Abstract
Objective: Thymidylate synthase (TYMS) constitutes a pivotal and potent target in the context of chemoresistance. However, the oncogenic role of TYMS has received insufficient attention. Methods: Leveraging data from the Cancer Genome Atlas (TCGA) and various public databases, we conducted an extensive investigation into the oncogenic role of TYMS across 33 cancer types. Subsequently, TYMS was inhibited using small interfering RNA (siRNA) in four different cell lines, and cell proliferation and migration were assessed using CellTiter-Glo and Transwell assays. Results: TYMS exhibited pronounced expression across a spectrum of cancers and demonstrated associations with clinical outcome in diverse cancer patient cohorts. Furthermore, genetic alterations were identified as potential influencers of overall survival in specific tumor types. Notably, the expression of thymidylate synthase correlated with tumor-infiltrating CD4+ cells in select cancers. Additionally, the functional mechanism of TYMS encompassed nucleotidase activity, chromosome segregation, and DNA replication progress. In vitro experiments further substantiated these findings, demonstrating that the suppression of TYMS impeded the cell growth and invasive capabilities of HeLa, A549, 786-O, and U87_MG cells. Conclusions: This study furnishes a comprehensive understanding of the oncogenic role played by TYMS in human tumors.
Keywords: Thymidylate synthase, tumor, TGCA, prognosis, tumor-infiltrating immune cell
Introduction
The exploration of novel oncogenes is crucial for achieving a comprehensive understanding of the mechanisms underlying malignant tumors and identifying therapeutic targets [1]. Using resources such as TCGA and various other publicly available analysis websites, which house valuable genomics and proteomics information [2], facilitates interactive analysis specifically focused on unraveling the oncogenic role of TYMS.
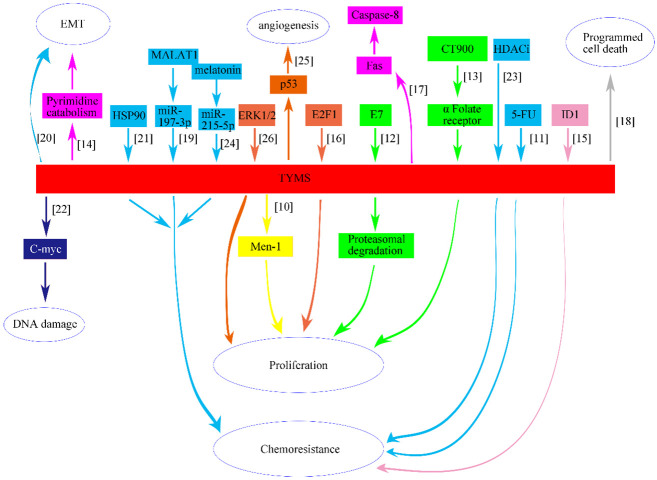
TYMS, a human nucleotide enzyme, plays a pivotal role in endogenous thymidylate synthesis, enabling de novo production of thymidylate. TYMS has been extensively studied in contexts such as hepatitis, rheumatic diseases, and neural development [3-5]. Elevated levels of TYMS mRNA and protein have been associated with worse prognosis for a wide range of hematologic and solid tumors [6]. Additionally, thousands of publications on PubMed link elevated TYMS levels in tumors to anti-cancer drug resistance and worse clinical outcome [7-9]. However, few studies have analyzed these articles to reveal the carcinogenic mechanisms of TYMS. This paper synthesizes findings from laboratory-based experiments involving cell or animal models, elucidating the intricate relationship between TYMS and various malignancies, including pancreatic, colorectal, ovarian, breast, esophageal, lung, kidney and skin tumors (Figure 1; Supplementary Table 1) [10-26].
Figure 1.
Schematic depicting the relationship between TYMS and eight different types of human cancer, including pancreatic (yellow), colorectal (blue), ovarian (green), breast (pink), esophageal (carneose), lung (brown), kidney (grey) and skin tumor (dark blue).
In our study, we used data from public databases to conduct a pan-cancer analysis of TYMS, covering gene expression, survival rate, genetic alterations, phosphorylation sites, immune cell infiltration, and related gene functions. Additionally, we conducted cell experiments involving the knockdown of TYMS to elucidate further its oncogenic features across different tumors. This comprehensive approach aims to provide a detailed understanding of the oncogenic mechanisms governed by TYMS.
Materials and methods
Gene location and protein structure analysis
The genomic location of the TYMS gene was determined based on the UCSC genome browser (GRCh38/hg38) [27]. Using the “HomoloGene” function of the National Center for Biotechnology Information (NCBI), conserved functional domain analysis of the TYMS protein across different species was conducted. The three-dimensional structure of TYMS was obtained using the cBioPortal web tool [28].
Gene expression analysis
Tumor Immune Estimation Resource, version 2 (TIMER2)
Differences in TYMS expression were assessed using the “Gene_DE” module of the TIMER2 [29]. In cases lacking contrast tissues or a sufficient contrast group, the Gene Expression Profiling Interactive Analysis, version 2 (GEPIA2) web server from Genotype-Tissue Expression (GTEx) was employed [30]. Violin plots depicting TYMS expression across various pathologic stages were generated using the “Pathological Stage Plot” module in GEPIA2, utilizing log2 [TPM (Transcripts per million) +1] transformed expression results with a log-scale test.
The Human Protein Atlas (HPA)
HPA database provided TYMS expression data in different cells, tissues, and plasma [31]. Plasma sample data were estimated through mass spectrometry-based proteomics, defining “low specificity” as “Normalized expression ≥1 in at least one tissue/cell type, but not elevated in any tissue/cell type”. Immunohistochemistry (IHC) images of TYMS in five pairs of normal and tumor tissues (breast invasive carcinoma (BRCA), colon adenocarcinoma (COAD), liver hepatocellular carcinoma (LIHC), and lung adenocarcinoma (LUAD)) were downloaded from HPA and analyzed.
Survival analysis
Using the Kaplan-Meier “Survival Map” module in GEPIA2, overall survival (OS) and disease-free survival (DFS) maps for TYMS were obtained. The expression threshold for categorizing high/low expression groups was set at 50%. Kaplan-Meier survival analysis in GEPIA2 generated survival plots using the log-rank test.
Genetic alteration analysis
Genetic alteration features, alteration frequency, mutation type, and mutated site information for TYMS were collected from the cBioPortal web. OS data for tumors with or without TYMS genetic alterations were collected, and Kaplan-Meier analysis with log-rank P-values was performed.
Phosphorylation features
The predicted phosphorylation features of TYMS at sites S6, T53, S114, S124, Y146, S151, Y153, S154, and T167 were obtained from the open-access PhosphoNET database by searching the protein name “TYMS”.
Immune infiltration cell analysis
The association between TYMS expression and immune infiltrates, specifically CD4+ T-cells, CD8+ T-cells, cancer-associated fibroblasts, and natural killer (NK) cells, was explored using the TIMER2 web tool. Purity-adjusted Spearman’s rank correlation test provided P-values and partial correlation (cor) values.
TYMS-related gene enrichment
The STRING website was employed to identify TYMS-binding proteins [32], with a low confidence score set at 0.7. Interaction types were based on the maximum number of interactors (≤50), full STRING network, and confidence.
Cell culture and transfection
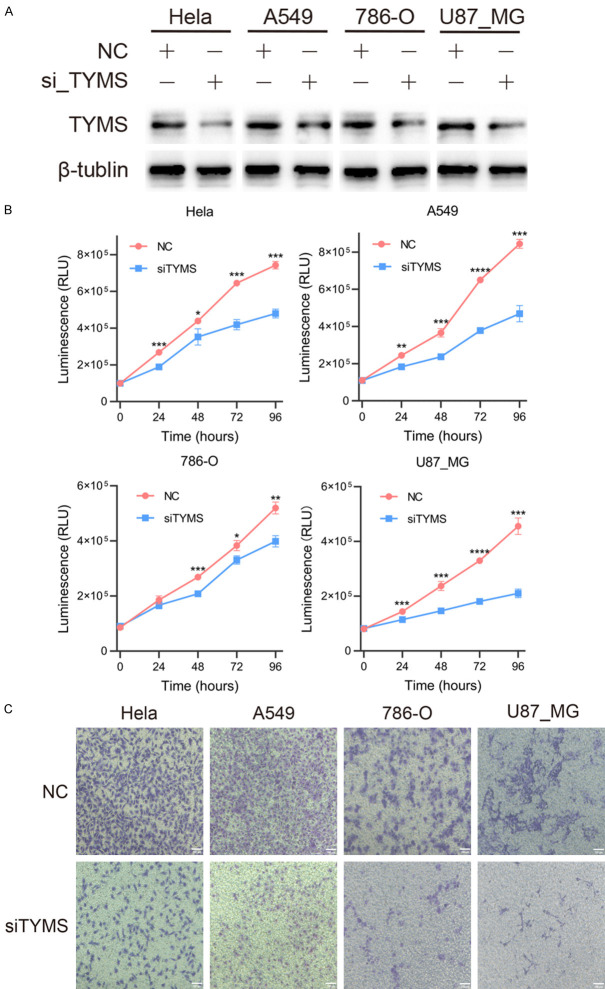
We procured four cell lines, namely HeLa, A549, 786-O, and U87_MG, from Wuhan Pricella Biotechnology Co., Ltd. These cells were cultured at 37°C, 95% humidity, and 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) sourced from Gibco, USA. The knockdown of TYMS in these cancer cell lines was achieved through the construction and transfection of siRNA-TYMS (siTYMS) and siRNA-NC (NC), serving as the corresponding control (obtained from Syngenbio, China). siTYMS sequence was 5’ to 3’ GCUACAGCCUGAGAGAUUATT/UAAUCUCUCAGGCUGUAGCTT. Upon reaching a cell density exceeding 80%, cells were seeded into 6-well plates, followed by transfection with siRNA-TYMS and siRNA-NC. The cells were then incubated in a stationary incubator. Transfection efficiency was evaluated by western blot analysis after 48 hours.
Western blot
Cellular proteins were extracted using RIPA lysate, and subsequently separated along with markers through electrophoresis utilizing SDS-PAGE gel (WSHT Biotechnology Inc., China), following the detailed method outlined in our previous article [33]. In brief, primary antibodies, including TYMS antibody (ab108995, Abcam, UK) and β-tubulin antibody (ab179513, Abcam, UK), were incubated overnight at 4°C. Following this, the membranes were subjected to three washes with TBST and then incubated with a secondary antibody for 1 hour at room temperature (Abcam, UK). The membranes were then analyzed for the expression of each group of protein bands using a ChemiDoc XRS machine (Bio-Rad, USA), and the results were analyzed using ImageJ (V 1.8.0).
Cell proliferation
The four types of transfected tumor cells were seeded into 96-well plates at a density of 2,000 cells per well. CellTiter-Glo assay (Promega, USA) was performed at 0, 24, 48, 72, and 96 hours after the cells had adhered to the wells. Luminescence (RLU) was measured using a microplate reader (TECAN Spark, Switzerland), and the results were analyzed for comparison between the NC and siTYMS groups.
Invasion
Transwell assays were performed to assess the impact of TYMS on the invasion of different cell types. Initially, the transwell inserts were coated with Matrigel (Corning Matrigel, USA) and incubated in a stationary incubator for 3 hours to allow for solidification. Subsequently, 5 × 104 cells were seeded into the upper chamber of the transwell inserts, which were equipped with 8 μm wells and filled with serum-free DMEM. The lower chamber was filled with DMEM containing 10% FBS. After 24 hours of incubation, the cells at the bottom of the upper chamber were fixed, stained, and then captured for analysis.
Statistical analysis
The R software (Version 4.2.3) along with the “ggplot2” and “enrichplot” packages were employed for bioinformatic analysis. GraphPad Prism 7.0 was utilized to analyze biological data and generate graphical representations. All experiments were independently performed three times. Statistical differences between groups were calculated using the t-test or ANOVA as appropriate. Significance was defined as a p-value less than 0.05.
Data availability
The datasets analyzed in this study are available in the online dataset. Requests for further access to the dataset can be directed to yibo.geng@ccmu.edu.cn.
Results
Gene location and protein structure
We investigated the oncogenic function of TYMS which is located on 18p11.32 and gene length was 15,926 bp (NM_001071 or NP_001062.1, Supplementary Figure 1A). The protein structure of TYMS was conserved across various species (Supplementary Figure 1B, 1C).
Gene expression analysis
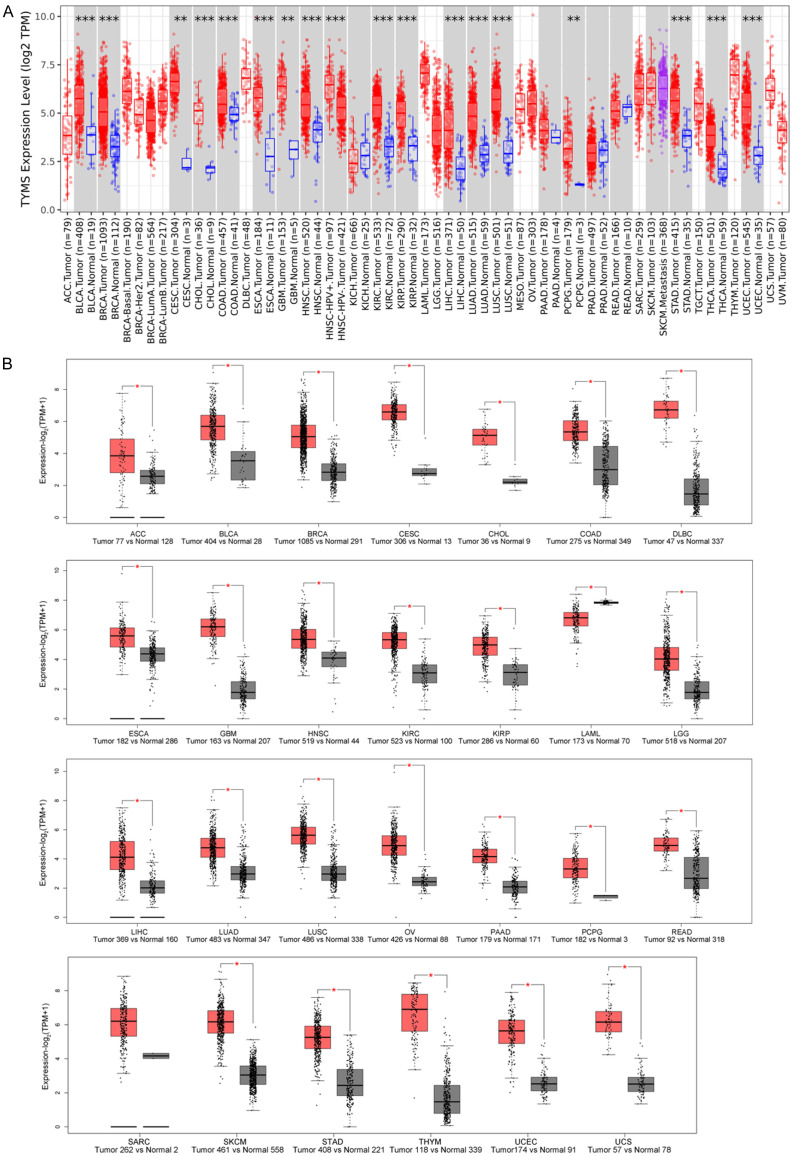
TYMS expression in multiple tumors, including bladder urothelial carcinoma (BLCA), BRCA, cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), cholangiocarcinoma (CHOL), COAD, esophageal carcinoma (ESCA), glioblastoma (GBM), head and neck squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), LIHC, LUAD, lung squamous cell carcinoma (LUSC), pheochromocytoma and paraganglioma (PCPG), stomach adenocarcinoma (STAD), thymoma (THYM), and uterine corpus endometrial carcinoma (UCEC), was higher than that in corresponding normal tissues (Figure 2A, P<0.01). Further analysis using the GTEx dataset revealed significant differences in expression levels between tumor and normal tissue in additional cancers (Figure 2B, P<0.05). However, TYMS expression in kidney chromophobe (KICH), prostatic adenocarcinoma (PRAD), testicular germ cell tumors (TGCT), or thyroid carcinoma (THCA) was similar to normal tissue (Supplementary Figure 2, P>0.05).
Figure 2.
TYMS expression difference across various tumors. A. TYMS expression between various tumors and comparable normal tissues through TCGA dataset. B. TYMS expression through TCGA and GTEx dataset. *P<0.05; **P<0.01; ***P<0.001. TCGA, the Cancer Genome Atlas; GTEx, genotype-tissue expression.
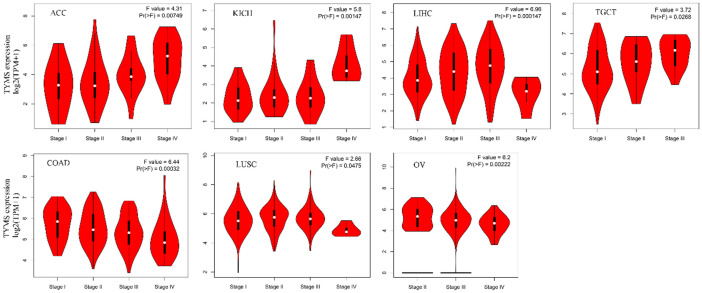
Positive correlations were found between TYMS expression and pathologic stage in adrenocortical carcinoma (ACC), KICH, LIHC, and TGCT. Conversely, COAD, LUSC, and ovarian serous cystadenocarcinoma (OV) showed negative correlations (Figure 3). No correlation was observed in other cancers (Supplementary Figure 3).
Figure 3.
TYMS expression difference across pathological stages in seven tumor types.
Immunohistochemical findings
Comparison of TYMS staining between normal and tumor tissues by IHC corroborated TYMS expression patterns in the HPA dataset. Medium or strong TYMS staining was observed in BRCA, COAD, LIHC, and LUAD, while low or negative staining was evident in normal comparable tissues (Figure 4).
Figure 4.
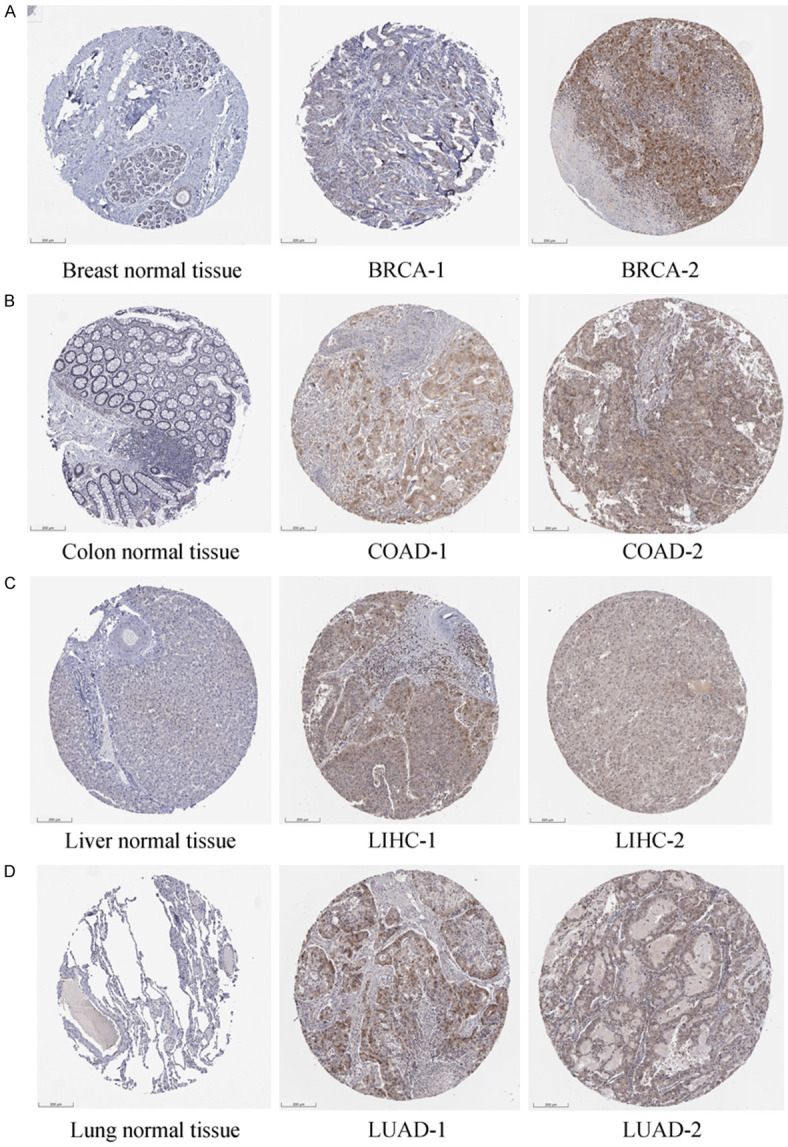
IHC difference between normal and tumor tissues in BRCA (A), COAD (B), LIHC (C) and LUAD (D). Negative or low IHC staining was obtained in the normal breast, colon, liver, and lung tissue, while medium or strong staining was obtained in the cancer tissue. IHC, immunohistochemistry; BRCA, breast invasive carcinoma; COAD, colon adenocarcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma. Scale bar: 200 um.
Survival analysis
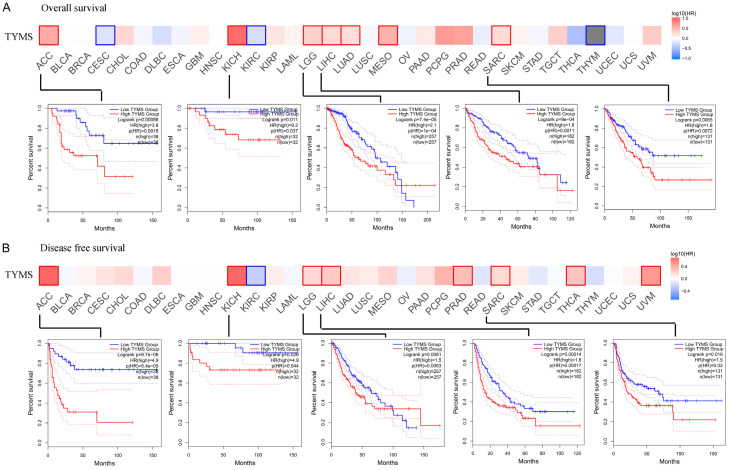
High TYMS expression correlated with poor overall survival in ACC (P<0.001, HR=3.8), KICH (P<0.05, HR=9.2), low grade glioma (LGG, P<0.001, HR=2.1), LIHC (P<0.001, HR=1.8), LUAD (P<0.001, HR=1.7), mesothelioma (MESO, P<0.001, HR=3.3) and sarcoma (SARC, P<0.01, HR=1.8) (Figure 5A). Similarly, it was associated with poor DFS in ACC (P<0.001, HR=4.9), KICH (P<0.05, HR=4.9), LGG (P<0.01, HR=1.5), LIHC (P<0.001, HR=1.8), PRAD (P<0.001, HR=2.0) and SARC (P<0.05, HR=1.5) (Figure 5B). While TYMS showed varied associations with outcome across different cancers, certain cancers, including ACC, KICH, LGG, LIHC, and SARC exhibited consistent tendencies in both OS and DFS (Figure 5).
Figure 5.
Correlation between TYMS expression and the survival outcome of tumors. OS (A) and DFS (B) are presented in the heat bar. A significantly similar trend was found between OS and DFS with ACC, KICH, LGG, LIHC and SARC, which is displayed in survival map (P<0.05). OS, overall survival; DFS, disease free survival; ACC, adrenocortical carcinoma; KICH, kidney chromophobe; LGG, low grade glioma; LIHC, liver hepatocellular carcinoma; SARC, sarcoma.
Genetic alteration
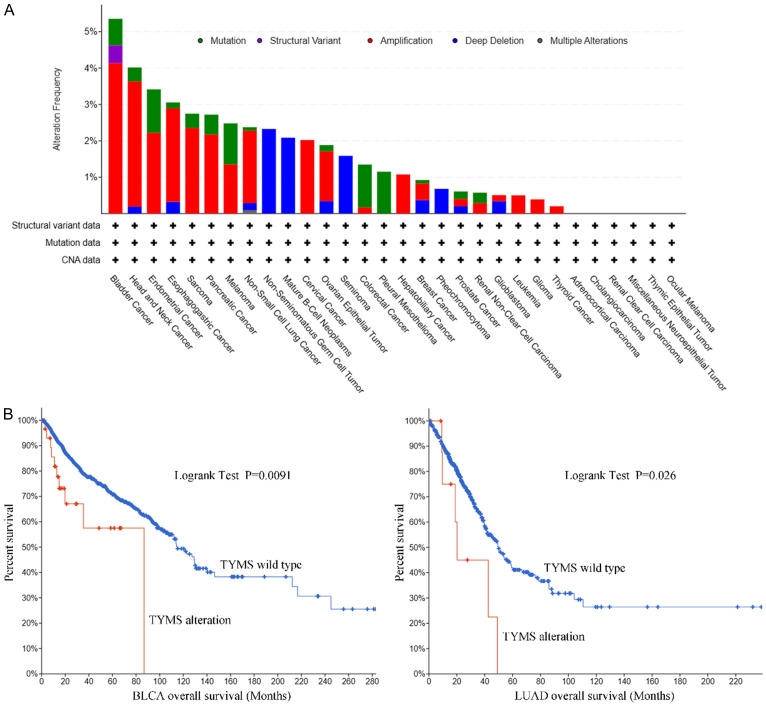
A genetic alteration of TYMS has been observed in distinct tumors. The highest alteration frequency of TYMS (>4%) found in patients with bladder cancer was amplification (Figure 6A). Overall survival in the TYMS alteration group was significantly shorter than in the wild type for both BLCA (P<0.01) and LUAD (Figure 6B, P<0.05).
Figure 6.
Mutation features of TYMS. A. The alteration frequency with mutation type is displayed. B. There are significant overall survival differences between TYMS alteration and wild-type in BLCA and LUAD (P<0.05). BLCA, bladder urothelial carcinoma; LUAD, lung adenocarcinoma.
Phosphorylation analysis
Phosphorylation site analysis of TYMS in mammals was achieved using the PhosphoNET database (Supplementary Figure 4). Unfortunately, limited studies explored phosphorylation differences between tumor and normal tissues (Supplementary File 1), preventing a comprehensive summary of the implications of TYMS phosphorylation in tumors.
Immune infiltration analysis
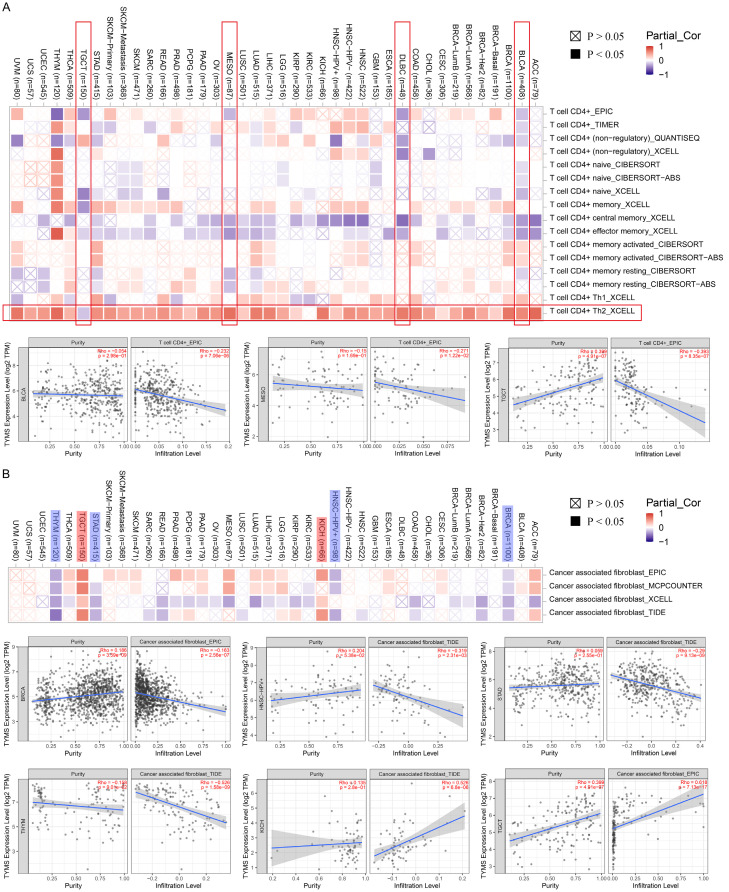
Tumor-infiltrating immune cells can enhance the development, progression, or metastasis of cancers [34]. A significant negative relationship was found between CD4+ T cells and TYMS expression in BLCA, diffuse large B-cell lymphoma (DLBC), and MESO and the whole types of tumors showed positive correlation between CD4+Th2 cells and TYMS expression (Figure 7A, marked as vertical and horizontal red borders). Additionally, a significant relationship between TYMS expression and cancer-associated fibroblasts was observed for BRCA, HNSC, KICH, STAD, TGCT, and THYM. Among these, BRCA, HNSC, STAD, and THYM exhibited a negative correlation (Figure 7B). However, similar trends were not observed in CD8+ and NK cell types (Supplementary Figure 5).
Figure 7.
Correlation between TYMS and CD4+ immune cells (A) and cancer associated fibroblast (B).
Enrichment analysis of TYMS-related genes
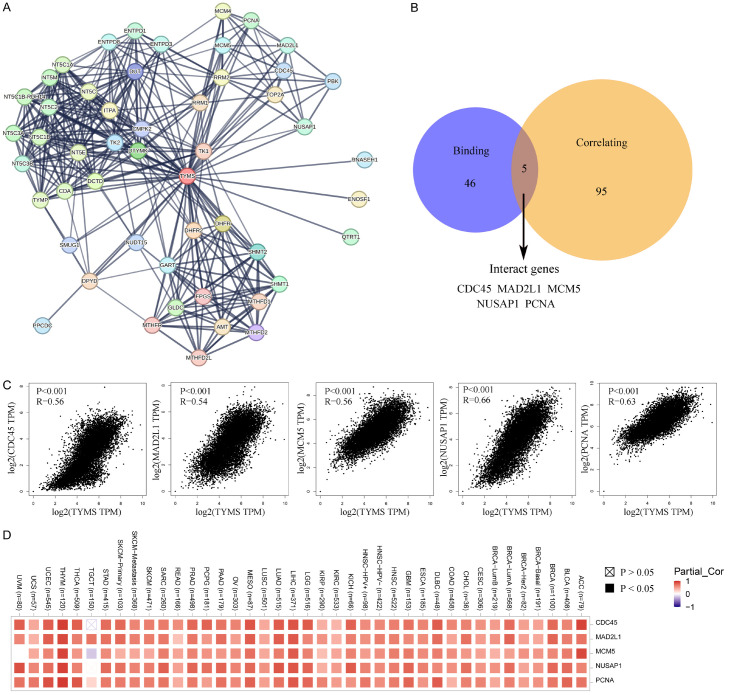
Fifty TYMS-binding proteins were identified through the STRING tool (Figure 8A). The top 100 TYMS-correlated targeting genes were summarized, with the top five being NDC80, EZH2, NUSAP1, WDR76, and MCM6 (Supplementary Table 2). Notably, five genes overlapped in the two datasets (Figure 8B). The expression of these interacting genes (CDC45, MAD2L1, MCM5, NUSAP1, and PCAN) positively correlated with TYMS expression across almost all types of cancer (Figure 8C, 8D).
Figure 8.
TYMS-related gene analysis. (A) Top 50 TYMS-binding proteins and their relationship. (B) Degree of overlap between the TYMS-binding and correlated genes. (C) Expression analysis between TYMS and the five interacting genes in (B). (C, D) Displayed through heatmap across cancers.
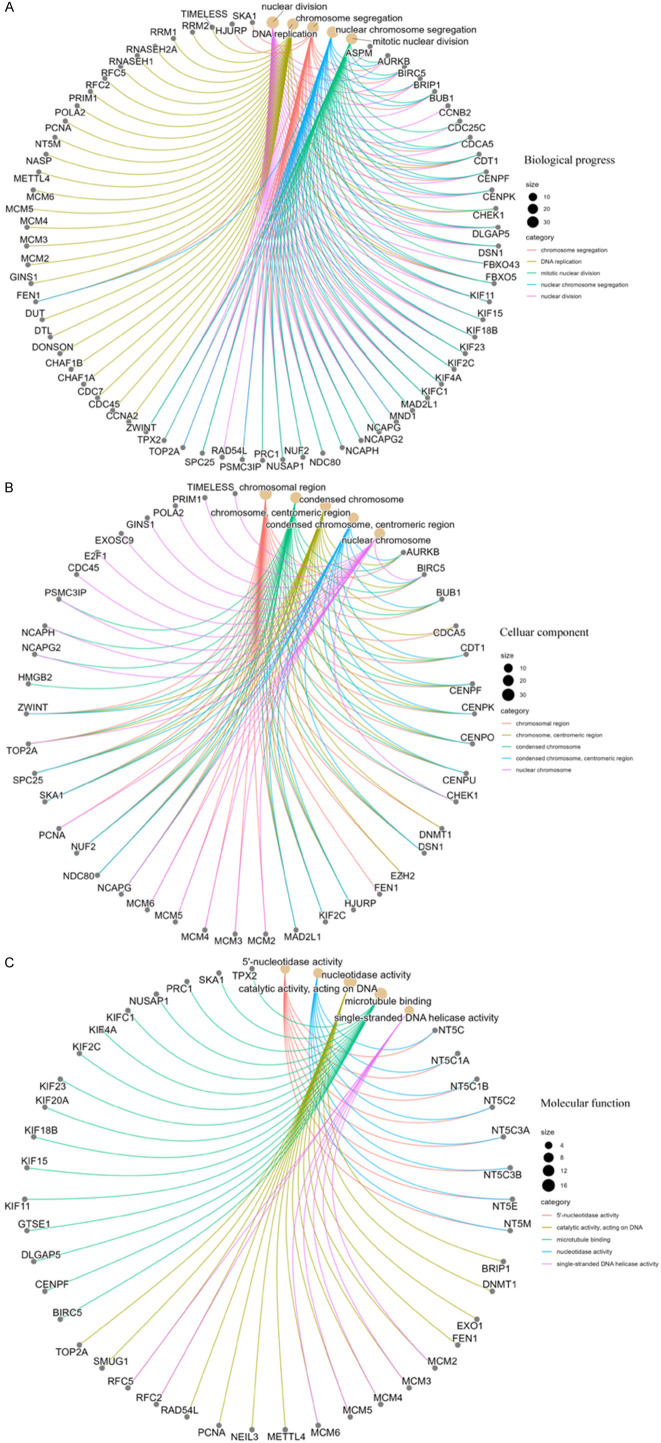
Next, gene ontology (GO) analysis for these genes indicated their predominant location in the chromosomal region and execution of nucleotidase activity (Figure 9). Furthermore, these genes were enriched in DNA replication, chromosome segregation, and nuclear division.
Figure 9.
GO analysis of TYMS-related genes. Biological process (A), cellular components (B), and molecular function (C) are displayed. GO, gene ontology.
TYMS promoted cell proliferation and invasion in multiple cancers
To further explore the oncogenic role of TYMS in human cancers, we utilized siRNA to reduce TYMS expression in four different types of human cancers, and the efficiency of knockdown was assessed by Western blot analysis. As depicted in Figure 10A, TYMS protein expression levels were markedly decreased in all tested cell lines (Hela, A549, 786O, and U87-MG) compared to control conditions.
Figure 10.
TYMS accelerated cell proliferation and migration of cervical carcinoma (Hela), NSCLC (A549), glioblastoma (U87_MG) and renal cell carcinoma (786-O). A. The protein expression levels of HeLa, A549, 786-O and U87_MG cells after transfecting TYMS-specific siRNAs. B. Hela, A549, 786-O and U87_MG cell growth was inhibited after TYMS knockdown. C. Migration of HeLa, A549, 786-O and U87_MG cells were inhibited after transfecting TYMS-specific siRNAs (magnificance 100 μm) *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. NSCLC, non-small cell lung carcinoma; siRNA, small interfering RNA.
Given that cell proliferation and invasion are two key characteristics of tumor oncogenic significance, we conducted experiments to assess these traits using CellTiter-Glo and transwell assays, respectively. The results revealed that reducing TYMS expression led to a notable decrease in tumor cell growth across cervical carcinoma, non-small cell lung carcinoma (NSCLC), GBM, and KIRC (Figure 10B). Furthermore, as shown in Figure 10C, TYMS knockdown significantly inhibited cell invasion in these tumors. Overall, the suppression of TYMS interfered with the malignant characteristics of human cancers.
Discussion
TYMS, a folate-dependent essential enzyme, plays a pivotal role in generating intracellular de novo deoxythymidine monophosphate (dTMP), critical for DNA synthesis and repair [35]. Its involvement extends beyond tumorigenesis, encompassing functions in coronary artery disease [36], virus replication, and congenital disorders [37,38]. This diversity underscores the significance of TYMS in various pathologic contexts. The expanding literature linking TYMS to tumors prompted our comprehensive analysis to elucidate its oncogenic role in diverse cancer types.
The conservation of TYMS protein structure across species suggests shared vital physiologic mechanisms. Notably, TYMS expression was elevated in tumor tissues compared to their normal counterparts, which underscores the potential influence of TYMS in various cellular processes, including genomic instability [9], epithelial-mesenchymal transition, and chemotherapy metabolism [39,40].
High TYMS expression consistently correlated with shorter overall survival and advanced tumor stage across multiple cancers. Recently, several studies have reported similar findings. Lai et al. suggested that the level of TYMS could serve as a predictive marker for longer overall survival in unresectable hepatocellular carcinoma patients [41]. Zhang et al. demonstrated that knockdown of TYMS reduced proliferation and invasion, and promoted apoptosis of liposarcoma through both bioinformatic and biological evidence [42]. This association could be attributed to TYMS’s dual role: directly promoting tumor cell proliferation and inducing chemoresistance. Additionally, TYMS contributed to multiple drug resistance, affecting responses to 5-fluorouracil (5-FU) in COAD [40], temozolomide in glioma [43], and platinum in NSCLC [44]. This resistance is due to distinct pathways, including AMPK-mTOR for temozolomide resistance, polymorphism for fluorouracil resistance and nucleic acid-biosynthetic pathway for pemetrexed resistance [45]. However, contrasting observations were noted in the survival analysis between TYMS and CESC or KIRC. The following studies may explain these discrepancies. 1) Zheng et al. studied patients post-COVID-19 infection, where the pandemic might have altered the immune system and influenced TYMS expression [46]; 2) Fu et al. utilized four GEO datasets and the TCGA database, which might lead to different results compared to our single database analysis [47]. This suggests that TYMS may engage in divergent pathways across different tumor types.
The investigation into TYMS gene mutations and their functional implications remains an area requiring further exploration. To date, only a few studies have focused on this aspect. For instance, Shimizu et al. suggested that TYMS gene amplification could predict pemetrexed resistance in NSCLC [48]. Moreover, TYMS gene deletion has been associated with shorter overall survival in gastric cancer, possibly due to 5-FU chemoresistance [49]. In summary, several types of TYMS mutation have been noted to correlate with clinical characteristics. However, the underlying mechanisms behind these associations require further investigation.
The interaction between TYMS and tumor-infiltrating immune cells highlights its role in the tumor microenvironment. A significant difference was found between TYMS and CD4+ T-cells in BLCA, DLBC, MESO and TGCT (Figure 7A), which was similar with the previous studies. Dersh et al indicated that TYMS inhibitor increased major histocompatibility complex class I (MHC-1) presentation in DLBC, which revealed the role of TYMS in manipulating immunosurveillance in cancers [50]. Wang et al. demonstrated that TYMS suppression impaired helper T cells (Th1 & Th17) differentiation and immune response [51]. Above all, these results provide evidence that TYMS has a complicated correlation with immune system and deserves further studies in tumor immunology and microenvironment.
Enrichment analysis combining TYMS-binding components and expression-related genes revealed the central involvement of “nucleotidase activity” and “DNA replication” in tumor cell proliferation. These processes are pivotal in the progression of tumors, emphasizing the need for further experimental validation to elucidate the specific oncogenic role of TYMS. Future studies should delve into the molecular mechanisms underlying TYMS-mediated tumor progression and its use as a therapeutic target.
Finally, a series of cell experiments utilizing various tumor cell lines were conducted to validate the oncogenic function of TYMS identified through bioinformatic methods. Interestingly, while several studies employing the same cell lines have reported TYMS’s oncogenic role, our results revealed an opposite trend. For instance, Fu et al. performed functional experiments and found that TYMS knockout could promote the proliferation, migration, and invasion of HeLa cells [47]. Conversely, Gotanda et al. demonstrated that TYMS decreased by miR-433 overexpression in HeLa cells resulted in inhibited cell proliferation [52], consistent with our findings. Notably, manipulation of TYMS expression in 786-O, U87_MG, or A549 cell lines has not been previously documented, suggesting significant gaps in TYMS research within the context of cancer. In our study, we found that suppression of TYMS also reversed the malignant characteristics of NSCLC, GBM and KIRC, a finding that has not been reported before. These findings underscore the complexity and variability of TYMS function across different tumor types and emphasize the need for further investigation in this field.
However, this study still presents several limitations that warrant improvement in future research endeavors. Firstly, our analysis of the oncogenic role was solely based on data derived from public databases, and thus, this conclusion needs to be validated in further clinical cohorts. Secondly, additional in vitro and in vivo experiments are necessary to elucidate the underlying mechanisms, particularly regarding the immune mechanisms involved. Thirdly, some of our results may be contradictory due to the inherent heterogeneity of data across multiple databases. These limitations underscore the need for continued research efforts to provide more comprehensive insight into the role of TYMS in cancer.
Conclusion
Based on a comprehensive analysis across various tumors, we found a factual association between TYMS expression and clinical outcome, protein phosphorylation and immune cell infiltration, as well as related genes and functions, which could help us understand the oncogenic role of TYMS.
Acknowledgements
This work was supported by the Beijing Municipal Natural Science Foundation (No. 7244344), Beijing Chaoyang Hospital Golden Seed foundation (No. CYJZ202201) and the inner-hospital foundation of Beijing Chaoyang Hospital. Besides, we would like to thank Xulei Huo for his technical supports.
Disclosure of conflict of interest
None.
Abbreviations
- ACC
Adrenocortical carcinoma
- BLCA
Bladder urothelial carcinoma
- BRCA
Breast invasive carcinoma
- CESC
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL
Cholangiocarcinoma
- COAD
Colon adenocarcinoma
- DFS
Disease free survival
- DLBC
Diffuse large B-cell lymphoma
- dTMP
deoxythymidine monophosphate
- ESCA
Esophageal carcinoma
- GBM
Glioblastoma
- GEPIA2
Gene expression profiling interactive analysis, version 2
- GO
Gene ontology
- GTEx
Genotype-tissue expression
- HNSC
Head and neck squamous cell carcinoma
- HPA
The human protein atlas
- IHC
Immunohistochemistry
- KICH
Kidney chromophobe
- KIRC
Kidney renal clear cell carcinoma
- KIRP
Kidney renal papillary cell carcinoma
- LAML
Acute myeloid leukemia
- LGG
Low grade glioma
- LIHC
Liver hepatocellular carcinoma
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- MESO
Mesothelioma
- MHC
Major histocompatibility complex
- NCBI
National center for biotechnology information
- NK
Natural killer
- NSCLC
Non-small cell lung carcinoma
- OS
Overall survival
- OV
Ovarian serous cystadenocarcinoma
- PAAD
Pancreatic adenocarcinoma
- PCPG
Pheochromocytoma and paraganglioma
- PRAD
Prostate adenocarcinoma
- READ
Prostate adenocarcinoma
- SARC
Sarcoma
- SKCM
Skin cutaneous melanoma
- STAD
Stomach adenocarcinoma
- TCGA
The cancer genome atlas
- TGCT
Testicular germ cell tumors
- THCA
Thyroid carcinoma
- THYM
Thymoma
- TIMER2
Tumor immune estimation resource, version 2
- TPM
Transcripts per million
- TYMS
Thymidylate synthase
- UCEC
Uterine corpus endometrial carcinoma
- UCS
Uterine carcinosarcoma
- UVM
Uveal melanoma
Supporting Information
References
- 1.Kontomanolis EN, Koutras A, Syllaios A, Schizas D, Mastoraki A, Garmpis N, Diakosavvas M, Angelou K, Tsatsaris G, Pagkalos A, Ntounis T, Fasoulakis Z. Role of oncogenes and tumor-suppressor genes in carcinogenesis: a review. Anticancer Res. 2020;40:6009–6015. doi: 10.21873/anticanres.14622. [DOI] [PubMed] [Google Scholar]
- 2.Blum A, Wang P, Zenklusen JC. SnapShot: TCGA-analyzed tumors. Cell. 2018;173:530. doi: 10.1016/j.cell.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Guan Z, Dong Y, Zhu Z, Wang J, Niu B. Inhibition of thymidylate synthase affects neural tube development in mice. Reprod Toxicol. 2018;76:17–25. doi: 10.1016/j.reprotox.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Z, He S, Yu X, Lai X, Tang S, Mariya M EA, Wang M, Yan H, Huang X, Zeng S, Zha D. Analysis and experimental validation of rheumatoid arthritis innate immunity gene CYFIP2 and pan-cancer. Front Immunol. 2022;13:954848. doi: 10.3389/fimmu.2022.954848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng Q, Li X, Xiong X. Identification of Hub genes associated with non-alcoholic steatohepatitis using integrated bioinformatics analysis. Front Genet. 2022;13:872518. doi: 10.3389/fgene.2022.872518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HS, Chen M, Kim JH, Kim WH, Ahn S, Maeng K, Allegra CJ, Kaye FJ, Hochwald SN, Zajac-Kaye M. Analysis of 320 gastroenteropancreatic neuroendocrine tumors identifies TS expression as independent biomarker for survival. Int J Cancer. 2014;135:128–137. doi: 10.1002/ijc.28675. [DOI] [PubMed] [Google Scholar]
- 7.Saviozzi S, Ceppi P, Novello S, Ghio P, Lo Iacono M, Borasio P, Cambieri A, Volante M, Papotti M, Calogero RA, Scagliotti GV. Non-small cell lung cancer exhibits transcript overexpression of genes associated with homologous recombination and DNA replication pathways. Cancer Res. 2009;69:3390–3396. doi: 10.1158/0008-5472.CAN-08-2981. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Wu Z, Wang Y, Chen C, Li Y, Dong H, Yao T, Jin G, Wang Z. TYMS knockdown suppresses cells proliferation, promotes ferroptosis via inhibits PI3K/Akt/mTOR signaling pathway activation in triple negative breast cancer. Cell Biochem Biophys. 2024 doi: 10.1007/s12013-024-01388-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Guijarro MV, Nawab A, Dib P, Burkett S, Luo X, Feely M, Nasri E, Seifert RP, Kaye FJ, Zajac-Kaye M. TYMS promotes genomic instability and tumor progression in Ink4a/Arf null background. Oncogene. 2023;42:1926–1939. doi: 10.1038/s41388-023-02694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijayakurup V, Maeng K, Lee HS, Meyer B, Burkett S, Nawab A, Dougherty MW, Jobin C, Mahmud I, Garrett TJ, Feely M, Lee KB, Kaye FJ, Guijarro MV, Zajac-Kaye M. Thymidylate synthase accelerates Men1-mediated pancreatic tumor progression and reduces survival. JCI Insight. 2022;7:e147417. doi: 10.1172/jci.insight.147417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar A, Singh AK, Singh H, Thareja S, Kumar P. Regulation of thymidylate synthase: an approach to overcome 5-FU resistance in colorectal cancer. Med Oncol. 2022;40:3. doi: 10.1007/s12032-022-01864-z. [DOI] [PubMed] [Google Scholar]
- 12.Costantino L, Ferrari S, Santucci M, Salo-Ahen OMH, Carosati E, Franchini S, Lauriola A, Pozzi C, Trande M, Gozzi G, Saxena P, Cannazza G, Losi L, Cardinale D, Venturelli A, Quotadamo A, Linciano P, Tagliazucchi L, Moschella MG, Guerrini R, Pacifico S, Luciani R, Genovese F, Henrich S, Alboni S, Santarem N, da Silva Cordeiro A, Giovannetti E, Peters GJ, Pinton P, Rimessi A, Cruciani G, Stroud RM, Wade RC, Mangani S, Marverti G, D’Arca D, Ponterini G, Costi MP. Destabilizers of the thymidylate synthase homodimer accelerate its proteasomal degradation and inhibit cancer growth. Elife. 2022;11:e73862. doi: 10.7554/eLife.73862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee S, Michalarea V, Ang JE, Ingles Garces A, Biondo A, Funingana IG, Little M, Ruddle R, Raynaud F, Riisnaes R, Gurel B, Chua S, Tunariu N, Porter JC, Prout T, Parmar M, Zachariou A, Turner A, Jenkins B, McIntosh S, Ainscow E, Minchom A, Lopez J, de Bono J, Jones R, Hall E, Cook N, Basu B, Banerji U. A Phase I trial of CT900, a novel alpha-folate receptor-mediated thymidylate synthase inhibitor, in patients with solid tumors with expansion cohorts in patients with high-grade serous ovarian cancer. Clin Cancer Res. 2022;28:4634–4641. doi: 10.1158/1078-0432.CCR-22-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui A, Gollavilli PN, Schwab A, Vazakidou ME, Ersan PG, Ramakrishnan M, Pluim D, Coggins S, Saatci O, Annaratone L, Hm Schellens J, Kim B, Asangani IA, Rasheed SAK, Marchio C, Sahin O, Ceppi P. Thymidylate synthase maintains the de-differentiated state of triple negative breast cancers. Cell Death Differ. 2019;26:2223–2236. doi: 10.1038/s41418-019-0289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Xu WW, Guan XY, Qin YR, Law S, Lee NP, Chan KT, Tam PY, Li YY, Chan KW, Yuen HF, Tsao SW, He QY, Cheung AL. Competitive binding between Id1 and E2F1 to Cdc20 regulates E2F1 degradation and thymidylate synthase expression to promote esophageal cancer chemoresistance. Clin Cancer Res. 2016;22:1243–1255. doi: 10.1158/1078-0432.CCR-15-1196. [DOI] [PubMed] [Google Scholar]
- 16.Huang CL, Liu D, Nakano J, Yokomise H, Ueno M, Kadota K, Wada H. E2F1 overexpression correlates with thymidylate synthase and survivin gene expressions and tumor proliferation in non small-cell lung cancer. Clin Cancer Res. 2007;13:6938–6946. doi: 10.1158/1078-0432.CCR-07-1539. [DOI] [PubMed] [Google Scholar]
- 17.Longley DB, Allen WL, McDermott U, Wilson TR, Latif T, Boyer J, Lynch M, Johnston PG. The roles of thymidylate synthase and p53 in regulating Fas-mediated apoptosis in response to antimetabolites. Clin Cancer Res. 2004;10:3562–3571. doi: 10.1158/1078-0432.CCR-03-0532. [DOI] [PubMed] [Google Scholar]
- 18.Rahman L, Voeller D, Rahman M, Lipkowitz S, Allegra C, Barrett JC, Kaye FJ, Zajac-Kaye M. Thymidylate synthase as an oncogene: a novel role for an essential DNA synthesis enzyme. Cancer Cell. 2004;5:341–351. doi: 10.1016/s1535-6108(04)00080-7. [DOI] [PubMed] [Google Scholar]
- 19.Matuszyk J. MALAT1-miRNAs network regulate thymidylate synthase and affect 5FU-based chemotherapy. Mol Med. 2022;28:89. doi: 10.1186/s10020-022-00516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciszewski WM, Chmielewska-Kassassir M, Wozniak LA, Sobierajska K. Thymidylate Synthase overexpression drives the invasive phenotype in colon cancer cells. Biomedicines. 2022;10:1267. doi: 10.3390/biomedicines10061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagaraju GP, Alese OB, Landry J, Diaz R, El-Rayes BF. HSP90 inhibition downregulates thymidylate synthase and sensitizes colorectal cancer cell lines to the effect of 5FU-based chemotherapy. Oncotarget. 2014;5:9980–9991. doi: 10.18632/oncotarget.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannava S, Moparthy KC, Wheeler LJ, Leonova KI, Wawrzyniak JA, Bianchi-Smiraglia A, Berman AE, Flanagan S, Shewach DS, Zeitouni NC, Gudkov AV, Mathews CK, Nikiforov MA. Ribonucleotide reductase and thymidylate synthase or exogenous deoxyribonucleosides reduce DNA damage and senescence caused by C-MYC depletion. Aging (Albany NY) 2012;4:917–922. doi: 10.18632/aging.100512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazzone W, Wilson PM, Labonte MJ, Lenz HJ, Ladner RD. Histone deacetylase inhibitors suppress thymidylate synthase gene expression and synergize with the fluoropyrimidines in colon cancer cells. Int J Cancer. 2009;125:463–473. doi: 10.1002/ijc.24403. [DOI] [PubMed] [Google Scholar]
- 24.Sakatani A, Sonohara F, Goel A. Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis. 2019;40:422–431. doi: 10.1093/carcin/bgy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li XY, Wang DP, Lu GQ, Liu KL, Zhang TJ, Li S, Mohamed O K, Xue WH, Qian XH, Meng FH. Development of a novel thymidylate synthase (TS) inhibitor capable of up-regulating P53 expression and inhibiting angiogenesis in NSCLC. J Adv Res. 2020;26:95–110. doi: 10.1016/j.jare.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko JC, Tsai MS, Chiu YF, Weng SH, Kuo YH, Lin YW. Up-regulation of extracellular signal-regulated kinase 1/2-dependent thymidylate synthase and thymidine phosphorylase contributes to cisplatin resistance in human non-small-cell lung cancer cells. J Pharmacol Exp Ther. 2011;338:184–194. doi: 10.1124/jpet.111.179663. [DOI] [PubMed] [Google Scholar]
- 27.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–W514. doi: 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W560. doi: 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponten F, Jirstrom K, Uhlen M. The human protein atlas--a tool for pathology. J Pathol. 2008;216:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- 32.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng Y, Zuo P, Li XO, Zhang L. PODNL1 promotes cell proliferation and migration in glioma via regulating Akt/mTOR pathway. J Cancer. 2020;11:6234–6242. doi: 10.7150/jca.46901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson PM, Danenberg PV, Johnston PG, Lenz HJ, Ladner RD. Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol. 2014;11:282–298. doi: 10.1038/nrclinonc.2014.51. [DOI] [PubMed] [Google Scholar]
- 36.Kim JO, Ryu CS, Lee JY, Ko EJ, Ha YH, Sung JH, Hwang TS, Kim IJ, Kim NK. Association of thymidylate synthase (TS) gene polymorphisms with incidence and prognosis of coronary artery disease. Int J Mol Sci. 2023;24:12591. doi: 10.3390/ijms241612591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desrosiers V, Barat C, Breton Y, Ouellet M, Tremblay MJ. Thymidylate synthase is essential for efficient HIV-1 replication in macrophages. Virology. 2021;561:47–57. doi: 10.1016/j.virol.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Yang W, Shaw N, Perloff S, Carmichael SL, Finnell RH, Shaw GM, Lammer EJ. Thymidylate synthase polymorphisms and risk of conotruncal heart defects. Am J Med Genet A. 2012;158A:2194–2203. doi: 10.1002/ajmg.a.35310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Ye J, Guo W, Zhang F, Wang L, Han A. TYMS-TM4SF4 axis promotes the progression of colorectal cancer by EMT and upregulating stem cell marker. Am J Cancer Res. 2022;12:1009–1026. [PMC free article] [PubMed] [Google Scholar]
- 40.Varghese V, Magnani L, Harada-Shoji N, Mauri F, Szydlo RM, Yao S, Lam EW, Kenny LM. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9:1505. doi: 10.1038/s41598-018-38017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Z, Huang Y, Wen D, Lin X, Kan A, Li Q, Wei W, Chen M, Xu L, He M, Shi M. One day versus two days of hepatic arterial infusion with oxaliplatin and fluorouracil for patients with unresectable hepatocellular carcinoma. BMC Med. 2022;20:415. doi: 10.1186/s12916-022-02608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Yan L, Cui C, Wang Z, Wu J, Zhao M, Dong B, Guan X, Tian X, Hao C. Identification of TYMS as a promoting factor of retroperitoneal liposarcoma progression: Bioinformatics analysis and biological evidence. Oncol Rep. 2020;44:565–576. doi: 10.3892/or.2020.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao M, Tan B, Dai X, Shao Y, He Q, Yang B, Wang J, Weng Q. DHFR/TYMS are positive regulators of glioma cell growth and modulate chemo-sensitivity to temozolomide. Eur J Pharmacol. 2019;863:172665. doi: 10.1016/j.ejphar.2019.172665. [DOI] [PubMed] [Google Scholar]
- 44.Agullo-Ortuno MT, Garcia-Ruiz I, Diaz-Garcia CV, Enguita AB, Pardo-Marques V, Prieto-Garcia E, Ponce S, Iglesias L, Zugazagoitia J, Lopez-Martin JA, Paz-Ares L, Nunez JA. Blood mRNA expression of REV3L and TYMS as potential predictive biomarkers from platinum-based chemotherapy plus pemetrexed in non-small cell lung cancer patients. Cancer Chemother Pharmacol. 2020;85:525–535. doi: 10.1007/s00280-019-04008-9. [DOI] [PubMed] [Google Scholar]
- 45.Sato Y, Tomita M, Soga T, Ochiai A, Makinoshima H. Upregulation of thymidylate synthase induces pemetrexed resistance in malignant pleural mesothelioma. Front Pharmacol. 2021;12:718675. doi: 10.3389/fphar.2021.718675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Z, Li X, Nie K, Wang X, Liang W, Yang F, Zheng K, Zheng Y. Identification of berberine as a potential therapeutic strategy for kidney clear cell carcinoma and COVID-19 based on analysis of large-scale datasets. Front Immunol. 2023;14:1038651. doi: 10.3389/fimmu.2023.1038651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu M, Pei Y, Lu F, Jiang H, Bi Y, Cheng J, Qin J. Identification of potential Hub genes and miRNA-mRNA pairs related to the progression and prognosis of cervical cancer through integrated bioinformatics analysis. Front Genet. 2021;12:775006. doi: 10.3389/fgene.2021.775006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu T, Nakagawa Y, Takahashi N, Hashimoto S. Thymidylate synthase gene amplification predicts pemetrexed resistance in patients with advanced non-small cell lung cancer. Clin Transl Oncol. 2016;18:107–112. doi: 10.1007/s12094-015-1359-y. [DOI] [PubMed] [Google Scholar]
- 49.Biagioni A, Staderini F, Peri S, Versienti G, Schiavone N, Cianchi F, Papucci L, Magnelli L. 5-fluorouracil conversion pathway mutations in gastric cancer. Biology (Basel) 2020;9:265. doi: 10.3390/biology9090265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dersh D, Phelan JD, Gumina ME, Wang B, Arbuckle JH, Holly J, Kishton RJ, Markowitz TE, Seedhom MO, Fridlyand N, Wright GW, Huang DW, Ceribelli M, Thomas CJ, Lack JB, Restifo NP, Kristie TM, Staudt LM, Yewdell JW. Genome-wide screens identify lineage- and tumor-specific genes modulating MHC-I- and MHC-II-restricted immunosurveillance of human lymphomas. Immunity. 2021;54:116–131. e110. doi: 10.1016/j.immuni.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Peng L, Zhang R, Zheng Z, Chen C, Cheung KL, Cui M, Bian G, Xu F, Chiang D, Hu Y, Chen Y, Lu G, Yang J, Zhang H, Yang J, Zhu H, Chen SH, Liu K, Zhou MM, Sikora AG, Li L, Jiang B, Xiong H. 5-fluorouracil targets thymidylate synthase in the selective suppression of TH17 cell differentiation. Oncotarget. 2016;7:19312–19326. doi: 10.18632/oncotarget.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gotanda K, Hirota T, Matsumoto N, Ieiri I. MicroRNA-433 negatively regulates the expression of thymidylate synthase (TYMS) responsible for 5-fluorouracil sensitivity in HeLa cells. BMC Cancer. 2013;13:369. doi: 10.1186/1471-2407-13-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in this study are available in the online dataset. Requests for further access to the dataset can be directed to yibo.geng@ccmu.edu.cn.