Abstract
Objective: To investigate the pro-apoptotic effects of Indirubin, a traditional Chinese medicine, on ovarian cancer SKOV3 cell line and to explore its underlying mechanisms. Methods: Ovarian cancer SKOV3 cells were divided into a control group (cells cultured normally) and an experimental group (cells cultured in medium containing Indirubin). SKOV3 cells at the logarithmic phase were treated with Indirubin at various concentrations. Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assay and 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) assay, while apoptosis was detected by flow cytometry, TdT-mediated dUTP Nick-End Labeling (TUNEL) staining, and immunofluorescence. Transcriptome sequencing was conducted to screen for apoptosis-related factors. qPCR and western blot were used to detect the mRNA and protein expression on added of molecules related to mitochondrial permeability transition pores. Results: MTT assay showed that Indirubin inhibited the growth of SKOV3 cells in both plate and sphere cultures, with IC50 values of 3.003 μM (plate culture) and 4.253 μM (sphere culture), respectively. Indirubin showed a lower inhibitory concentration than cisplatin (IC50 3.687 μM in plate culture and 7.023 μM in sphere culture), and its effect was comparable to adriamycin. Flow cytometry revealed an increase in apoptosis rates in SKOV3 cells treated with Indirubin. Transcriptome sequencing indicated significant changes in the transcription of various apoptosis-related genes, particularly those involved in the mitochondrial apoptosis pathway, such as Bcl-2-associated X protein (Bax) and Bcl2 associated agonist of cell death (Bad). After Indirubin treatment, the mRNA and protein expression levels of mitochondrial channel-related genes Cyclophilin D (CyPD), adenine nucleotide translocator 1 (ANT1), and voltage-dependent anion channel (VADC) were significantly elevated (all P < 0.05). By regulating the mitochondrial membrane permeability through the Bcl-2 family members, Indirubin promoted apoptosis in SKOV3 cells. Conclusion: Indirubin inhibits the proliferation and promotes the apoptosis of ovarian cancer cells, exerting an anti-tumor effect. Its pro-apoptotic action is closely related to the mitochondrial apoptosis pathway.
Keywords: Ovarian cancer, apoptosis, Indirubin
Introduction
Ovarian cancer is one of the major malignancies affecting the female reproductive system. Due to its insidious onset, 70-80% of ovarian cancer patients are diagnosed at an advanced stage, leading to the highest mortality rate among gynecological malignancies [1]. Epithelial ovarian cancer, the most common pathological type, accounts for approximately 95% of malignant ovarian tumors [2]. The management of epithelial ovarian cancer involves a combination of surgical intervention and platinum-based chemotherapy. Despite achieving complete remission with first-line treatment, approximately 70-85% of ovarian cancer patients experience relapse, with a median survival time ranging from 12 to 24 months in cases of recurrence [3]. The advent of molecular diagnosis and targeted therapy has significantly improved the median survival time and overall survival time of ovarian cancer patients [4]. Nevertheless, the application of targeted therapy depends on breast cancer susceptibility (BRCA) gene mutation and homologous recombination deficiency (HRD) status. Nearly 50% of patients cannot benefit from BRAC gene mutation and therefore derive limited benefit from targeted therapy, particularly in cases of recurrence and drug resistance.
Indirubin, an active ingredient isolated from the traditional Chinese medicine Qing Dai, is a bisindole anti-cancer drug that has demonstrated inhibitory effects on various types of tumor xenografts in animal models [5]. Indirubin has a variety of biological functions, including immunosuppression, anti-inflammatory response, and tumor inhibition. It has been applied in the treatment of leukemia and has also shown therapeutic potential in breast cancer. The role of Indirubin in the treatment of ovarian cancer has been preliminarily explored and studied. Indirubin exerts its anti-tumor effects by inhibiting cell cycle-related kinases such as cyclin-dependent kinases (CDKs) and glycogen synthase kinase-3β (GSK-3β), thereby suppressing tumor cell proliferation and metastasis, and promoting apoptosis through multiple pathways [5,6]. While its anti-cancer effects may involve multiple synergistic mechanisms, the precise pathways by which Indirubin inhibits ovarian cancer remain unclear.
Our previous study found that Indirubin could promote the apoptosis of ovarian cancer SKOV3 cell line [7]. However, the specific mechanism remains elusive. This study focuses on the effects of Indirubin on SKOV3 cell line and to explore the mitochondrial pathway of apoptosis, aiming to elucidate the potential mechanisms by which Indirubin induces apoptosis.
Materials and methods
Cells, instruments, and reagents
Cells: Ovarian cancer SKOV3 cell line was purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences.
Instruments: Refrigerators for targeted temperatures of -80°C, -20°C, and 4°C (Thermo, USA); a biosafety cabinet; autoclave; cell culture dishes; centrifuge tubes; cryopreservation tubes; cell collection plates; hemocytometer; fluorescence inverted microscope; room temperature centrifuge; low temperature centrifuge; flow cytometer; cell technology instrument; electrophoresis apparatus; and membrane transfer device.
Reagents: Indirubin was purchased from GLPBIO (Good Laboratory Technology Inc., San, USA, Cat. No.: GC12975). RPMI 1640 medium, fetal bovine serum (FBS), and trypsin were purchased from Gibco (USA). CCK-8 (Cat. No.: C0037) and TUNEL apoptosis assay kits (Cat. No.: C1089) were purchased from Beyotime Biotechnology (Shanghai, China). Primary antibodies were purchased from BIOSS (Beijing, China), including rabbit anti-VDAC1 antibody (Cat. No.: bsm-52251R), rabbit anti-VDAC antibody (Cat. No.: bs-23683R), rabbit anti-ANT-1 antibody (Cat. No.: bs-6794R), rabbit anti-Cyclophilin 40/PPID antibody (Cat. No.: bs-9878R) and rabbit anti-GAPDH antibody (Cat. No.: bsm-52262R). Anti-mouse IgG peroxidase conjugate was purchased from Zhongshan Jinqiao Biotechnology Co., Ltd., (Beijing, China, Cat. No.: ZF-0317).
Cell culture
The SKOV3 cell line was cultured in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin in a 37°C incubator with 5% CO2. Cells were passaged when they reached 80-90% confluence. Due to their strong adherence, cells were passaged using 0.25% trypsin. Drug interventions were performed when the cell density reached 50-60%.
Drug preparation
Indirubin powder was dissolved in dimethyl sulfoxide (DMSO) to prepare a 10 mM stock solution by incubating it at 37°C for 10 minutes and ultrasonication for 2 minutes. The stock solution was stored at -20°C. For experimental use, the stock solution was diluted with complete RPMI 1640 medium to achieve final concentrations of 0, 0.1, 0.5, 1, 2, 3, 4, 5, 6, 7, and 10 μM. Cisplatin was prepared in concentrations of 0, 0.1, 0.5, 1, 5, 10, 15, 20, 25, and 30 μM. Adriamycin as prepared in a concentration gradient of 0, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 3, 4, 5, 6, 7, 8, 10 μM. Paclitaxel was prepared in a concentration gradient of 0, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, and 20 μM.
Transcriptome sequencing
SKOV3 cells were seeded in 25 ml culture flasks, with control (normal culture) and experimental (Indirubin treatment) groups, each having three replicates. After 24 hours of incubation, cells were treated with complete medium or medium containing 10 μmol/L Indirubin for 4 hours. Cells were collected, lysed with 1 ml Trizol (TaKaRa, Dalian, China) per flask, and stored in liquid nitrogen before being sent to Novogene for sequencing.
CCK-8 cell viability assay and MTT assay
SKOV3 cells were seeded in 96-well plates at a density of 3 × 103 cells per well and cultured for 24 hours to allow adherence. Cells were then incubated with Indirubin, Cisplatin, Adriamycin and Paclitaxel at gradient concentrations. 20 μl CCK-8 solution was added to each well of the plate containing Cisplatin, Adriamycin, and Paclitaxel medium. 20 μl MTT solution was added to each well of the plate containing indirubin medium. The 96-well plate containing CCK-8 solution was incubated in an incubator until the OD value of the control group was between 0.8 and 1.2. The 96-well plate containing MTT solution was incubated in the incubator for 3 hours, then immediately removed and added with 100 μl of the solution and placed on the shaker for 5 minutes before detection. For the CCK-8 assay, the OD value of cells at 450 nm was detected by microplate reader. For the MTT assay, the OD value of cells at 490 nm was detected. Cell viability was calculated using the formula: cell viability (%) = (A (substance) - A (blank))/(A (0 substance) - A (blank)) × 100.
Flow cytometry
SKOV3 cells were seeded and incubated until they reached 50-60% confluence, then treated with Indirubin at gradient concentrations (0, 2, 5 μM) for 24 hours. Cells were digested with EDTA-free trypsin to prepare a suspension of 1 × 106 cells/ml. After three washes with PBS, the cells were stained using the TUNEL one-step apoptosis assay kit according to the manufacturer’s instructions, with 50 μl of staining solution added per sample and incubated at 37°C in the dark for 30 minutes. After three PBS washes, the cells were resuspended in 100 μl PBS and analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using CellQuest Pro and BD associated software. The apoptosis rate was calculated as: apoptosis rate = (early apoptosis cells) + (late apoptosis cells)/(total cells) × 100%.
Immunofluorescence
SKOV3 cells were seeded on a round cell slide, with the control group (Indirubin concentration: 0 μM) and the experimental group (Indirubin concentration: 10 μM). The cells were permeabilized using Triton X-100. Apoptosis was observed under a fluorescence microscopy after staining with Cy3 (red fluorescence) and DAPI (blue fluorescence).
qRT-PCR
SKOV3 cells were seeded and incubated until they reached 50-60% confluence, followed by treatment with Indirubin at gradient concentrations (0, 2, 5, 10, 20 μM) for 24 hours. Total RNA was extracted using the TRIzol kit, and 2 μg of RNA was reverse-transcribed into cDNA. β-actin was used as the reference gene. The qRT-PCR mixture (20 μl) contained 10 μl 2× TB Green Premix Ex Taq II, 100 ng cDNA, 0.8 μl forward and reverse primers (10×), and sterile enzyme-free water to a final volume of 20 μl. The amplification conditions were as follows: 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds and 60°C for 20 seconds. CT values were recorded, and gene expression levels were analyzed using the 2-ΔΔCT method. Specific primer sequences for the target genes are listed in Table 1.
Table 1.
Primer sequences for the target genes
| Oligo name | Sequence (5’ to 3’) |
|---|---|
| GAPDH | F: GGAGCGAGATCCCTCCAAAAT |
| R: GGCTGTTGTCATACTTCTCATGG | |
| Cyclophilin D, CypD | F: CGCTTTCCTGACGAGAACTTT |
| R: TCTTTGACGTGACCGAACACA | |
| Adenine nucleotide translocase 1, ANT1 | F: CTCTCCTTCTGGAGGGGTAAC |
| R: GAACTGCTTATGCCGATCCAC | |
| Voltage-dependent anion channel 1, VDAC1 | F: CTGACCTTCGATTCATCCTTCTC |
| R: CTCCCGCTTGTACCCTGTC | |
| Voltage-dependent anion channel 2, VDAC2 | F: GGCGTGGAATTTTCAACGTCC |
| R: AGACCATACTCACACCACTTGTA |
Western blot analysis
Cells were lysed to extract total protein for western blot analysis as previously described [7]. Primary antibodies used for immunodetection included anti-CyPD, anti-ANT1, anti-VDAC1, anti-VDAC, and secondary antibodies were anti-rabbit and anti-mouse IgG peroxidase conjugate. GAPDH was used as a loading control. The results were quantified by densitometry, using Image J software (NIH, Bethesda, MD). Results were expressed as a ratio of the protein of interest to GAPDH to correct for variations in sample loading.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, California, USA). Data were presented as mean ± standard deviation. Analysis of variance (ANOVA) was used to assess differences in cell viability and apoptosis. For multiple group comparisons, one-way ANOVA was used, followed by Tukey’s and Dunnett’s post-hoc tests for pairwise comparisons. A P-value of < 0.05 was considered statistically significant.
Results
Indirubin inhibited SKOV3 cell viability
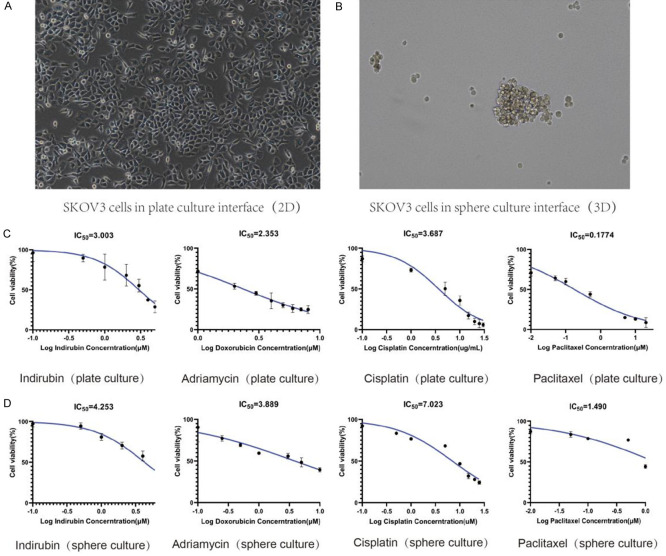
SKOV3 cell line was incubated with different concentrations of Indirubin and the first-line chemotherapeutic drugs (Paclitaxel, Cisplatin, Adriamycin) commonly used in ovarian cancer therapy. MTT assay showed that Indirubin inhibited the viability of SKOV3 cells in both plate and sphere cultures (Figure 1A, 1B). The IC50 of Indirubin was 3.003 μM for plate culture and 4.253 μM for sphere culture, which were lower than the IC50 values of Cisplatin (IC50 of 3.687 μM in plate culture and 7.023 μM in sphere culture), demonstrating superior efficacy over Cisplatin in inhibiting SKOV3 cells (Figure 1C, 1D).
Figure 1.
The inhibitory effect of Indirubin and commonly used chemotherapeutic drugs on the proliferation of SKOV3 ovarian cancer cells under different culture methods. A: SKOV3 cells in plate culture interface (×200). B: SKOV3 cells in sphere culture interface (×200). C: MTT assay showed the inhibitory effect of Indirubin, paclitaxel, cisplatin and adriamycin on SKOV3 cells by plate culture. D: MTT assay showed the inhibitory effect of Indirubin, paclitaxel, cisplatin and adriamycin on SKOV3 cells by sphere culture.
Indirubin promoted cell apoptosis
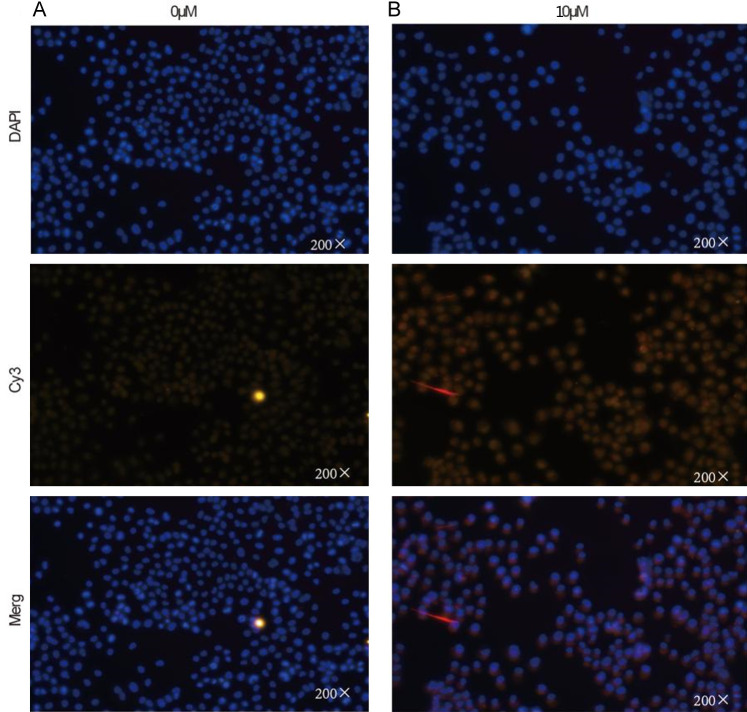
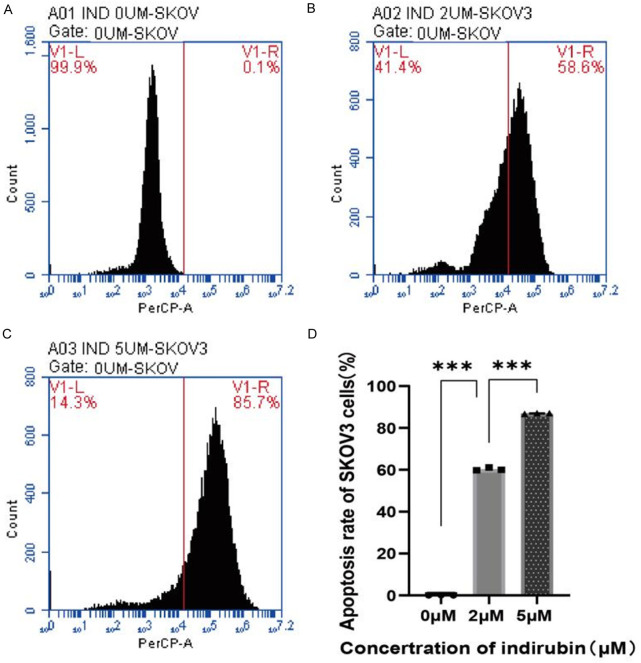
Cells were seeded in 6-well plates and allowed to adhere. After adhesion, the cells were incubated with complete medium and 10 μmol/L Indirubin for 24 hours. In situ staining was performed using the Elabscience TUNEL kit (red fluorescence), followed by DAPI counterstaining (blue fluorescence). As shown in Figure 2, the number of apoptotic cells increased significantly after Indirubin incubation. SKOV3 cells were seeded in 10 cm dishes, and after adhesion, SKOV3 cells were treated with various concentrations of Indirubin (0, 2, 5 μmol/L) for 24 hours. Cells were stained with the TUNEL kit and analyzed by flow cytometry. The results showed a significant increase in apoptosis with increasing concentrations of Indirubin (Figure 3). Compared to the control group, the proportion of apoptotic cells was significantly higher in the 2 μmol/L and higher concentration groups (P < 0.001). Additionally, compared to the 2 μmol/L group, the apoptosis rate increased in the 5 μmol/L groups (P < 0.001).
Figure 2.
The effect of Indirubin on SKOV3 cell apoptosis detected by immunofluorescence techniques. A: Immunofluorescent staining of SKOV3 cells incubated with complete medium after 24 hours (×200). B: Immunofluorescent staining of SKOV3 cells incubated with Indirubin after 24 hours (×200).
Figure 3.
The effect of Indirubin at different concentrations on SKOV3 cell apoptosis detected by flow cytometry. A: Apoptosis at concentration of 0 μmol/L; B: Apoptosis at concentration of 2 μmol/L; C: Apoptosis at concentration of 5 μmol/L; D: Quantified results of cell apoptosis. ***P < 0.001.
Indirubin induced mitochondrial apoptosis
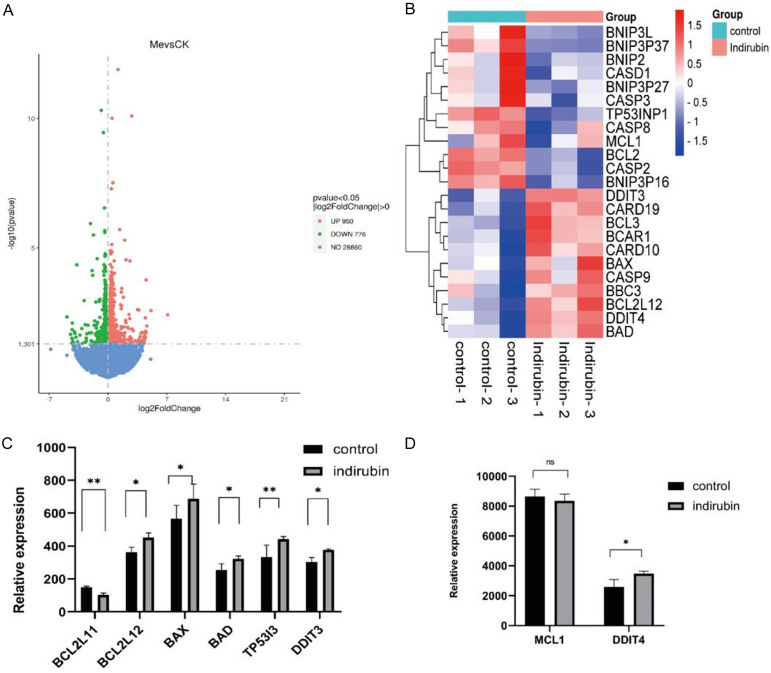
As shown in Figure 4, transcriptome sequencing revealed significant changes in the expression of several apoptosis-related genes after 4-hour incubation with 10 μmol/L Indirubin. Hierarchical analysis of these genes revealed a significant impact on the mitochondrial apoptosis pathway. Specifically, the expression levels of BCL2L11 were significantly higher in the experimental group (150.19 ± 6.07) compared to the control group (103.83 ± 10.57). Additionally, expressions of BCL2L12, BAX, BAD, DDIT3, DDIT4, and the tumor suppressor gene TP53I3 were all elevated (FC=2, all P < 0.05).
Figure 4.
Transcriptome sequencing analysis of the impact of Indirubin on the expression of apoptosis-related genes. A: Effects of Indirubin on gene expression in ovarian cancer SKOV3 cell line analyzed by Transcriptome sequencing analysis; B: Hierarchical clustering analysis of genes with differential expression; C, D: The effects of Indirubin on the expression of mitochondrial apoptosis associated genes. *P < 0.05, **P < 0.01.
Indirubin promoted apoptosis by altering mitochondrial membrane permeability
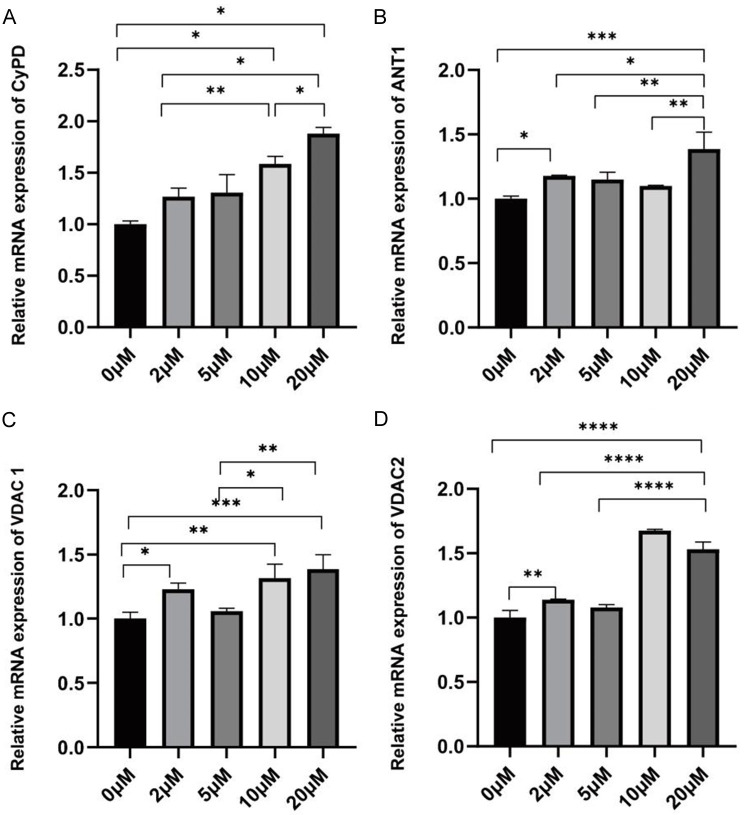
qPCR results showed that the mRNA expression levels of CypD, ANT1, VADC1, and VADC2 in SKOV3 cells were significantly elevated in the Indirubin-treated groups (0, 2, 5, 10, 20 μmol/L) compared to the control group (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001), displaying a dose-dependent relationship, as illustrated in Figure 5.
Figure 5.
Effects of Indirubin on the expression of mitochondrial membrane permeability associated genes. A: mRNA expression of CypD; B: mRNA expression of ANT1; C: mRNA expression of VADC1; D: mRNA expression of VADC2. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
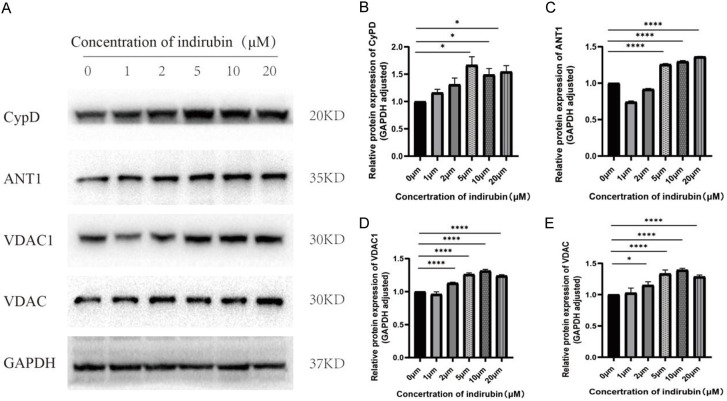
Western blot was used to examine the protein expression of CypD, ANT1, VADC1, and VADC. As shown in Figure 6, dose-dependent increase in the expression of these proteins was observed in SKOV3 cells after treatment with escalating concentrations of Indirubin (0 μm, 1 μm, 2 μm, 5 μm, 10 μm, 20 μm) for 6 hours.
Figure 6.
Effects of Indirubin on the expression of mitochondrial membrane permeability associated proteins. (A) Representative western blots for CypD, ANT1, VADC1, and VADC in SKOV3 cells treated with various concentrations of Indirubin (0, 1, 2, 5, 10, 20 μmol/L); (B-E) Quantified expression levels of CyPD (B), ANT1 (C), VDAC1 (D) and VDAC (E). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Discussion
Ovarian cancer is one of the three major malignant tumors of the female reproductive system, with an incidence rate second only to cervical and endometrial cancer. Globally, approximately 31,400 new cases and 207,000 deaths occur annually worldwide due to ovarian cancer [8]. The disease is characterized by insidious onset and rapid progression, making it the deadliest gynecological malignancy [9,10].
In the 1980s, Indirubin was first used in China for the treatment of chronic myeloid leukemia (CML), with over 50% of patients achieving partial or complete remission, comparable to standard treatment with cytotoxic agent busulfan [11,12]. Numerous studies have demonstrated that Indirubin has excellent anti-cancer, anti-inflammatory, and neuroprotective properties [13,14]. Indirubin and its derivatives regulate the expression of key molecules such as CDKs, GSK-3β, Bax, Bcl-2, c-Myc, matrix metalloproteinases (MMPs), and focal adhesion kinase (FAK), primarily through signaling pathways like PI3K/AKT/mTOR, NF-κB, MAPK, and JAK/STAT3, thereby exerting anti-cancer effects [5]. Nam et al. found that Indirubin and its derivatives (E564, E728, and E804) inhibited the proliferation and promoted the apoptosis of breast and prostate cancer cells by inhibiting STAT3 [15]. These drugs may inhibit oncogenes such as c-Myc and activate Bax, exerting anti-tumor effects. Additionally, Li et al. reported that Indirubin promoted apoptosis by altering the expression of proteins such as Bax, Bcl-2, and PARP, while also inducing autophagy by upregulating the expression of LC3BI, LC3BII, ATG12, and ATG5 [16].
It is well established that the activation of mitochondrial apoptosis pathway is a primary mechanism through which cells undergo damage in response to external stressors or drugs [17]. Our previous studies found that Indirubin can inhibit the proliferation of ovarian cancer cells and promote their apoptosis [7]. To further explore the mechanism of Indirubin-induced apoptosis, we performed transcriptome sequencing and hierarchical analysis of apoptosis-related genes. We discovered that Indirubin significantly affected mitochondrial apoptosis-related molecules, leading to increased expression of pro-apoptotic genes such as BCL2L12, BAX, and BAD after Indirubin treatment.
The Bcl-2 family is an important group of mitochondrial apoptosis regulators, divided into two categories based on their function and structure: anti-apoptotic members (e.g., Bcl-2, Bcl-xl, Bcl-w, Mcl-1) and pro-apoptotic members (e.g., Bax, Bad, Bid, Bak) [18-22]. Bcl-2 proteins are located in the mitochondria, endoplasmic reticulum, and nuclear outer membrane, primarily on the mitochondrial membrane, where they regulate transmembrane channels or the mitochondrial permeability transition pore (MPTP), activating caspases and leading to apoptosis [23,24]. The mitochondrial PT pore is composed of proteins such as adenine nucleotide translocator (ANT) in the inner mitochondrial membrane and voltage-dependent anion channel (VDAC) in the outer membrane, regulated by multiple mitochondrial proteins [25,26]. Cyclophilin D (CypD), a peptidyl-prolyl cis-trans isomerase, plays a crucial role in MPTP formation and regulation [27]. CypD is a convergence point for various cell death pathways, making it a key regulator of apoptosis across different disease conditions. Our qPCR analysis of mitochondrial channel proteins ANT1, VDAC1, VDAC2, and CypD revealed an increasing trend in gene expression, suggesting that Indirubin may promote apoptosis by upregulating the expression of pro-apoptotic Bcl-2 family members such as Bax and Bid, thereby regulating mitochondrial membrane permeability.
In conclusion, Indirubin inhibits the proliferation and induces the apoptosis of ovarian cancer SKOV3 cell line. The underlying mechanism may involve altering mitochondrial membrane permeability, thereby inducing mitochondrial pathway apoptosis. This study provides valuable experimental evidence for the potential use of Indirubin in the treatment of ovarian cancer.
Acknowledgements
This study was supported by the Fujian Provincial Health Technology Project (grant number 2018-CX-28), and Joint Funds for the Innovation of Science and Technology, Fujian Province (grant numbers 2019Y9130 and 2023Y9051).
Disclosure of conflict of interest
None.
Abbreviations
- CCK-8
Cell Counting Kit-8
- TUNEL
TdT-mediated dUTP Nick-End Labeling
- CDKs
cyclin-dependent kinases
- GSK-3β
glycogen synthase kinase-3β
- FBS
fetal bovine serum
- CST
Cell Signaling Technology
- ANOVA
analysis of variance
- CML
chronic myeloid leukemia
- MMPs
matrix metalloproteinases
- FAK
focal adhesion kinase
- ANT
adenine nucleotide translocator
- VDAC
voltage-dependent anion channel
- CypD
Cyclophilin D
References
- 1.Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Webb PM, Jordan SJ. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. 2024;21:389–400. doi: 10.1038/s41571-024-00881-3. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Nag S, Aggarwal S, Rauthan A, Warrier N. Maintenance therapy for recurrent epithelial ovarian cancer: current therapies and future perspectives - a review. J Ovarian Res. 2019;12:103. doi: 10.1186/s13048-019-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans T, Matulonis U. PARP inhibitors in ovarian cancer: evidence, experience and clinical potential. Ther Adv Med Oncol. 2017;9:253–267. doi: 10.1177/1758834016687254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Li X, Huang W, Rao X, Lai Y. Pharmacological properties of indirubin and its derivatives. Biomed Pharmacother. 2022;151:113112. doi: 10.1016/j.biopha.2022.113112. [DOI] [PubMed] [Google Scholar]
- 6.Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, Eisenbrand G, Meijer L. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Wang J, Wu J, Zheng Q, Hu J. Indirubin suppresses ovarian cancer cell viabilities through the STAT3 signaling pathway. Drug Des Devel Ther. 2018;12:3335–3342. doi: 10.2147/DDDT.S174613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sideris M, Menon U, Manchanda R. Screening and prevention of ovarian cancer. Med J Aust. 2024;220:264–274. doi: 10.5694/mja2.52227. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SR, Gore ME. Caelyx: phase II studies in ovarian cancer. Eur J Cancer. 2001;37(Suppl 9):S8–14. doi: 10.1016/s0959-8049(01)00329-x. [DOI] [PubMed] [Google Scholar]
- 10.Knisely AT, St Clair CM, Hou JY, Collado FK, Hershman DL, Wright JD, Melamed A. Trends in primary treatment and median survival among women with advanced-stage epithelial ovarian cancer in the US from 2004 to 2016. JAMA Netw Open. 2020;3:e2017517. doi: 10.1001/jamanetworkopen.2020.17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, Niederberger E, Tang W, Eisenbrand G, Meijer L. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 12.Nam S, Scuto A, Yang F, Chen W, Park S, Yoo HS, Konig H, Bhatia R, Cheng X, Merz KH, Eisenbrand G, Jove R. Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling. Mol Oncol. 2012;6:276–283. doi: 10.1016/j.molonc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosuge Y, Saito H, Haraguchi T, Ichimaru Y, Ohashi S, Miyagishi H, Kobayashi S, Ishige K, Miyairi S, Ito Y. Indirubin derivatives protect against endoplasmic reticulum stress-induced cytotoxicity and down-regulate CHOP levels in HT22 cells. Bioorg Med Chem Lett. 2017;27:5122–5125. doi: 10.1016/j.bmcl.2017.10.069. [DOI] [PubMed] [Google Scholar]
- 14.Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 2006;281:23425–23435. doi: 10.1074/jbc.M602627200. [DOI] [PubMed] [Google Scholar]
- 15.Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, Jove R. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci U S A. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Wang H, Wei J, Han L, Guo Z. Indirubin exerts anticancer effects on human glioma cells by inducing apoptosis and autophagy. AMB Express. 2020;10:171. doi: 10.1186/s13568-020-01107-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A, Arif T. Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front Oncol. 2017;7:154. doi: 10.3389/fonc.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 20.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalier L, Vallette F, Manon S. Bcl-2 family members and the mitochondrial import machineries: the roads to death. Biomolecules. 2022;12:162. doi: 10.3390/biom12020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Orsi B, Mateyka J, Prehn JHM. Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem Int. 2017;109:162–170. doi: 10.1016/j.neuint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Vander Heiden MG, Thompson CB. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol. 1999;1:E209–216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Jia P, Huang Z, Liu S, Miao J, Guo Y, Wu N, Jia D. Lycopene protects against myocardial ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Drug Des Devel Ther. 2019;13:2331–2342. doi: 10.2147/DDDT.S194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182–1191. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- 26.Hu T, Zou HX, Le SY, Wang YR, Qiao YM, Yuan Y, Liu JC, Lai SQ, Huang H. Tanshinone IIA confers protection against myocardial ischemia/reperfusion injury by inhibiting ferroptosis and apoptosis via VDAC1. Int J Mol Med. 2023;52:109. doi: 10.3892/ijmm.2023.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou D, Hu F, Mao Y, Yan L, Zhang Y, Zheng Z, Wu A, Forouzanfar T, Pathak JL, Wu G. Cationic antimicrobial peptide NRC-03 induces oral squamous cell carcinoma cell apoptosis via CypD-mPTP axis-mediated mitochondrial oxidative stress. Redox Biol. 2022;54:102355. doi: 10.1016/j.redox.2022.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]