Abstract
Objective: To investigate the prognostic value of interleukin (IL)-10 and glial fibrillary acidic protein (GFPA) in patients with brain glioma following surgery. Methods: The clinical data of 75 patients with brain glioma (Observation group) who were treated at the Central Hospital Affiliated with Shandong First Medical University were retrospectively analyzed. Additionally, 40 healthy volunteers who underwent physical examination during the same period were selected as the control group. The serum levels of IL-10 and GFPA were compared between the observation group and the control group as well as across different clinicopathological characteristics of the glioma patients. Multivariate logistic regression analysis was used to identify factors influencing postoperative brain injury in patients with brain gliomas. Receiver operating characteristic (ROC) curve was used to analyze the predictive value of preoperative IL-10 and GFPA levels for patient prognosis. Results: Serum levels of IL-10 and GFPA in the observation group were significantly higher than those in the control group (all P<0.05). Before surgery, there was no significant differences in serum IL-10 and GFPA levels between the mild- and moderate-severity group; however, postoperatively, both markers were elevated in the moderate-severity group compared to the mild group (all P<0.05). Patients with World Health Organization (WHO) grade III-IV, mutant protein 53 (P53) genotype, and high Ki-67 expression exhibited higher IL-10 levels than their counter parts. However, the trends were conversely observed for GFAP. Pearson correlation analysis showed that IL-10 and GFPA levels were negatively associated with Glasgow Coma Scale (GCS) scores after surgery. Logistic regression analysis showed that IL-10 and GFPA were the independent risk factors for postoperative brain injury in glioma patients. The area under the curve (AUC) of IL-10 and GFPA for predicting the postoperative brain injury in glioma patients were 0.718 and 0.745, respectively; while their combined detection achieved an AUC of 0.835, which was significantly higher than each single detection. Conclusion: Elevated serum IL-10 and GFPA levels in patients with brain glioma are associated with postoperative brain injury. IL-10 and GFPA can serve as valuable indicators for the prognostic evaluation of patients with brain gliomas.
Keywords: Brain glioma, Interleukin-10, glial fibrillary acidic protein, prognosis
Introduction
Glioma is a primary neurological tumor characterized by symptoms such as headache, epilepsy and neurological disorders [1]. Studies showed that the incidence rate of glioma in China ranges from 5 to 8 per 100,000, with a 5-year morbidity and mortality rate ranked third among systemic tumors, following pancreatic cancer and lung cancer [2]. In recent years, advancements in surgical techniques, chemotherapy, radiotherapy, and biological agents have improved the average survival rate of patients with malignant gliomas. Currently, surgical resection remains the primary treatment; however, the unique location of glioma lesions, coupled with high degree of infiltration, complicates complete removal, as the boundary of the lesions can be difficult to distinguish visually. Additionally, intraoperative stretching and electrocoagulation may lead to secondary brain injury, resulting in abnormal neurological function and adversely affecting treatment outcomes and prognosis [3]. Studies found that the grade of malignancy of gliomas is closely related to the therapeutic effect and prognosis [4]. Histopathological methods to assess the therapeutic effects often face challenges in material acquisition. Therefore, timely assessment of postoperative brain damage in gliomas is crucial for predicting treatment efficacy and prognosis, making the search for specific markers a key focus of current research.
Interleukin-10 (IL-10) is produced by lymphocytes, macrophages, and other immune cells, and is highly expressed in several tumors. It has strong anti-inflammatory effects and can play an immunosuppressive role by inhibiting antigen presentation and dendritic cell (DC) differentiation. This suppression hampers the body’s ability to recognize cancer cells, leading to uncontrolled cell proliferation, immune escape, and mediating the process of tumorigenesis and progression [5]. Some studies reported that IL-10 mainly plays an immunosuppressive role in the occurrence and progression of gliomas [6]. Monitoring IL-10 levels may provide insights into neurological damage associated with conditions like traumatic brain injury and ischemic stroke [7]. Glial fibrillary acidic protein (GFAP) serves as a specific marker for astrocytes, which proliferate and differentiate following neuronal injury, leading to elevated GFAP levels [8]. It has been reported that GFAP-positive expression can reliably reflect the grade of glioma [9], and at the microscopic level, it can reflect the local metabolic status of neural tissue, making GFAP a potential biomarker for nerve injury [10]. However, the predictive value of IL-10 and GFPA for postoperative prognosis in patients with glioma remains under-explored. This study aims to investigate the predictive value of IL-10 and GFPA for postoperative brain injury in glioma patients, providing clinical evidence for treatment and prognosis evaluation.
Material and methods
General information
This retrospective study involved 75 glioma patients (observation group) who were treated at Central Hospital Affiliated to Shandong First Medical University between June 2021 and January 2022. Additionally, 40 healthy subjects who underwent physical examination during the same period were selected as the control group. The Ethics Committee of the Central Hospital Affiliated with Shandong First Medical University approved this research (No. 2020-218).
Inclusion criteria: ① Patients were diagnosed with glioma according to established criteria from previous studies [11], confirmed through imaging and pathological examinations. Healthy subjects were included as controls. ② Patients were first diagnosed and underwent elective tumor resection. ③ Patients had not received the radiotherapy and chemotherapy. ④ Complete medical records were available. Exclusion criteria: ① History of craniocerebral trauma. ② Accompanying cerebral infarction. ③ Severe liver or kidney dysfunction. ④ Severe coagulation disorders. ⑤ Presence of mental disorders. ⑥ Incomplete medical records, including current and past medical history.
After surgery, according to the Glasgow Coma Scale (GCS) scores [12], these patients were divided into a mild group (GCS: 9-15 scores, N=25) and a moderate-severity group (GCS: 3-8 scores, N=50). The GCS was scored based on three subscale scores including verbal (score range 1-5), eye-opening (score range 1-4) and motor responses (score range 1-6), with total scores ranging from 3 to 15. Total scores reflects the severity of injury, with scores of 3-8 indicating severe injury, 9-12 moderate injury, and 13-15 mild injury.
Methods
The clinical data of all patients were collected from medical records, including age, body mass index (BMI), smoking status, hypertension, alcohol consumption, diabetes, and hyperlipaemia, tumor location, tumor diameter, Karnofsky Performance Scale (KPS) scores, World Health Organization (WHO) classification, and expression levels of P53 and Ki-67 (also known as Molecular Immunology Borstel-1).
Detection of serum levels of IL-10 and GFPA: In the morning of the first day after admission (T0) and at 3 days (T1) and 7 days (T2) after surgery, 5 mL of fasting peripheral venous blood were obtained from all patients and then centrifuged at the rate of 3000 r/min for 10 min to acquire the serum. Enzyme-linked immunosorbent assay (ELISA) was used to detect the serum levels of IL-10 (Lot numbers: PI528, Shanghai Beyotime Biotech. Inc) and GFPA (Lot numbers: XG-E988974, Shanghai Sig Biotechnology Co., Ltd.). The test was performed according to the kit instructions.
Statistical methods
All the clinical data collected in this study were analyzed using Statistic Package for Social Science (SPSS) version 22.0. The measurement data were presented as Mean ± Standard deviation, and the comparison was conducted by independent t test. The One-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests was used to identify significant differences among three or more groups. The count data was presented as percentages/cases, and the comparison among groups was performed using χ2 test. The relationship of IL-10 and GFPA levels with GCS was assessed using Pearson correlation analysis. Multiple Logistic regression models, using the forward LR method, were used to identify significant effects of IL-10 and GFPA levels in predicting postoperative brain injury in glioma patients, following previously reported methods [13]. The predictive values of IL-10 and GFPA for postoperative brain injury, including specificity and sensitivity, were calculated based on the previous studies [14]. Receiver operating characteristic (ROC) curve was used to assess the predictive ability of variables with P<0.05 in multiple logistic regression. P<0.05 indicated significant statistical differences.
Results
Comparison of general information between the control group and observation group
As described in Table 1, there was no significant difference in age, BMI, gender, smoking and alcohol consumption, hypertension, diabetes and hyperlipidemia between the observation group and the control group (all P>0.05), indicating that the groups were comparable.
Table 1.
Comparison of general information between the observation group and control group
| Parameters | Control group (N=40) | Observation group (N=75) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 52.15±6.21 | 51.48±5.82 | 1.718 | 0.090 |
| BMI (kg/m2) | 22.19±2.67 | 22.03±2.54 | 0.438 | 0.658 |
| Gender (Male/Female) | 24/16 | 49/26 | 0.490 | 0.473 |
| Smoking (%) | 16 (40.00%) | 33 (44.00%) | 1.850 | 0.067 |
| Drinking (%) | 15 (37.50%) | 26 (34.67%) | 0.648 | 0.419 |
| Hypertension (%) | 14 (35.00%) | 30 (40.00%) | 0.619 | 0.462 |
| Diabetes (%) | 11 (27.50%) | 23 (30.67%) | 0.086 | 0.717 |
| Hyperlipaemia (%) | 8 (20.00%) | 17 (22.67%) | 0.213 | 0.594 |
Note: BMI: Body mass index.
Comparison of serum IL-10 and GFAP levels between the control group and observation group
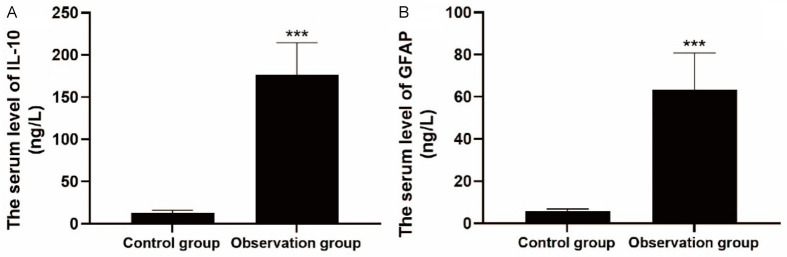
As shown in Figure 1, at the first day after admission, the serum levels of IL-10 and GFAP in the observation group were 176.23±38.52 ng/L and 63.26±17.51 ng/L, respectively, which were significantly higher than those in the control group (all P<0.001).
Figure 1.
Comparison of serum levels of IL-10 (A) and GFAP (B) between the two groups. Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein. ***P<0.001.
Comparison of serum levels of IL-10 and GFAP between the mild and the moderate-severity group
In both groups, the serum levels of IL-10 and GFAP first increased and then decreased compared to their levels at the T0 (all P<0.05). No significant differences were observed in the serum levels of IL-10 and GFAP between the mild and moderate-severity groups at the T0 time. However, at T1 and T2, the serum levels of IL-10 and GFAP in the moderate-severe group were significantly higher than those in the mild group (all P<0.001), as shown in Table 2.
Table 2.
Comparison of serum levels of IL-10 and GFAP at different time points between the two groups
| Groups | IL-10 | GFAP | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| T0 | T1 | T2 | T0 | T1 | T2 | |
| Mild group (n=25) | 170.45±22.63 | 217.03±36.42 | 102.37±21.68 | 60.41±15.72 | 80.35±19.67 | 30.36±8.95 |
| Moderate-severity group (n=50) | 179.12±32.71 | 259.81±38.77 | 145.27±24.73 | 64.69±17.53 | 110.83±27.39 | 55.27±10.12 |
| t value | 0.625 | 6.782 | 12.413 | 0.438 | 5.796 | 6.784 |
| P value | 0.532 | <0.001 | <0.001 | 0.662 | <0.001 | <0.001 |
Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; T0: The first day after hospital admission; T1: 3 days after surgery; T2: 7 days after surgery.
Comparison of serum levels of IL-10 and GFAP among glioma patients with different clinicopathological features
Comparison of serum IL-10 and GFAP levels in glioma patients based on gender, age, tumor location, tumor diameter, and KPS scores showed no significant differences. However, the serum IL-10 level was significantly higher in patients with WHO grade III-IV (t=3.550, P<0.001), mutant P53 genotype (t=6.034, P<0.001), and high Ki-67 expression (t=3.491, P<0.001) than their counterparts. Conversely, the serum level of GFAP was significantly higher in patients with WHO grade I-II (t=6.064, P<0.001), wild-type P53 genotype (t=6.050, P<0.001), and low expression of Ki-67 (t=4.126, P<0.001) than their counterparts, as seen Table 3.
Table 3.
Comparison of serum levels of IL-10 and GFAP among glioma patients with different clinicopathological features
| Parameters | Cases | IL-10 (ng/L) | t/χ2 value | P value | GFAP (ng/L) | t/χ2 value | P value | |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | 39 | 171.45±36.28 | 1.417 | 0.159 | 59.14±12.05 | 1.133 | 0.260 |
| Female | 36 | 181.41±38.71 | 67.72±14.64 | |||||
| Age | ≤60 years old | 41 | 172.63±27.28 | 0.850 | 0.397 | 57.28±10.92 | 0.827 | 0.410 |
| >60 years old | 34 | 180.57±30.15 | 70.47±11.83 | |||||
| Location | Loubus fromatis | 41 | 170.73±39.06 | 0.551 | 0.583 | 59.06±11.04 | 1.102 | 0.273 |
| Temporal lobe | 21 | 171.93±28.07 | 57.14±10.85 | |||||
| Parietal lobe | 7 | 171.71±27.94 | 58.29±11.73 | |||||
| Others | 6 | 183.26±34.21 | 66.37±13.29 | |||||
| Tumor diameter | ≤3 cm | 11 | 170.38±21.74 | 1.378 | 0.171 | 57.87±9.87 | 1.111 | 0.269 |
| 3-5 cm | 35 | 175.22±23.58 | 59.23±11.26 | |||||
| >5 cm | 29 | 181.47±26.39 | 65.31±13.85 | |||||
| KPS scores | <70 points | 38 | 175.57±24.67 | 0.837 | 0.405 | 61.26±12.24 | 0.845 | 0.399 |
| ≥70 points | 37 | 177.21±26.35 | 65.38±13.73 | |||||
| WHO Class | I and II grading | 37 | 163.86±19.27 | 3.550 | <0.001 | 71.69±13.95 | 6.064 | <0.001 |
| III and IV grading | 38 | 188.63±37.94 | 54.83±9.69 | |||||
| Genotype of P53 | wild type | 44 | 157.19±26.38 | 6.034 | <0.001 | 73.95±16.17 | 6.050 | <0.001 |
| Mutant type | 31 | 203.25±39.76 | 55.73±9.88 | |||||
| Ki-67 expression | Low | 42 | 165.17±20.94 | 3.491 | <0.001 | 69.65±12.18 | 4.126 | <0.001 |
| High | 33 | 190.25±40.17 | 58.19±11.75 |
Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; KPS: Karnofsky Performance Scale; WHO: World Health Organization; P53: Protein 53.
The relationship between IL-10, GFAP and GCS
Pearson correlation analysis showed that there was no correlation between serum IL-10, GFAP levels and GCS before surgery. However, serum levels of IL-10 and GFAP were negatively correlated with GCS at 3d (IL-10: r=-0.618, P<0.001; GFAP: r=-0.481, P<0.001) and 7d (IL-10: r=-0.411, P<0.001; GFAP: r=-0.437, P<0.001) after surgery, as shown in Table 4.
Table 4.
Pearson correlation analysis for the relationship of IL-10 and GFAP with GCS
| Parameters | IL-10 | GFAP | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Before surgery | 3 d after surgery | 7 d after surgery | Before surgery | 3 d after surgery | 7 d after surgery | ||
| GCS | r value | -0.046 | -0.618 | -0.411 | -0.185 | -0.481 | -0.437 |
| P value | 0.701 | <0.001 | <0.001 | 0.140 | <0.001 | <0.001 | |
Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; GCS: Glasgow Coma Scale.
Multiple Logistic regression analysis of IL-10 and GFPA for assessing postoperative brain injury in glioma patients
As seen in Table 5, the results of multiple Logistic regression analysis showed IL-10 [OR=2.496, 95% CI: 1.302-5.025] and GFPA [OR=3.704, 95% CI: 1.615-8.712] were independent risk factors for postoperative brain injury in glioma patients.
Table 5.
Multivariate Logistic regression analysis of IL-10 and GFPA for predicting postoperative brain injury in glioma patients
| Parameters | β | SE | Wald | P | OR (95% CI) |
|---|---|---|---|---|---|
| IL-10 (≤228.61=0, >228.61=1) | 0.893 | 0.339 | 7.275 | 0.006 | 2.496 (1.302-5.025) |
| GFPA (≤87.67=0, >87.67=1) | 1.294 | 0.435 | 8.796 | 0.002 | 3.704 (1.615-8.712) |
Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; SE: Standard error; OR: Odds Ratio; CI: Confidence interval.
Predictive value of IL-10 and GFAP for assessing the severity of postoperative brain injury
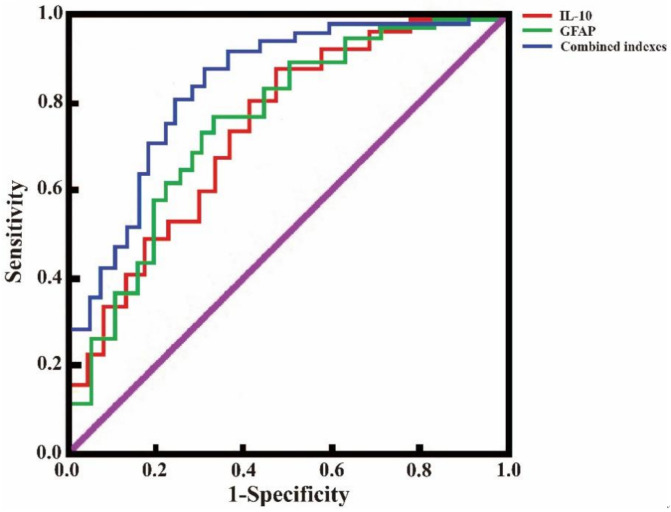
As shown in Table 6 and Figure 2, serum levels of IL-10 or GFAP demonstrated some predictive value for assessment of postoperative brain injury in glioma patients. The AUC values of IL-10 and GFAP were 0.718 and 0.745, respectively. Their combined detection achieved an AUC value of 0.835, with sensitivity and specificity of 92.00% and 66.00%, respectively.
Table 6.
The predictive value of IL-10, GFAP and their combined detection for ostoperative brain injury
| Parameters | AUC | 95% CI | P value | Cut-off value (ng/L) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| IL-10 | 0.718 | 0.625-0.871 | <0.001 | 228.61 | 76.00 (19/25) | 66.00 (33/50) |
| GFAP | 0.745 | 0.617-0.884 | <0.001 | 87.67 | 84.00 (21/25) | 58.00 (29/50) |
| Jointed indexes | 0.835 | 0.776-0.962 | <0.011 | - | 92.00 (23/25) | 66.00 (33/50) |
Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; AUC: Area under the curve; CI: Confidence interval.
Figure 2.
ROC curves of IL-10 and GFAP in predicting postoperative brain injury in glioma patients. Note: IL-10: Interleukin-10; GFPA: Glial fibrillary acidic protein; ROC: Relative operating characteristic.
Discussion
Gliomas are mainly derived from neuroglia, ventricular canal membranes and other neural mesenchymal cells, making them clinically refractory neurological tumors. The lesions mainly occur in the neural ectoderm and are highly invasive, often leading to infiltrative growth, which in turn induces cerebral edema, elevates intracranial pressure and provokes inflammatory reactions to the peripheral nerves, and ultimately exacerbates brain tissue damage [15]. During tumor resection, defining the boundary between the lesion and the surrounding normal brain tissue is a significant challenge. To maximize the preservation of normal brain tissue and blood vessels, it is difficult to completely resect the lesion. Moreover, intraoperative maneuvers such as pulling, electrocoagulation, along with factors like body stress, postoperative intracranial edema, and hemorrhage, can further aggravate postoperative brain injury. Therefore, accurate assessment of postoperative brain injury is crucial for evaluating surgical efficacy.
Given that the GCS scale is primarily used to assess patients’ neurological status, its inherent subjectivity and various confounding factors can lead to significant deviations in the assessment process, complicating the accurate determination of postoperative brain injury and adversely affecting the prognosis [16]. As a glioma marker protein, GFAP plays multiple biological roles, including cell proliferation, differentiation, blood-brain barrier protection, neurotransmitter balance and autophagy. Studies have confirmed that GFAP is abnormally elevated in patients with neurological impairment due to trauma or tumors [17]. This study found that the serum level of GFAP in the observation group was significantly higher compared to the control group. Similarly, Piña-Medina AG et al. [18] reported elevated serum GFAP concentrations in glioma tissues compared to intracranial non-glioma tissues and normal brain tissues, indicating its diagnostic relevance for glioma. Further analysis revealed that the GFAP levels were lower in patients with WHO grade III-IV, mutant P53 genotype, and high Ki-67 expression compared to those with grade I-II, wild-type P53 genotype, and low Ki-67 expression, indicating that the serum GFAP level decreases as tumor malignancy increases. Moreover, our findings indicated that GFAP levels increased at 3 days post-surgery before decreasing by 7 days in both groups. This initial rise may be attributed to the adverse effects of tumor resection, which can damage local brain tissue and lead to cerebral edema, thereby exacerbating brain injury. Over time, as intracranial edema resolves, GFAP levels decrease, falling below preoperative levels and indicating improved surgical efficacy. Notably, GFAP and GCS were negatively correlated at T1 and T2. The AUC value for GFAP predicting postoperative brain injury in glioma patients was 0.745, with a sensitivity and specificity of 84.0% and 58.0%, respectively. These results suggest that GFAP holds predictive value for assessing postoperative brain injury in glioma, aligning with findings from Wiberg S et al. [19], who reported sensitivity and specificity of 82.40% and 77.2% for GFAP, respectively.
IL-10 not only mediates the inflammatory response to promote tumor cell growth and metastasis, but also inhibits anti-tumor factors and tumor cell apoptosis [20]. Studies reported that serum IL-10 was significantly higher in patients with high-grade gliomas than that those with low-grade gliomas, and was also elevated in gliomas patients relative to healthy subjects [21], suggesting that IL-10 could serve as an important indicator for diagnosing gliomas and assessing their differentiation. In our study, we found that the IL-10 level in the observation group was significantly higher than that in the control group. Additionally, IL-10 levels at T1 and T2 time points in both groups showed a pattern of initial increase followed by a decrease. This increase at 3 days post-surgery can be attributed to aggravated brain damage immediately after tumor resection. Over time, as the effects of the surgery became apparent, specifically, the reduction of tumor tissue, the synthesis of lesion cells decreased, leading to a reduction in inflammatory reactions and gradual recovery from secondary brain damage. Consequently, IL-10 levels were significantly lower by 7 days post-surgery than preoperative levels. Moreover, our findings showed that IL-10 and GCS were negatively correlated at T1 and T2. The AUC value of IL-10 for prediction of postoperative brain injury in glioma was 0.718, with a sensitivity and specificity of 76.00% and 66.00%, respectively.
Considering that postoperative brain injury after glioma surgery can results from multiple factors and mechanisms, IL-10 and GFAP may work synergistically in this process [9,22]. The results of this study showed that combined assessment of IL-10 and GFAP provided the highest predictive value for postoperative brain injury in glioma patients, with an AUC value of 0.835, sensitivity and specificity of 92.00% and 66.00%, respectively. Therefore, dynamic monitoring of IL-10 and GFAP levels can offer timely insights into the clinical outcomes of glioma patients, guiding subsequent treatment decisions.
In summary, both IL-10 and GFAP are independent risk factors for postoperative brain injury after glioma resection, and their combined detection offers enhanced accuracy in predicting brain injury following glioma surgery. Thus, their application in clinical practice is encouraged. There are still some limitations to this study, including it bine a single-center study, with a small sample size, lack of long-term follow-up, absence of subgroups comparison and insufficient exploration of the related mechanism. Future research should involve multicenter, controlled studies with larger sample sizes and extended follow-up periods to validate these findings.
Disclosure of conflict of interest
None.
References
- 1.Sledzinska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. 2021;22:10373. doi: 10.3390/ijms221910373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, Chang SM. Primary brain tumours in adults. Lancet. 2023;402:1564–1579. doi: 10.1016/S0140-6736(23)01054-1. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, Wen PY, Chang SM, Dirven L, Lim M, Monje M, Reifenberger G. Glioma. Nat Rev Dis Primers. 2024;10:33. doi: 10.1038/s41572-024-00516-y. [DOI] [PubMed] [Google Scholar]
- 4.Yin X, Wu Q, Hao Z, Chen L. Identification of novel prognostic targets in glioblastoma using bioinformatics analysis. Biomed Eng Online. 2022;21:26. doi: 10.1186/s12938-022-00995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salkeni MA, Naing A. Interleukin-10 in cancer immunotherapy: from bench to bedside. Trends Cancer. 2023;9:716–725. doi: 10.1016/j.trecan.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widodo SS, Dinevska M, Furst LM, Stylli SS, Mantamadiotis T. IL-10 in glioma. Br J Cancer. 2021;125:1466–1476. doi: 10.1038/s41416-021-01515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Yue S, Wang P, Wen B, Zhang X. Systemic inflammation in traumatic brain injury predicts poor cognitive function. Immun Inflamm Dis. 2022;10:e577. doi: 10.1002/iid3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu PH, Chen SC, Chen HY, Wu CB, Huang WT, Chiang HY. Astrocyte-associated fibronectin promotes the proinflammatory phenotype of astrocytes through beta1 integrin activation. Mol Cell Neurosci. 2023;125:103848. doi: 10.1016/j.mcn.2023.103848. [DOI] [PubMed] [Google Scholar]
- 9.Ali H, Harting R, de Vries R, Ali M, Wurdinger T, Best MG. Blood-based biomarkers for glioma in the context of gliomagenesis: a systematic review. Front Oncol. 2021;11:665235. doi: 10.3389/fonc.2021.665235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konnova EA, Deftu AF, Chu Sin Chung P, Pertin M, Kirschmann G, Decosterd I, Suter MR. Characterisation of GFAP-expressing glial cells in the dorsal root ganglion after spared nerve injury. Int J Mol Sci. 2023;24:15559. doi: 10.3390/ijms242115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvani A. New perspectives: glioma in adult patients. Tumori. 2023;109:350–355. doi: 10.1177/03008916231159716. [DOI] [PubMed] [Google Scholar]
- 12.Zhou JH, Wang C, Yang D, Wu YX, Feng DY, Qin H, Wang JL, Wei MH. Clinical features and treatment of apoplectic intratumoral hemorrhage of glioma. BMC Neurol. 2024;24:254. doi: 10.1186/s12883-024-03753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie J, Qiao L, Deng G, Liang N, Xing L, Zhang J. PCGF1 is a prognostic biomarker and correlates with tumor immunity in gliomas. Ann Transl Med. 2022;10:227. doi: 10.21037/atm-22-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song D, Yang Q, Li L, Wei Y, Zhang C, Du H, Ren G, Li H. Novel prognostic biomarker TBC1D1 is associated with immunotherapy resistance in gliomas. Front Immunol. 2024;15:1372113. doi: 10.3389/fimmu.2024.1372113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przybylowski CJ, Hervey-Jumper SL, Sanai N. Surgical strategy for insular glioma. J Neurooncol. 2021;151:491–497. doi: 10.1007/s11060-020-03499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansari A, Zoghi S, Khoshbooei A, Mosayebi MA, Feili M, Yousefi O, Niakan A, Kouhpayeh SA, Taheri R, Khalili H. Development of a novel neurological score combining GCS and FOUR scales for assessment of neurosurgical patients with traumatic brain injury: GCS-FOUR scale. World Neurosurg. 2024;182:e866–e871. doi: 10.1016/j.wneu.2023.12.064. [DOI] [PubMed] [Google Scholar]
- 17.Amalia L. Glial Fibrillary Acidic Protein (GFAP): neuroinflammation biomarker in acute ischemic stroke. J Inflamm Res. 2021;14:7501–7506. doi: 10.2147/JIR.S342097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pina-Medina AG, Diaz NF, Molina-Hernandez A, Mancilla-Herrera I, Camacho-Arroyo I. Effects of progesterone on the cell number of gliomaspheres derived from human glioblastoma cell lines. Life Sci. 2020;249:117536. doi: 10.1016/j.lfs.2020.117536. [DOI] [PubMed] [Google Scholar]
- 19.Wiberg S, Holmgaard F, Zetterberg H, Nilsson JC, Kjaergaard J, Wanscher M, Langkilde AR, Hassager C, Rasmussen LS, Blennow K, Vedel AG. Biomarkers of cerebral injury for prediction of postoperative cognitive dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2022;36:125–132. doi: 10.1053/j.jvca.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Batchu RB, Gruzdyn OV, Kolli BK, Dachepalli R, Umar PS, Rai SK, Singh N, Tavva PS, Weaver DW, Gruber SA. IL-10 signaling in the tumor microenvironment of ovarian cancer. Adv Exp Med Biol. 2021;1290:51–65. doi: 10.1007/978-3-030-55617-4_3. [DOI] [PubMed] [Google Scholar]
- 21.Ansari T, Dutta G, Srivastava AK, Jagetia A, Singh D, Singh H, Bharti R, Prakash A, Kumar A. Serum cytokines in astrocytic brain tumors: a prospective study. Br J Neurosurg. 2023;37:35–40. doi: 10.1080/02688697.2020.1859461. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Shi Y, Liu Y, Huang H, Che J, Shi J, Xu C. Exosome-transmitted podoplanin promotes tumor-associated macrophage-mediated immune tolerance in glioblastoma. CNS Neurosci Ther. 2024;30:e14643. doi: 10.1111/cns.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]