Abstract
Understanding the pathobiology of critical illness is essential for patients’ prognosis. Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. As part of the host response, procoagulant responses, one of the most primitive reactions in biology, start at the very beginning of diseases and can be monitored throughout the process. Currently, we can achieve near-complete monitoring of the coagulation process, and procoagulant responses serve as indicators of the severity of host response in critically ill patients. However, the rapid interpretation of the complex results of various biomarkers remains a challenge for many clinicians. The indicators commonly used for coagulation assessment are complex, typically divided into three categories for clarity: process index, functional index, and outcome index. Monitoring and understanding these indicators can help manage procoagulant responses. The intervention of procoagulant response should be part of the bundle therapy, alongside the treatment for primary disease, management for hemodynamics, and controlling for host response. Early intervention for procoagulant response mainly includes anti-inflammation, antiplatelet and anticoagulant therapy, as well as management of primary disease. In this review, we systemically introduce the onset, assessment and intervention of procoagulant response.
Keywords: Procoagulant response, critically ill patients, antiplatelet therapy, anticoagulant therapy
Introduction
Experts in Intensive Care Unit (ICU) have engaged in discussion to redefine critical illness, from syndromes to underlying biological changes [1]. Understanding the pathobiology of critical illness is essential for patient’s outcomes. In this conceptual framework, the latest definition of sepsis, as a life-threatening organ dysfunction caused by a dysregulated host response to infection, captures the complexity of critical illness and establishes a bridge between diagnosis and therapy [2]. Research demonstrated that critical illness represents a stress-related decompensation syndrome mediated by neural, endocrine, bioenergetic, and immune systems, which is another form of dysregulation [3]. Recent studies also confirmed that the dysregulated host response are not limited to sepsis, but also occur in other critical conditions, such as coronavirus disease 2019 [4,5], severe acute pancreatitis [6], and traumatic brain injury [7,8]. Gradually, dysregulated host response to any stress is increasingly recognized as a core pathobiological change in critically ill patient. Some patients with a systemic inflammatory response also present with coagulation abnormalities, which are common in the ICU and require a clinicopathological approach to ensure appropriate therapy [9]. Procoagulant response is one of the oldest and most primitive reactions in biology. For instance, the bold of Horseshoe crab, an ancient species lived on earth for 500 million years, clots to limit bacterial spread when infected with gram negative bacilli, illustrating the ancient procoagulant response seen in lower organisms. Nowadays, procoagulant response acts as part of the host response and plays an important role in bundle therapy in ICU [10], as it can be monitored throughout the process. If not well controlled, procoagulant response may progress into coagulation disorders, coagulopathy, and disseminated intravascular coagulation. In this review, we will systematically describe the pathophysiological process, monitoring, and potential therapy of procoagulant response in critically ill patients.
Inflammation, immunity and coagulation (Figure 1)
Figure 1.
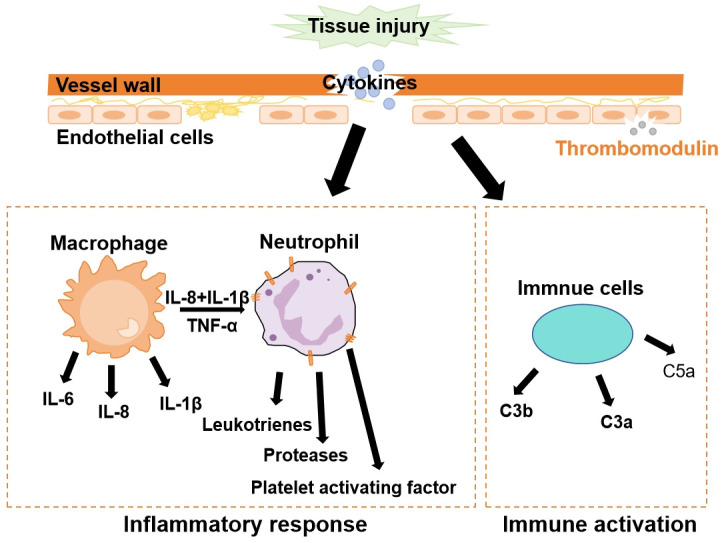
Inflammation, immunity and coagulation.
Platelets and endothelial cells (ECs) are crucial components in both critical illness and coagulation. Circulating platelets activate proinflammatory cells and release chemokine. Through biochemical interactions, platelets and ECs can activate and adversely affect one another. Endothelial activation is one of the consequences upon host response, which plays an important role in coagulopathies. Although endothelial dysfunction pathways differ between 2019 coronavirus disease (COVID-19) and non-COVID-19 patients, endothelial activation is observable in all cases [5]. ECs and platelets produce a variety of cytokines that modulate both inflammatory response and immune response [11].
Inflammation and coagulation
Thrombotic complications are common in both acute infections and chronic inflammatory diseases. ECs cover the surface of all blood vessels, promoting the recruitment of inflammatory cells and preventing the uncontrolled activation of coagulation [12]. Following extensive tissue and endothelial damage, damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines are released, amplifying thrombin production, clot formation, and endothelial lesions [13]. The endothelium responds to stressors with morphological and functional changes, resulting in increased platelet adhesion [14]. During the proliferative phase, platelets adhere to the damaged endothelium, providing a procoagulant surface for coagulation activation and amplifying thrombin generation on activated platelets. Activated leukocytes and platelets form platelet-leukocyte aggregates, while activated neutrophils and macrophages release extracellular traps that promote platelet aggregation and thrombin formation [15]. Inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and Interleukin (IL)-1, induce the expression of several important adhesion molecules of ECs and promote various coagulation processes. Independently, thrombin released by ECs stimulates the production of E-selectin IL-8, further promoting fibrinogen formation and platelet aggregation [16]. Endothelial protection plays a vital role in reducing both thrombotic events and mortality [17]. A positive correlation exists between platelet activation and disease severity [18]. Following injury to the blood vessel wall, platelets adhere to the damaged EC surface, leading to an increase in tissue factor (TF)-related procoagulant activity. Surface receptors on platelets mediate platelet aggregation by binding fibrinogen and von Willebrand factor, while leukocytes form a monolayer over aggregated platelets [19]. Leukocytes binding to P-selectin exposed on the surfaces of platelets promote the conversion of fibrinogen to fibrin [20]. Monocytic cells can directly activate factor X [21]. Inflammatory processes, such as ischemia-reperfusion injury and acute lung injury, are also associated with platelets and ECs [22,23]. As has been demonstrated, the direct invasion and destruction of vascular ECs by novel coronavirus is a primary characteristic contributing to the high incidence of thrombotic events during COVID-19 pandemic, with underlying mechanism remaining consistent [24].
Immune system and coagulation
Both the innate and adaptive immune systems are activated after viral and bacterial infection, which can lead to coagulopathy. Viral infections, including severe acute respiratory syndrome coronavirus (SARS) [25], Middle East respiratory syndrome coronavirus (MERS) [26], and COVID-19 [27], cause severe lymphopenia, indicating dysregulation of immune response. This may be due to a sharp increase in the number of circulating lymphocytes bounded to platelets, which occurs during homeostatic deregulation caused by both infectious and noninfectious diseases [28]. It has been revealed that nonspecific antibodies may be produced and released into the circulation system after the immune activation, potentially affecting coagulation. Zhang et al. identified a significant elevation of antiphospholipid antibodies in COVID-19 patients with coagulopathy [29]. Other contributing factors for coagulopathy activated by dysregulated immune response include abnormal platelet activation [30], endothelial dysfunction [31], and the interference of non-specific antibodies with bioactive phospholipids of signaling cascades that activate procoagulant response [32].
Assessment for the procoagulant response
Inflammation and immune assessment
Many biomarkers reflect the inflammation state, which are mainly categorized into four groups: white blood cells (WBC), erythrocyte sedimentation rate (ESR), acute-phase protein (APP), and cytokines. Neutrophils are vital components of the immune system especially during bacterial infection. Upon immune system activation, leukocytosis occurs, leading to an increased proportion of neutrophils. However, in the early stage of viral infection, the total WBC count may not increase significantly, while the proportion of lymphocyte increases. ESR is a nonspecific indicator of inflammatory response, which can be accelerated in infectious inflammatory diseases, tissue necrosis and injury, malignant tumors, multiple causes of hyperglobulinemia, hypercholesterolemia, and other diseases.
APP refers to the protein whose plasma concentration change by at least 25% during inflammatory disorders, due to hepatocyte production alterations [33]. Common clinical indicators include C3 and C4 from the complement system, fibrinogen and D-dimer from the coagulation and fibrinolytic system, C-reactive protein, and albumin. APP is induced by cytokines in inflammatory processes, with pro-inflammatory cytokines such as IL-6, IL-1β, IL-17, TNF-α, interferon-γ (IFN-γ), transforming growth factor β (TGF-β), and IL-8 playing key roles [34,35]. IL-1β induces autocrine secretion, stimulating the synthesis of TNFα, IL-6, IL-8, and RANTES chemokine [36]. TNF-α acts synergistically with IL-1β, activating similar intracellular signaling pathways that enhance inflammation [37-39]. IL-6 is recognized as a potent activator of the immune system and is elevated in critical illnesses such as sepsis, acute respiratory distress syndrome (ARDS), and, most recently, COVID-19. While IL-6 is known for its pro-inflammatory effects, it can also function as an anti-inflammatory or protective molecule. Zhang et al. demonstrated a negative correlation between IL-6 levels and platelet counts, while pro-inflammatory factors were positively associated with coagulation parameters, particularly a strong correlation between IL-6 and the International Normalized Ratio (INR) (r2 = 0.444, P < 0.001) [40].
Numerous cytokines, such as IL-4, IL-10, IL-13 and IL-1RA, are classified as anti-inflammatory cytokines that limit systemic off-target effects. Regulation of IL-4 can decrease the levels of IL-1β, TNFα, and IL-6, as well as the secretion of other inflammatory mediators, such as cyclooxygenase-2, phospholipase A2, and inducible nitric oxide synthase [41,42]. In mice with collagen-induced arthritis, inflammation was shown to enhance the synthesis of anti-inflammatory IL-10 and TGF-β, as well as exert inhibitory or excitatory effects on other immune cells [43]. IL-10 inhibits the production of TNFα, IL-1, IL-6, and IL-12, while down-regulating antigen presentation [44]. Further, it can also suppress the proliferation of Th17 lymphocytes, which synthesize and secrete proinflammatory IL-17 [45].
Nowadays, cytokines are routinely examined in many hospitals. At Peking Union Medical College Hospital, we routinely test 12 cytokines, including proinflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, IFN-γ, TNF-α), anti-inflammatory cytokines (IL-4, IL-10), and other cytokines (IL-2, IL-5, IL-12p70, IFN-α). However, few studies have established links between cytokines and the routine coagulation test, warranting further exploration. For immune system, the most commonly used assessment tool is blood routine and peripheral lymphocyte subsets [27].
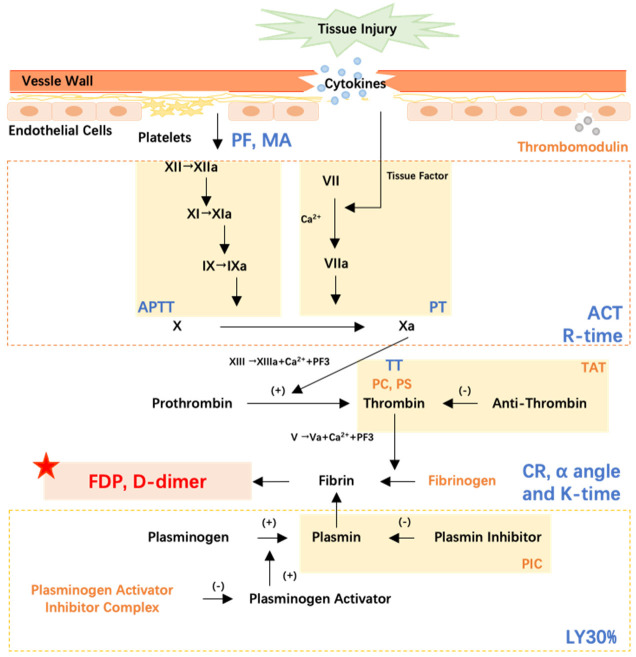
Coagulation tests (Figure 2)
Figure 2.
Coagulation process and different kinds of indicators. Yellow stands for process indexes, blue stands for functional indexes, and red stands for outcome indexes. ACT, activated clotting time; APTT, activated partial prothrombin time; CR, clot rate; FDP, fibrin degradation products; PAI-1, plasminogen activator inhibitor 1; PC, protein C; PF, platelet function; PIC, α2-plasmininhibitor-plasmin complex; PT, prothrombin time; TAT, thrombin antithrombin complex; TT, thrombin time.
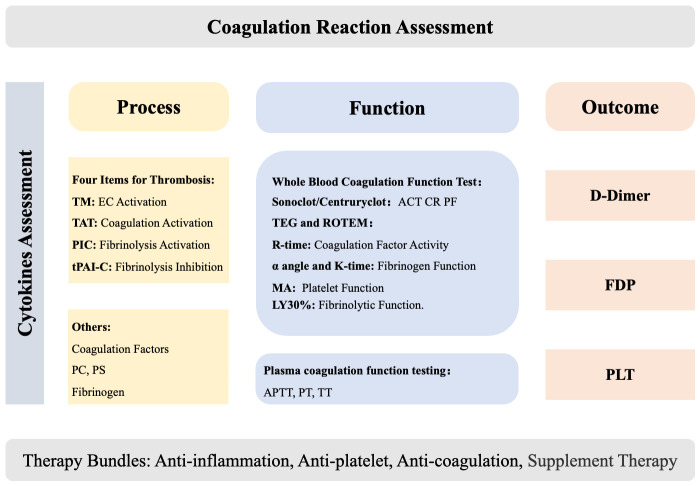
At present, we can monitor nearly the entire coagulation process, with the procoagulant response serving as an indicator of the severity of host response in critically ill patients. However, quickly interpreting the complex results from various biomarkers remains challenging. To simplify this, we propose categorizing the biomarkers into three categories: process index, functional index, and outcome index. The process index forms the foundation of coagulation function, while the outcome index needs to be carefully considered to achieve ultimate therapeutic goal. If the outcome index is outside the normal range, each functional indicator in the coagulation process should be checked to pinpoint the source of the coagulopathy. When the functional indexes are abnormal, the first step is to find the corresponding process indexes and correct them. This allows for a comprehensive and detailed evaluation of the procoagulant response. Process indicators include platelet count, coagulation factors, thrombomodulin (TM), thrombin-antithrombin complex (TAT), α2-plasmin inhibitor-plasmin complex (PIC), tissue plasminogen activator inhibitor complex (t-PAIC), protein C (PC), protein S (PS), and fibrinogen (Fbg). Functional indicators include point-of-care (POC) test indicators, prothrombin time (PT), activated partial prothrombin time (APTT) and thrombin time (TT). FDP and D-dimer are taken as the outcome indicators, as they reflect the result of the procoagulant response. In our previous research, only 11.1% out of the 9,261 ICU patients had normal D-dimer levels. Sensitivity analysis showed that these patients had either undergone uncomplicated high-risk surgery or had very low sequential organ failure assessment (SOFA) score (average SOFA score: 3.3) [46].
Process indexes
Key process indicators for thrombosis (TM, TAT, PIC, t-PAIC)
The four key indicators for thrombogenesis are thrombomodulin (TM), thrombin-antithrombin complex (TAT), α2-plasmin inhibitor-plasmin complex (PIC), and tissue plasminogen activator inhibitor complex (t-PAIC). Each reflects a specific aspect of thrombosis process.
TM, a transmembrane glycoprotein expressed on the surface of endothelial cells, has long been considered a potential biomarker of endothelial injury [47]. It inhibits thrombus formation by modulating the procoagulant effects of thrombin [47]. Under certain conditions, such as sepsis and inflammation, endogenous soluble TM (sTM) is produced through proteolytic cleavage and released into bloodstream [48]. When endothelial cells undergo further injury or rupture, TM is released into blood, forming sTM. As the result, compared to other biomarkers, sTM indicates more severe endothelial injury. Studies have shown that sepsis patients exhibit higher serum sTM level than healthy people [49]. sTM is an early indicator of vascular endothelial injury, triggering microcirculation dysfunction, promoting infection spread to multiple organs, and aggravating the disease [50]. Elevated sTM level can predict the morbidity of septic shock, sepsis-induced coagulopathy, and patient prognosis [51,52]. Monterio et al. demonstrated a strong association between TM elevation and increased mortality (Hazard Ratio [HR] = 1.003) and organ failure in mechanically ventilated children [53]. Despite the detection of TM anticoagulant activity in soluble fragments, a recombinant form (rTM) was developed, which has been shown to inhibit thrombin generation by activating protein C and inactivating factor Va [54,55]. Yoshimura et al. reported that the administering rTM was significantly associated with reduced mortality among patients with a high mortality risk (HR = 0.281, 95% CI: 0.093-0.850; P = 0.025) [56].
Factors Xa, Va, calcium, and phospholipid membrane convert prothrombin into thrombin. The TAT test provides a comprehensive view of both EC and pathway activation, directly reflecting the activation of coagulation system. It has been used as a marker of coagulation initiation. Asakura et al. recommended using TAT to assess coagulation disorders in sepsis-related disseminated intravascular coagulation (DIC) [57].
PIC is formed by plasmin and fibrinolytic system α2 plasmin inhibitors, reflecting the degree of fibrinolytic activation. PIC levels vary across different diseases and are typically used as an indicator of plasmin generation [58]. PIC can help predict the formation of thrombus, assist the diagnosis of DIC, and most importantly, guide antifibrinolytic treatment [59].
The fibrinolytic system is modulated by plasminogen activator inhibitor 1 (PAI-1), which forms t-PAIC by combining with tissue plasminogen activator (t-PA). When t-PA binds to PAI-1, it becomes inactivated, and thus, t-PAIC inhibits fibrinolysis [60]. Studies have shown that t-PAIC is closely related to endothelial injury, in addition to its role as an early marker of fibrinolytic inhibition [61]. t-PAIC has also been linked to organ failure caused by microthrombus formation [62].
Other indexes
Quantity and activity of coagulation factors can help identify the cause of coagulation abnormalities accurately. Thrombin binds to TM in the presence of PC receptor on ECs, acquiring anticoagulant effect by activating PC [63]. Activated protein C (APC) inhibits FVa and FVIIIa to degrade prothrombinase in the presence of PS, which slows thrombin generation processes [64]. In patients with sepsis, PC deficiency may result from enhanced consumption, liver dysfunction, or vascular leakage [65]. PC deficiency in sepsis is associated with hypercoagulable state and increased mortality [66].
Fibrinogen is routinely tested in blood coagulation assessment. During coagulation process, thrombin activates factor XIII, which converts fibrinogen into fibrin. In the presence of platelets, a stable thrombus is formed. Clinically, fibrinogen is also a vital biomarker to predict prognosis. Lower fibrinogen levels indicate higher mortality rates among healthy adults, COVID-19 patients, dialysis patients and critically ill patients [67-70]. Conversely, higher preoperative fibrinogen levels may reduce the possibility of postsurgical bleeding [71]. Meanwhile, fibrinogen is directly involved in inflammatory processes [72].
Functional indexes
Key functional indicators (PT, APTT, TT)
After the activation of ECs, inflammation storm starts, accompanied by the initiation of coagulation process. Coagulation factors play a critical role throughout procoagulant response. Every coagulation factor can now be quantified. Variations in the quantity and quality of these factors ultimately lead to functional changes. Prothrombin time (PT) and activated partial thromboplastin time (APTT) reflect the activation of extrinsic and intrinsic pathways, respectively. Prolonged PT and APTT indicate a deficiency in coagulation factors or the presence of factor antibodies in the serum. A mixing test can help identify the etiology. However, one drawback is that the testing time for PT and APTT is relatively long. TT assesses the ability of fibrinogen to convert into fibrin after addition of human thrombin. Clinically, TT is sensitive to anticoagulation therapy with thrombin (FIIa) inhibitors like heparin or dabigatran [73]. TT has also been reported to be elevated in COVID-19 patients, especially those with critical illness [74].
Indexes of POC test
Viscoelastic coagulation test uses whole blood as the test sample to reflect the entire coagulation process. It is simple, fast, and real-time, which is suitable for bedside monitoring of coagulation function in ICU. On the contrary, traditional coagulation tests (such as PT, APTT, or INR) are less sensitive and take longer to yield results [75,76]. Currently, there are two approaches: the Sonoclot/Centuaryclot Analyzer and Thrombelastogram (TEG)/Rotational Thromboelastometry (ROTEM). TEG and ROTEM share the same detection principle, though their detection parameters differ slightly. Both approaches provide a comprehensive evaluation of the coagulation process and the interaction between coagulation factors and platelets. Most importantly, they can monitor coagulation function dynamically and immediately as needed. These methods have been widely used in the fields of liver transplantation, cardiac surgery, and neurosurgery.
The main indices of TEG include R-time for coagulation factor activity, α angle and K-time for fibrinogen function, MA (maximum amplitude) for platelet function, and LY30% for fibrinolytic function. Whereas Sonoclot/Centuaryclot Analyzer has three main indicators: activated clotting time (ACT) for coagulation factor function, clot rate (CR) for fibrinogen function, and PF for platelet function. To data, no study has specifically focused on the association between cytokines and viscoelastic coagulation test parameters. A meta-analysis of 1893 studies on the relationship between TEG and post-injury hypercoagulability and thrombosis suggested that an MA > 66.7 mm could be used as a diagnostic criterion for trauma-induced hypercoagulopathy [77]. In addition, a retrospective study of 983 trauma patients found that 582 (85.1%) patients developed hypercoagulοpathy at admission, while 99 (14.5%) were diagnosed with deep venous thrombosis (DVT) by ultrasound. The incidence of DVT was significantly higher in trauma patients with hypercoagulopathy based on TEG than in patients without hypercoagulopathy [odds ratio (OR) 2.41, 95% CI: 1.11-5.24, P = 0.026] [78]. Thus, the viscoelastic coagulation test has been recommended for detecting trauma-induced hyper-coagulopathy [79]. Viscoelastic coagulation tests are also used to identify hypocoagulopathy [80]. POC test is a fast and reliable method for evaluation of the entire coagulation process.
The parameters of POC test are associated with inflammation and prognosis in ICU. Calvet et al. demonstrated that besides D-dimer, lysis index at 60 minutes had good prediction effect on mortality and/or intubation [81]. In a study of 50 patients with severe sepsis, MA on admission was demonstrated as an independent predictor for 28-day mortality (HR = 4.29, 95% CI: 1.35 to 13.65) [82]. Hypocoagulable status has also been shown to be an independent risk factor for 30-day mortality (OR = 4.1, 95% CI: 1.4 to 11.9) [83]. As research continues, it is possible that more POC test parameters will be found to predict clinical outcomes. To data, few studies have explored the connections between the cytokines and POC coagulation test results.
Outcome indexes
FDP/D-dimer
In the presence of tissue plasminogen activator (tPA), fibrinogen is activated and converted into fibrinolytic enzymes, initiating the fibrinolytic process. These enzymes degrade fibrin into various soluble fragments, forming fibrin degradation products (FDP). FDP mainly consists of X-oligomer, D-dimer, intermediate fragments, and fragment E. D-dimer is widely used for the diagnosis of DVT and pulmonary embolus (PE). The level of D-dimer mainly reflects the degradation of fibrin, serving as an outcome indicator for the secondary hyperfibrinolysis. Quantitative assays for D-dimer are commonly used as continuous markers of thrombolytic activity in patients diagnosed with a procoagulant response. However, there are several different methods for D-dimer measurement, and these methods are not standardized [84]. Thus, D-dimer test reports should include the measurement method to ensure accurate interpretation by physicians [85]. Our research group has focused on the relationship between D-dimer and prognosis in critical ill patients. A study of 9,261 patients revealed that elevated D-dimer levels can serve as a warning sign of disease severity, as D-dimer levels at ICU admission are linked to an increased risk of prolonged ventilation time and extended ICU stay [46].
Platelet count
A regular blood test offers information on platelet count. While platelet function may be a more sensitive indicator of platelet health, a lower platelet count is still significant, often indicating a poorer prognosis in sepsis [86]. Approximately 80% of septic patients experience some degree of coagulopathy, with platelet consumption being the primary cause of low platelet count. In cases of disseminated intravascular coagulation (DIC) in septic patients, the uncontrolled systemic activation of the clotting cascade consumes an extremely large number of platelets.
Intervention and therapy
As part of the host response, managing procoagulant response should be integrated into bundle therapy, alongside treatment for the primary disease, hemodynamic management, and controlling the overall host response. Here, we emphasize on the intervention of procoagulant response. Assessment and therapy bundles for procoagulant response are summarized in Figure 3.
Figure 3.
Assessment and therapy bundles for procoagulant response.
For hemorrhagic conditions, guidelines have proposed clear protocols for blood transfusion and using blood products, such as recombinant activated factor VII and prothrombin complex concentrates [87-89]. Meanwhile, tranexamic acid, a natural antagonist of fibrinolytic enzymes, is widely used as a hemostatic agent. Above biomarkers offer a clear direction for treatment. POC tests help clinicians pinpoint coagulation abnormalities, whether related to platelets or clotting pathways, allowing for targeted therapy. For thromboembolic diseases, various drugs have been developed for anticoagulant, antiplatelet, or thrombolytic therapy (Table 1). Antiplatelet drugs mainly include thromboxane A2 (TXA2) inhibitors (e.g., aspirin), P2Y12 receptor antagonists (e.g., clopidogrel, ticagrelor), and glycoprotein (GP) I b/III a receptor inhibitor (e.g., abciximab, tirofiban). In addition to vitamin K antagonists such as warfarin, anticoagulants targets either factor X and or thrombin. However, in ICU patients with abnormal coagulation function, the timing and approach to treatment remain uncertain, except in cases with clear evidence of thromboembolism. COVID-19, a unique form of virus-related sepsis, is widely recognized to be associated with thrombotic events [24]. Bacterial sepsis, the most common and classic cause of sepsis in ICU, is also associated with a high incidence of coagulation dysfunction. Thrombotic phase of DIC and multiorgan dysfunction are notable features in bacterial sepsis [90]. Based on the two diseases, we intend to propose a therapy framework for managing procoagulant response.
Table 1.
The most commonly used antiplatelet and anticoagulant agents
| Type | Sub-type | Representative agents |
|---|---|---|
| Antiplatelet agents | Thromboxane A2 inhibitors | Aspirin |
| P2Y12 receptor antagonists | Clopidogrel/Ticagrelor | |
| Glycoprotein II b/III a receptor inhibitor | Asimumab | |
| Tirofiban | ||
| Anticoagulant agents | Vitamin K antagonist | Warfarin |
| Indirect thrombin inhibitor | Heparin | |
| Low Molecular Weight Heparin | ||
| Direct thrombin inhibitors | Argatroban | |
| Dabigatran | ||
| Factor Xa inhibitor | Fondaparinux | |
| Rivaroxaban/apixaban/Edoxaban | ||
| Fibrinolytic enzyme inhibitor | Tranexamic Acid |
In the early phase COVID-19, when the infection is localized to the lungs, microthrombosis is also confined within the lungs. Patients typically exhibit no clinical manifestations in this phase, though inflammation markers and D-dimer levels are elevated [91]. Other biomarkers, such as platelet count and PT are often in the normal range [92]. Efforts have been made to suppress inflammation and cytokine storms to prevent ECs dysfunction and platelet activation, interrupting procoagulant response at this early stage. As the disease progresses, the procoagulant response, regardless of pathogen or etiology, tends to follow a consistent pattern [46]. D-dimer level is a potential indicator of disease severity in COVID-19 patients [93]. Results from randomized clinical trials suggest that administering therapeutic dose of low molecular weight of heparin (LMWH) can reduce major thromboembolic events and mortality in non-critical patients with D-dimer levels more than 4 times the upper limit of normal [94]. In terms of anticoagulation, therapeutic dose of heparin can increase in-hospital survival rate and reduce the need for organ support [95]. However, there are also studies with negative results regarding anti-coagulation therapy, especially in those critically ill patients [95-97]. As mentioned above, endothelial dysfunction and platelet activation play important roles in promoting procoagulant response, which has been proved by studies from Yale school of Medicine in COVID-19 patients [91,98]. Based on this, a daily dose of aspirin (81 mg) was added to the guidelines for all hospitalized COVID-19 patients, regardless of critical illness, which was proved to be associated with a lower cumulative incidence of in-hospital mortality (HR 0.522 [0.336-0.812]) compared to patients who did not receive antiplatelet therapy [99]. Anti-platelets and anti-coagulation therapies are two important parts in the control of procoagulant response, although the effect of anti-platelet therapy is not so prominent in COVID-19 patients. Further studies are needed to demonstrate the importance of antiplatelet therapy in specific patient groups. The outcome index, D-dimer, remains a key target for both ani-platelet and anti-coagulation therapies during the pandemic. After integrating various findings, the International Society on Thrombosis and Hemostasis (ISTH) published guidelines to standardize the use of anticoagulants and antiplatelet agents in different groups [100]. These initiatives confirm the necessity of tailored interventions for managing the coagulation response in patients.
Sepsis-induced DIC is regarded as the late stage of thrombo-inflammatory response, which is part of the host-organ response [90]. In contrast, sepsis-induced coagulopathy (SIC) is considered an earlier phase of DIC [101]. In the late stage of DIC, coagulation substrates are essential for achieving hemostasis. The treatments of SIC include anti-infection to interrupt procoagulant response and correct coagulopathy. Similar therapeutic targets apply to COVID-19 patients. In addition to heparin and LMWH, other methods such as antithrombin, APC, recombinant thrombomodulin, and recombinant tissue factor pathway inhibitor are recommended to inhibit early procoagulant response, conserve coagulation substrates, and ultimately prevent DIC [102].
The therapy for coagulopathy in sepsis and COVID-19 highlights the entire procoagulant response, from initial EC dysfunction to thrombosis. In COVID-19, successful interventions in non-critical patients indicate that earlier anticoagulation therapy may improve the prognosis. The procoagulant response in these patients is moderate and can be managed to prevent organ failure and improve prognosis. However, in patients with multiple organ failure caused by thromboembolism, anticoagulation therapy may increase the risk of bleeding while offering limited benefits to the compromised organs. Therefore, anticoagulation therapy should be carried out before organ failure. The therapeutic experience can be extended to both critical and non-critical patients.
Conclusion
Procoagulant response is an integral part of host response. With advancements in medical laboratory technology, we can now monitor the entire procoagulant response. Early intervention with anti-inflammation and anti-coagulation therapy can help disrupt the procoagulant response, thereby preventing organ failure and the progression to DIC. The therapeutic strategies developed for COVID-19 may be applicable to a broader patient population.
However, this study still has some limitations. Coagulation dysfunction is a broad term encompassing any alteration or impairment of hemostasis, which can be caused by various factors leading to either bleeding, clotting, or both. For example, hemorrhagic and thrombotic coagulopathies arise from different underlying diseases. This study mainly focuses on the inflammatory and immune aspects, as well as related biomarkers, in the procoagulant response. Besides, in the management of coagulopathies, more specific intervention or therapies tailored to the underlying cause should be considered.
Acknowledgements
We thank Professor Dawei Liu and all CCUSG trainers for their contribution to this work. This study is supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-B-026) and Key Project of Central Health Care Scientific Research (2020ZD08).
Disclosure of conflict of interest
None.
Abbreviations
- ACT
activated clotting time
- APC
activated protein C
- APP
acute-phase protein
- APTT
activated partial prothrombin time
- ARDS
acute respiratory distress syndrome
- CI
confidence interval
- COVID 19
2019 coronavirus disease
- CR
clot rate
- DIC
disseminated intravascular coagulation
- DVT
deep venous thrombosis
- ECs
endothelial cells
- ESR
erythrocyte sedimentation rate
- Fbg
Fibrinogen
- FDP
Fibrin Degradation Products
- GP
glycoprotein
- HR
Hazard Ratio
- ICU
Intensive Care Unit
- IL
Interleukin
- IFN-γ
interferon-γ
- INR
International Normalized Ratio
- ISTH
International Society on Thrombosis and Haemostasis
- MERS
Middle East respiratory syndrome coronavirus
- OR
Odds Ratio
- PAI-1
plasminogen activator inhibitor 1
- PC
Protein C
- PIC
α2-Plasmininhibitor-Plasmin Complex
- POC
point-of-care
- PS
Protein
- PT
prothrombin time
- ROTEM
Rotational Thromboelastometry
- SARS
severe acute respiratory syndrome coronavirus
- SOFA
sequential organ failure assessment
- TAT
Thrombin Antithrombin complex
- TEG
Thrombelastogram
- TF
tissue factor
- TM
Thrombomodulin
- TNF-α
tumor necrosis factor α
- t-PA
tissue plasminogen activator
- t-PAIC
tissue Plasminogen Activator Inhibitor Complex
- TT
Thrombin Time
- TXA2
thromboxane A2
- WBC
white blood cells
References
- 1.Maslove DM, Tang B, Shankar-Hari M, Lawler PR, Angus DC, Baillie JK, Baron RM, Bauer M, Buchman TG, Calfee CS, Dos Santos CC, Giamarellos-Bourboulis EJ, Gordon AC, Kellum JA, Knight JC, Leligdowicz A, McAuley DF, McLean AS, Menon DK, Meyer NJ, Moldawer LL, Reddy K, Reilly JP, Russell JA, Sevransky JE, Seymour CW, Shapiro NI, Singer M, Summers C, Sweeney TE, Thompson BT, van der Poll T, Venkatesh B, Walley KR, Walsh TS, Ware LB, Wong HR, Zador ZE, Marshall JC. Redefining critical illness. Nat Med. 2022;28:1141–1148. doi: 10.1038/s41591-022-01843-x. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuesta JM, Singer M. The stress response and critical illness: a review. Crit Care Med. 2012;40:3283–3289. doi: 10.1097/CCM.0b013e31826567eb. [DOI] [PubMed] [Google Scholar]
- 4.Bermejo-Martin JF, González-Rivera M, Almansa R, Micheloud D, Tedim AP, Domínguez-Gil M, Resino S, Martín-Fernández M, Ryan Murua P, Pérez-García F, Tamayo L, Lopez-Izquierdo R, Bustamante E, Aldecoa C, Gómez JM, Rico-Feijoo J, Orduña A, Méndez R, Fernández Natal I, Megías G, González-Estecha M, Carriedo D, Doncel C, Jorge N, Ortega A, de la Fuente A, Del Campo F, Fernández-Ratero JA, Trapiello W, González-Jiménez P, Ruiz G, Kelvin AA, Ostadgavahi AT, Oneizat R, Ruiz LM, Miguéns I, Gargallo E, Muñoz I, Pelegrin S, Martín S, García Olivares P, Cedeño JA, Ruiz Albi T, Puertas C, Berezo JÁ, Renedo G, Herrán R, Bustamante-Munguira J, Enríquez P, Cicuendez R, Blanco J, Abadia J, Gómez Barquero J, Mamolar N, Blanca-López N, Valdivia LJ, Fernández Caso B, Mantecón MÁ, Motos A, Fernandez-Barat L, Ferrer R, Barbé F, Torres A, Menéndez R, Eiros JM, Kelvin DJ. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju PK, Morrell ED, Zelnick L, Sathe NA, Chai XY, Sakr SS, Sahi SK, Sader A, Lum DM, Liu T, Koetje N, Garay A, Barnes E, Lawson J, Cromer G, Bray MK, Pipavath S, Kestenbaum BR, Liles WC, Fink SL, West TE, Evans L, Mikacenic C, Wurfel MM. Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID-19: a prospective observational cohort study. Crit Care. 2021;25:148. doi: 10.1186/s13054-021-03547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Y, Cui N, Miao B, Zhao E. Immune dysregulation in patients with severe acute pancreatitis. Inflammation. 2011;34:36–42. doi: 10.1007/s10753-010-9205-4. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Fan M, Lu W, Zhu W, Meng L, Lu S. Emerging roles of T helper cells in non-infectious neuroinflammation: savior or sinner. Front Immunol. 2022;13:872167. doi: 10.3389/fimmu.2022.872167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moro F, Pischiutta F, Portet A, Needham EJ, Norton EJ, Ferdinand JR, Vegliante G, Sammali E, Pascente R, Caruso E, Micotti E, Tolomeo D, di Marco Barros R, Fraunberger E, Wang KKW, Esser MJ, Menon DK, Clatworthy MR, Zanier ER. Ageing is associated with maladaptive immune response and worse outcome after traumatic brain injury. Brain Commun. 2022;4:fcac036. doi: 10.1093/braincomms/fcac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370:2153. doi: 10.1056/NEJMc1403768. [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Liu D, Zhang H, Ding X, Wang X Critical Care Ultrasound Study Group (CCUSG) Focus on host/organ unregulated response: a common cause of critical illness. Chin Med J (Engl) 2023;136:108–110. doi: 10.1097/CM9.0000000000002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16:231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 12.Martín-Fernández M, Tamayo-Velasco Á, Aller R, Gonzalo-Benito H, Martínez-Paz P, Tamayo E. Endothelial dysfunction and neutrophil degranulation as central events in sepsis physiopathology. Int J Mol Sci. 2021;22:6272. doi: 10.3390/ijms22126272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J, Iba T, Connors JM, Gando S, Levi M, Meziani F, Levy JH. How to manage coagulopathies in critically ill patients. Intensive Care Med. 2023;49:273–290. doi: 10.1007/s00134-023-06980-6. [DOI] [PubMed] [Google Scholar]
- 16.Kaplanski G, Fabrigoule M, Boulay V, Dinarello CA, Bongrand P, Kaplanski S, Farnarier C. Thrombin induces endothelial type II activation in vitro: IL-1 and TNF-alpha-independent IL-8 secretion and E-selectin expression. J Immunol. 1997;158:5435–5441. [PubMed] [Google Scholar]
- 17.Okada H, Yoshida S, Hara A, Ogura S, Tomita H. Vascular endothelial injury exacerbates coronavirus disease 2019: the role of endothelial glycocalyx protection. Microcirculation. 2021;28:e12654. doi: 10.1111/micc.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theuerkauf K, Obach-Schröck C, Staszyk C, Moritz A, Roscher KA. Activated platelets and platelet-leukocyte aggregates in the equine systemic inflammatory response syndrome. J Vet Diagn Invest. 2022;34:448–457. doi: 10.1177/10406387221077969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrottmaier WC, Mussbacher M, Salzmann M, Assinger A. Platelet-leukocyte interplay during vascular disease. Atherosclerosis. 2020;307:109–120. doi: 10.1016/j.atherosclerosis.2020.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Green D. Coagulation cascade. Hemodial Int. 2006;10(Suppl 2):S2–4. doi: 10.1111/j.1542-4758.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 21.Barnard MR, Linden MD, Frelinger AL 3rd, Li Y, Fox ML, Furman MI, Michelson AD. Effects of platelet binding on whole blood flow cytometry assays of monocyte and neutrophil procoagulant activity. J Thromb Haemost. 2005;3:2563–2570. doi: 10.1111/j.1538-7836.2005.01603.x. [DOI] [PubMed] [Google Scholar]
- 22.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 23.Tabuchi A, Kuebler WM. Endothelium-platelet interactions in inflammatory lung disease. Vascul Pharmacol. 2008;49:141–150. doi: 10.1016/j.vph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Iba T, Warkentin TE, Thachil J, Levi M, Levy JH. Proposal of the definition for COVID-19-associated coagulopathy. J Clin Med. 2021;10:191. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z, Zhao C, Dong Q, Zhuang H, Song S, Peng G, Dwyer DE. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, Hawa H, Alothman A, Khaldi A, Al Raiy B. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gros A, Ollivier V, Ho-Tin-Noé B. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front Immunol. 2015;5:678. doi: 10.3389/fimmu.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habets KL, Trouw LA, Levarht EW, Korporaal SJ, Habets PA, de Groot P, Huizinga TW, Toes RE. Anti-citrullinated protein antibodies contribute to platelet activation in rheumatoid arthritis. Arthritis Res Ther. 2015;17:209. doi: 10.1186/s13075-015-0665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy EM, Moreno-Martinez D, Wilkinson FL, McHugh NJ, Bruce IN, Pauling JD, Alexander MY, Parker B. Microparticle subpopulations are potential markers of disease progression and vascular dysfunction across a spectrum of connective tissue disease. BBA Clin. 2016;7:16–22. doi: 10.1016/j.bbacli.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayar Z, Moll R, Isenberg D, Cohen H. Thrombotic antiphospholipid syndrome: a practical guide to diagnosis and management. Thromb Res. 2021;198:213–221. doi: 10.1016/j.thromres.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 34.Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- 35.Wigmore SJ, Fearon KC, Maingay JP, Lai PB, Ross JA. Interleukin-8 can mediate acute-phase protein production by isolated human hepatocytes. Am J Physiol. 1997;273:E720–726. doi: 10.1152/ajpendo.1997.273.4.E720. [DOI] [PubMed] [Google Scholar]
- 36.Aigner T, McKenna L, Zien A, Fan Z, Gebhard PM, Zimmer R. Gene expression profiling of serum- and interleukin-1 beta-stimulated primary human adult articular chondrocytes--a molecular analysis based on chondrocytes isolated from one donor. Cytokine. 2005;31:227–240. doi: 10.1016/j.cyto.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Gao Y, Lin T. Expression of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) in non-small cell lung cancer and its relationship with the occurrence and prognosis of cancer pain. Ann Palliat Med. 2021;10:12759–12766. doi: 10.21037/apm-21-3471. [DOI] [PubMed] [Google Scholar]
- 38.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Zhou X, Yan S, Tian R, Su L, Ding X, Xiao M, Chen Y, Zhao H, Chen H, Zhang H, Li Z, Li Q, Xu Y, Yan X, Li Y, Zhang S. Correlation between cytokines and coagulation-related parameters in patients with coronavirus disease 2019 admitted to ICU. Clin Chim Acta. 2020;510:47–53. doi: 10.1016/j.cca.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rai MF, Graeve T, Twardziok S, Schmidt MF. Evidence for regulated interleukin-4 expression in chondrocyte-scaffolds under in vitro inflammatory conditions. PLoS One. 2011;6:e25749. doi: 10.1371/journal.pone.0025749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Meegeren ME, Roosendaal G, Jansen NW, Wenting MJ, van Wesel AC, van Roon JA, Lafeber FP. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage. 2012;20:764–772. doi: 10.1016/j.joca.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Fu J, Zhang L, Song S, Sheng K, Li Y, Li P, Song S, Wang Q, Chu J, Wei W. Effect of bone marrow-derived CD11b(+)F4/80 (+) immature dendritic cells on the balance between pro-inflammatory and anti-inflammatory cytokines in DBA/1 mice with collagen-induced arthritis. Inflamm Res. 2014;63:357–367. doi: 10.1007/s00011-014-0707-7. [DOI] [PubMed] [Google Scholar]
- 44.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, Koretzky GA. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264–2277. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lian H, Cai H, Zhang H, Ding X, Wang X, Zhang S. The prediction value of D-dimer on prognosis in intensive care unit among old patients (≥ 65 years): a 9-year single-center retrospective study of 9261 cases. Oxid Med Cell Longev. 2022;2022:2238985. doi: 10.1155/2022/2238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito T, Thachil J, Asakura H, Levy JH, Iba T. Thrombomodulin in disseminated intravascular coagulation and other critical conditions-a multi-faceted anticoagulant protein with therapeutic potential. Crit Care. 2019;23:280. doi: 10.1186/s13054-019-2552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loghmani H, Conway EM. Exploring traditional and nontraditional roles for thrombomodulin. Blood. 2018;132:148–158. doi: 10.1182/blood-2017-12-768994. [DOI] [PubMed] [Google Scholar]
- 49.Lipowsky HH, Lescanic A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvasc Res. 2013;90:80–85. doi: 10.1016/j.mvr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Xue M, Chen Y, Liu C, Kuang Z, Mu S, Wei W, Yin J, Xiang H, Hu Y, Long X, Fang S, Sun S, Wang B, Tong C, Song Z. Identification of soluble thrombomodulin and tissue plasminogen activator-inhibitor complex as biomarkers for prognosis and early evaluation of septic shock and sepsis-induced disseminated intravascular coagulation. Ann Palliat Med. 2021;10:10170–10184. doi: 10.21037/apm-21-2222. [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues AT, Rodrigues JT, Rodrigues CT, Volpe CMO, Rocha-Silva F, Nogueira-Machado JA, Alberti LR. Association between thrombomodulin and high mobility group box 1 in sepsis patients. World J Crit Care Med. 2020;9:63–73. doi: 10.5492/wjccm.v9.i4.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue L, Deng X, Yang M, Li X. Elevated B-type natriuretic peptide (BNP) and soluble thrombomodulin (sTM) indicates severity and poor prognosis of sepsis. Ann Palliat Med. 2021;10:5561–5567. doi: 10.21037/apm-21-1048. [DOI] [PubMed] [Google Scholar]
- 53.Monteiro ACC, Flori H, Dahmer MK, Sim MS, Quasney MW, Curley MAQ, Matthay MA, Sapru A BALI Study Investigators of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Thrombomodulin is associated with increased mortality and organ failure in mechanically ventilated children with acute respiratory failure: biomarker analysis from a multicenter randomized controlled trial. Crit Care. 2021;25:271. doi: 10.1186/s13054-021-03626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohlin AK, Larsson K, Hansson M. Soluble thrombomodulin activity and soluble thrombomodulin antigen in plasma. J Thromb Haemost. 2005;3:976–982. doi: 10.1111/j.1538-7836.2005.01267.x. [DOI] [PubMed] [Google Scholar]
- 55.Mohri M, Sugimoto E, Sata M, Asano T. The inhibitory effect of recombinant human soluble thrombomodulin on initiation and extension of coagulation--a comparison with other anticoagulants. Thromb Haemost. 1999;82:1687–1693. [PubMed] [Google Scholar]
- 56.Yoshimura J, Yamakawa K, Ogura H, Umemura Y, Takahashi H, Morikawa M, Inoue Y, Fujimi S, Tanaka H, Hamasaki T, Shimazu T. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care. 2015;19:78. doi: 10.1186/s13054-015-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2:20. doi: 10.1186/2052-0492-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, Morishita E, Yamazaki M, Aoshima K, Nakao S. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29:1164–1168. doi: 10.1097/00003246-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 59.Kawakami M, Kawagoe M, Harigai M, Hara M, Hirose T, Hirose W, Norioka K, Suzuki K, Kitani A, Nakamura H. Elevated plasma levels of alpha 2-plasmin inhibitor-plasmin complex in patients with rheumatic diseases. Possible role of fibrinolytic mechanism in vasculitis. Arthritis Rheum. 1989;32:1427–1433. doi: 10.1002/anr.1780321112. [DOI] [PubMed] [Google Scholar]
- 60.Urano T, Suzuki Y. Parameters related to fibrinolysis and their meanings. Rinsho Byori. 2011;59:703–708. [PubMed] [Google Scholar]
- 61.Eržen B, Šabovič M. In young post-myocardial infarction male patients elevated plasminogen activator inhibitor-1 correlates with insulin resistance and endothelial dysfunction. Heart Vessels. 2013;28:570–577. doi: 10.1007/s00380-012-0287-9. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe R, Wada H, Miura Y, Murata Y, Watanabe Y, Sakakura M, Okugawa Y, Nakasaki T, Mori Y, Nishikawa M, Gabazza EC, Shiku H, Nobori T. Plasma levels of total plasminogen activator inhibitor-I (PAI-I) and tPA/PAI-1 complex in patients with disseminated intravascular coagulation and thrombotic thrombocytopenic purpura. Clin Appl Thromb Hemost. 2001;7:229–233. doi: 10.1177/107602960100700309. [DOI] [PubMed] [Google Scholar]
- 63.Esmon CT. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989;264:4743–4746. [PubMed] [Google Scholar]
- 64.Kisiel W. Human plasma protein C: isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979;64:761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ten Cate H. Pathophysiology of disseminated intravascular coagulation in sepsis. Crit Care Med. 2000;28(Suppl):S9–11. doi: 10.1097/00003246-200009001-00003. [DOI] [PubMed] [Google Scholar]
- 66.Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29(Suppl):S90–94. doi: 10.1097/00003246-200107001-00028. [DOI] [PubMed] [Google Scholar]
- 67.Yu J, Lin T, Huang N, Xia X, Li J, Qiu Y, Yang X, Mao H, Huang F. Plasma fibrinogen and mortality in patients undergoing peritoneal dialysis: a prospective cohort study. BMC Nephrol. 2020;21:349. doi: 10.1186/s12882-020-01984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang G, Zhang L, Si S, Jiang T, Xia Y, Zhu Y, Zhang X, Yao C, Chen M, Chen S. Fibrinogen and antithrombin III are associated with in-hospital mortality among critically ill patients with acute kidney injury. Ren Fail. 2022;44:1938–1947. doi: 10.1080/0886022X.2022.2142138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Zhang Y, Lavie CJ, Tabung FK, Xu J, Hu Q, He L, Zhang Y. Associations of C-reactive protein and fibrinogen with mortality from all-causes, cardiovascular disease and cancer among U.S. adults. Prev Med. 2020;139:106044. doi: 10.1016/j.ypmed.2020.106044. [DOI] [PubMed] [Google Scholar]
- 70.Celikkol A, Dogan M, Guzel EC, Erdal B, Yilmaz A. A novel combined index of D-dimer, fibrinogen, albumin, and platelet (FDAPR) as mortality predictor of COVID-19. Niger J Clin Pract. 2022;25:1418–1423. doi: 10.4103/njcp.njcp_1633_21. [DOI] [PubMed] [Google Scholar]
- 71.Fricault P, Piot J, Estève C, Savan V, Sebesteyn A, Durand M, Chavanon O, Albaladejo P. Preoperative fibrinogen level and postcardiac surgery morbidity and mortality rates. Ann Card Anaesth. 2022;25:485–489. doi: 10.4103/aca.aca_103_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sui J, Noubouossie DF, Gandotra S, Cao L. Elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front Cell Infect Microbiol. 2021;11:734005. doi: 10.3389/fcimb.2021.734005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bronić A, Coen Herak D, Margetić S, Milić M. Croatian society of medical biochemistry and laboratory medicine: national recommendations for blood collection, processing, performance and reporting of results for coagulation screening assays prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen and D-dimer. Biochem Med (Zagreb) 2019;29:020503. doi: 10.11613/BM.2019.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guevara-Noriega KA, Lucar-Lopez GA, Nuñez G, Rivera-Aguasvivas L, Chauhan I. Coagulation panel in patients with SARS-CoV2 infection (COVID-19) Ann Clin Lab Sci. 2020;50:295–298. [PubMed] [Google Scholar]
- 75.Kander T, Larsson A, Taune V, Schött U, Tynngård N. Assessment of haemostasis in disseminated intravascular coagulation by use of point-of-care assays and routine coagulation tests, in critically ill patients; a prospective observational study. PLoS One. 2016;11:e0151202. doi: 10.1371/journal.pone.0151202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Long B, Long DA, Koyfman A. Emergency medicine misconceptions: utility of routine coagulation panels in the emergency department setting. Am J Emerg Med. 2020;38:1226–1232. doi: 10.1016/j.ajem.2020.01.057. [DOI] [PubMed] [Google Scholar]
- 77.Brown W, Lunati M, Maceroli M, Ernst A, Staley C, Johnson R, Schenker M. Ability of thromboelastography to detect hypercoagulability: a systematic review and meta-analysis. J Orthop Trauma. 2020;34:278–286. doi: 10.1097/BOT.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 78.Brill JB, Badiee J, Zander AL, Wallace JD, Lewis PR, Sise MJ, Bansal V, Shackford SR. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg. 2017;83:413–419. doi: 10.1097/TA.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 79.Song JC, Yang LK, Zhao W, Zhu F, Wang G, Chen YP, Li WQ Chinese People’s Liberation Army Professional Committee of Critical Care Medicine and Chinese Society of Thrombosis, Hemostasis and Critical Care, Chinese Medicine Education Association. Chinese expert consensus on diagnosis and treatment of trauma-induced hypercoagulopathy. Mil Med Res. 2021;8:25. doi: 10.1186/s40779-021-00317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min J, Wan P, Liu G, Yu M, Su L. Sonoclot signature analysis: a new point-of-care testing method for defining heat stroke-induced coagulopathy. Int J Gen Med. 2021;14:6925–6933. doi: 10.2147/IJGM.S321982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calvet L, Thouy F, Mascle O, Sapin AF, Grapin K, Liteaudon JM, Evrard B, Bonnet B, Adda M, Souweine B, Dupuis C. Hypercoagulability in critically ill patients with COVID 19, an observational prospective study. PLoS One. 2022;17:e0277544. doi: 10.1371/journal.pone.0277544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ostrowski SR, Windeløv NA, Ibsen M, Haase N, Perner A, Johansson PI. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: a prospective study. J Crit Care. 2013;28:317, e1–11. doi: 10.1016/j.jcrc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Adamzik M, Langemeier T, Frey UH, Görlinger K, Saner F, Eggebrecht H, Peters J, Hartmann M. Comparison of thrombelastometry with simplified acute physiology score II and sequential organ failure assessment scores for the prediction of 30-day survival: a cohort study. Shock. 2011;35:339–342. doi: 10.1097/SHK.0b013e318204bff6. [DOI] [PubMed] [Google Scholar]
- 84.Lippi G, Tripodi A, Simundic AM, Favaloro EJ. International survey on D-dimer test reporting: a call for standardization. Semin Thromb Hemost. 2015;41:287–293. doi: 10.1055/s-0035-1549092. [DOI] [PubMed] [Google Scholar]
- 85.Oude Elferink RF, Loot AE, Van De Klashorst CG, Hulsebos-Huygen M, Piersma-Wichers M, Oudega R. Clinical evaluation of eight different D-dimer tests for the exclusion of deep venous thrombosis in primary care patients. Scand J Clin Lab Invest. 2015;75:230–238. doi: 10.3109/00365513.2014.993697. [DOI] [PubMed] [Google Scholar]
- 86.Greco E, Lupia E, Bosco O, Vizio B, Montrucchio G. Platelets and multi-organ failure in sepsis. Int J Mol Sci. 2017;18:2200. doi: 10.3390/ijms18102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The Lancet Haematology. Updates on blood transfusion guidelines. Lancet Haematol. 2016;3:e547. doi: 10.1016/S2352-3026(16)30172-7. [DOI] [PubMed] [Google Scholar]
- 88.Berková J. Prehospital blood and blood products administration. Rozhl Chir. 2019;98:481–487. doi: 10.33699/PIS.2019.98.12.481-487. [DOI] [PubMed] [Google Scholar]
- 89.Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, Holcomb JB, Duchesne JC. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern association for the surgery of trauma. J Trauma Acute Care Surg. 2017;82:605–617. doi: 10.1097/TA.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 90.Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. doi: 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 91.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levi M. COVID-19 coagulopathy vs disseminated intravascular coagulation. Blood Adv. 2020;4:2850. doi: 10.1182/bloodadvances.2020002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E, Cohen M, Sharifova G, Lund JM, Tafur A, Lewis PA, Cohoon KP, Rahman H, Sison CP, Lesser ML, Ochani K, Agrawal N, Hsia J, Anderson VE, Bonaca M, Halperin JL, Weitz JI HEP-COVID Investigators. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT, Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M, McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan BA, Brooks MM, Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A, Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S, Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM, Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E, Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M, Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ, Tritschler T, Turner AM, van Bentum-Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ, Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA, Farkouh ME, Hochman JS, Zarychanski R. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopes RD, de Barros E Silva PGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, Barbosa LM, de Aveiro Morata J, Ramacciotti E, de Aquino Martins P, de Oliveira AL, Nunes VS, Ritt LEF, Rocha AT, Tramujas L, Santos SV, Diaz DRA, Viana LS, Melro LMG, de Alcântara Chaud MS, Figueiredo EL, Neuenschwander FC, Dracoulakis MDA, Lima RGSD, de Souza Dantas VC, Fernandes ACS, Gebara OCE, Hernandes ME, Queiroz DAR, Veiga VC, Canesin MF, de Faria LM, Feitosa-Filho GS, Gazzana MB, Liporace IL, de Oliveira Twardowsky A, Maia LN, Machado FR, de Matos Soeiro A, Conceição-Souza GE, Armaganijan L, Guimarães PO, Rosa RG, Azevedo LCP, Alexander JH, Avezum A, Cavalcanti AB, Berwanger O ACTION Coalition COVID-19 Brazil IV Investigators. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, Baucom AL, Kirwan BA, Eng H, Martin D, Zaharris E, Everett B, Castro L, Shapiro NL, Lin JY, Hou PC, Pepine CJ, Handberg E, Haight DO, Wilson JW, Majercik S, Fu Z, Zhong Y, Venugopal V, Beach S, Wisniewski S, Ridker PM ACTIV-4B Investigators. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA. 2021;326:1703–1712. doi: 10.1001/jama.2021.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT, Campbell RA. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meizlish ML, Goshua G, Liu Y, Fine R, Amin K, Chang E, DeFilippo N, Keating C, Liu Y, Mankbadi M, McManus D, Wang SY, Price C, Bona RD, Ochoa Chaar CI, Chun HJ, Pine AB, Rinder HM, Siner JM, Neuberg DS, Owusu KA, Lee AI. Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis. Am J Hematol. 2021;96:471–479. doi: 10.1002/ajh.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulman S, Sholzberg M, Spyropoulos AC, Zarychanski R, Resnick HE, Bradbury CA, Broxmeyer L, Connors JM, Falanga A, Iba T, Kaatz S, Levy JH, Middeldorp S, Minichiello T, Ramacciotti E, Samama CM, Thachil J International Society on Thrombosis and Haemostasis. ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost. 2022;20:2214–2225. doi: 10.1111/jth.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iba T, Levy JH, Raj A, Warkentin TE. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8:728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]