Abstract
Objectives: This study aimed to evaluate the therapeutic effect of the QingreHuoxue formula on mice with Idiopathic Membranous Nephropathy (IMN) and its impact on Th17 cells and Tregs. Methods: A mouse model of IMN was established, and the mice were treated with traditional Chinese medicine, western medicine, or a combination of both. The efficacy and immunomodulatory effects of the QingreHuoxue formula were evaluated by examining renal pathology, urinary protein levels, peripheral blood Th17 and Treg cell counts, and comparing the expression levels of IL-17 and transforming growth factor-β1 in renal tissues. Results: Compared to the untreated IMN model group, the IMN mice treated with TCM, western medicine, or the combination showed significant improvements in proteinuria, renal pathology, peripheral T lymphocyte counts, and IL-17 expression in renal tissues. Notably, the group treated with a combination of Chinese and western medicine exhibited better outcomes than the group treated with western medicine alone. Conclusions: The QingreHuoxue formula was effective in reducing proteinuria, modulating T cell immune function, and protecting renal tissue in mice with IMN.
Keywords: QingreHuoxue formula, idiopathic membranous nephropathy
Introduction
Membranous nephropathy is a glomerular disease of unknown etiology that can develop at any age. It is the most common cause of nephrotic syndrome in adults, and when no obvious secondary factors are present, it is termed idiopathic membranous nephropathy (IMN). IMN accounts for approximately 30-40% of all cases of primary nephrotic syndrome. Pathologically, IMN is characterized by the deposition of immune complexes beneath the glomerular basement membrane epithelium and diffuse thickening of the membrane. Diagnosis of IMN is made after excluding secondary factors. For patients with IMN, immunosuppressive therapy with cyclophosphamide and corticosteroids has been shown to significantly reduce the need for renal replacement therapy [1,2]. However, due to the carcinogenic risks associated with these treatments, calcineurin inhibitors, such as cyclosporin A and tacrolimus, are more commonly used in clinical practice [3,4].
T cells play a crucial role in assisting B cell differentiation, antibody secretion, inflammatory responses, and cytotoxicity, and are significant for autoimmunity. Research indicates that Th2, Th17, Treg cells, and their signature cytokines are involved in the pathogenesis of IMN. Among these, Th17 cells - a subset of CD4+ T cells are known for their role as inducers of immune responses, capable of secreting various pro-inflammatory cytokines, including granulocyte-macrophage colony-stimulating factor, interleukin-22 (IL-22), interferon-gamma, and tumor necrosis factor-alpha. Previous studies have shown that Th17 cells can induce the expression of C-C chemokine ligand 20 (CCL20) and CXCL5 in glomerular mesangial and renal tubular epithelial cells through the secretion of IL-17. Furthermore, research has shown that Th17 cells and neutrophils can migrate to the kidneys througjh distinct biologic pathways: Th17 cells through the C-C chemokine receptor 6 (CCR6)/CCL20 axis, and neutrophils through the CXCR2/CXCL5 axis. This migration promotes tissue damage, with Th17 cells also inducing the infiltration of other CCR6-expressing white blood cells into the kidneys [5]. Regulatory T cells (Tregs) are another subset of T cells that modulate immune responses by inhibiting B cells, macrophages, natural killer cells, CD8+ T cells, and dendritic cells. Tregs play a crucial immunosuppressive role through direct regulation of tumor necrosis factor receptor superfamily members 4, cytotoxic T lymphocyte-associated antigen 4, and glucocorticoid-associated tumor necrosis factor receptor, which in turn influences the secretion of TGF-β1 and IL-10. The balance between Th17 cells and Tregs, which are mutually inhibiting, is critical for maintaining organ-specific immunity and managing kidney disease. Previous studies have revealed that in cases of IMN, Th17 cell counts and IL-17 expression are notably increased, while Treg cell counts and TGF-β1 expression levels are reduced [6]. Additionally, research has shown that IL-17 and IL-21 are upregulated in IMN patients compared to healthy individuals [7]. These findings suggest that the balance between Th17 and Treg cells plays a significant role in the pathogenesis of IMN.
Traditional Chinese Medicine (TCM) also plays a crucial role in the treatment of IMN [8]. Previous studies have demonstrated that Astragalus, a key component of the QingreHuoxue formula, can enhance antibody production, an effect linked to increased T cell activity [9,10]. Therefore, this study aimed to evaluate the effects of the QingreHuoxue formula in mice with IMN, specifically focusing on the counts of Th17 cells and Tregs.
Materials and methods
Animal models
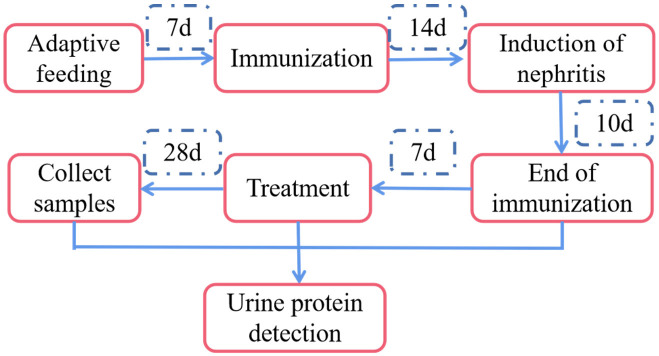
Fifty male ICR mice from the Central Laboratory of Animal Science at Jiaxing University School of Medicine, China, aged 8 weeks or older, were kept under controlled light conditions at 22±2°C and 60% humidity, with unrestricted access to food and water. All procedures involving animals were approved by the Animal Ethics Committee of Jiaxing Hospital of Traditional Chinese Medicine, Zhejiang Chinese Medical University.
After a one-week acclimation period on a normal diet, the mice were randomly divided into two groups: sham operation group (n=10) and model group (n=40). For the model group, nephritis was induced by injecting an emulsion containing 0.1 mg of cBSA (2 mg/ml) mixed with an equal dose of complete Freund’s adjuvant [11,12]. Two weeks after immunization, nephritis was further induced by administering 0.2 ml (400 µg) of cBSA (2 mg/ml) solution through the tail vein, five times every other day [11,12]. Urine protein levels in the mice were qualitatively assessed during modeling using a dipstick (URIT Medical Electronic Co., Ltd., Guilin, Guangxi, China). For both the initiation and conclusion of treatment, mice were placed in metabolic cages and fasted without access to drinking water [11,12]. A 16-hour urine sample was collected, and urine protein levels were measured using a mouse albumin ELISA kit (Figure 1). Mice were considered nephrotic if the total albumin content exceeded 1 mg [11,12]. Reagents for the IMN model were obtained from Chondrex, Inc. (Woodinville, WA, USA).
Figure 1.
Protocol of animal experiments.
Treatments
One week after the formal immunization, routine feeding was resumed, and the mice in the model group were randomly divided into four treatment groups (n=10 per group): i. IMN group: Mice were treated with normal saline. ii. TCM group (C Group): Mice were treated with the QingreHuoxue formula, consisting of Radix Codonopsis (1.35 g/kg), Atractylodes macrocephala koidz (1.08 g/kg), Salvia miltiorrhiza bunge (2.70 g/kg), Herba hedyotis (2.70 g/kg), Scutellaria baicalensis georgi (1.08 g/kg), Folium pyrrosiae (1.35 g/kg), Herba plantaginis (2.70 g/kg), Polyporus (1.35 g/kg), Angelicae sinensis radix (1.08 g/kg), Herba leonuri (1.35 g/kg), and Radix astragali (2.70 g/kg). The dosage was calculated according to the Methodology on Chinese Medicinal Pharmacology. iii. Western Medicine Group (W Group): Mice were treated with prednisone acetate (0.045 mg/kg/day; Zhejiang Xianju Pharmaceutical Co., Ltd., Taizhou, Zhejiang, China) and cyclosporine A (25 mg/kg/day; Hangzhou Zhongmei East China Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China). iv. TCM plus Western Medicine Group (C+W Group): Mice were treated with both the QingreHuoxue formula and the Western medicine regimen.
The intragastric administration volume for each mouse was controlled at 1 mL, and all treatments were administered continuously for four weeks. Then, the mice were anesthetized with 1% pentobarbital sodium (50 mg/kg, i.p.), and blood samples (~1.5 ml per mouse) were collected by eyeball blood sampling. The mice were then euthanized with a higher dose of 1% pentobarbital sodium (100 mg/kg body weight). Once death was confirmed by the absence of breathing or heartbeat, the bilateral kidneys were excised.
Histopathologic examinations
After isolating the kidneys, a portion of the kidney tissue was immediately immersed in 4% paraformaldehyde, fixed in phosphate-buffered saline (PBS, pH 7.4), embedded in paraffin, and sectioned into 5 μm slices. Immunohistochemical staining was performed using the streptavidin-peroxidase method. Endogenous peroxidase activity was blocked by incubating the sections with 3% hydrogen peroxide for 30 minutes. The sections were then incubated overnight at 4°C with primary antibodies against IL-17 (1:500 dilution; Abcam, Cambs, UK) and TGF-β1 (1:500 dilution; Abcam, Cambs, UK). Following this, the tissue sections were treated with a Histostain Plus Kit and stained with hematoxylin for counterstaining according to the reagent specifications. Rabbit IgG or PBS-treated tissue sections were used as controls for the immunostaining procedures. Other sections were stained with hematoxylin-eosin (H&E) and periodic acid-Schiff (PAS) for further histological analysis. Additionally, renal tissues were fixed with formaldehyde, immersed in sugar water, and then embedded in optimal cutting temperature (OCT) compound to create frozen sections. The deposits were labeled with FITC-conjugated anti-mouse IgG (1:100; Sigma-Aldrich, Darmstadt, Germany), and the tissue specimens were examined under a fluorescence microscope. Fluorescence intensity was recorded semi-quantitatively, ranging from negative to strongest staining (0 to 4+). A fluorescence intensity of ≥ 1+ was considered positive, while 3+ and 4+ were classified as strongly positive. Finally, renal tissues were prefixed with 2% glutaraldehyde-paraformaldehyde and postfixed with 1% osmium tetroxide. Following dehydration, embedding, and sectioning, the samples were double-stained with 5% aqueous uranyl acetate and lead citrate. Glomerular podocytes and the glomerular basement membrane were then observed using a transmission electron microscope.
Flow cytometry
Flow cytometry was performed on blood samples collected on the day of analysis. Th17 cells and Tregs were labeled using specific staining kits for mouse Th17 and Treg cells (Multisciences, Hangzhou, Zhejiang, China). Th17 cells were stained with the markers CD3ε-FITC, CD4-PerCP-Cy5.5, and IL-17A-PE. Tregs were labeled with CD4-FITC, CD25-APC, and FoxP3-PE.
Protein preparation and immunoblotting assay
A portion of kidney tissue was cut into small pieces and homogenized in a protein inhibitor radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) using ultrasound at 4°C. The homogenate was then centrifuged at 12,000 rpm for 30 minutes at 4°C, and the supernatant was collected. Protein concentration was determined using the Lowry method. The supernatant was diluted with an equal volume of 2× electrophoresis sample buffer and boiled for 9 minutes. Proteins were separated by gel electrophoresis and transferred to a polyvinylidene fluoride (PVDF) membrane (MilliporeSigma, Burlington, MA, USA).
The membrane was blocked at room temperature with 5% nonfat milk in Tris-buffered saline with Tween-20 (TBST). It was then incubated overnight at 4°C with primary antibodies against IL-17 and TGF-β1 (diluted 1:1000; Abcam, Cambs, UK). Following primary antibody incubation, the membrane was washed and incubated with horseradish peroxidase-conjugated secondary antibodies (diluted 1:500; Biorbyt, Ltd., Cambridge, UK).
Western blotting was performed using luminol reagents, and samples were measured using a chemiluminescence/fluorescence image analysis system (Tanon-5200 Multi; Tianneng Technology Co., Ltd., Shanghai, China). The semi-quantitative density of the bands was analyzed using ImageJ software (Version 1.8.0).
qPCR
Total RNA was extracted from kidney specimens using the RNAiso Plus kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer’s instructions. The RNA concentration and purity were assessed using a microplate reader, with the absorbance ratio between 260 nm and 280 nm ranging from 1.8 to 2.0.
The total RNA was then reverse transcribed into complementary DNA (cDNA) in a 20 μl reaction mixture using the PrimeScript™ RT kit. Quantitative PCR (qPCR) amplification was performed with sequence-specific primers for IL-17, TGF-β, and GAPDH (see Table 1). qPCR was conducted using the SYBR® PrimeScript™ RT-PCR kit (Takara Bio, Inc., Otsu, Japan). To control for potential contamination, a negative control reaction was performed with distilled water instead of cDNA, which showed no amplification. To ensure the reliability of the results, the qPCR experiments were repeated at least three times.
Table 1.
Primer sequences used for fluorescence quantitative PCR
| Gene | Primer | Nucleotide sequence (5’-3’) | Product size, bp |
|---|---|---|---|
| IL-17 | F | TTCAGGGTCGAGAAGATGCT | 112 |
| R | AAACGTGGGGGTTTCTTAGG | ||
| TGF-β1 | F | GAGAGCCCTGGATACCAACTACTGC | 93 |
| R | CAACCCAGGTCCTTCCTAAAGTCA | ||
| GAPDH | F | CCTTCCGTGTTCCTACCCC | 131 |
| R | GCCCAGGATGCCCTTTAGTG |
Note: IL-17, interleukin-17; TGF-β1, transforming growth factor-β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Results
Effect of treatment regimen on urinary protein
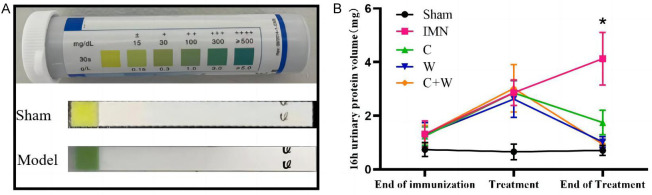
Following nephritis induction, all mice exhibited proteinuria, confirming the successful establishment of the nephritis model. After treatment, significant differences were observed between the treatment groups and the model group (P < 0.05). Notably, the 16-hour urinary protein levels were significantly reduced in the C+W group compared to the model group. However, no significant differences in urinary protein levels were observed among the C, W, and C+W groups (P > 0.05; Figure 2).
Figure 2.
Urine protein at baseline and 16-hours. A. Urine protein test dipsticks on day 1 of treatment; B. 16-hour urinary protein volume (mg). *P < 0.05 C, W, C+W vs. IMN. Western Medicine (W), Traditional Chinese Medicine plus Western Medicine (C+W), idiopathic membranous nephropathy (IMN).
Histopathologic findings
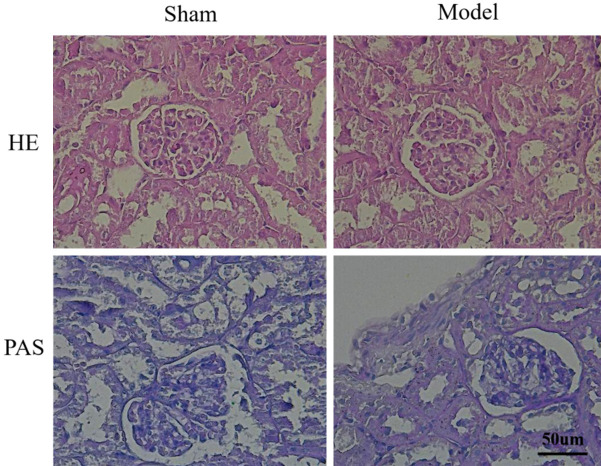
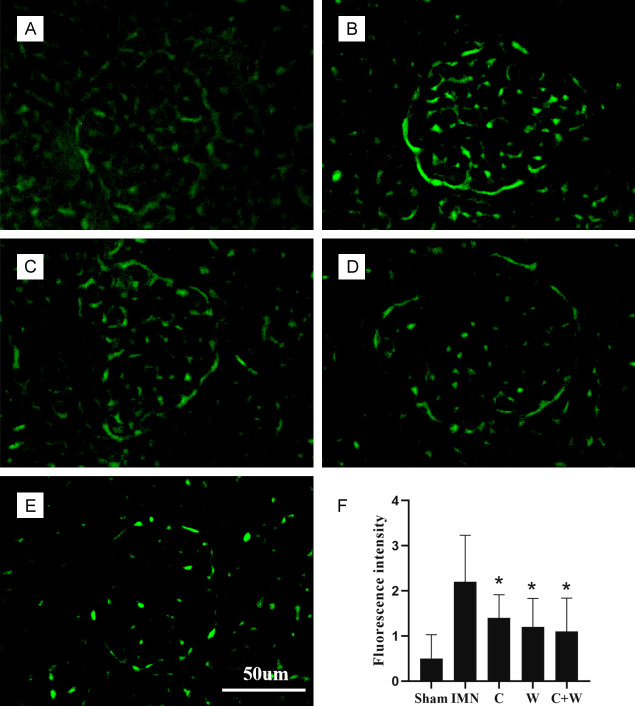
The morphologic changes in renal tissues of both the sham and model groups were assessed using light microscopy. H&E staining revealed improvements in glomerular hypercellularity and basement membrane thickening in the C, W, and C+W groups compared to the IMN group, indicating that these interventions were effective in alleviating IMN-related renal pathology. Additionally, PAS staining showed basement membrane thickening and granular degeneration in the renal tubular epithelial cells of IMN mice (Figure 3). Immunofluorescence staining revealed a significant upregulation of IgG in the glomeruli of the IMN group compared to the treatment groups (P < 0.05; Figure 4). Notably, IgG expression levels were significantly reduced in the C+W group, suggesting that treatment with TCM and Western medicine improved renal injury in mice with IMN.
Figure 3.
H&E and PAS staining results.
Figure 4.
Immunofluorescence staining. (A) Sham group, (B) IMN group, (C) C group, (D) W group, (E) C+W group, (F) Fluorescence intensity (n=10). *P < 0.05 vs. IMN group. Western Medicine (W), Traditional Chinese Medicine plus Western Medicine (C+W), idiopathic membranous nephropathy (IMN).
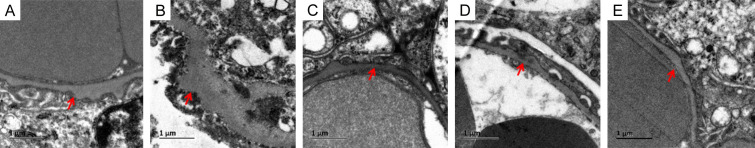
Observation under the transmission electron microscope revealed that in the IMN group, the basement membrane was thickened and exhibited protrusions, accompanied by immune complex deposition beneath the epithelium and extensive fusion of foot processes. These pathologic features are consistent with the typical signs of membranous nephropathy. In contrast, the pathological manifestations in the C, W, and C+W groups were milder. Specifically, the basement membrane protrusions were less pronounced, and there were fewer electron-dense deposits under the epithelium (Figure 5). These findings indicate an improvement in renal injury in the IMN mice following treatment, as evidenced by the reduced severity of the pathological changes.
Figure 5.
Renal specimens under transmission electron microscope. (A) Sham group, (B) IMN group: BGM thickening + immune complex protrusion, (C) C group: a small number of immune complexes protrude below the epithelium, (D) W group: a small number of immune complexes protrude below the epithelium, (E) C+W group: a small number of immune complexes protrude below the epithelium. Western Medicine (W), Traditional Chinese Medicine plus Western Medicine (C+W), idiopathic membranous nephropathy (IMN).
Effect of QingreHuoxue formula on TH17 cell and Treg counts in peripheral blood
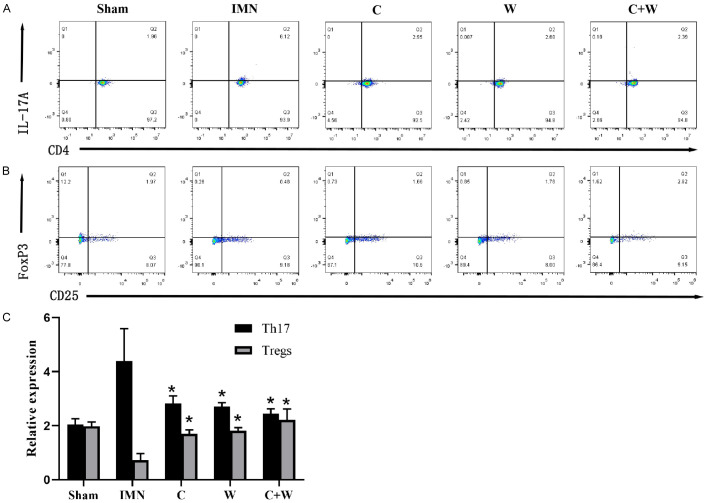
Flow cytometric analysis revealed an increased number of Th17 cells and a reduced number of Tregs in the IMN group, while the Th17 cell count decreased and the Treg count increased in the treatment groups (P < 0.05), with the most pronounced changes observed in the C+W group (Figure 6). This suggests that the drug interventions effectively modulated T cell populations in the peripheral blood of IMN mice.
Figure 6.
Flow cytometry results. A. The proportion of Th17 cells in peripheral blood. B. The proportion of Treg in peripheral blood. C. Relative cell proportion of Th17 cells and Treg. *P < 0.05 vs. IMN group. Western Medicine (W), Traditional Chinese Medicine plus Western Medicine (C+W), idiopathic membranous nephropathy (IMN).
Effect of QingreHuoxue formula on IL-17 and TGF-β1 expression in renal tissues
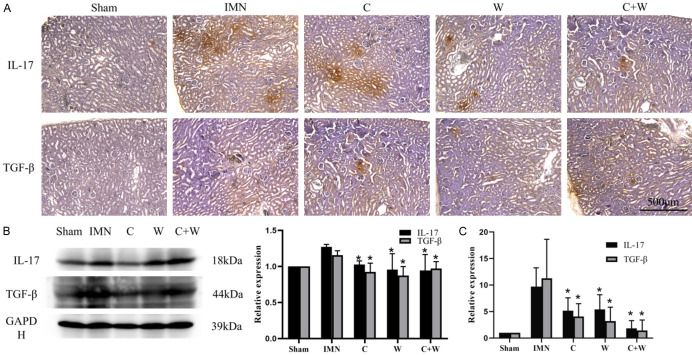
The immunohistochemical staining results indicated that IL-17 was significantly upregulated in the renal tissues of the IMN group. After drug intervention, IL-17 expression levels were restored in the kidney tissues in the C, W, and C+W groups, with the most notable reduction observed in the C+W group. Additionally, TGF-β1 expression was slightly elevated in the kidney tissues of IMN mice. Following treatment, TGF-β1 levels were reduced in the C, W, and C+W groups. Western blot and qPCR analyses corroborated the immunohistochemical findings, showing consistent expression levels of IL-17 and TGF-β1. These results suggest that integrated Chinese and Western medicine interventions can protect kidney function by modulating T lymphocyte-mediated immune responses (Figure 7).
Figure 7.
Expression of IL-17 and TGF-β1 in renal specimens. A. Immunohistochemical staining of IL-17 and TGF-β1. B. Results of immunoblotting. C. Results of q-PCR. *P < 0.05 vs. IMN group. Western Medicine (W), Traditional Chinese Medicine plus Western Medicine (C+W), idiopathic membranous nephropathy (IMN).
Discussion
In this study, we established an IMN mouse model, and the mice were subsequently treated with TCM and/or Western medicine. Following treatment, the number of Th17 cells and Tregs in peripheral blood, as well as the expression levels of IL-17 and TGF-β1 in renal tissues, were assessed. The results indicated that the QingreHuoxue formula effectively modulated T cell immune function and provided protection to kidney tissues in mice with IMN.
It has been reported that IMN is closely associated with T cells, as evidenced by an increased CD4/CD8 ratio in patients with IMN, whether or not they have nephrotic syndrome [13]. Additionally, research has suggested that evaluating Tregs in IMN patients could predict their early response to CD20-targeted therapies, such as rituximab [14]. These studies indicate that T cells play a significant role in the pathogenesis of IMN.
In the present study, similar findings were observed in mice with IMN. Specifically, the number of Th17 cells was decreased, and the number of Tregs was increased in the peripheral blood of IMN mice. Following intervention with the QingreHuoxue formula and/or Western medicine, the Th17 cell count was reduced, and the Treg count was elevated. These changes in both Th17 and Treg cell counts after treatment further support the notion that the QingreHuoxue formula effectively modulates T lymphocytes and provides a protective effect in mice with IMN.
The hallmark feature of IMN is the accumulation of discrete deposits containing immunoglobulins and antigens, which can form and expand beneath the basal surface of podocytes [14]. It has been reported that IMN is associated with a dysregulated overall immunophenotype [15,16], characterized by a decreased proportion of Tregs in untreated patients [14]. Li et al. demonstrated that enhanced Th2- and Th17-mediated immune responses in IMN patients were positively associated with disease activity, highlighting a significant role of Th17 cells in IMN pathogenesis and progression [17]. In this study, flow cytometry was used to measure the number of Th17 cells and Tregs in the peripheral blood of IMN mice. Consistent with previous findings, the Th17 cell count was increased, indicating that Th17 cells are significant mediators of immune responses in IMN. Conversely, the number of Tregs was reduced, leading to a loss of effective regulation of T cell-mediated immune responses and contributing to disease progression.
Emerging evidence suggests that the combination of cyclophosphamide and glucocorticoids can slow the progression of renal failure in patients with IMN [1]. However, this therapy is associated with significant adverse effects, including infections, central obesity, osteoporosis, bone marrow suppression, abnormal liver function, and gonadal suppression. Consequently, the 2012 KDIGO guidelines do not recommend the unrestricted use of this therapy for IMN patients [18]. In addition, cyclophosphamide-based therapy should be avoided in patients at high risk for renal failure [19]. The introduction of novel immunosuppressants, such as calcineurin inhibitors (CNIs), has offered a promising alternative with potentially lower toxicity. Experimental studies have demonstrated that CNIs can directly target podocytes, leading to a reduction in proteinuria [20]. Additionally, CNIs have been shown to increase the likelihood of achieving partial or complete remission of proteinuria (72-75%, compared to 22%) [21], with remission rates at 12-month follow-up comparable to or slightly higher than those achieved with cyclophosphamide. However, a major limitation is that most patients experience relapse after discontinuing CNIs.
It has also been reported that TCM can be effective in treating IMN. For example, Zheng et al. demonstrated that astragaloside IV could mitigate podocyte injury induced by the complement membrane attack complex by downregulating extracellular regulated protein kinase, thereby promoting remission of IMN [22]. TCM not only reduces the side effects and drug dependence associated with a large number of immunosuppressants but also addresses inflammation, oxidative stress, immune responses, and the TGF-β/Smad and Wnt/β-catenin signaling pathways [23-26]. Increasing evidence suggests that Chinese herbal medicine can enhance the efficacy of immunosuppressive agents. For instance, one study found that Wushi capsules significantly increased FK506 blood concentration in hospitalized patients with IMN [27]. Although these studies support the efficacy of herbal medicine for treating IMN and alleviating proteinuria, the precise mechanisms underlying these effects have not been fully elucidated.
The TCM QingreHuoxue formula has been shown to be effective in treating IMN and reducing proteinuria. This study aimed to further elucidate the underlying mechanisms of the QingreHuoxue formula. The results indicated that proteinuria was significantly reduced in the TCM treatment group compared to the model group. Notably, the reduction in proteinuria was more pronounced in the TCM plus Western medicine group, suggesting that QingreHuoxue formula has a synergistic effect with cyclosporine A on reducing proteinuria. Additionally, IgG expression was lower in the TCM group compared to the model group, indicating that the QingreHuoxue formula can modulate immune responses. Flow cytometric analysis further revealed that the Th17 cell count was elevated, while the Treg count was decreased in the model group. Following the interventions, the number of Th17 cells decreased and the number of Tregs increased in the TCM group, indicating the restoration of the abnormal T lymphocyte counts observed in IMN. These results suggest that TCM exerts an immunomodulatory effect by regulating the number of Th17 cells and Tregs in vivo.
The expression levels of IL-17 and TGF-β1 in renal tissues of IMN mice were also assessed. The results showed that IL-17 expression in renal tissues correlated with the number of Th17 cells in peripheral blood, being significantly upregulated in the model group. However, IL-17 was notably downregulated in the renal tissues of mice across all treatment groups. Conversely, TGF-β1 expression was more pronounced in the renal tissues of the model group. Surprisingly, following treatment, TGF-β1 levels were downregulated in all three treatment groups. This unexpected finding may be attributed to TGF-β1’s role as a multi-potent cytokine with a broad range of actions and distributions. TGF-β1 is involved in various physiologic and pathologic processes and can be secreted and expressed by both innate and infiltrating inflammatory cells in the kidneys. Previous studies have reported increased TGF-β1 levels in renal tissues during immune responses, inflammation, and ischemia [27,28]. Thus, multiple factors could interfere with the detection of TGF-β1, and errors during experimental measurement cannot be ruled out. Further studies are required to confirm these findings and elucidate the underlying mechanisms.
Disclosure of conflict of interest
None.
References
- 1.Zou H, Jiang F, Xu G. Effectiveness and safety of cyclophosphamide or tacrolimus therapy for idiopathic membranous nephropathy. Ren Fail. 2019;41:673–681. doi: 10.1080/0886022X.2019.1637758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofstra JM, Wetzels JF. Introduction of a cyclophosphamide-based treatment strategy and the risk of ESRD in patients with idiopathic membranous nephropathy: a nationwide survey in the Netherlands. Nephrol Dial Transplant. 2008;23:3534–3538. doi: 10.1093/ndt/gfn350. [DOI] [PubMed] [Google Scholar]
- 3.Liu D, Yang Y, Kuang F, Qing S, Hu B, Yu X. Risk of infection with different immunosuppressive drugs combined with glucocorticoids for the treatment of idiopathic membranous nephropathy: a pairwise and network meta-analysis. Int Immunopharmacol. 2019;70:354–361. doi: 10.1016/j.intimp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Li X, Huang T, Xu G. Efficacy and safety of 12 immunosuppressive agents for idiopathic membranous nephropathy in adults: a pairwise and network meta-analysis. Front Pharmacol. 2022;13:917532. doi: 10.3389/fphar.2022.917532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Z, Liu W, Gao H, Chen W, Ge W, Li F, Deng Y. Traditional Chinese Medicine as an adjunct therapy in the treatment of idiopathic membranous nephropathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251131. doi: 10.1371/journal.pone.0251131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CX, Liu Y, Zhang YZ, Li JC, Lai J. Astragalus polysaccharide: a review of its immunomodulatory effect. Arch Pharm Res. 2022;45:367–389. doi: 10.1007/s12272-022-01393-3. [DOI] [PubMed] [Google Scholar]
- 10.Song B, Li P, Yan S, Liu Y, Gao M, Lv H, Lv Z, Guo Y. Effects of dietary astragalus polysaccharide supplementation on the Th17/treg balance and the gut microbiota of broiler chickens challenged with necrotic enteritis. Front Immunol. 2022;13:781934. doi: 10.3389/fimmu.2022.781934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomas NM, Dehde S, Meyer-Schwesinger C, Huang M, Hermans-Borgmeyer I, Maybaum J, Lucas R, von der Heide JL, Kretz O, Köllner SMS, Seifert L, Huber TB, Zahner G. Podocyte expression of human phospholipase A2 receptor 1 causes immune-mediated membranous nephropathy in mice. Kidney Int. 2023;103:297–303. doi: 10.1016/j.kint.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Jao TM, Wu CZ, Cheng CW, Guo CH, Bai CY, Chang LC, Fang TC, Chen JS. Urokinase plasminogen activator deficiency aggravates cationic bovine serum albumin-induced membranous nephropathy through T helper cell type 2-prone immune response in mice. Lab Invest. 2023;103:100146. doi: 10.1016/j.labinv.2023.100146. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Zuo K, Wu Y, Huang Q, Qin WS, Zeng CH, Li LS, Liu ZH. Correlation between B lymphocyte abnormality and disease activity in patients with idiopathic membranous nephropathy. J Int Med Res. 2011;39:86–95. doi: 10.1177/147323001103900111. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, Klatzmann D, Ronco P. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017;92:227–237. doi: 10.1016/j.kint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Cremoni M, Brglez V, Perez S, Decoupigny F, Zorzi K, Andreani M, Gérard A, Boyer-Suavet S, Ruetsch C, Benzaken S, Esnault V, Seitz-Polski B. Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol. 2020;11:574997. doi: 10.3389/fimmu.2020.574997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motavalli R, Etemadi J, Soltani-Zangbar MS, Ardalan MR, Kahroba H, Roshangar L, Nouri M, Aghebati-Maleki L, Khiavi FM, Abediazar S, Mehdizadeh A, Hojjat-Farsangi M, Mahmoodpoor A, Kafil HS, Zolfaghari M, Ahmadian Heris J, Yousefi M. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine. 2021;141:155452. doi: 10.1016/j.cyto.2021.155452. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Wu H, Guo Q, Yu H, Xu Y, Yu J, Wang Z, Yi H. Myeloid-derived suppressor cells promote the progression of primary membranous nephropathy by enhancing Th17 response. Front Immunol. 2020;11:1777. doi: 10.3389/fimmu.2020.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, Somers MJ, Trachtman H, Waldman M. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–441. doi: 10.1053/j.ajkd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 19.van den Brand JA, van Dijk PR, Hofstra JM, Wetzels JF. Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol. 2014;25:150–158. doi: 10.1681/ASN.2013020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Logt AE, Hofstra JM, Wetzels JF. Pharmacological treatment of primary membranous nephropathy in 2016. Expert Rev Clin Pharmacol. 2016;9:1463–1478. doi: 10.1080/17512433.2016.1225497. [DOI] [PubMed] [Google Scholar]
- 22.Zheng R, Deng Y, Chen Y, Fan J, Zhang M, Zhong Y, Zhu R, Wang L. Astragaloside IV attenuates complement membranous attack complex induced podocyte injury through the MAPK pathway. Phytother Res. 2012;26:892–898. doi: 10.1002/ptr.3656. [DOI] [PubMed] [Google Scholar]
- 23.Chen DQ, Cao G, Zhao H, Chen L, Yang T, Wang M, Vaziri ND, Guo Y, Zhao YY. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and β-catenin pathway in AKI-to-CKD continuum. Ther Adv Chronic Dis. 2019;10:2040622319869116. doi: 10.1177/2040622319869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen DQ, Feng YL, Chen L, Liu JR, Wang M, Vaziri ND, Zhao YY. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFκB/Nrf2 axis. Free Radic Biol Med. 2019;134:484–497. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Feng YL, Chen DQ, Vaziri ND, Guo Y, Zhao YY. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med Res Rev. 2020;40:54–78. doi: 10.1002/med.21596. [DOI] [PubMed] [Google Scholar]
- 26.Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. doi: 10.1016/j.arr.2020.101063. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Lu X, Dong L, Ma J, Fan X. Clinical observation on the effect of Wuzhi soft capsule on FK506 concentration in membranous nephropathy patients. Medicine (Baltimore) 2019;98:e18150. doi: 10.1097/MD.0000000000018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroo I, Stokman G, Teske GJ, Raven A, Butter LM, Florquin S, Leemans JC. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]