of C-reactive protein (CRP), D-dimer, and homocysteine (Hcy) were significantly increased (P<0.05 or 0.01). Logistic regression analysis found that the levels of hemoglobin and Hcy were independent risk factors for frailty in acute cerebral infarction patients, with the ROC curve areas of 0.707 and 0.751, respectively. The ROC curve area for predicting frailty by combining hemoglobin and Hcy levels was 0.799. Conclusion: The incidence of frailty in patients with acute cerebral infarction is high, and serum markers of hemoglobin and Hcy have certain value in determining frailty in patients with acute cerebral infarction.
Keywords: Cerebral infarction, frailty, serum, markers
Introduction
Frailty is a clinical syndrome related to aging, characterized by increases in the body’s fragility and decreases in its ability to maintain homeostasis. The core characteristic is the decrease in the functional reserves of multiple physiological systems, which is a preliminary manifestation of many chronic diseases. This can increase the incidence of clinical adverse outcomes such as disability in patients, and contributes to various acute events or chronic diseases leading to frailty [1]. Research has shown that frail patients are prone to stroke, and frailty is also an independent risk factor affecting the recovery and poor prognosis of stroke patients [2-4]. At the same time, stroke patients can also have a frailty status which can accelerate the decline of their body functions, seriously affecting their quality of life [5,6]. Therefore, early assessment of the frailty status of acute stroke patients will help clinicians to intervene and ameliorate the disability rate of patients.
So far, there has been no gold standard for evaluating frailty, and the main method for evaluating frailty is through scales. There are still some shortcomings in evaluating frailty status solely using scales, mainly due to the subjectivity and learning nature of scales. Therefore, clinicians need some objective clinical indicators to screen and evaluate frailty. At present, some scholars use biomarkers to identify frailty in elderly people. For example, Ju SY [7] found that low levels of 25(OH)D in the blood could be a potential target for screening or intervention in frailty patients. Zuliani G et al. [8] believed that serum albumin and high-density lipoprotein cholesterol levels had certain value in assessing the frailty of older nursing-home residents. Some studies found that levels of inflammatory factors could be used as prognostic factors for adverse outcomes; such as C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-1 (IL-1), leukocytes, lymphocytes, and tumor necrosis factor α (TNF α), which were significantly increased in stroke patients complicated with hypertension, diabetes, atrial fibrillation and other chronic diseases, thus affecting the prognosis of stroke patients [9,10]. Gnanenthiran et al. found that a decrease in hemoglobin levels was associated with the occurrence of frailty syndrome and an increase in cardiovascular events [11,12]. However, there were different opinions on selecting specific biomarkers [13-17], and no single biomarker could predict or diagnose frailty [18]. Despite the close relationship between frailty and cerebral infarction, there is still a lack of serum markers for evaluating the frailty status of cerebral infarction in clinical practice.
Therefore, this study intends to prospectively select elderly patients with acute cerebral infarction and screen for multiple serum markers that reflect the functional status of the body’s multiple systems. The aim is to discover the frailty-related serum markers in patients with acute cerebral infarction and determine their diagnostic value for frailty, in a hope to help understand the occurrence and development of the relationship between frailty and acute cerebral infarction and provide more objective basis for the evaluation of frailty.
Materials and methods
Materials
Prospective selection of acute cerebral infarction patients was made for patients admitted to the Hangzhou Third People’s Hospital from January 2021 to December 2023. We calculated the sample size using GPower3.1 software [19]. By selecting effect size =0.3, α=0.05 (two-tails), and Power =0.9, the required sample size was calculated to be 109 cases. Assuming a dropout rate of 20%, the total sample size was at least 131 cases.
Inclusion criteria: Age ≥18 years; Within 48 hours of stroke onset; Based on the patient’s medical history, clinical symptoms, signs, and imaging examination, the diagnosis was confirmed to be acute cerebral infarction with the first onset. The diagnosis of cerebral infarction was based on the diagnostic criteria of the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke [20], and confirmed by cranial magnetic resonance imaging (MRI). The patient had clear consciousness and stable vital signs and could cooperate with the assessment of frailty.
Exclusion criteria: (1) Patients with transient ischemic attacks; (2) Unable to determine whether the patient had frailty or not when they were admitted; (3) Severe kidney and liver dysfunction; (4) Severe anxiety, depression and mental disorders; (5) Patients or family members did not agree to join the study.
This research protocol was approved by the ethics committee of Hangzhou Third people’s Hospital (Number: 2020KA015), and all patients signed informed consent forms.
Methods
Clinical data collection
The baseline information of the enrolled patients, including their age, gender, and risk factors related to cerebral infarction (coronary heart disease, atrial fibrillation, diabetes, hypertension, hyperlipidemia, etc.) was collected. Clinical data included the Instrumental Activities of Daily Living (IADL) score, the National Institutes of Health Stroke Scale (NIHSS) score, and the Modified Rankin Scale (mRS) score at admission, all collected by qualified neurologists.
Detection of biomarkers in peripheral blood
All enrolled patients had fasting venous blood drawn in the morning of the second day of admission. We extracted 2 ml of fasting venous blood from each enrolled patient, mixed it evenly in an Ethylenediaminetetraacetic acid (EDTA) anticoagulant tube, and used a fully automated blood cell analyzer (Sysmex XN-1000, China’s Mindray) to measure the white blood cell count and hemoglobin content of each blood sample. We extracted 2 ml of venous blood from each enrolled patient into a tube anticoagulated with sodium citrate and tested the D-dimer levels with turbidimetric inhibition immunoassay using an automated coagulation analyzer (Sysmex CS-5100). We extracted 10 ml of fasting venous blood and centrifuged it under a sterile condition at 3000 r/min for 10 minutes. We took serum samples and used a fully automated biochemical analyzer (Hitachi 7600-220, Japan) to detect levels of CRP, triglycerides, total cholesterol, low-density lipoprotein, high-density lipoprotein, albumin, globulin, total protein, bilirubin, total bile acid, urea nitrogen, creatinine, blood glucose, blood uric acid, and homocysteine (Hcy). Using high-performance liquid chromatography, the content of glycated hemoglobin (HBA1C) was detected using a fully automated glycated hemoglobin biochemical analyzer (ARKRAYHA-8180, Japan). The levels of Total Triiodothyronine (TT3), Total Thyroxine (TT4), Thyroid Stimulating Hormone (TSH), and B-type natriuretic peptide (BNP) were detected with a chemiluminescence method using a fully automated chemiluminescence immunoassay analyzer (Siemens Atellica IM 1600, USA). All tests were strictly carried out in accordance with the instructions of the test kits. The test kits were purchased from Sysmex in Japan, Mindray Biotechnology Co. Ltd. in China, Beijing Haomai Biotechnology Co. Ltd. in China, Wako Pure Chemical in Japan, Sekisui Medical Co. Ltd. in Japan and Siemens Healthcare Diagnostic Inc. in the United States.
Assessment of frailty status
The patient’s frailty status was evaluated within 24 hours after admission using the Edmonton Scale [21], which included 9 items: cognition, basic health status, independence, social support, medication use, nutrition, emotions, incontinence, and self-reported symptoms. Each item was scored 0-2 points, with a total score of ≥7 points indicating frailty. The Clinical frailty Scale (CFS) [22] was used to assess the severity of frailty in patients, and frailty patients were divided into mild, moderate, and severe frailty groups based on the scores.
Statistics analysis
SPSS 17.0 software was used for data processing and statistical analysis. The Shapiro-Wilk test was used to distinguish continuous variables between normal and abnormal distributions. The data that conformed to a normal distribution were expressed as mean ± standard deviation. Independent Samples t-test was used for comparison between frailty and non-frailty groups, and one-way analysis of variance (ANOVA) was used for comparison between mild, moderate, and severe frailty groups, using the Bonferroni approach to correct multiple comparisons. Abnormally distributed data was described using median (interquartile range) {M (Q1-Q3)}. The Mann-Whitney U test was used for comparison between two groups, and the Kruskal-Wallis H test was used for comparison between multiple groups. Count data was represented by frequency, and inter-group comparisons were performed using chi square or Fisher’s tests. We selected serum markers with statistical differences in univariate analysis for binary logistic regression analysis, and adjusted them for clinical factors (gender, age, severity of infarction, etc.) which could possibly affect their values [23-25]. We created receiver operating characteristic (ROC) curves and area under ROC curves for serum indicators related to frailty. A difference of P<0.05 was taken as statistically significant.
Results
Comparison of general clinical data between the frailty and non-frailty groups
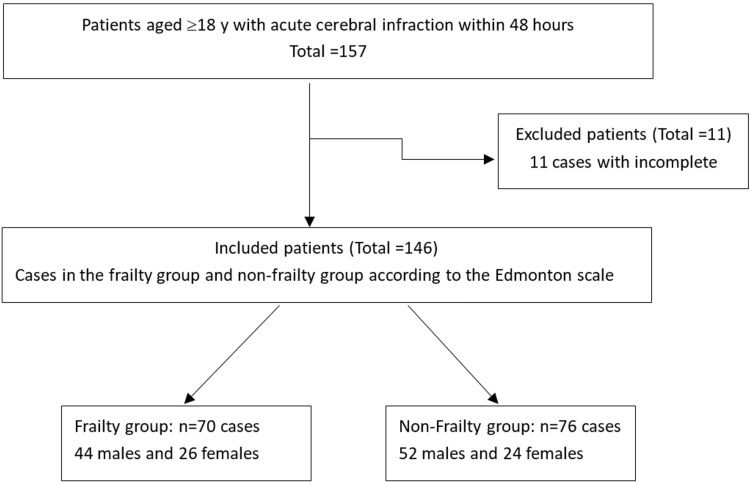
During the study period, 157 cases were included, and 11 cases with incomplete data were excluded. The final number of enrolled cases was 146 (See Figure 1). Among the 146 patients, 96 were males and 50 females, aged 35-91 years (68.7±14.0 years). According to the Edmonton scale score, there were 70 cases (47.9%) in the frailty group, including 44 males and 26 females, aged 55-91 years (76.8±9.8), and 76 cases (52.1%) in the non-frailty group, including 52 males and 24 females, aged 35-84 years (61.2±13.2).
Figure 1.
Study flowchart.
There was no statistically significant difference in gender, concomitant coronary heart disease, atrial fibrillation, and hyperlipidemia between the two groups of patients (P>0.05). Age, concomitant diabetes and hypertension, NHISS score, mRS score, Edmonton score of the frailty group were significantly higher than those of the non-frailty group, and IADL score was significantly lower than that of the non-frailty group (P<0.05, P<0.01) (Table 1).
Table 1.
Comparison of baseline characteristics between the two groups of patients
| Frailty group (n=70) | Non-frailty group (n=76) | t/x2 | P | |
|---|---|---|---|---|
| Age (years) | 76.8±9.8 | 61.2±13.2 | 8.041 | 0.001 |
| Gender (cases) | ||||
| Male | 44 | 52 | 0.502 | 0.479 |
| Female | 26 | 24 | ||
| Concomitant diseases (cases) | ||||
| Coronary heart disease | 10 | 6 | 1.525 | 0.217 |
| Atrial fibrillation | 8 | 11 | 0.299 | 0.585 |
| Diabetes | 16 | 7 | 5.113 | 0.024 |
| Hypertension | 24 | 13 | 5.685 | 0.017 |
| Hyperlipidemia | 7 | 12 | 1.079 | 0.299 |
| NIHSS score | 5.9±4.0 | 4.5±2.6 | 2.443 | 0.016 |
| mRS score | 1.9±1.0 | 1.4±0.8 | 3.335 | 0.001 |
| IADL score | 17.7±4.4 | 21.2±3.4 | 5.371 | 0.001 |
| Edmonton score | 10.3±3.2 | 2.4±1.4 | 19.500 | 0.001 |
Abbreviations: NIHSS, the National Institutes of Health Stroke scale; mRS, Modified Rankin Scale; IADL, Instrumental Activities of Daily Living.
Comparison of levels of serum markers between the frailty and non-frailty groups
Compared with patients in the non-frailty group, patients in the frailty group had significantly lower levels of hemoglobin, triglycerides, low-density lipoprotein, and albumin, while levels of CRP, D-dimer, and Hcy were significantly increased. The differences were statistically significant (P<0.05, P<0.01) (Table 2).
Table 2.
Comparison of levels of serum markers between the frailty and non-frailty groups with acute cerebral infarction
| Frailty group (n=70) | Non-frailty group (n=76) | t | P | |
|---|---|---|---|---|
| White blood cell count (×109/L) | 7.0±2.0 | 7.1±2.1 | 0.201 | 0.841 |
| Hemoglobin (g/L) | 124.9±19.0 | 136.6±19.9 | 3.643 | 0.001 |
| CRP (mg/L) | 4.1 (0.5-13.1) | 0.95 (0.5-4.9) | 3.379 | 0.001 |
| Triglycerides (mmol/L) | 1.2±0.6 | 1.5±1.0 | 2.692 | 0.008 |
| Total cholesterol (mmol/L) | 3.8±0.9 | 4.1±1.4 | 1.766 | 0.080 |
| Low-density lipoprotein (mmol/L) | 2.2±0.8 | 2.6±1.3 | 2.360 | 0.019 |
| High-density lipoprotein (mmol/L) | 1.2±0.3 | 1.1±0.2 | 1.021 | 0.472 |
| D-dimer (mg/L) | 0.45 (0.28-0.96) | 0.34 (0.12-0.47) | 2.823 | 0.005 |
| Albumin (g/L) | 33.2±3.4 | 35.2±3.3 | 3.473 | 0.001 |
| Globulin (g/L) | 27.8±4.7 | 26.4±3.3 | 1.418 | 0.601 |
| Total protein (g/L) | 60.8±6.3 | 60.6±4.8 | 0.221 | 0.825 |
| Bilirubin (μmol/L) | 12.8±7.3 | 13.9±7.2 | 0.884 | 0.378 |
| Total bile acid (μmol/L) | 7.3±5.3 | 5.2±3.7 | 1.940 | 0.055 |
| Urea nitrogen (mmol/L) | 4.9±1.9 | 5.0±1.9 | 0.240 | 0.811 |
| Creatinine (μmol/L) | 69.5±23.2 | 77.2±24.5 | 1.954 | 0.063 |
| Glucose (mmol/L) | 7.1±2.1 | 6.7±2.6 | 1.449 | 0.149 |
| HBA1C (%) | 7.2±2.3 | 6.7±1.8 | 1.126 | 0.262 |
| BNP (pg/ml) | 243.0 (125.0-871.8) | 296.3 (102.0-606.0) | 0.802 | 0.425 |
| Blood uric acid (μmol/L) | 300.6±102.1 | 303.8±79.7 | 0.207 | 0.836 |
| Hcy (mmol/L) | 21.7±11.4 | 14.9±7.9 | 4.073 | 0.001 |
| TT3 (nmol/L) | 1.5±0.3 | 1.5±0.3 | 0.168 | 0.867 |
| TT4 (nmol/L) | 107.4±28.8 | 102.6±24.4 | 1.162 | 0.333 |
| TSH (mIu/L) | 1.6 (0.9-3.3) | 1.8 (1.2-2.9) | 0.799 | 0.425 |
Abbreviations: CRP, C-reactive protein; HBA1C, glycated hemoglobin; BNP, B-type natriuretic peptide; Hcy, homocysteine; TT3, Total Triiodothyronine; TT4, Thyroxine; TSH, Thyroid Stimulating Hormone.
Comparison of levels of serum markers between different degrees of frailty groups
According to the CFS score, among the 70 frailty patients, there were 26 with mild frailty, 20 with moderate frailty, and 24 with severe frailty. Compared with the mild and moderate frailty groups, the severe frailty group showed a significant decrease in hemoglobin levels, and a significant increase in CRP and Hcy levels (P<0.05 and P<0.01, respectively). Compared with the mild frailty group, the levels of CRP and Hcy were significantly increased in the moderate frailty group (P<0.05). There was no statistically significant difference in the levels of triglycerides, total cholesterol, low-density lipoprotein, high-density lipoprotein, D-dimer, albumin, globulin, and total protein among the groups, as shown in Table 3.
Table 3.
Comparison of the levels of serum markers between different degrees of frailty groups
| Mild frailty group (n=26) | Moderate frailty group (n=20) | Severe frailty group (n=24) | F | P | |
|---|---|---|---|---|---|
| White blood cell count (×109/L) | 7.1±2.1 | 6.6±1.3 | 7.4±2.4 | 0.790 | 0.458 |
| Hemoglobin (g/L) | 128.9±26.8 | 129.9±4.5 | 116.3±13.0**,## | 4.103 | 0.021 |
| CRP (mg/L) | 1.7 (0.5-5.4) | 4.5 (0.5-13.1)* | 8.6 (3.3-53.7)**,## | 3.389 | 0.029 |
| Triglycerides (mmol/L) | 1.2±0.5 | 1.2±0.5 | 1.2±0.7 | 0.015 | 0.985 |
| Total cholesterol (mmol/L) | 3.7±1.2 | 4.0±0.7 | 3.5±0.7 | 1.826 | 0.227 |
| Low-density lipoprotein (mmol/L) | 2.2±1.0 | 2.5±0.7 | 2.0±0.7 | 2.701 | 0.075 |
| High-density lipoprotein (mmol/L) | 1.2±0.3 | 1.3±0.3 | 1.1±0.2 | 1.429 | 0.337 |
| D-dimer (mg/L) | 0.4 (0.3-0.9) | 0.7 (0.3-1.0) | 0.4 (0.2-0.9) | 2.297 | 0.108 |
| Albumin (g/L) | 32.6±3.6 | 33.7±3.5 | 33.5±3.0 | 0.688 | 0.506 |
| Globulin (g/L) | 26.1±3.6 | 28.7±4.9 | 29.0±5.2 | 3.042 | 0.054 |
| Total protein (g/L) | 58.7±4.6 | 62.4±7.3 | 61.6±6.6 | 2.385 | 0.100 |
| Bilirubin (μmol/L) | 13.2±8.3 | 12.4±5.3 | 12.8±7.8 | 0.072 | 0.931 |
| Total bile acid (μmol/L) | 8.0±5.1 | 6.4±5.3 | 7.3±5.0 | 0.180 | 0.836 |
| Urea nitrogen (mmol/L) | 4.5±1.3 | 4.8±2.1 | 5.5±2.2 | 1.887 | 0.160 |
| Creatinine (μmol/L) | 62.7±7.8 | 65.3±17.3 | 70.3±33.3 | 1.438 | 0.216 |
| Glucose (mmol/L) | 6.2±2.1 | 5.9±1.8 | 6.3±2.4 | 0.180 | 0.836 |
| HBA1C (%) | 6.3±1.3 | 6.5±1.3 | 7.4±2.5 | 1.978 | 0.148 |
| BNP (pg/ml) | 461.4 (229.3-1388.0) | 301.1 (128.7-799.5) | 314.2 (199.0-1028.2) | 0.217 | 0.806 |
| Blood uric acid (μmol/L) | 278.2±97.7 | 313.5±138.1 | 314.2±65.0 | 0.999 | 0.374 |
| Hcy (mmol/L) | 19.3±9.4 | 22.1±8.3* | 24.7±16.9**,# | 8.148 | 0.001 |
| TT3 (nmol/L) | 1.5±0.3 | 1.7±0.2 | 1.3±0.3 | 1.099 | 0.339 |
| TT4 (nmol/L) | 103.9±29.5 | 119.8±37.6 | 103.9±20.0 | 1.717 | 0.188 |
| TSH (mIu/L) | 2.0±1.6 | 2.9±1.8 | 2.6±2.3 | 0.949 | 0.393 |
Abbreviations: CRP, C-reactive protein; HBA1C, glycated hemoglobin; BNP, B-type natriuretic peptide; Hcy, homocysteine; TT3, Total Triiodothyronine; TT4, Thyroxine; TSH, Thyroid Stimulating Hormone.
Compared with mild frailty group;
P<0.05;
P<0.01.
Compared with moderate frailty group;
P<0.05;
P<0.01.
Multivariate logistic regression analysis
Using the occurrence of frailty as the dependent variable, a multivariate logistic regression analysis was conducted using levels of serum markers (hemoglobin, CRP, triglycerides, low-density lipoprotein, D-dimer, albumin, and Hcy) as independent variables (Model 1), which was significantly different (P<0.05) between the frailty group and the non-frailty group in univariate analysis. The results showed that hemoglobin and Hcy were independent risk factors for predicting frailty in patients with cerebral infarction. However, CRP, triglycerides, low-density lipoprotein, D-dimer, albumin were not independent risk factors (P>0.05). After adjusting for potential confounding factors such as age, gender and severity of infarction (Model 2), hemoglobin and Hcy remained independent risk factors for predicting frailty in patients with cerebral infarction (Table 4).
Table 4.
Multivariate logistic regression analysis
| Variable | β | SE | Wald | OR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Model 1 | Hemoglobin | -0.032 | 0.012 | 7.168 | 0.969 | 0.947-0.992 | 0.007 |
| CRP | 0.034 | 0.019 | 3.397 | 1.035 | 0.998-1.074 | 0.065 | |
| Triglyceride | -0.282 | 0.330 | 0.727 | 0.755 | 0.395-1.441 | 0.394 | |
| Low-density lipoprotein | -0.476 | 0.254 | 3.521 | 0.621 | 0.378-1.021 | 0.061 | |
| D-dimer | 0.519 | 0.402 | 1.667 | 1.681 | 0.764-3.697 | 0.197 | |
| Albumin | 0.033 | 0.076 | 0.191 | 1.034 | 0.890-1.201 | 0.662 | |
| Hcy | 0.125 | 0.033 | 14.493 | 1.134 | 1.063-1.209 | 0.001 | |
| Model 2 | Hemoglobin | -0.018 | 0.014 | 8.805 | 0.983 | 0.956-1.010 | 0.003 |
| CRP | 0.045 | 0.024 | 3.574 | 1.046 | 0.998-1.097 | 0.059 | |
| Triglyceride | -0.227 | 0.443 | 0.263 | 1.255 | 0.526-2.992 | 0.608 | |
| Low-density lipoprotein | -0.902 | 0.304 | 1.580 | 0.406 | 0.224-0.736 | 0.209 | |
| D-dimer | 0.093 | 0.427 | 0.047 | 1.097 | 0.475-2.534 | 0.828 | |
| Albumin | 0.150 | 0.102 | 2.141 | 1.162 | 0.950-1.419 | 0.143 | |
| Hcy | 0.128 | 0.038 | 11.085 | 1.137 | 1.054-1.226 | 0.001 |
Note: Model 1 has not been corrected; Model 2 corrects for factors such as age, gender and severity of infarction.
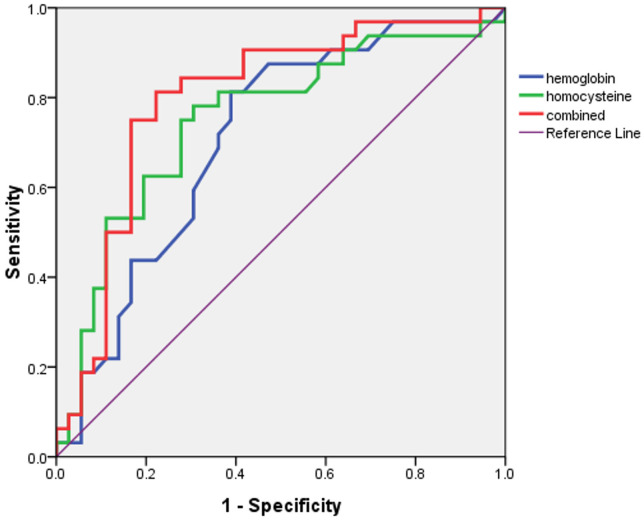
The areas under the ROC curves for hemoglobin and Hcy to predict frailty were 0.707 and 0.751, respectively with a 95% CI of 0.622-0.793 and 0.666-0.836, respectively. The area under the ROC curve of hemoglobin combined with Hcy to determine frailty was 0.799 (95% CI 0.721-0.878) (Figures 2, 3 and 4).
Figure 2.
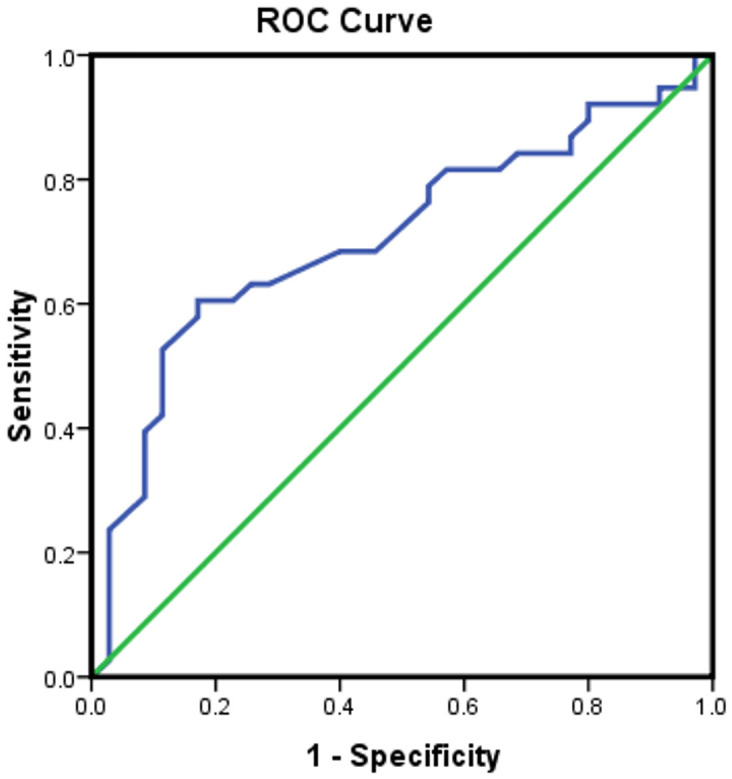
Establishing a predicted frailty ROC curve based on hemoglobin levels, with an area under the ROC curve of 0.707.
Figure 3.
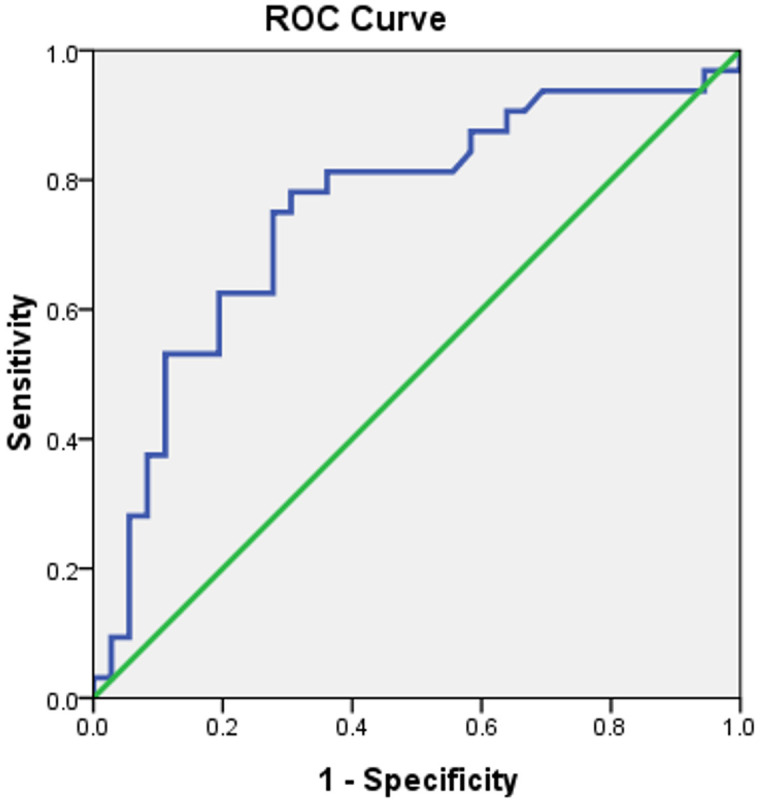
Establishing a predicted frailty ROC curve based on Hcy levels, with an area under the ROC curve of 0.751.
Figure 4.
The ROC curve for predicting frailty was established by combining hemoglobin and Hcy levels, with an area under the ROC curve of 0.799.
Discussion
Frailty is a common syndrome in the elderly population, which means there is an accumulated decline of multiple biological system functions which leads to the decrease in reserve capacity and stress resistance, and the increase in fragility when facing stress, thereby increasing the risk of adverse consequences, including falls, delirium, disability, cognitive decline, etc. [26,27]. Frailty has become one of the major focuses of health and medical research. Currently, frailty is mainly screened and evaluated through scales such as the Tilburg frailty index, Edmonton frailty scale, Groningen frailty index (GFI), frailty phenotype, frailty scale (FRAIL), frailty index (FI), clinical frailty scale (CFS), etc. Each scale has different characteristics; for example, the Edmonton scale is easy to operate and can be used by non-geriatric researchers to quickly identify frailty status of patient [21], and CFS belongs to the frailty rating scale, which is a comprehensive health assessment of patients, based on their activities of daily living (ADL) and disease severity. The higher the grading, the more severe the frailty. Previous studies have shown that both these scales can effectively evaluate the frailty status of patients and grade frailty, especially in identifying frailty patients in early stages. In the past, patients with a history of stroke were often excluded from studies related to frailty phenotype [28], hence our study mainly focused on evaluating frailty in stroke patients. Since, the FRAIL and FI scales evolved from frailty phenotypes, these scales were not used in this study to evaluate the frailty status of stroke patients. However, CFS can classify clinical frailty levels, which is a characteristic that other scales do not possess, therefore, this study simultaneously used the Edmonton Scale and the CFS to evaluate the frailty status of patients with acute cerebral infarction.
Cerebral infarction is a common disease that seriously endangers the health of the elderly, with high disability and mortality rates [29]. Research has shown that frailty increased the risk of stroke and was one of the independent risk factors for stroke [30]. Compared to non-frailty stroke patients, frail stroke patients had an increased risk of death within 30 days and 1 year [31,32]. A large-scale prospective multicenter study conducted by the UK Stroke Clinical Network Evaluation Study groups also found that severe pre-stroke frailty could predict mortality within 1 year after stroke [33]. Current research suggests that the prognosis of cerebral infarction is not only related to the development of the disease itself, but also to the overall state of the patient. The presence of frailty can reflect the overall functional level of the patient, thereby affect their prognosis. Among the 146 patients with acute cerebral infarction in this study, 70 cases had frailty, accounting for 47.9%, which is consistent with previous studies that reported 54% prevalence of frailty in acute cerebral infarction patients [34]. Further evidence suggests that frailty is closely related to the occurrence of cerebral infarction, and that early identification and corresponding intervention are extremely important, which can improve the prognosis and quality of life of patients.
At present, there are some shortcomings in the assessment of frailty solely using scales by clinicians. The main reason is that the scales have a certain degree of subjectivity and learning ability, which may lead to some deviation in the assessment of frailty. Therefore, objective indicators are needed for clinicians to screen for frailty. This study detected and analyzed the levels of serum markers commonly used in clinical practice to reflect the functional status of multiple systems. The results showed that hemoglobin and homocysteine were independent risk factors for frailty in patients with acute cerebral infarction. At the same time, there were significant differences in hemoglobin and homocysteine levels among patients with different degrees of frailty, indicating that hemoglobin and homocysteine can be used as objective indicators for evaluating frailty in patients with acute cerebral infarction. Landi F et al. [35] found that anemia and hemoglobin concentration were associated with mortality in frail elderly people, while Zara S et al. [36] found that hemoglobin concentration was closely related to frailty, and the pathogenesis may be related to nutritional status and chronic inflammation. Anemia can lead to disability, decreased mobility, and decreased muscle strength in elderly patients [37]. Dynamic detection of hemoglobin levels can identify physiological decline in early disease course and is also a sensitive indicator for early identification of frailty. Improving the nutritional status of patients early in the disease course may have a positive effect on improving the prognosis of frail patients and reducing their mortality rate. Homocysteine is a risk factor for cardio-cerebrovascular diseases, and its metabolic pathways are closely related to folate, vitamin B12, and vitamin B6. The results of this study suggest that the levels of homocysteine in the frailty group is higher than that in the non-frailty group. Compared with the mild and moderate frailty groups, the levels of homocysteine in the severe frailty group is significantly higher. Ma et al. found that high homocysteine was also a biomarker for predicting frailty in high-risk elderly people [38]. Early intervention is needed for elderly people with high homocysteine to prevent frailty. Previous studies have also found that homocysteine levels gradually increased in the non-frailty group, pre-frailty group, and frailty group. The pathogenesis of this may be through inducing inflammation, increasing muscle protein hydrolysis, and reducing muscle regeneration ability, leading to muscle atrophy, decreased physical function, and ultimately frailty [39], Therefore, monitoring homocysteine levels in patients with acute cerebral infarction is equally important for early assessment of frailty. Further analysis of the ROC curve reveals that the combined detection levels of hemoglobin and homocysteine has a higher predictive value for acute cerebral infarction with frailty, with the area under the ROC curve of 0.799. Therefore, regular detection of the levels of hemoglobin and homocysteine is crucial for monitoring frailty patients with acute cerebral infarction. Although the ROC curve in this study suggested that CRP, Low-density lipoprotein, Albumin, and D-dimer were not independent risk factors for frailty, there were significant differences between patients in the frailty group and those in the non-frailty group. It is suggested that inflammation, metabolic markers, hyperfibrinolysis and malnutrition have important effects on the occurrence and development of frailties, which is consistent with the conclusions of other studies [40].
Currently, the impact of frailty on secondary prevention after stroke has received little attention. Frailty may be an intervention target for stroke prognosis, and improving the risk factors of frailty may reduce the adverse outcomes after stroke [41]. This study found that high Hcy and low hemoglobin levels are independent risk factors for frailty in acute stroke patients. Therefore, early intervention in Hcy and hemoglobin levels in stroke patients may improve their prognosis. In the meantime, the value of these two serum markers in predicting the prognosis of stroke patients can be explored further in the future.
This is a small sample size and a single center study. In the future, the sample size should be expanded and multi-center joint studies should be conducted to reduce bias of patient selection. The lack of baseline data on nutritional status, cognitive function, body mass index, and other factors that may affect patient’s frailty may have some impact on the results. Meanwhile, the serum markers of patients were not dynamically observed, and no follow-up was conducted on patients. Patients with severe anxiety, depression and mental disorders were excluded in our study, mainly based on the following factors. Firstly, many studies have shown that depression/anxiety/mental disorders were closely related to frailty, and the probability of frailty in individuals with depression/anxiety/mental disorders was significantly higher than in the normal population [42-44]. Hence, in order to better align with clinical practice and prevent selection bias among included patients, this study excluded those patients with severe anxiety, depression, and mental disorders. Secondly, in this study, the Edmonton/CFS scales were chosen to assess the frailty status of patients. The measurement of the scales has a certain degree of subjectivity, and the accuracy of the scale may be insufficient when evaluating individuals with depression/anxiety/mental disorders, which may lead to bias in the accuracy of the assessment.
In conclusion, the incidence of frailty in patients with acute cerebral infarction is high. Hemoglobin and homocysteine are independent risk factors to predict frailty. The decreased levels of hemoglobin and the increased levels of homocysteine can identify the frailty status of acute cerebral infarction early at the disease course, and can be used as biological markers for early identification of frailty. Clinicians should pay close attention to the dynamic monitoring of the levels of hemoglobin and homocysteine.
Acknowledgements
We acknowledge the support from the Hangzhou Health Science and Technology Plan Projects (A20200219) and Zhejiang Province Traditional Chinese Medicine Science and Technology Project (2024ZL129).
Disclosure of conflict of interest
None.
References
- 1.Junius-Walker U, Onder G, Soleymani D, Wiese B, Albaina O, Bernabei R, Marzetti E ADVANTAGE JA WP4 group. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10. doi: 10.1016/j.ejim.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Winovich DT, Longstreth WT Jr, Arnold AM, Varadhan R, Zeki Al Hazzouri A, Cushman M, Newman AB, Odden MC. Factors associated with ischemic stroke survival and recovery in older adults. Stroke. 2017;48:1818–1826. doi: 10.1161/STROKEAHA.117.016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer K, Vetrano DL, Padua L, Romano V, Rivoiro C, Scelfo B, Marengoni A, Bernabei R, Onder G. Frailty syndromes in persons with cerebrovascular disease: a systematic review and meta-analysis. Front Neurol. 2019;10:1255. doi: 10.3389/fneur.2019.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanai M, Noguchi M, Kubo H, Nozoe M, Kitano T, Izawa KP, Mase K, Shimada S. Pre-stroke frailty and stroke severity in elderly patients with acute stroke. J Stroke Cerebrovasc Dis. 2020;29:105346. doi: 10.1016/j.jstrokecerebrovasdis.2020.105346. [DOI] [PubMed] [Google Scholar]
- 5.Wei J, Wang J, Chen J, Yang K, Liu N. Stroke and frailty index: a two-sample Mendelian randomisation study. Aging Clin Exp Res. 2024;36:114. doi: 10.1007/s40520-024-02777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi M, Kubo H, Kanai M, Nozoe M, Shimada S. Relationship between pre-stroke frailty status and short-term functional outcome in older patients with acute stroke-a mediation analysis. Arch Gerontol Geriatr. 2021;94:104370. doi: 10.1016/j.archger.2021.104370. [DOI] [PubMed] [Google Scholar]
- 7.Ju SY, Lee JY, Kim DH. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: a systematic review and dose-response meta-analysis. BMC Geriatr. 2018;18:206. doi: 10.1186/s12877-018-0904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuliani G, Volpatol S, Romagnoni F, Soattin L, Bollini C, Leoci V, Fellin R. Combined measurement of serum albumin and high-density lipoprotein cholesterol strongly predicts mortality in frail older nursing-home residents. Aging Clin Exp Res. 2004;16:472–475. doi: 10.1007/BF03327404. [DOI] [PubMed] [Google Scholar]
- 9.Tack RWP, Amboni C, van Nuijs D, Pekna M, Vergouwen MDI, Rinkel GJE, Hol EM. Inflammation, anti-inflammatory interventions, and post-stroke cognitive impairment: a systematic review and meta-analysis of human and animal studies. Transl Stroke Res. 2023 doi: 10.1007/s12975-023-01218-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Couch C, Mallah K, Borucki DM, Bonilha HS, Tomlinson S. State of the science in inflammation and stroke recovery: a systematic review. Ann Phys Rehabil Med. 2022;65:101546. doi: 10.1016/j.rehab.2021.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnanenthiran SR, Ng ACC, Cumming RG, Brieger DB, le Couteur DG, Waite LM, Seibel M, Handelsman DJ, Naganathan V, Kritharides L, Blyth FM. Hemoglobin, frailty, and long-term cardiovascular events in community-dwelling older men aged ≥70 years. Can J Cardiol. 2022;38:745–753. doi: 10.1016/j.cjca.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Hou C, Yao L, Ma Y, Li Y, Li J, Gui M, Wang M, Zhou X, Lu B, Fu D. The association between chronic heart failure and frailty index: a study based on the national health and nutrition examination survey from 1999 to 2018. Front Cardiovasc Med. 2023;9:1057587. doi: 10.3389/fcvm.2022.1057587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King KE, Fillenbaum GG, Cohen HJ. A cumulative deficit laboratory test-based frailty index: personal and neighborhood associations. J Am Geriatr Soc. 2017;65:1981–1987. doi: 10.1111/jgs.14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, Kirkwood TB. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi: 10.1186/s12916-015-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchis J, Núñez E, Ruiz V, Bonanad C, Fernández J, Cauli O, García-Blas S, Mainar L, Valero E, Rodríguez-Borja E, Chorro FJ, Hermenegildo C, Núñez J. Usefulness of clinical data and biomarkers for the identification of frailty after acute coronary syndromes. Can J Cardiol. 2015;31:1462–1468. doi: 10.1016/j.cjca.2015.07.737. [DOI] [PubMed] [Google Scholar]
- 16.Kane AE, Sinclair DA. Frailty biomarkers in humans and rodents: current approaches and future advances. Mech Ageing Dev. 2019;180:117–128. doi: 10.1016/j.mad.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuiper LM, Polinder-Bos HA, Bizzarri D, Vojinovic D, Vallerga CL, Beekman M, Dollé ET, Ghanbari M, Voortman T, Reinders MJT, Verschuren WMM, Slagboom PE, van den Akker EB, van Meurs JBJ. Epigenetic and metabolomic biomarkers for biological age: a comparative analysis of mortality and frailty risk. J Gerontol A Biol Sci Med Sci. 2023;78:1753–1762. doi: 10.1093/gerona/glad137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutiérrez-Robledo LM, Rockwood K, Rodríguez Artalejo F, Serviddio G, Vega E FOD-CC group (Appendix 1) Searching for an operational definition of frailty: a delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:17. doi: 10.3352/jeehp.2021.18.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Han S, Qin H, Zheng H, Jiang B, Cao Y, Gao Y, Guan L, Jia Q, Jiang Y, Jiao Y, Li S, Li Y, Li Z, Liu W, Ru X, Sun D, Sun H, Wang P, Wang T, Zong L, Guo L, Xie X, Xu Y, Xu Y, Yang X, Yang Y, Zhou M, Wang W Chinese Stroke Association Stroke Council Guideline Writing Committee. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of the management of high-risk population. Stroke Vasc Neurol. 2020;5:270–278. doi: 10.1136/svn-2020-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the edmonton frail scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YH. Biostatistics 202: logistic regression analysis. Singapore Med J. 2004;45:149–153. [PubMed] [Google Scholar]
- 24.Harris JK. Primer on binary logistic regression. Fam Med Community Health. 2021;9(Suppl 1):e001290. doi: 10.1136/fmch-2021-001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson RP, Jin R, Grunkemeier GL. Understanding logistic regression analysis in clinical reports: an introduction. Ann Thorac Surg. 2003;75:753–757. doi: 10.1016/s0003-4975(02)04683-0. [DOI] [PubMed] [Google Scholar]
- 26.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward DD, Ranson JM, Wallace LMK, Llewellyn DJ, Rockwood K. Frailty, lifestyle, genetics and dementia risk. J Neurol Neurosurg Psychiatry. 2022;93:343–350. doi: 10.1136/jnnp-2021-327396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Feigin VL, Mensah GA, Norrving B, Murray CJ, Roth GA GBD 2013 Stroke Panel Experts Group. Atlas of the global burden of stroke (1990-2013): the GBD 2013 study. Neuroepidemiology. 2015;45:230–236. doi: 10.1159/000441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renedo D, Acosta JN, Koo AB, Rivier C, Sujijantarat N, de Havenon A, Sharma R, Gill TM, Sheth KN, Falcone GJ, Matouk CC. Higher hospital frailty risk score is associated with increased risk of stroke: observational and genetic analyses. Stroke. 2023;54:1538–1547. doi: 10.1161/STROKEAHA.122.041891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XM, Jiao J, Zhu C, Guo N, Liu Y, Lv D, Wang H, Jin J, Wen X, Zhao S, Wu XJ, Xu T. Cognitive frailty and 30-day mortality in a national cohort of older Chinese inpatients. Clin Interv Aging. 2021;16:389–401. doi: 10.2147/CIA.S294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XM, Jiao J, Xu T, Wu XJ. The association between frailty of older stroke patients during hospitalization and one-year all-cause mortality: a multicenter survey in China. Int J Nurs Sci. 2022;9:162–168. doi: 10.1016/j.ijnss.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myint PK, O Bachmann M, Loke YK, D Musgrave S, Price GM, Hale R, Metcalf AK, Turner DA, Day DJ, A Warburton E, Potter JF. Important factors in predicting mortality outcome from stroke: findings from the anglia stroke clinical network evaluation study. Age Ageing. 2017;46:83–90. doi: 10.1093/ageing/afw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warburton EA, Romero-Ortuno R, Wallis SJ, To B, Wall J, Evans NR. Clinical frailty independently predicts early mortality after ischaemic stroke. Age Ageing. 2020;49:588–591. doi: 10.1093/ageing/afaa004. [DOI] [PubMed] [Google Scholar]
- 35.Landi F, Russo A, Danese P, Liperoti R, Barillaro C, Bernabei R, Onder G. Anemia status, hemoglobin concentration, and mortality in nursing home older residents. J Am Med Dir Assoc. 2007;8:322–327. doi: 10.1016/j.jamda.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Steinmeyer Z, Delpierre C, Soriano G, Steinmeyer A, Ysebaert L, Balardy L, Sourdet S. Hemoglobin concentration; a pathway to frailty. BMC Geriatr. 2020;20:202. doi: 10.1186/s12877-020-01597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penninx BW, Pahor M, Cesari M, Corsi AM, Woodman RC, Bandinelli S, Guralnik JM, Ferrucci L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J Am Geriatr Soc. 2004;52:719–724. doi: 10.1111/j.1532-5415.2004.52208.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma T, Sun XH, Yao S, Chen ZK, Zhang JF, Xu WD, Jiang XY, Wang XF. Genetic variants of homocysteine metabolism, homocysteine, and frailty - Rugao longevity and ageing study. J Nutr Health Aging. 2020;24:198–204. doi: 10.1007/s12603-019-1304-9. [DOI] [PubMed] [Google Scholar]
- 39.Carrillo-Vico A, Lardone PJ, Cruz-Chamorro I, Rodríguez-Mañas L, García-García FJ, Guerrero JM, Álvarez-Ríos AI, Álvarez-Sánchez N. Homocysteine and C-reactive protein levels are associated with frailty in older spaniards: the Toledo study for healthy aging. J Gerontol A Biol Sci Med Sci. 2020;75:1488–1494. doi: 10.1093/gerona/glz168. [DOI] [PubMed] [Google Scholar]
- 40.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the cardiovascular health study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 41.Evans NR, Todd OM, Minhas JS, Fearon P, Harston GW, Mant J, Mead G, Hewitt J, Quinn TJ, Warburton EA. Frailty and cerebrovascular disease: concepts and clinical implications for stroke medicine. Int J Stroke. 2022;17:251–259. doi: 10.1177/17474930211034331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang R, Noble S, Rosenblatt M, Dai W, Ye J, Liu S, Qi S, Calhoun VD, Sui J, Scheinost D. The brain structure, inflammatory, and genetic mechanisms mediate the association between physical frailty and depression. Nat Commun. 2024;15:4411. doi: 10.1038/s41467-024-48827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan M, Bhanu C, Frost R. The association between frailty and anxiety: a systematic review. Int J Geriatr Psychiatry. 2023;38:e5918. doi: 10.1002/gps.5918. [DOI] [PubMed] [Google Scholar]
- 44.Mutz J, Choudhury U, Zhao J, Dregan A. Frailty in individuals with depression, bipolar disorder and anxiety disorders: longitudinal analyses of all-cause mortality. BMC Med. 2022;20:274. doi: 10.1186/s12916-022-02474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]