Abstract
Objective: To assess the efficacy of combining acupuncture with pricking blood therapy to treat chronic spontaneous urticaria (CSU) and compare its outcomes with those of second-generation H1-antihistamines. Methods: Seventy CSU patients were enrolled and randomly assigned to receive treatment with either oral loratadine tablets or a combination of acupuncture and pricking blood therapy (n=35 each). Evaluations were conducted at baseline, at the conclusion of the 4-week treatment period, and at the 4th and 8th weeks post-treatment time points. We used six scales to gauge the severity of the skin lesions, itchiness, quality of life, and emotional and sleep states of the CSU patients. Results: Upon completion of the treatment, both groups demonstrated a significant reduction in the scores of six scales from the baseline (P < 0.05). Notably, the scores of the itch visual analog scale (VAS), Hamilton Anxiety Scale, and Hamilton Depression Scale in the acupuncture and pricking blood therapy group were lower than those in the loratadine group (all P < 0.05). At the 8-week follow-up, the scores for all six scales were reduced from baseline in both groups (all P < 0.05), though the acupuncture and pricking blood therapy group showed significantly lower scores on the 7-day urticaria activity scale, with the Dermatology Life Quality Index, and the itchiness VAS compared with the loratadine group (all P < 0.05). Conclusions: Acupuncture combined with pricking blood therapy significantly ameliorated skin lesions, itchiness, and the associated psychological distress in CSU patients. This integrative approach not only matched the short-term efficacy of oral loratadine but surpassed its long-term benefits.
Keywords: Chronic spontaneous urticaria, acupuncture, pricking blood, bloodletting, randomized controlled trial
Introduction
Chronic spontaneous urticaria (CSU) is defined as the spontaneous manifestation of wheals and/or angioedema without identifiable provoking factors, with episodes occurring at least twice weekly and persisting for more than 6 consecutive weeks [1,2]. The hallmark symptoms include daily or intermittent emergence of wheals accompanied by pronounced itching. Notably, angioedema is the primary symptom in approximately 10% of cases, with skin lesions typically enduring from 4 to 24 hours [3,4]. The disease is characterized by frequent recurrence of the lesions, which are often numerous and intensely itchy, predominantly during the nocturnal hours. These symptoms lead to a significantly higher incidence of anxiety, depression, and sleep disturbances among CSU patients compared with the general population [5]. The course of CSU is often protracted, lasting from 2 to 5 years, though approximately 20% of patients experience symptoms for more than 5 years [6-8]. The propensity for relapse is considerable: potential recurrences can occur months or years following complete remission. This pattern of prolonged and recurrent episodes results in increased healthcare expenditures and diminished occupational productivity, thereby exerting a considerable economic strain on both families and societal resources [9]. CSU may also increase the risk of other diseases, such as thyroid disease, asthma, and tumors, as well as the risk of disability [10,11]. Studies have shown that the prevalence rate of CSU in China is 1.29%, with a slightly higher incidence in female individuals compared with male individuals [12]. Despite regional variations in prevalence rates, there is a consistent upward trend in incidence rates across all age groups [13]. Thus, the manifestation of CSU significantly compromises the physical functionality and subjective well-being of the patient while placing a growing burden on the social healthcare system [13-16].
Contemporary medical treatment for CSU primarily follows guidelines recommending second-generation H1-antihistamines as the first-line pharmacotherapy [1,2]. However, research indicates that a certain proportion of CSU patients exhibit a suboptimal response to these antihistamines, necessitating the use of novel biologics, such as omalizumab, and, in some instances, immunosuppressants, such as cyclosporine, to effectively manage disease flare ups [17]. Given the frequent and prolonged nature of CSU symptom episodes, patients who wish to control the disease have to endure long-term drug applications and face the potentially unknown risks posed by drugs while also suffering from the disease’s effects on normal life, including great psychological pressure [18]. For pregnant CSU patients in particular, although some studies have shown that the use of H1-antihistamines within the first 3 months of pregnancy is not associated with an overall increased risk of neonatal malformations, spontaneous abortions, preterm labor, stillbirth, or low birth weight have occurred [19], while more persuasive data are needed to substantiate these findings, as most of the available evidence is derived from small-sample studies and meta-analyses [20]. Therefore, the Chinese urticaria treatment guidelines still recommend avoiding antihistamines during pregnancy, especially early pregnancy [1]. For breastfeeding CSU patients, the American Academy of Pediatrics considers second-generation H1-antihistamines to be relatively safe because the drug concentration in breast milk is much lower than its concentration in maternal plasma; however, studies monitoring the infant response are still needed because possible harm to the infant is unknown [21]. Furthermore, the instructions for commonly used second-generation H1-antihistamines, such as loratadine, cetirizine, and ebastine, state that they are not recommended for use during lactation [20].
Our team previously conducted an in-depth randomized controlled trial to address the real-world challenges encountered in CSU treatment considering the positive outcomes from recent research on various acupuncture techniques, such as filiform needle acupuncture, bloodletting, cupping, acupoint injection, and scalp acupuncture, for managing CSU [22-29]. The trial was designed to investigate the precise efficacy of acupuncture therapy for CSU patients and assess how it compares to the efficacy of second-generation H1-antihistamines. By contrast, in this study, we selected acupuncture and pricking blood therapy from the various acupuncture methods as the treatment modalities for the treatment group and compared them with second-generation H1-antihistamines because acupuncture and pricking blood therapy each have advantages: acupuncture can stimulate acupoints to harmonize the flow of qi and blood throughout the body via the acupoint-meridian-organ system, thereby restoring the balance of yin and yang, and pricking blood therapy can stimulate acupoints and release the body’s stasis, thereby activating the blood to dissipate blood stasis and smoothing the whole body’s qi and blood circulation [30]. When used together, the two methods complement each other, resulting in better therapeutic effects. In this study, the control group received orally administered loratadine, a second-generation H1-antihistamine endorsed by urticaria treatment guidelines. Research indicates that oral loratadine significantly improves rashes in patients with CSU with no observed drug-induced side effects, except for minor sedative effects and dry mouth symptoms in a few patients [31]. Given the recommendations in the clinical guidelines and the results of previous studies, this research administered loratadine to the control group, setting it against a regimen of acupuncture in conjunction with pricking blood therapy in the treatment group. This comprehensive and scale-based assessment of the therapeutic efficacy of these interventions will furnish robust, evidence-based support for the utility of acupuncture combined with pricking blood therapy for managing CSU, thereby enriching the repertoire of clinical treatment options for this condition.
Methods
Study design
This randomized controlled clinical trial was conducted over 15 weeks, including a 2-week washout period, a 1-week baseline assessment period, a 4-week treatment period, and an 8-week follow-up period. The study was approved by the Medical Ethics Committee of Hebei University of Chinese Medicine (Approval Report Number: YXLC2021031) and registered in the Chinese Clinical Trial Registry (ChiCTR 2100050384), and all participants voluntarily signed an informed consent form.
To assign the patients to the groups, the numbers from 1 to 70 were written on individual cards and placed in closed opaque envelopes, which were kept and distributed by specialized personnel at the study sites. The envelopes were opened according to the order of patient visits. Patients who received odd numbers were assigned to the control group, and those who received even numbers were assigned to the treatment group. During the trial, the indicator evaluators, data managers, and data analysts were not aware of the groupings.
Study population
Patients who visited the acupuncture and dermatology outpatient clinics of the First Affiliated Hospital of Hebei University of Chinese Medicine between March 2021 and December 2022 for CSU treatment were recruited for this exploratory study. Oral loratadine was used as the control group treatment, and acupuncture combined with pricking blood therapy was used as the treatment group therapy. The Pearson chi-square test was used to test the differences in the immediate and long-term efficacy of the two therapies between the two groups. We estimated that the efficacy of the treatment group was approximately 65% and that of the control group was approximately 30%. With an α level of 0.05 and a β of 0.2 (i.e., power =80%), we calculated the necessary sample size (with a 1:1 parallel design) to be n1=n2=29. Considering a dropout rate of no more than 15%, we finalized the sample size as 35 cases in each group. The formula for this calculation is as follows [32].
Note: p1=0.65, p2=0.3, k=1, ε=p1-p2=0.35, α=0.05, Zα/2=1.960, β=0.2, Zβ=0.842.
Diagnostic criteria
According to the International Urticaria Guidelines and the Chinese Urticaria Guidelines [1,2], CSU was defined as (a) spontaneous attacks of urticaria wheals without clear inducement (excluding chronic induced urticaria caused by cold, heat, sunlight, pressure, and other inducements), (b) with or without angioedema, (c) with wheals attacks ≥ 2 times/week; and (d) with recurrent attacks for > 6 weeks.
Inclusion criteria
Patients were included if they (a) met the diagnostic criteria for CSU, (b) were aged 18 years to < 70 years, (c) had used neither antihistamines within 2 weeks before enrollment nor steroids or immunosuppressants within 1 month before enrollment, (d) had normal liver and kidney function, (e) were not participating in other ongoing clinical studies, and (f) were willing to participate and provide written informed consent.
Exclusion criteria
Patients were excluded from the study if they were (a) unconscious or unable to express subjective discomfort or mental illness symptoms, (b) had an additional severe consumptive disease or acute and critical illness, (c) had a serious primary disease, such as any disease of the cardiovascular, digestive, or hematopoietic system, (d) were currently pregnant or lactating or preparing for pregnancy, (e) were allergic to loratadine, or (f) worked as a driver or in any position requiring working at a height.
Criteria for trial termination
The trial was to be terminated if severe adverse reactions occurred during the study that made it inappropriate to continue treatment, or if other serious diseases were discovered or other unexpected events occurred that made further observation inappropriate during treatment.
Criteria for withdrawal and dropout
Participants were removed from the study if they (a) used any of the treatment methods prohibited by this trial or switched to other treatment methods partway through the trial, (b) voluntarily withdrew or were lost to follow-up, (c) failed to undergo treatment as prescribed, or (d) provided incomplete data affecting the evaluation of efficacy and safety.
Intervention measures
Control group
The control group received oral loratadine tablets (Hainan Haishen Tongzhou Pharmaceuticals Co., Ltd., National Medical Products Administration approval number H20040797, batch number 2100071, specification: 10 mg*12 tablets/box) at a dose of 10 mg once daily for 4 weeks.
Treatment group
The treatment group received both acupuncture and pricking blood therapy.
(1) Acupuncture with filiform needles: Points used: Zhongwan (CV12), Guanyuan (CV4), Tianshu (ST25), Zusanli (ST36), Sanyinjiao (SP6), Xuehai (SP10), Quchi (LI11), Hegu (LI4), Taichong (LR3), Baihui (GV20), Shenting (GV24). Procedure: The patient laid on their back with the area of skin to be treated fully exposed. The practitioner disinfected their hands and the area around the acupuncture points on the patient with 75% alcohol on a cotton ball. The practitioner used disposable acupuncture needles sized 0.25 mm × 40 mm (Ma’anshan Bond Medical Instrument Co., Ltd., registration number: Wan Medical Device Registration 20172270055, batch number 2021021) for direct insertion (Baihui and Shenting are needled horizontally backward) to a depth of 25-40 mm and performed even reinforcing and reducing techniques upon achieving de qi, leaving the needles in place for 30 minutes. This procedure was performed once daily for 4 weeks.
(2) Pricking blood therapy: Points used: Dazhui (GV14), Geshu (BL17). Procedure: The patient was positioned prone with the treatment area fully exposed. The practitioner disinfected their hands and the local area around the acupuncture points on the patient with 75% alcohol on a cotton ball. A 0.5 × 20-mm disposable syringe needle (Zhejiang Kangdelai Medical Instrument Co., Ltd., registration number: National Medical Device Registration No. 20193141818, batch number 20210929) was used to rapidly prick Dazhui and Geshu (both sides) 3-5 times per point, with a pricking depth of approximately 4-7 mm. Immediately after pricking, cupping was applied to the areas, and the cups were left in place for 10 minutes before being removed. The blood was then wiped clean, and the area was disinfected and covered with sterile gauze. The amount of blood drawn from each point was approximately 1 ml. The procedure was performed every other day for 4 weeks.
Efficacy evaluation
Efficacy evaluation criteria
The 7-day urticaria activity score (UAS7), which is internationally recognized to indicate the severity of CSU symptoms, was used as the final efficacy evaluation criterion [33,34]. The UAS7 mainly records the number of wheals and degree of itchiness each day for 1 week, and the daily total score for wheals and itchiness is used to assess and detect the disease activity and response to treatment in CSU patients. Efficacy evaluations were performed at the end of treatment and 8 weeks later (i.e., the second follow-up) to evaluate the short- and long-term efficacy differences between the two therapies.
Clinical efficacy was evaluated based on the UAS7 and the Symptom Score Reduction Index (SSRI), where the SSRI = (UAS7 before treatment - UAS7 after treatment)/UAS7 before treatment × 100%. The efficacy was categorized according to the SSRI grading standards: cured, SSRI ≥ 90%; significantly effective, 60% ≤ SSRI < 90%; effective, 20% ≤ SSRI < 60%; ineffective, SSRI < 20%.
The overall response rate was calculated as (number of cured cases + number of significantly effective cases + number of effective cases)/total number of cases in the group × 100%.
Primary observation indicators
The primary outcome indicators were the UAS7, the itch visual analog scale (VAS) score, and the Dermatology Life Quality Index (DLQI) score. These three indicators were assessed once at baseline, four times during and at the end of treatment (once a week), and twice during the follow-up period (at the 4th and 8th week after the end of treatment), for a total of seven evaluations.
The daily scoring range for the UAS7 is 0 to 6 points. The scores for the evaluations over 7 consecutive days are summed to calculate the total score for the week (range, 0-42 points). A weekly score of less than 7 points indicates low CSU activity, whereas a score greater than 28 points indicates high activity and a severe condition.
The itch VAS score is determined based on the patient’s perception, using a scale from 0 to 10 to represent the intensity of itching experienced in the past 24 hours. A score of 0 indicates no itching, 1-3 points indicates mild itching, 4-6 points indicates moderate itching, 7-9 points signifies severe itching, and 10 points denotes extreme itching. The score is evaluated daily over 7 consecutive days, and an average score is calculated for the week.
The DLQI is designed to comprehensively assess the impact of CSU skin symptoms on the patient’s life, specifically their daily living, work, and social interactions. The questionnaire consists of 10 items, each scored according to the severity level, with a total scoring range is 0-30 points. A score of 0 or 1 indicates that CSU has no impact on the patient’s quality of life, 2-5 points suggest a small impact, 6-10 points indicate a moderate impact, 11-20 points signify a significant impact, and 21-30 points denote a severe impact on the patient’s quality of life.
Secondary observation indicators
The secondary outcome indicators included scores from the Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), and Pittsburgh Sleep Quality Index (PSQI). These three indicators were assessed once at baseline and once at the end of treatment.
The HAMA was used to comprehensively assess whether skin symptoms triggered anxiety symptoms in patients. The scale evaluates anxious mood and tension, with a scoring range of 0-56 points. A score of < 7 points indicates no anxiety symptoms, ≥ 7 points suggests possible anxiety symptoms, ≥ 14 points confirms the presence of anxiety, ≥ 21 points confirms significant anxiety, and ≥ 29 points suggests severe anxiety.
The HAMD was used to evaluate whether skin symptoms led to depressive symptoms in the patients. It considers depressive mood and suicidal tendencies and has a total scoring range of 0-56 points. A score of < 7 points indicates no depressive symptoms, ≥ 7 points suggests possible depression, ≥ 17 points confirms the presence of depression, and ≥ 24 points indicates severe depression.
The PSQI was used to evaluate the impact of skin symptoms on patient sleep, considering both sleep quality and latency. The scoring range is 0-21 points. Scores of 0-5 indicate very good sleep quality, 6-10 signify fair sleep quality, 11-15 denote average sleep quality, and 16-21 indicate very poor sleep quality.
Adverse event management and safety evaluation
During the study, if participants experienced clinical symptoms such as fatigue, dizziness, edema, nausea, abdominal pain, fainting from needles, subcutaneous hemorrhage (bruising), or any abnormal laboratory findings indicative of adverse events, the researchers promptly and thoroughly documented the timing and cause of these events, along with necessary interventions. We assessed whether the incidents warranted the immediate termination of the trial, and we analyzed and evaluated the safety of the research protocol based on the recorded adverse events after the conclusion of the study.
Data analysis and processing
SPSS 26.0 and GraphPad Prism 8.0 software were used to analyze and plot the obtained data. Descriptive statistics were generated for quantitative data with a normal distribution; paired sample t-tests were performed for within-group comparisons, and independent sample t-tests were used for between-group comparisons. The rank-sum test was applied for data not following a normal distribution. For categorical data expressed as rates, the chi-square test was used for between-group comparisons. P-values less than 0.05 were considered statistically significant.
Results
Baseline characteristics
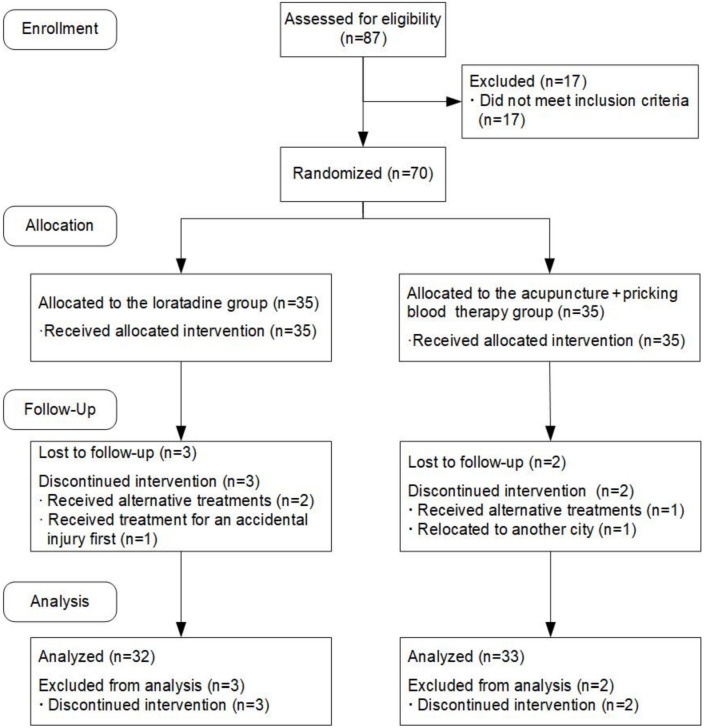
Seventy patients were enrolled in the study, 65 of whom completed all the trials and five of whom dropped out. In the loratadine group, three participants dropped out, resulting in 32 participants completing the study; in the acupuncture + pricking blood therapy group, two participants dropped out, leaving 33 participants who completed the study. Data from the dropped cases were not included in the final statistical analysis (Figure 1).
Figure 1.
Flowchart of the study.
There were no statistically significant differences in sex, age, duration of illness, history of other diseases, and history of smoking and drinking between the two groups (Table 1).
Table 1.
Comparison of general data between the two groups of patients
| Factors | Loratadine (n=32) | Acupuncture + pricking blood therapy (n=33) | χ2 value | P value |
|---|---|---|---|---|
| Age | 40.41±13.68 | 41.06±14.31 | -0.126 | 0.901 |
| Sex | 0.371 | 0.543 | ||
| Male | 13 | 11 | ||
| Female | 19 | 22 | ||
| Course of illness (months) | 45.29±25.45 | 42.47±24.87 | 0.229 | 0.821 |
| Thyroid disorder history | 1.001 | 0.317 | ||
| Yes | 1 | 3 | ||
| No | 31 | 30 | ||
| Gastritis history | 0.167 | 0.683 | ||
| Yes | 5 | 4 | ||
| No | 27 | 29 | ||
| Smoking history | 0.548 | 0.459 | ||
| Yes | 6 | 4 | ||
| No | 26 | 29 | ||
| Drinking history | 0.032 | 0.857 | ||
| Yes | 10 | 11 | ||
| No | 22 | 22 |
Efficacy
Overall response rate comparison
The short-term efficacy evaluation results showed that at the end of treatment, the overall response rate was similar between the two groups (χ2=0.167, P=0.683). The results of the long-term efficacy evaluation showed that the total effective rate of acupuncture + pricking blood therapy was higher than that of loratadine at the second follow-up (χ2=4.481, P=0.034) (Tables 2 and 3).
Table 2.
Comparison of the efficacy of loratadine versus acupuncture + pricking blood therapy at the end of treatment
| Grouping | Recovered | Highly Effective | Effective | Invalid | Effectiveness Rate |
|---|---|---|---|---|---|
| Loratadine (n=32) | 5 (15.63%) | 17 (53.13%) | 5 (15.63%) | 5 (15.63%) | 27 (84.38%) |
| Acupuncture + pricking blood therapy (n=33) | 2(6.06%) | 14 (42.42%) | 13 (39.39%) | 4 (12.12%) | 29 (87.88%) |
| χ2/Z value | -1.445 | 0.167 | |||
| P value | 0.149 | 0.683 |
Table 3.
Comparison of the efficacy of loratadine versus acupuncture + pricking blood therapy at the second follow-up
| Grouping | Recovered | Highly Effective | Effective | Invalid | Effectiveness Rate |
|---|---|---|---|---|---|
| Loratadine (n=32) | 0 (0%) | 4 (12.50%) | 7 (21.88%) | 21 (65.62%) | 11 (34.38%) |
| Acupuncture + pricking blood therapy (n=33) | 0 (0%) | 9 (27.27%) | 11 (33.33%) | 13 (39.39%) | 20 (60.61%) |
| χ2/Z value | -2.134 | 4.481 | |||
| P value | 0.033 | 0.034 |
Primary observation indicators
The results of repeated measures ANOVA indicated that the differences in UAS7 scores at different time points were statistically significant (F=71.392, P=0.000). There was an interaction effect between the two variables, time point and group (F=9.265, P=0.000). Although the overall difference in UAS7 scores between the two groups over the specified time span was not statistically significant (F=0.079, P=0.781), post hoc pairwise comparisons using Bonferroni correction revealed that at the first, second, third, and fourth weeks of treatment, as well as at the first and second follow-ups, the UAS7 scores in both groups were significantly lower than the baseline scores (loratadine: P=0.000, P=0.000, P=0.000, P=0.000, P=0.001, P=0.036; acupuncture + pricking blood therapy: all P=0.000). The UAS scores of the two groups were similar before treatment, at the second, third, and fourth weeks of treatment (P=0.818, P=0.195, P=0.884, P=0.356), as well as at the first follow-up (P=0.085). The UAS7 scores in the acupuncture + pricking blood group were higher than those in the loratadine group after the first week of treatment (P=0.015); however, at the second follow-up, the UAS7 scores in the acupuncture + pricking blood therapy group were lower than those in the loratadine group (P=0.001) (Figures 2 and 3).
Figure 2.
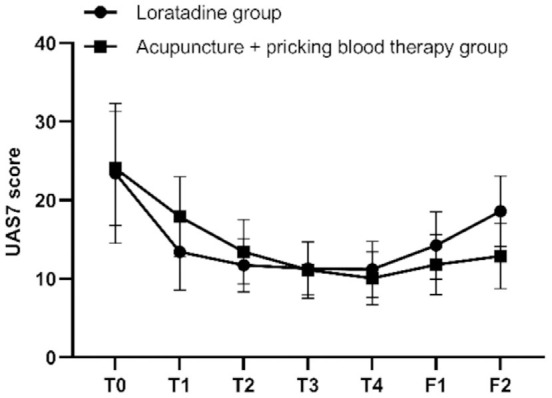
Longitudinal study of the 7-day urticaria activity (UAS7) scores. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment).
Figure 3.
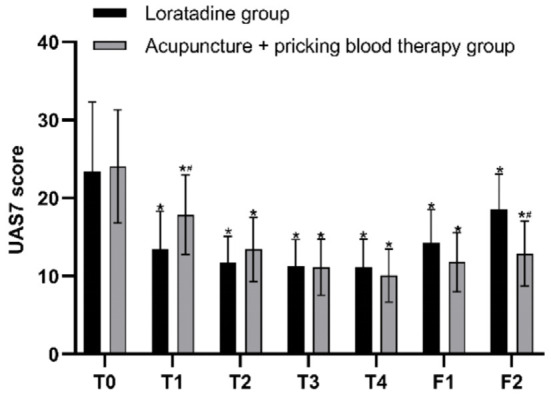
Seven-day urticaria activity (UAS7) scores before and after treatment in both groups. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment). *P < 0.05 compared with baseline; #P < 0.05, comparison between the two groups.
The results of repeated measures ANOVA indicated that the differences in itch VAS scores at different time points were statistically significant (F=120.495, P=0.000). There was an interaction effect between the two variables, time point and group (F=6.022, P=0.001). Although the overall difference in VAS scores between the two groups over the specified time span was not statistically significant (F=2.827, P=0.102), post hoc pairwise comparisons using Bonferroni correction revealed that at the first, second, third, and fourth weeks of treatment, as well as at the first and second follow-ups, the itch VAS scores in both groups were significantly lower than the baseline scores (all P=0.000 in both groups). The itch VAS scores of the two groups were similar before treatment, at the first, second, and third weeks of treatment (P=0.907, P=0.748, P=0.550, P=0.087); however, after the fourth week of treatment and at both follow-up assessments, the itch VAS scores in the acupuncture + pricking blood group were lower than those in the loratadine group (P=0.007, P=0.015, P=0.004) (Figures 4 and 5).
Figure 4.
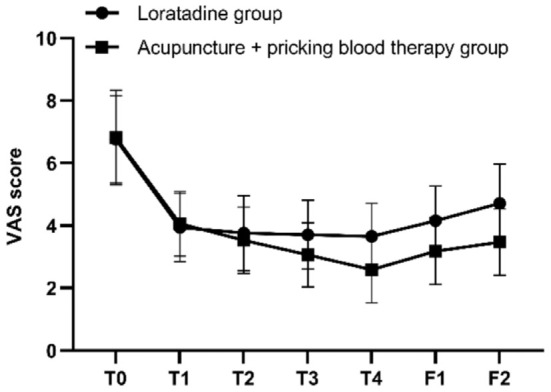
Longitudinal study of the itch visual analog scale (VAS) scores. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment).
Figure 5.
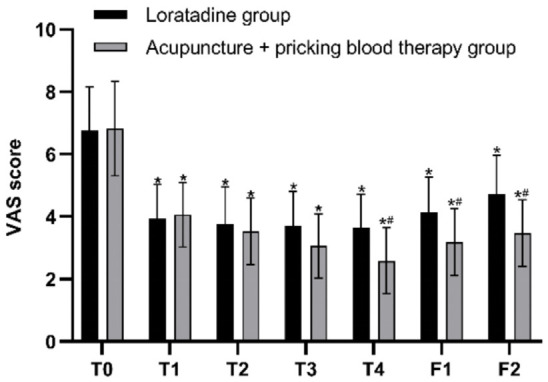
Itch visual analog scale (VAS) scores before and after treatment in both groups. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment). *P < 0.05 compared with baseline; #P < 0.05, comparison between the two groups.
The results of repeated measures ANOVA indicated that the differences in DLQI scores at different time points were statistically significant (F=44.708, P=0.000). There was an interaction effect between the two variables, time point and group (F=4.275, P=0.006). Although the overall difference in DLQI scores between the two groups over the specified time span was not statistically significant (F=0.012, P=0.913), post hoc pairwise comparisons using Bonferroni correction revealed that at the first, second, third, and fourth weeks of treatment, as well as at the first follow-up, the DLQI scores in both groups were significantly lower than the baseline scores (all P=0.000 in both groups). At the second follow-up, the difference in DLQI scores from baseline was not statistically significant in the loratadine group (P=0.087), whereas the DLQI scores in the acupuncture + pricking blood group significantly lower than the baseline (P=0.000). The DLQI scores of the two groups were similar before treatment, at the first, second, third, and fourth weeks of treatment (P=0.930, P=0.145, P=0.585, P=0.867, P=0.827), as well as at the first follow-up (P=0.208); however, at the second follow-up, the DLQI scores in the acupuncture + pricking blood therapy group were lower than those in the loratadine group (P=0.047) (Figures 6 and 7).
Figure 6.
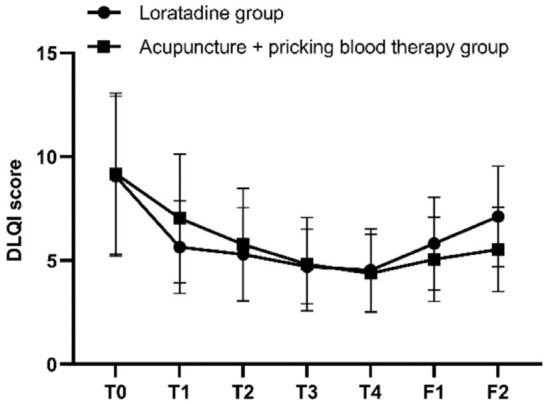
Longitudinal study of the Dermatology Life Quality Index (DLQI) scores. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment).
Figure 7.
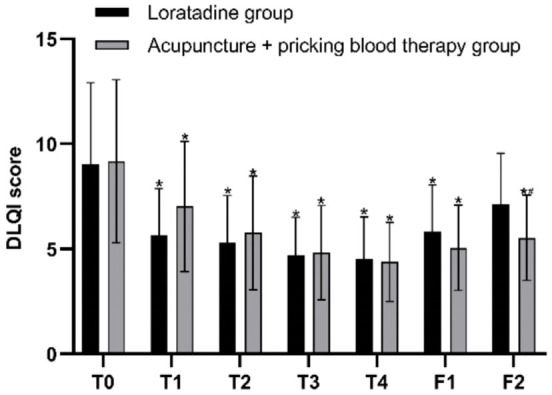
Dermatology Life Quality Index (DLQI) scores before and after treatment in both groups. Note: T0= baseline, T1-T4= the four treatment weeks, F1= follow-up 1 (4 weeks after the end of treatment), F2= follow-up 2 (8 weeks after the end of treatment). *P < 0.05, compared with baseline; #P < 0.05, comparison between the two groups.
Secondary observation indicators
The HAMA scores for both groups were significantly lower at the end of treatment than at baseline (both P=0.000). At the end of treatment, the HAMA scores in the acupuncture + pricking blood therapy group were lower than those in the loratadine group (P=0.008) (Figure 8).
Figure 8.
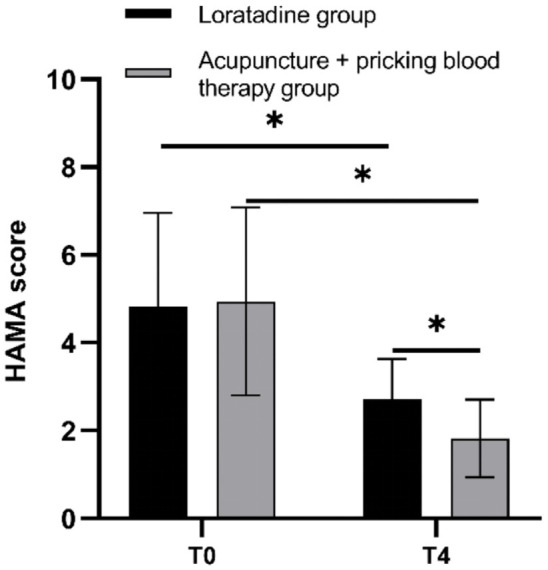
Hamilton Anxiety Scale (HAMA) scores before and after treatment in both groups. Note: T0= baseline, T4= end of treatment. *P < 0.05.
Similarly, at the end of treatment, the HAMD scores for both groups were significantly lower compared with baseline (loratadine: P=0.001; acupuncture + pricking blood therapy: P=0.000), and those in the acupuncture + pricking blood therapy group were lower than those in the loratadine group (P=0.004) (Figure 9).
Figure 9.
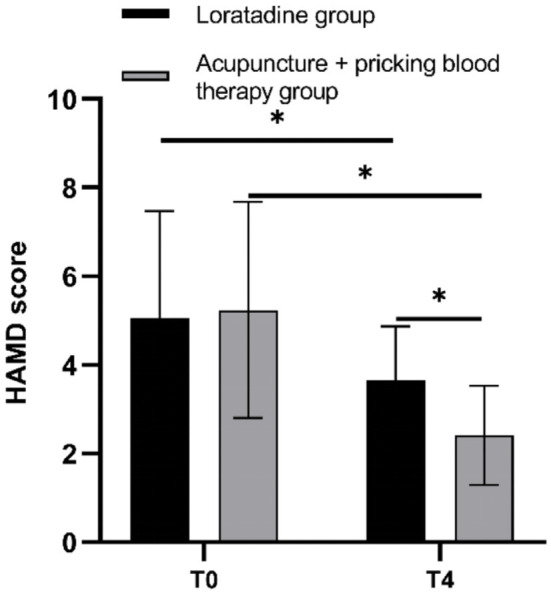
Hamilton Depression Scale (HAMD) scores before and after treatment in both groups. Note: T0= baseline, T4= end of treatment. *P < 0.05.
The PSQI scores were also significantly lower for both groups after versus before treatment (both P=0.000). However, the PSQI scores of the two groups were similar at the end of treatment (P=0.127) (Figure 10).
Figure 10.
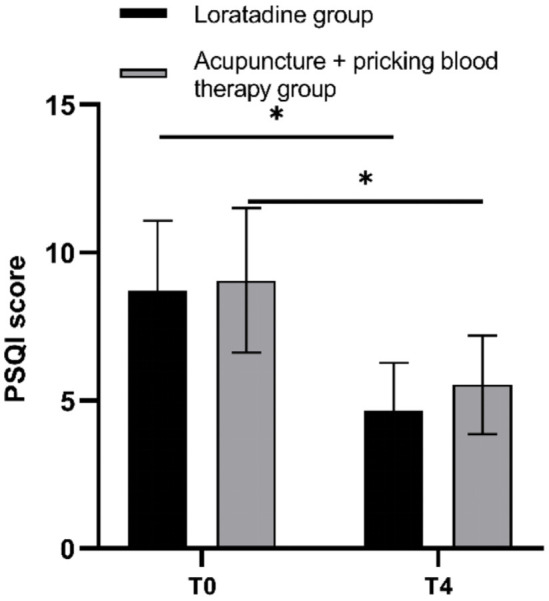
Pittsburgh Sleep Quality Index (PSQI) scores before and after treatment in both groups. Note: T0= baseline, T4= end of treatment. *P < 0.05.
Safety evaluation
In the loratadine group, four patients experienced fatigue and drowsiness after taking the loratadine tablets. Two additional patients in this group developed mild swelling of the extremities and face; a dermatologist examined the patients, confirmed that this was a possible side effect of the medication, and indicated that they could continue taking the medication unless other discomforts arose. In the acupuncture + pricking blood therapy group, three patients exhibited local mild subcutaneous bleeding and bruising after needle removal. After careful examination and good communication with the patients, no special treatment was deemed necessary, and their condition was expected to resolve on its own. There was no significant difference in the incidence of adverse reactions between the two groups (χ2=1.271, P=0.260) (Table 4).
Table 4.
Comparison of complications among patients in the loratadine versus acupuncture + pricking blood therapy groups
| Grouping | Fatigue | Edema | Purpura | All reactions |
|---|---|---|---|---|
| Loratadine (n=32) | 4 (12.5%) | 2 (6.25%) | 0 (0.00%) | 6 (18.75%) |
| Acupuncture + pricking blood therapy (n=33) | 0 (3.03%) | 0 (0.00%) | 3 (9.09%) | 3 (9.09%) |
| χ2 value | 4.395 | 2.128 | 3.050 | 1.271 |
| P value | 0.053 | 0.145 | 0.081 | 0.260 |
Discussion
In this study, we performed a comparative analysis between acupuncture combined with pricking blood therapy and oral loratadine for CSU. Employing six distinct scales, we aimed to comprehensively and multidimensionally evaluate primary skin lesions, itching symptoms, associated emotional disturbances, sleep disruptions, and the overall quality of life in patients with CSU. We extensively compared the differences in scores across various time points between the two treatment approaches, which enabled us to uncover the precise efficacy of acupuncture combined with pricking blood therapy in treating different symptoms in CSU patients compared with the current first-line pharmacotherapy.
The UAS7, itch VAS, and DLQI scores were the primary efficacy evaluation indicators in this study because they are crucial for assessing the frequency and severity of patients’ symptoms as well as their overall quality of life. The assessments were carried out at regular intervals, specifically before the commencement of treatment; in the first, second, and third weeks of treatment; at the conclusion of treatment; and at two follow-up visits. With this schedule, we aimed to monitor the efficacy onset, speed, and duration of the two therapies as well as differences in efficacy between the patient groups at various time points. Initially, both groups showed significant improvements in UAS7 scores after treatment, but there was no statistically significant difference between the groups. Notably, observation of the scores at other time points revealed differences in the onset time and trend of efficacy between the two therapies. During the treatment period, the loratadine group experienced a rapid decline in scores within the first week of medication use and maintained a steady state thereafter with no significant further decrease. By contrast, the acupuncture and pricking blood therapy group showed a gradual downward trend in symptom scores over time and with increased treatment sessions, reaching the lowest scores by the end of treatment, indicating a difference in the rapidity of effect onset between the two therapies. During the follow-up period, the UAS7 scores for the loratadine group rebounded quickly after medication cessation. For the acupuncture and pricking blood therapy group, symptom scores also increased after intervention cessation but remained lower than those in the loratadine group, with a more gradual upward trend. These results suggest that second-generation H1-antihistamines, such as loratadine, have a quick onset of action that rapidly suppresses symptoms but a higher recurrence rate after the end of treatment. Acupuncture combined with pricking blood therapy, on the other hand, is characterized by a slower onset of effect that gradually controls symptom outbreaks and a lower chance of symptom recurrence after treatment cessation compared with oral antihistamines. The DLQI scores showed trends and differences between the two groups similar to those observed in the UAS7 scores, highlighting the direct impact of urticaria symptoms on patient quality of life. Furthermore, both groups showed significant improvement in itch VAS scores after treatment, though the acupuncture and pricking blood therapy group outperformed the loratadine group. The trends in itch VAS scores also differed between the two groups. After the first week of treatment, both groups showed a significant decrease in VAS scores. In the loratadine group, the scores then stabilized and did not change significantly through the end of treatment; by contrast, the acupuncture and pricking blood therapy group continued to show decreasing scores over time and with the number of treatments, reaching the lowest point at the end of the treatment. During the follow-up period, the loratadine group experienced a quicker rebound in scores after stopping the medication, whereas the acupuncture and pricking blood therapy group saw a more gradual rebound, with scores remaining lower than those in the loratadine group. These findings indicate that acupuncture combined with pricking blood therapy significantly reduces itchiness in CSU patients, with a quicker onset and longer duration of effect. Thus, in terms of controlling itching symptoms, we found this therapy to be superior to oral loratadine. A comprehensive analysis of UAS7 and itch VAS scores showed that although acupuncture and pricking blood therapy controlled CSU skin lesions as effectively as oral loratadine, it had a distinct advantage in improving patients’ itchiness. Clinical observations during the treatment process further revealed that the occurrence of itchiness does not always accompany the appearance of wheals, suggesting that patients could experience rashes without significant itching after the combined therapy. This finding indicates that although the occurrence and transmission mechanism of itchiness is closely related to the outbreak of urticaria lesions, it still has unique influencing factors. Past research reveals that acupuncture has a potential antipruritic (itch-relieving) effect [35]. Some studies suggest that acupuncture can reduce itch-induced activation of the insula and prefrontal cortex areas, thereby inhibiting itch transmission [36]. Others propose that acupuncture may suppress itchiness by promoting the selective release of opioid substances in the spinal cord, which block the itch-related impulses transmitted via slow-conducting C fibers from the periphery [37]. Further research on acupuncture’s inhibition of itchiness has focused on the gastrin-releasing peptide receptor (GRPR), which is considered a core mediator of itch transmission and an effective target for antipruritic intervention [38]. Specifically, acupuncture is thought to suppress itch symptoms by inhibiting GRPR expression and intervening in itch transmission at the spinal level-another possible mechanism for its effectiveness [39,40]. Given the results of previous studies and the significant reduction of itch symptoms in CSU patients who underwent acupuncture combined with pricking blood therapy in this study, acupuncture has a definitive antipruritic effect. Further in-depth exploration and investigation into the specific mechanisms should be conducted to provide more evidence for acupuncture’s role in intervening in itch transmission.
The HAMA, HAMD, and PSQI scores were used as secondary efficacy indicators to assess the emotional and sleep-related disturbances that accompany skin lesion symptoms in CSU patients. These metrics provided supplemental data for evaluating the clinical efficacy of two distinct therapies, primarily focusing on pre-treatment versus post-treatment conditions. Initially, the analysis of the HAMA scores, indicative of patient anxiety levels, and HAMD scores, which reflected their depression levels, revealed varying extents of anxiety and depression across the patient cohorts prior to treatment. Nonetheless, the post-treatment observations demonstrated notable enhancements in both anxiety and depression in both groups. Remarkably, the group that received acupuncture combined with pricking blood therapy exhibited more pronounced improvements compared with the group treated with loratadine. This observation, together with our detailed evaluation of the patients’ UAS7 scores, suggests that though emotional issues are intricately linked to the manifestation of urticarial skin lesions, their onset is also influenced by myriad other factors. When it comes to mitigating emotional disturbances, acupuncture and pricking blood therapy present superior benefits over oral loratadine, markedly reducing the emotional stress experienced by patients. Prior research has highlighted notable progress in understanding the suppressive impact of acupuncture on anxiety and depression symptoms. Clinical investigations have documented significant benefits of acupuncture in ameliorating anxiety symptoms related to anxiety disorders and headaches [41,42]. Furthermore, existing evidence indicates that acupuncture can diminish anxiety and depression like behaviors in mice with atopic dermatitis by counteracting the upsurge in pCREB, ΔFosB, BDNF, and pDARPP-32 levels within the reward regions of the mouse brain while decreasing the expression of TH and D1R proteins [43]. Though the underlying mechanisms warrant further investigation, our study offers robust clinical proof supporting the efficacy of acupuncture paired with pricking blood therapy in improving symptoms of anxiety and depression among CSU patients. Similarly, the patients’ PSQI scores revealed different extents of sleep disturbances in both groups before treatment. With the mitigation of skin lesions and itching symptoms, significant enhancements in sleep quality were observed post-treatment, with no discernible statistical difference in the effectiveness of the two therapeutic approaches. This finding suggests that acupuncture combined with pricking blood therapy not only offers sedative and anti-itch benefits but also rivals the sedative effects commonly associated with antihistamine use. Thus, acupuncture and pricking blood therapy emerge as a credible alternative to antihistamines for the amelioration of sleep disturbances. The literature notes that the co-occurrence of insomnia with depression and anxiety ranges from 80% to 90% [44]. Insomnia both exacerbates the symptoms of these comorbid mental health issues and poses challenges to their treatment [45]. Acupuncture is known to regulate the levels of central neurotransmitters, including amines, amino acids, and peptides, affecting the sleep architecture [46,47]. It enhances sleep quality through the upregulation of serum γ-aminobutyric acid (GABA) and serotonin (5-HT) levels [48]. Additionally, research has found that acupuncture can promote the synthesis of TNF-α and IL-1β in the hypothalamus, reducing the inhibition of 5-HT and its metabolites caused by p-chlorophenylalanine, and thus alleviates the symptoms of insomnia in rats [49,50]. Overall, this study substantiates the potential of acupuncture and pricking blood therapy to improve sleep disturbances, and prior research findings shed light on the potential mechanisms through which acupuncture helps rectify sleep-related disorders.
In conclusion, our results confirm the efficacy of acupuncture combined with pricking blood therapy for treating CSU. However, this study has some limitations. First, as this was only a single-center randomized controlled trial, more clinical data are needed to verify our results. Second, the sample size was small, and the treatment time was relatively short, which might also lead to a discrepancy between the final study results and our initial expectations. Finally, at the second follow-up, the overall response rate of the acupuncture and pricking blood group was significantly higher than that of the loratadine group; however, the overall response rate of the acupuncture and pricking blood group itself was still decreased compared to the end of treatment. Therefore, we hope to carry out a follow-up multicenter randomized controlled study and appropriately extend the treatment time of the included cases to obtain further improvements in long-term clinical efficacy and refine our conclusions.
Conclusions
Acupuncture combined with pricking blood therapy significantly improved CSU symptoms, on par with oral loratadine’s short-term effectiveness and exceeding its long-term efficacy. This combined therapy significantly alleviated itching symptoms, anxiety, and depression in CSU patients, with superior efficacy compared with oral loratadine. Further exploration and research are needed to elucidate its mechanisms of action and provide insights and guidance for the treatment of other pruritic skin conditions. Acupuncture combined with pricking blood therapy also significantly enhanced patient sleep quality, demonstrating efficacy equivalent to oral loratadine.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (No. 2017YFC1703605) and Key Research and Development Program of the Hebei Provincial Department of Science and Technology (No. 21377766D). The authors thank Li-Yun He of the China Academy of Chinese Medical Sciences and Ying Li of the Chengdu University of Traditional Chinese Medicine for their contribution to the direction of the study. The authors thank all 70 CSU patients enrolled in the study for their contribution to the completion of the study.
Disclosure of conflict of interest
None.
References
- 1.Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, Ballmer-Weber B, Bangert C, Ben-Shoshan M, Bernstein JA, Bindslev-Jensen C, Brockow K, Brzoza Z, Chong Neto HJ, Church MK, Criado PR, Danilycheva IV, Dressler C, Ensina LF, Fonacier L, Gaskins M, Gaspar K, Gelincik A, Gimenez-Arnau A, Godse K, Goncalo M, Grattan C, Grosber M, Hamelmann E, Hebert J, Hide M, Kaplan A, Kapp A, Kessel A, Kocaturk E, Kulthanan K, Larenas-Linnemann D, Lauerma A, Leslie TA, Magerl M, Makris M, Meshkova RY, Metz M, Micallef D, Mortz CG, Nast A, Oude-Elberink H, Pawankar R, Pigatto PD, Ratti Sisa H, Rojo Gutierrez MI, Saini SS, Schmid-Grendelmeier P, Sekerel BE, Siebenhaar F, Siiskonen H, Soria A, Staubach-Renz P, Stingeni L, Sussman G, Szegedi A, Thomsen SF, Vadasz Z, Vestergaard C, Wedi B, Zhao Z, Maurer M. The international EAACI/GA(2)LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734–766. doi: 10.1111/all.15090. [DOI] [PubMed] [Google Scholar]
- 2.Chinese Dermatologist Association Urticaria Research Center of the Chinese medical Association. Chinese guidelines for the diagnosis and treatment of urticaria 2022 edition. Chinese Journal of Dermatology. 2022;55:1041–1049. [Google Scholar]
- 3.Kaplan AP, Ferrer M. An algorithm for the diagnosis, pathogenesis and treatment of chronic spontaneous urticaria, 2024 update. Allergy. 2024;79:2567–2569. doi: 10.1111/all.16113. [DOI] [PubMed] [Google Scholar]
- 4.Sabroe RA, Seed PT, Francis DM, Barr RM, Black AK, Greaves MW. Chronic idiopathic urticaria: comparison of the clinical features of patients with and without anti-fcepsilonri or anti-ige autoantibodies. J Am Acad Dermatol. 1999;40:443–450. doi: 10.1016/s0190-9622(99)70495-0. [DOI] [PubMed] [Google Scholar]
- 5.Hoskin B, Ortiz B, Paknis B, Kavati A. Exploring the real-world profile of refractory and non-refractory chronic idiopathic urticaria in the USA: clinical burden and healthcare resource use. Curr Med Res Opin. 2019;35:1387–1395. doi: 10.1080/03007995.2019.1586222. [DOI] [PubMed] [Google Scholar]
- 6.Nebiolo F, Bergia R, Bommarito L, Bugiani M, Heffler E, Carosso A, Castiglioni G, Guida G, Badiu I, Pizzimenti S, Mietta S, Ferrero N, Rolla G. Effect of arterial hypertension on chronic urticaria duration. Ann Allergy Asthma Immunol. 2009;103:407–410. doi: 10.1016/S1081-1206(10)60360-2. [DOI] [PubMed] [Google Scholar]
- 7.Toubi E, Kessel A, Avshovich N, Bamberger E, Sabo E, Nusem D, Panasoff J. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy. 2004;59:869–873. doi: 10.1111/j.1398-9995.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 8.Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969;81:588–597. doi: 10.1111/j.1365-2133.1969.tb16041.x. [DOI] [PubMed] [Google Scholar]
- 9.Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, Canonica GW, Church MK, Godse KV, Grattan CE, Greaves MW, Hide M, Kalogeromitros D, Kaplan AP, Saini SS, Zhu XJ, Zuberbier T. Unmet clinical needs in chronic spontaneous urticaria. A ga (2) len task force report. Allergy. 2011;66:317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 10.Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144:35–39. doi: 10.1001/archdermatol.2007.5. [DOI] [PubMed] [Google Scholar]
- 11.Kim BR, Yang S, Choi JW, Choi CW, Youn SW. Epidemiology and comorbidities of patients with chronic urticaria in Korea: a nationwide population-based study. J Dermatol. 2018;45:10–16. doi: 10.1111/1346-8138.14075. [DOI] [PubMed] [Google Scholar]
- 12.Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282:232–247. doi: 10.1111/imr.12632. [DOI] [PubMed] [Google Scholar]
- 13.Maurer M, Staubach P, Raap U, Richter-Huhn G, Bauer A, Rueff F, Jakob T, Yazdi AS, Mahler V, Wagner N, Lippert U, Hillen U, Schwinn A, Pawlak M, Behnke N, Chaouche K, Chapman-Rothe N. H1-antihistamine-refractory chronic spontaneous urticaria: it’s worse than we thought - first results of the multicenter real-life AWARE study. Clin Exp Allergy. 2017;47:684–692. doi: 10.1111/cea.12900. [DOI] [PubMed] [Google Scholar]
- 14.Maurer M, Staubach P, Raap U, Richter-Huhn G, Baier-Ebert M, Chapman-Rothe N. ATTENTUS, a German online survey of patients with chronic urticaria highlighting the burden of disease, unmet needs and real-life clinical practice. Br J Dermatol. 2016;174:892–894. doi: 10.1111/bjd.14203. [DOI] [PubMed] [Google Scholar]
- 15.Maurer M, Abuzakouk M, Berard F, Canonica W, Oude Elberink H, Gimenez-Arnau A, Grattan C, Hollis K, Knulst A, Lacour JP, Lynde C, Marsland A, Mcbride D, Nakonechna A, Ortiz de Frutos J, Proctor C, Sussman G, Sweeney C, Tian H, Weller K, Wolin D, Balp MM. The burden of chronic spontaneous urticaria is substantial: real-world evidence from ASSURE-CSU. Allergy. 2017;72:2005–2016. doi: 10.1111/all.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncalo M, Gimenez-Arnau A, Al-Ahmad M, Ben-Shoshan M, Bernstein JA, Ensina LF, Fomina D, Galvan CA, Godse K, Grattan C, Hide M, Katelaris CH, Khoshkhui M, Kocaturk E, Kulthanan K, Medina I, Nasr I, Peter J, Staubach P, Wang L, Weller K, Maurer M. The global burden of chronic urticaria for the patient and society. Br J Dermatol. 2021;184:226–236. doi: 10.1111/bjd.19561. [DOI] [PubMed] [Google Scholar]
- 17.Saini SS, Kaplan AP. Chronic spontaneous urticaria: the Devil’s itch. J Allergy Clin Immunol Pract. 2018;6:1097–1106. doi: 10.1016/j.jaip.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antia C, Baquerizo K, Korman A, Bernstein JA, Alikhan A. Urticaria: a comprehensive review: epidemiology, diagnosis, and work-up. J Am Acad Dermatol. 2018;79:599–614. doi: 10.1016/j.jaad.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Etwel F, Faught LH, Rieder MJ, Koren G. The risk of adverse pregnancy outcome after first trimester exposure to H1 antihistamines: a systematic review and meta-analysis. Drug Saf. 2017;40:121–132. doi: 10.1007/s40264-016-0479-9. [DOI] [PubMed] [Google Scholar]
- 20.Kocaturk E, Podder I, Zenclussen AC, Kasperska Zajac A, Elieh-Ali-Komi D, Church MK, Maurer M. Urticaria in pregnancy and lactation. Front Allergy. 2022;3:892673. doi: 10.3389/falgy.2022.892673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–789. [PubMed] [Google Scholar]
- 22.Zheng H, Xiao XJ, Shi YZ, Zhang LX, Cao W, Zheng QH, Zhong F, Hao PS, Huang Y, Chen ML, Zhang W, Zhou SY, Wang YJ, Wang C, Zhou L, Chen XQ, Yang ZQ, Zou ZH, Zhao L, Liang FR, Li Y. Efficacy of Acupuncture for chronic spontaneous urticaria: a randomized controlled trial. Ann Intern Med. 2023;176:1617–1624. doi: 10.7326/M23-1043. [DOI] [PubMed] [Google Scholar]
- 23.Liu RS, Wang ZY. Clinical observation on treatment of chronic spontaneous urticaria by acupuncture. Journal of Modern Medicine & Health. 2018;34:3156–3157. [Google Scholar]
- 24.Duan YW. Curative effect analysis of acupuncture combined with bleeding therapy in the treatment of chronic spontaneous urticaria. Chinese Community Doctors. 2018;34:102–103. [Google Scholar]
- 25.Zou Y, Lv XT, Tang QT. Effects of acupuncture combined with autohemotherapy in treatment of chronic urticaria (blood-deficiency and wind-dryness type) and influence on UAS score and expression of T lymphocyte STAT3 mRNA in peripheral blood. Chinese Archives of Traditional Chinese Medicine. 2019;37:1781–1784. [Google Scholar]
- 26.Ni W, Liu ZJ, Wu X. Therapeutic observation of scalp plus body acupuncture for urticaria. Shanghai Journal of Acupuncture and Moxibustion. 2017;36:77–80. [Google Scholar]
- 27.Wang YJ, Guo HC, Sun B, Hu HJ, Du YZ, Li YH. Clinical efficacy of combination therapy of Needle-pot on chronic urticaria: based on Xuanfu theory. Hebei Journal of Traditional Chinese Medicine. 2021;43:1874–1878. [Google Scholar]
- 28.Li HK, Shi F, Sun MX, Wang YJ. Acupuncture-cupping therapy for chronic urticaria based on xuanfu theory. Journal of Clinical Acupuncture and Moxibustion. 2021;37:1–4. [Google Scholar]
- 29.Cao WJ, Wang SJ, Li CC, Wang ZY, Wang YJ. “Mind-Body Holistic” acupuncture method for treating 55 cases of chronic urticaria. Chinese Acupuncture & Moxibustion. 2021;41:169–170. [Google Scholar]
- 30.Wang FC. Acupuncture and Moxibustion Techniques. Beijing: China Traditional Chinese Medicine Press; 2021. [Google Scholar]
- 31.Monroe EW, Fox RW, Green AW, Izuno GT, Bernstein DI, Pleskow WW, Willis I, Brigante JR. Efficacy and safety of loratadine (10 mg once daily) in the management of idiopathic chronic urticaria. J Am Acad Dermatol. 1988;19:138–139. doi: 10.1016/s0190-9622(88)80230-5. [DOI] [PubMed] [Google Scholar]
- 32.Chow SC, Shao J, Wang H, Lokhnygina Y. Sample size calculations in clinical research. New York: Chapman and Hall/CRC; 2017. [Google Scholar]
- 33.Mlynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777–780. doi: 10.1111/j.1398-9995.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 34.Hawro T, Ohanyan T, Schoepke N, Metz M, Peveling-Oberhag A, Staubach P, Maurer M, Weller K. Comparison and interpretability of the available urticaria activity scores. Allergy. 2018;73:251–255. doi: 10.1111/all.13271. [DOI] [PubMed] [Google Scholar]
- 35.Weisshaar E, Szepietowski JC, Dalgard FJ, Garcovich S, Gieler U, Gimenez-Arnau AM, Lambert J, Leslie T, Mettang T, Misery L, Savk E, Streit M, Tschachler E, Wallengren J, Stander S. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469–506. doi: 10.2340/00015555-3164. [DOI] [PubMed] [Google Scholar]
- 36.Napadow V, Li A, Loggia ML, Kim J, Schalock PC, Lerner E, Tran TN, Ring J, Rosen BR, Kaptchuk TJ, Pfab F. The brain circuitry mediating antipruritic effects of acupuncture. Cereb Cortex. 2014;24:873–882. doi: 10.1093/cercor/bhs363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Xu Y, Liu H, Cai H, Jiang Y, Xu HE, Yin W. Molecular recognition of itch-associated neuropeptides by bombesin receptors. Cell Res. 2023;33:184–187. doi: 10.1038/s41422-022-00743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li HP, Wang XY, Chen C, Li JJ, Yu C, Lin LX, Yu ZE, Jin ZY, Zhu H, Xiang HC, Hu XF, Cao J, Jing XH, Li M. 100 Hz Electroacupuncture alleviated chronic itch and GRPR expression through activation of Kappa Opioid receptors in spinal dorsal horn. Front Neurosci. 2021;15:625471. doi: 10.3389/fnins.2021.625471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu JJ, Li X, Guo J, Yu S, Yang S. Role of GRPR in Acupuncture intervention in the “itch-scratch vicious cycle” spinal circuit of chronic pruritus. Chin Med. 2023;18:2. doi: 10.1186/s13020-022-00706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amorim D, Brito I, Caseiro A, Figueiredo JP, Pinto A, Macedo I, Machado J. Electroacupuncture and acupuncture in the treatment of anxiety - A double blinded randomized parallel clinical trial. Complement Ther Clin Pract. 2022;46:101541. doi: 10.1016/j.ctcp.2022.101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiller J, Niederer D, Kellner T, Eckhardt I, Egen C, Zheng W, Korallus C, Achenbach J, Ranker A, Sturm C, Vogt L, Gutenbrunner C, Fink MG, Karst M. Effects of acupuncture and medical training therapy on depression, anxiety, and quality of life in patients with frequent tension-type headache: a randomized controlled study. Cephalalgia. 2023;43:3331024221132800. doi: 10.1177/03331024221132800. [DOI] [PubMed] [Google Scholar]
- 43.Yeom M, Ahn S, Jang SY, Jang JH, Lee Y, Hahm DH, Park HJ. Acupuncture attenuates comorbid anxiety- and depressive-like behaviors of atopic dermatitis through modulating neuroadaptation in the brain reward circuit in mice. Biol Res. 2022;55:28. doi: 10.1186/s40659-022-00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan NY, Chan JWY, Li SX, Wing YK. Non-pharmacological approaches for management of insomnia. Neurotherapeutics. 2021;18:32–43. doi: 10.1007/s13311-021-01029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riemann D, Krone LB, Wulff K, Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45:74–89. doi: 10.1038/s41386-019-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, Zhao CH, Li ZJ, Chen XY, Hu TJ, Sheng YX. Effects of auriculo-acupuncture on monoamine neuro-transmitter and IL-1β in the hypothalamus of rats with sleep deprivation after cerebral ischemia reperfusion injury. Journal of Jinan University Natural Science & Medicine Edition. 2017;38:223–227. [Google Scholar]
- 47.Wei Y, Liang W, Guo YW, Li YQ, Luo BH. Study on the effects of acupuncture on Neiguan points combined with insomnia points on the hypothalamic Glu, GABA neurotransmitter content in PCPA-induced insomnia rats. Shizhen Guoyi Guoyao. 2019;30:2279–2281. [Google Scholar]
- 48.Li ZW, Yang L, Song XJ, Du Li, Zhu YH. Effects of shenmen (HT7) and sanyinjiao (SP6) acupoint compatibility on sleep quality, serum GABA and 5-HT in insomnia. World Science and Technology-Modernization of Traditional Chinese Medicine. 2022;24:860–866. [Google Scholar]
- 49.Tang L, You F, Hu X, Li YF. Electroacupuncture improves insomnia by down-regulating peripheral benzodiazepine receptor expression in hippocampus, and up-regulating 5-HT, 5-HIAA, TNF-alpha and IL-1beta contents in hypothalamus in insomnia rats. Zhen Ci Yan Jiu. 2019;44:560–565. doi: 10.13702/j.1000-0607.180610. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Zhao H, Shi K, Wang M. Treatment of insomnia based on the mechanism of pathophysiology by acupuncture combined with herbal medicine: a review. Medicine (Baltimore) 2023;102:e33213. doi: 10.1097/MD.0000000000033213. [DOI] [PMC free article] [PubMed] [Google Scholar]