Abstract
Objective: To investigate and analyze the clinical efficacy and safety of polymyxin B sulfate in the treatment of carbapenem-resistant gram-negative bacteria (CR-GNB) in sepsis; in order to provide reference for the clinical diagnosis, treatment and prognosis evaluation of sepsis. Methods: The clinical data of 76 patients with CR-GNB sepsis treated with polymyxin B sulfate combined with an anti-infection regimen in the First Affiliated Hospital of Gannan Medical University from January 2020 to February 2024 were retrospectively studied. To analyze and discuss the clinical characteristics, results of the bacterial culture and drug sensitivity, clinical efficacy and prognosis of CR-GNB patients, efficacy comparison of different doses of polymyxin B sulfate treatment regimens, efficacy comparison of different combination regimens based on polymyxin B sulfate, changes in clinical indexes before and after treatment of polymyxin B sulfate, adverse drug reactions and adverse events of polymyxin B sulfate were investigated. Results: A total of 76 patients with CR-GNB sepsis were included in this study, with 55 males and 21 females, with an average age of 59.86 years old, 44 of which were (57.89%) were > 60 years old. All patients included in this study were treated with polymyxin B based combination therapy, 49 cases (64.47%) received the two-drug combination regimen, 27 cases (35.53%) received the three-drug or more combination regimen, and all the patients had the above treatment followed by systematic symptomatic supportive treatment. Patients in this study received polymyxin B for an average of (8.6±4.3) days, there were 60 (78.95%) patients with effective clinical treatment, and 49 patients (64.47%) achieved pathogen (bacterial) clearance of infection. Twenty-two cases (28.95%) died within 28 days, 31 cases (40.79%) died within 90 days, and the remaining 23 cases (30.26%) survived. There were statistically significant differences in the therapeutic effective rates and bacterial clearance rates among different courses of treatment or different initial doses of polymyxin B (all P < 0.05). Moreover, there were significant differences in APACHE II score, WBC, NE, HGB, platelet count, albumin, NT-proBNP and CRP before and after polymyxin B treatment (all P < 0.001). In this study, 7 cases (9.21%) developed drug-related kidney injury, which recovered or decreased below the pre-medication level after discontinuation or dose adjustment and infection control. Skin darkening (melanin deposition) occurred in 5 cases (6.58%), and the above patients basically returned to normal several months after withdrawal of the drug, but there was still a certain degree of skin pigmentation. Meanwhile, 3 cases (3.95%) had neurotoxic reactions, mainly manifested as numbness at the extremities, and the neurotoxic symptoms were improved after reducing the dosage. Accordingly, there was no statistically significant difference in the prognosis of CR-GNB sepsis patients between different age and gender groups (all P > 0.05), while the treatment course and dosage of polymyxin B had statistically significant effects on the prognosis of CR-GNB sepsis patients (all P < 0.05). Conclusion: A Polymyxin B sulfate based combination regimen is an effective choice for CR-GNB sepsis, which can maximize the survival and prognosis benefits of sepsis patients.
Keywords: Polymyxin B sulfate, sepsis, carbapenem-resistant gram-negative bacteria (CR-GNB), clinical effect, prognosis
Introduction
Sepsis is becoming a serious public health problem globally, as it has complex infectious factors that lead to a high mortality rate in humans. Due to the extremely complex pathogenesis of sepsis, including the body’s inflammatory response imbalance, coagulation, immune dysfunction, mitochondrial function damage and other pathophysiological processes, it eventually leads to systemic circulation and organ dysfunction, and the development of septic shock, all of which seriously threaten the lives of patients [1,2]. Bacterial infection is the most common cause of sepsis, and the fatality rate of hospitalized patients can reach 30% to 60%. Among bacterial infections, gram-negative bacteria dominate, followed by microorganisms such as fungus and viruses [3,4]. Studies have found that more than half of sepsis patients are infected with gram-negative bacteria [5,6]. However, the increasingly serious problem of bacterial drug resistance, especially carbapenem-resistant gram-negative bacteria (CR-GNB) has brought great challenges to the treatment of sepsis [7,8].
Since the nephrotoxicity and neurotoxicity of polymyxin has been disclosed, it was replaced by other antimicrobials, and there is now the dilemma of slow progress in the research and development of new antimicrobials. With polymyxin as the last line of defense in the treatment of gram-negative bacteria infection, it has been given more attention again. Polymyxin is a group of polypeptide antibiotics. Currently, polymyxin B and polymyxin E are used clinically [9]. In the treatment of CR-GNB infection, polymyxin B has been increasingly used in clinics due to its unique chemical structure, mechanism of action, excellent pharmacokinetic effect and high antibacterial activity. The combination regimen based on polymyxin for the treatment of CR-GNB infection has been recommended by various guidelines and expert consensus [10,11]. This study retrospectively analyzed the clinical efficacy of polymyxin B sulfate combined with other antibiotics in the treatment of CR-GNB sepsis, aiming to provide reference and important clinical guidance value for the treatment of sepsis caused by CR-GNB infection.
Materials and methods
Clinical baseline data
The clinical data of 76 patients with CR-GNB related sepsis who received polymyxin B sulfate via injection combined with anti-infective therapy at the First Affiliated Hospital of Gannan Medical University from January 2020 to February 2024 were collected and collated. The 76 cases included in this study were from the ICU (42 cases), Respiratory Department (17 cases), Department of Oncology (8 cases), Department of Nephrology (4 cases), Department of Gastroenterology (3 cases), and the Department of Hematology (2 cases). Among the patients included in this study, 55 were males (72.37%) and 21 were females (27.63%). The patients ranged in age from 21 to 89 years old, with an average age of 59.86 years old. This study complied with the norms of medical ethics and was reviewed and approved by the Ethics Committee of the hospital (approval number: LLSC2023266). All patients’ families were informed and agreed to be included in this study. Written informed consent was obtained from the patient or the patient’s family for the publication of this clinical research article and the accompanying images.
Research criteria
Inclusion criteria: All patients meet the diagnostic criteria of Sepsis 3.0 [12]: the score of sepsis related Organ Failure Evaluation (SOFA) caused by infection or suspected infection is scored ≥ 2; CR-GNB was identified by etiological examination and considered as a pathogen. Patients were intravenously treated with polymyxin B sulfate > 3 days. Site infections meet the diagnostic criteria of the U.S. Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) [13]: (1) Pulmonary infection was positive for respiratory secretions culture, including fever (> 38.0°C), white blood cell count (WBC) < 4×109/L or ≥ 12×109/L, new or worsening cough, new purulent sputum or changes in sputum properties, increased respiratory secretions, pulmonary stethoscopy of moist rale, dyspnea and other symptoms. (2) Blood flow infection meets at least one of the three symptoms of blood culture positive, fever (> 38.0°C), chills, systolic blood pressure ≤ 90 mmHg. (3) Urinary tract infection met urine culture positive, fever (> 38.0°C), frequent urination, urgent urination, dysuria, pubic tenderness and other symptoms. (4) Abdominal infection met two of the symptoms: positive drainage culture, fever (> 38.0°C), nausea, vomiting, abdominal pain or jaundice. (5) Intracranial infection met two of the symptoms: cerebrospinal fluid culture positive, headache, fever (> 38.0°C), local nervous system signs, altered level of consciousness or confusion. (6) Skin and soft tissue infection met one of the symptoms of a positive culture of secretions, such as pain or tenderness, local swelling, redness, or fever.
Exclusion criteria: (1) Children < 14 years of age; (2) Pregnant women; (3) Treatment regimen of polymyxin B sulfate < 3 days; (4) Patients with intramuscular injection, single intrathecal injection or aerosol administration were excluded.
Therapeutic drugs
The batch number and manufacturer information of the drugs used by the patients included in this study were as follows: Polymyxin B sulfate (H31022631, SPH No. 1 Biochemical & Pharmaceutical Co., Ltd., China), Tigacycline (H20160471-2, Wyeth Lederle S.R.L. Co., Ltd., Italy), Imipenem-cilastatin sodium (H20130347, Merck Sharp & Dohme Co., Ltd., Germany), Meropenem (H20163391-2, Luoxin Pharmaceutical Group Stock Co., Ltd., China), Piperacillin tazobactam (H20133353, Shandong Anxin Pharmaceutical Co., Ltd., China), Cefoperazone and sulbactam sodium (H20020597, Pfizer Pharmaceutical Co., Ltd., U.S.A.), Ciprofloxacin (H10910038, GuangZhou BaiYunShan Pharmaceutical Holdings Co., Ltd., China), Ceftazidime (H20150185, Hanmi Pharm. Co., Ltd., Korea), Cefepime (H20052616, Shenzhen Lijian Pharmaceutical Co., Ltd., China).
Treatment plan (regimens)
In this study, all patients were treated with polymyxin B sulfate treatment combined with other antibiotics, including tigacycline, carbapenems (imipenem, meropenem) and other antibacterial drugs (piperacillin tazobactam, cefoperazone sulbactam, ciprofloxacin, ceftazidime, cefepime, and so on). Administration: polymyxin B 1.5-2.5 mg/(kg·d), q12h; The first dose of tigecycline was 100 mg, followed by 50 mg, q12h; Meropenem was given once at 1 g, 8 h and maintained for 4 h each time. Imipenem once at 1 g, q8h; Piperacillin tazobactam 4.5 g, q8h; Cefoperazone and sulbactam 3 g, q8h; Ciprofloxacin 0.2 g, q12h; Etimicin 200 mg once; Ceftazidime 4 g, q12h; Cefepime 2 g, q12h. Patients with renal insufficiency had reduced dosage according to the instructions of the drug according to the creatinine clearance results.
Observation and monitoring indicators
General baseline data, underlying diseases, infection sites, specimen sources, laboratory test results before and after treatment, bacterial culture and drug susceptibility results, basic medication and course of treatment, life support methods, and comorbidities of patients were collected. Comprehensive and in-depth evaluation of acute physiology and chronic health evaluation II (APACHE II) score, biochemical indexes, infection index, microbial evaluation, efficacy evaluation, safety evaluation, adverse reactions before and after treatment of polymyxin B sulfate.
Clinical efficacy evaluation
According to the body temperature, symptoms, signs, laboratory indicators and etiological examination results of patients before and after treatment, the curative effect was divided into cure, obvious effect and ineffective. Cure: The patient’s infection was controlled, and the clinical symptoms, signs, inflammatory indicators and etiological results were normal. Obvious effect: The infection of the patient was improved, and the clinical symptoms, signs, inflammatory indicators, laboratory indicators, and etiological results were improved. Ineffective: The patient’s infection did not change or worsen, and clinical symptoms, signs, inflammatory indicators, laboratory indicators, and etiological results did not improve or even worsen. Effective rate = (number of cured cases + number of obvious effective cases)/total number of cases × 100%.
Evaluation of pathogen removal effect
Clear: No bacteria were detected for 3 consecutive measures. Replacement: The results of the three consecutive bacterial cultures showed that other bacteria grew, and the original drug-resistant bacteria disappeared. Not removed: the amount of bacteria is not reduced or the amount of bacteria is reduced but not completely removed. Total clearance rate = (number of cleared cases + number of replaced cases)/total number of cases × 100%.
Definition of contraindications and relative contraindications
Contraindications: Patients allergic to polymyxin and their related components.
Relative contraindications: Due to the common adverse reactions of polymyxin, the following conditions are relative contraindications and should be used with caution: (1) Pregnant women should avoid it; (2) Avoid combination with tubocurarine muscle relaxants and other neurotoxic drugs; (3) Avoid combination with aminoglycosides, vancomycin and other nephrotoxic drugs.
Security assessment
During treatment, adverse reactions related to drug use should be actively monitored and recorded, and the relationship with drug use should be evaluated. Among them, the adverse reactions were mainly liver and kidney function injury, neurotoxicity, contact dermatitis, skin pigmentation, pruritus, drug fever and other allergic reactions. While creatinine exceeding the pre-medication level of 88.4 μmol/L and excluding other causes was defined as polymyxins B-related kidney injury [14].
Prognostic follow-up
The electronic inpatient record system was used to follow the indicators of patients’ and any return to the hospital for re-examination and hospitalization status, and the follow-up was conducted by telephone contact. The follow-up date was March 1, 2024. The overall survival (OS) was defined as the interval between the date of diagnosis and the onset of death or loss of follow-up (the end point of follow-up). Subsequently, all patients included in this study were divided into an age comparison group and gender comparison group according to clinical baseline data. Besides, the patients were also divided into dose comparison group and course comparison group according to the clinical treatment, and we aimed to compare the difference in survival and prognosis between the groups.
Statistical analysis
SPSS 26.0 software was used for statistical analysis. Measurement data conforming to a normal distribution were expressed as mean ± standard deviation, and comparison between the two groups was performed by T-test (or corrected T-test). Measurement data that did not conform to normal distribution were represented by median (quartile), and Mann-Whitney U test was used for two groups of independent samples. Count data were expressed as cases or cases (%) using χ2 test (Fisher’s precision probability test). The survival curve was plotted by Kaplan-Meier method. P < 0.05 indicated that the difference was statistically significant.
Results
Baseline data and clinical features
Among the 76 patients with CR-GNB sepsis included in this study, 55 were males and 21 were females, with an average age of 59.86 years old, with 44 cases (57.89%) being > 60 years old. Polymyxin B sulfate was used for more than 3 days in all patients. The main causes of infection were pulmonary infection (59.21%). The clinical characteristics and baseline data of other specific patients are shown in Table 1.
Table 1.
Baseline characteristics of patients with CR-GNB sepsis
| Characteristics | Results |
|---|---|
| Gender | |
| Male | 55 (72.37%) |
| Female | 21 (27.63%) |
| Age/years | 59.86±11.32 |
| Sites of infection | |
| Respiratory system | 45 (59.21) |
| Bloodstream infection | 12 (15.79) |
| Abdominal infection | 7 (9.21) |
| Urinary system | 4 (5.26) |
| Intracranial or nervous System | 3 (3.95) |
| Skin and soft tissues | 2 (2.63) |
| Others | 3 (3.95) |
Bacteriological examination and clearance
All patients included in this study received targeted treatment after confirming the etiology. A total of 98 strains of pathogens were detected. In terms of specimen sources, 53 strains (54.08%) were detected in sputum culture, 23 strains (23.47%) were detected in blood culture, 9 strains (9.18%) were detected in urine, 7 strains (7.14%) were detected in pleural effusion or peritoneal effusion, 4 strains (4.08%) were detected in the catheter and drainage fluid, and one strain (1.02%) was detected in the cerebrospinal fluid and skin soft tissue secretions, respectively. In terms of pathogen clearance and efficacy, 48 strains (48.98%) of Acinetobacter baumannii, 37 strains were cleared after treatment. Tweny-nine strains (29.59%) of Klebsiella pneumoniae, and 18 were cleared after treatment. Seventeen strains (17.35%) of Pseudomonas aeruginosa, and 13 strains were cleared after treatment. Four strains (4.08%) of other pathogens, and an additional 2 strains were cleared after treatment. A total of 70 strains (71.43%) of pathogens were cleared after treatment.
Treatment options
The 76 patients included in this study were treated with polymyxin B based combination therapy, of which 52 cases (68.42%) were treated with carbapenems for initial anti-infection therapy, and the median time of use was 14.5 days. Nineteen cases (25.0%) were treated with cefoperazone and sulbactam sodium for a median of 12 days. Five cases (6.58%) were combined with other antibiotics as the initial drug. In this study, 49 cases (64.47%) received the two-drug combination regimen, 27 cases (35.53%) received the three-drug or more combination regimen, and all the patients had the above treatment followed by systematic symptomatic supportive treatment. The main combination regimen for the patients in this study (excluding monotherapy, post-alternate or replacement therapy) is detailed in Table 2.
Table 2.
Major combination therapy based on polymyxin B in patients with CR-GNB sepsis
| Antimicrobial agents combined with polymyxin B | Cases (%) |
|---|---|
| Imipenem-cilastatin sodium | 14 (18.42) |
| Meropenem | 11 (14.47) |
| Cefoperazone and sulbactam sodium | 9 (11.84) |
| Tigacycline | 11 (14.47) |
| Piperacillin tazobactam | 2 (2.63) |
| Ceftazidime and avibactam | 2 (2.63) |
| Imipenem-cilastatin sodium + Tigacycline | 15 (19.74) |
| Meropenem + Tigecycline | 8 (10.53) |
| Cefoperazone sodium sulbactam sodium + Tigacycline | 2 (2.63) |
| Meropenem + Cefoperazone sodium sulbactam sodium + Tigacycline | 2 (2.63) |
Clinical efficacy and prognosis
All patients in this study received a combination regimen based on polymyxin B. There were 60 (78.95%) of patients with effective clinical treatment, of which 16 cases were cured and 43 cases were improved. Forty-nine patients (64.47%) achieved pathogen (bacterial) clearance of infection. Twenty-two cases (28.95%) died within 28 days, 31 cases (40.79%) died within 90 days, and 23 cases (30.26%) survived. In this study, 76 patients all received polymyxin B for 3 to 27 days, with an average of (8.6±4.3) days, of which 24 cases (31.58%) were treated for 3 to 5 days, 30 cases (39.47%) were treated for 6 to 10 days, 17 cases (22.37%) were treated for 11 to 15 days, and 5 cases (6.58%) were treated for more than 15 days. As shown in Table 3, the longer the use time of polymyxin B, the higher the therapeutic effective rate and bacterial clearance rate, and there were statistically significant differences in the therapeutic effective rate and bacterial clearance rate among different courses of treatment (P < 0.05).
Table 3.
Efficacy of polymyxin B in terms of treatment duration in patients with CR-GNB sepsis
| Treatment duration | Clinical efficacy rate/% (n/N) | Bacterial eradication rate/% (n/N) |
|---|---|---|
| 3-5 d | 58.33 (14/24) | 45.83 (11/24) |
| 6-10 d | 83.33 (25/30) | 60.0 (18/30) |
| 11-15 d | 94.12 (16/17) | 88.23 (15/17) |
| > 15 d | 100.0 (5/5) | 100.0 (5/5) |
| χ2 value (Fisher) | 8.642 | 10.575 |
| P value | 0.027 | 0.011 |
Clinical efficacy of different doses of polymyxin B
As shown in Table 4, the 76 patients included in this study were given the initial dose of polymyxin B as follows: The maintenance dose for 12 patients (15.79%) was 100 mg, q12h; for 16 patients (21.05%) it was 75 mg, q12h; for 43 cases (56.58%) it was 50 mg, q12h and the maintenance dose of 5 cases (6.58%) was < 50 mg, q12h. There were significant differences in the bacterial clearance rate among different doses (P < 0.01), that is, the higher the daily dose, the higher the bacterial clearance rate. However, there was no statistical significance in the treatment effective rate among different doses (P > 0.05), which may be related to the different degrees of organ damage caused by pathogen infection in the early stages of patients and the delayed recovery of the body.
Table 4.
Efficacy of polymyxin B in terms of daily doses in patients with CR-GNB sepsis
| Total daily dose | Clinical efficacy rate/% (n/N) | Bacterial eradication rate/% (n/N) |
|---|---|---|
| 200 mg | 75.0 (9/12) | 91.67 (11/12) |
| 150 mg | 68.75 (11/16) | 87.50 (14/16) |
| 100 mg | 83.72 (36/43) | 51.16 (22/43) |
| < 100 mg | 80.0 (4/5) | 40.0 (2/5) |
| χ2 value (Fisher) | 2.068 | 12.293 |
| P value | 0.577 | 0.004 |
Efficacy of different polymyxin B-based combinations
As shown in Table 5, all patients included in this study were treated with polymyxin B based combination therapy, that is, polymyxin B sulfate combined with carbapenem antibiotics, tigacycline, cefoperazone sulbactam sodium, carbapenems + tigacycline, cefoperazone sulbactam sodium + tigacycline and other antibacterial drugs, respectively. Unfortunately, there were no statistically significant differences in treatment effectiveness and bacterial clearance among all groups (P > 0.05), which may be related to the small number of cases included in each group.
Table 5.
Efficacy of different polymyxin B-based combinations in patients with CR-GNB sepsis
| Antimicrobial agents combined with polymyxin B | Clinical efficacy rate/% (n/N) | Bacterial eradication rate/% (n/N) |
|---|---|---|
| Carbapenem antibiotics | 80.0 (20/25) | 72.0 (18/25) |
| Tigacycline | 72.73 (8/11) | 54.55 (6/11) |
| Cefoperazone and sulbactam sodium | 77.78 (7/9) | 55.56 (5/9) |
| Carbapenem antibiotics + Tigacycline | 82.61 (19/23) | 73.91(17/23) |
| Cefoperazone sulbactam sodium + Tigacycline + other antibacterial agents | 100 (2/2) | 50.0 (1/2) |
| Other regimens | 66.67 (4/6) | 33.33 (2/6) |
| χ2 value (Fisher) | 1.788 | 5.315 |
| P value | 0.919 | 0.372 |
Changes of clinical variables before and after the treatment of polymyxin B
Subsequently, by sorting out and analyzing various clinical laboratory indicators and data of CR-GNB sepsis patients before and after polymyxin B treatment, there were significant differences in APACHE II score, WBC, NE, HGB, platelet count, Albumin, NT-proBNP and CRP (all P < 0.001), while there were no statistically significant differences in creatinine, ALT, AST and PCT (P > 0.05), as shown in Table 6 for details.
Table 6.
Changes of clinical variables after the treatment of polymyxin B in patients with CR-GNB sepsis
| Variables | Before treatment | After treatment | t/Z value | P value |
|---|---|---|---|---|
| WBC/(×109/L) | 24.15±6.23 | 13.07±4.51 | 12.559 | < 0.001 |
| NE/(%) | 83.06±12.72 | 64.83±9.85 | 9.879 | < 0.001 |
| HGB/(g·L) | 77.26±8.65 | 86.93±10.57 | -6.172 | < 0.001 |
| PLT/(×109/L) | 127.18±20.34 | 149.84±13.98 | -8.004 | < 0.001 |
| Albumin/(g·L) | 22.47±7.36 | 33.81±5.08 | -11.055 | < 0.001 |
| Creatinine/(μmol/L) | 110.00 (68.00, 248.75) | 97.00 (62.25, 187.75) | -0.535 | 0.592 |
| ALT/(U/L) | 25.50 (12.25, 62.75) | 27.50 (17.00, 39.75) | -0.346 | 0.729 |
| AST/(U/L) | 37.00 (24.25, 63.00) | 35.50 (26.25, 62.50) | -0.128 | 0.898 |
| NT-proBNP/(pg·mL) | 905.02±29.82 | 472.59±37.41 | 78.800 | < 0.001 |
| PCT/(ng·mL) | 2.30 (0.61, 7.48) | 1.06 (0.58, 3.08) | -1.944 | 0.052 |
| CRP/(mg·L) | 30.40 (15.72, 102.20) | 11.57 (7.97, 27.45) | -4.463 | < 0.001 |
| APACHE II score | 21.92±6.04 | 12.48±5.17 | 10.351 | < 0.001 |
Note: WBC, white blood cell count; NE, neutrophils; HGB, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; AST, asparate aminotransferase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin; CRP, C-reactive protein; APACHE II, acute physiology and chronic health evaluation II.
Adverse reactions and adverse events
Of all the patients included in this study, 7 cases (9.21%) developed drug-related kidney injury, which recovered or decreased below the pre-medication level after discontinuation or dose adjustment and infection control. Skin darkening (melanin deposition) occurred in 5 cases (6.58%), and the above patients basically returned to normal several months after withdrawal of the drug, but there was still a certain degree of skin pigmentation. Meanwhile, 3 cases (3.95%) had neurotoxic reactions, mainly manifested as numbness at the extremity, and the neurotoxic symptoms could be improved after reducing the dosage. No other adverse reactions such as drug allergy were reported in this study.
Follow-up and prognostic analysis
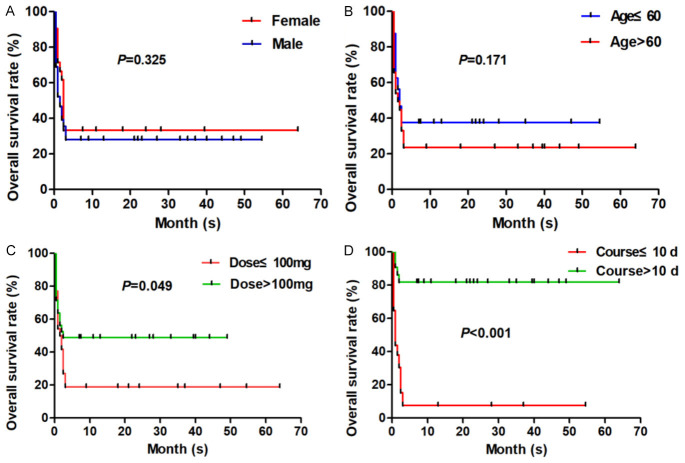
The median follow-up time was 17.5 (range, 0.5-64) months, and at the latest follow-up (March 1, 2024), 23 cases of 76 patients with CR-GNB sepsis were still alive, while 53 patients died in our study, all of which were due to uncontrollable progression of the disease and failed treatment. All patients incorporated in this study were classified and compared based on clinical baseline data. Firstly, based on gender classification, there were 39 deaths (39/55, 70.91%) among males and 14 deaths (14/21, 66.67%) among females, and no statistical significance existed between the two groups (P > 0.05). Secondly, in terms of age classification, there were 20 deaths (20/32, 62.50%) aged ≤ 60 years old and 33 deaths (33/44, 75.0%) aged > 60 years old, and no statistical significance was found between the two groups (P > 0.05). Then, regarding the classification and comparison of clinical treatment, 39 deaths (39/48, 81.25%) occurred when the dose of polymyxin B was ≤ 100 mg, and 14 deaths (14/28, 50.0%) occurred when the dose of polymyxin B was > 100 mg, the difference between the two groups was statistically significant (P = 0.049). Finally, the patients were classified and compared in accordance with the duration of treatment, 49 patients (49/54, 90.74%) with a treatment duration of ≤ 10 days died, and 4 patients (4/22, 18.18%) with a treatment course of > 10 days died, the difference between the two groups was statistically significant (P < 0.001). Our study showed that there is no statistical significance in the different age and gender groups of the patients with CR-GNB sepsis (all P > 0.05), while the treatment course and dosage of polymyxin B sulfate have statistical significance in the prognostic outcome of patients with CR-GNB sepsis (all P < 0.05). The survival curve of all patients with CR-GNB sepsis in this study is shown in Figure 1.
Figure 1.
Overall survival (OS) rate of patients with CR-GNB sepsis in this study. A. Patients with CR-GNB sepsis were divided into gender comparison (male or female) group, and the OS was compared. B. Patients with CR-GNB sepsis were divided into age comparison (≤ 60 years or > 60 years) group, and the OS was compared. C. Patients with CR-GNB sepsis were divided into dose comparison (≤ 100 mg or > 100 mg) group, and the OS was compared. D. Patients with CR-GNB sepsis were divided into course comparison (≤ 10 d or > 10 d) group, and the OS was compared.
Discussion
Sepsis is a systemic inflammatory response caused by a serious infection, while sepsis could be defined as septic shock if hypotension still exists after full volume resuscitation [15]. There is no doubt that bacterial infection is a common cause of this disease, and the fatality rate of hospitalized patients can reach 30% to 60%, making sepsis a serious and notorious disease that threatens people’s lives [16].
Carbapenem antibiotics have been regarded as one of the most effective drugs against multi-resistant gram-negative bacteria. With the aggravation of multi-drug resistance of bacteria, the resistance of gram-negative bacteria to carbapenem antibiotics has become a very important and difficult clinical problem. In the global priority list of antibiotic resistant bacteria published by the World Health Organization (WHO) in 2017, four pathogenic microorganisms were designated as the focus of research and development of new antibiotics [17]. Carbapenem-resistant enterobacter, carbapenem-resistant Klebsiella pneumoniae (KP), carbapenem-resistant Pseudomonas aeruginosa (PA) and carbapenem-resistant Acinetobacter baumannii (AB) are the most common carbapenem-resistant bacteria, which undoubtedly brings unprecedented challenges to the treatment prognosis of patients with sepsis and septic shock [18]. In the 76 patients with sepsis in this study, drug-resistant strains of these pathogens were detected.
Because CR-GNB sepsis patients are usually critically ill, the selection of reasonable antibiotics is the key to successful treatment, and it is also a major clinical problem. Due to adverse effects such as nephrotoxicity and neurotoxicity, polymyxin was replaced by other antibacterial agents a long time ago [19]. However, in the face of the slow progress in the research and development of new antibacterial drugs, polymyxin B has been re-used in the clinic because of its unique bactericidal mechanism and good antibacterial activity, and it is regarded as the last line of defense in the treatment of gram-negative bacteria infection. Up to now, polymyxin B and polymyxin E have been clinically used, while polycolistin B has special and excellent pharmacokinetic effects [20].
Currently, it is believed that the antibacterial mechanism of polymyxin may be the combination of polycationic rings in its drug molecules with phosphoric acid groups on the cell membrane of Gram-negative bacilli, resulting in increased membrane permeability, leakage of small molecules such as purine and pyrimidine in the cell, and bacterial expansion, dissolution and death [21]. At the same time, polymyxin can also cross components between the inner and outer membranes of cells through vesicle contact, causing osmotic imbalance, resulting in bacterial expansion and dissolution [22]. In addition, polymyxins have the effect of neutralizing endotoxins, and can also cause oxidative stress, which leads to the accumulation of hydroxyl free radicals and damage the DNA of bacteria [23,24]. It should be noted that polymyxin B belongs to a narrow spectrum of antibacterial drugs, which have good pharmacological activity against most gram-negative bacilli, including pseudomonas aeruginosa, escherichia coli, enterobacter, klebsiella, acinetobacter, aeromonas, stenotrophomonas maltophilia, citrobacter, and so on [25,26]. However, it is frustrating that polymyxin B has poor antibacterial activity against haemophilus influenzae, bacillus pertussis, legionella pneumophila, salmonella and shigella. In addition, polymyxin B is basically ineffective against all gram-positive bacteria, anaerobic bacteria, and some gram-negative bacteria (neisseria gonorrhoeae, neisseria meningitidis), mycoplasma, chlamydia, proteus, morganella, serratia, burkella, and parasites [27-30].
In this study, all patients with CR-GNB sepsis were treated with the combined treatment regimen based on polymyxin B sulfate. The results showed that the effective rate of clinical treatment was 78.95% and the bacterial clearance rate was 64.47%, indicating that the bacterial clearance rate and clinical efficacy could be significantly improved by the combined treatment of polymyxin B in the early stage after CR-GNB infection. There was no statistically significant difference in treatment effectiveness between different doses of polymyxin B sulfate, which may be related to the different degree of organ damage caused by early pathogen infection and the slow recovery degree of the body. While we analyzed and compared the efficacy of polymyxin B sulfate based treatment combined with other different antibiotics, unfortunately, there was no statistically significant difference in the therapeutic effect and bacterial clearance rate among the groups, which may be related to the small number of cases included in the different treatment regimens. However, fortunately, through the analysis of indicators before and after polymyxin B administration, it was found that APACHE II score, WBC, NE, HGB, platelet count, Albumin, NT-proBNP, CRP and other indicators were significantly improved, and these results suggest that polymyxin B is an uncontroversial option for the clinical treatment of CR-GNB sepsis.
It is worth noting that in clinical practice, the traditional drug sensitivity test is based on blood culture, and its time span is mostly 48 to 72 h, or even longer, which may be likely to cause patients to worsen or even miss the best treatment opportunity and eventually die. In addition, most clinical studies have used the 28-day or 30-day mortality associated with polymyxin B as an indicator of whether clinical treatment is effective or ineffective [31]. This will undoubtedly make it difficult to obtain reliable clinical pharmacodynamic data of polymyxin B therapy in clinical practice. However, it is gratifying that current studies have confirmed the clinical benefits of using polymyxin-based combination therapy and demonstrated the adverse consequences of synergistic killing and suppression of multidrug resistance and extensive drug resistance [32].
Nevertheless, Studies have shown that the early use of polymyxin B after infection could reduce the mortality of bloodstream infections caused by carbapenemase-producing enterobacillus and carbapenem-resistant Klebsiella pneumoniae, suggesting that the application of polymyxin B in the early stage of CR-GNB infection could quickly achieve effective blood and tissue concentrations, facilitate the elimination of bacteria, and benefit the prognosis of patients [33,34]. Since the exact pharmacodynamic mechanism of polymyxin B is not completely clear, sufficient treatment course and dose can ensure the realization and completion of pharmacological effects, and then the polymyxin can enter the bacteria smoothly, effectively eliminate bacteria, and obtain satisfactory curative effect. The results of this study showed that polymyxin B combined with antibiotics could increase drug sensitivity, and it has shown satisfactory effects in terms of total bacterial clearance, clinical treatment efficiency, and reduction of patient mortality, which undoubtedly indicates that the antibacterial activity and clinical efficacy of antibiotics increased after the combination of polymyxin B with other antibiotics. It is suggested that polymyxin B-based combination medication may help to improve the bactericidal rate and prevent polymyxin resistance. However, it should be noted that polymyxin B monotherapy may not show its therapeutic advantages, so it is urgent to combine polymyxin B with other antibiotics in clinical practice to improve the prognosis of CR-GNB sepsis patients to the greatest extent.
It is worth noting that the common adverse reactions (side effects) associated with the clinical application of polymyxin B mainly include nephrotoxic injury, neurotoxicity and skin pigmentation, but the number of cases is small. There is no evidence that the adverse reactions are caused by polymyxin B or other combined antibacterial agents, and the above adverse reactions are basically reversible [35,36]. When the drug is stopped or the dose of polymyxin B is reduced, it can basically return to normal. A very small number of patients in this study unfortunately experienced these adverse reactions (side effects), which were most likely related to the dosage and course of polymyxin B. By contrast, it was found that patients with renal and liver dysfunction may not need to adjust the initial dose of polymyxin B [37].
Admittedly, the 2019 international guideline consensus on polymyxin suggested that the daily maintenance dose of polymyxin B (1.25-1.50 mg/kg, q12h) should not be adjusted to increase in patients with renal insufficiency [38]. While in this study, we recommend that patients with renal insufficiency should be given a low dose of 1.5 mg/(kg·d), a daily dose of 50-100 mg, and no impairment of renal function was observed during treatment. In general, this study did not find any correlation between long course of treatment and high dose and adverse reactions, indicating that the adverse reactions of polymyxin B may be weakly correlated with course of treatment and dose, which may also be caused by the relationship between a single center study and limited sample size, which is worthy of our team’s subsequent efforts to include more cases and conduct more in-depth research.
Conclusion
In conclusion, polymyxin B-based combination regimen with sufficient dosage and full duration is a safe and effective method for the treatment of CR-GNB sepsis, which can maximize the survival and prognosis benefits of sepsis patients. In clinical practice, the dose of polymyxin B should be adjusted in real time according to therapeutic drug monitoring (TDM), blood biochemical indicators and clinical rehabilitation of the patients, and an anti-infection regimen should be optimized in real time to improve clinical efficacy and reduce adverse reactions. In addition, in terms of drug combination, tigacycline, carbapenems, piperacillin tazobactam, cefoperazone sulbactam and other antibiotics can be used as reliable and nice options for polymyxin B combination therapy.
Acknowledgements
We would like to thank our colleagues from the Department of Intensive Medicine (Comprehensive ICU) and the Department of Emergency, as well as the First Affiliated Hospital of Gannan Medical University for their academic assistance in the publication of this paper. The study was supported by the Science and Technology Project of Jiangxi Provincial Administration of Traditional Chinese Medicine (No.2023A0351).
Disclosure of conflict of interest
None.
References
- 1.Mandal L, Rijal G, Singh R, Tiwari B, Jahan F, Lama D, Shrestha S, Kurmi RN, Das U. Sepsis among patients admitted to the intensive care unit of a tertiary care centre. JNMA J Nepal Med Assoc. 2023;61:691–694. doi: 10.31729/jnma.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia X, Guo H, Sun H. Association between serum magnesium trajectory and in-hospital mortality in hospitalized patients with sepsis: an analysis of the MIMIC-IV database. Magnes Res. 2023;36:37–48. doi: 10.1684/mrh.2023.0520. [DOI] [PubMed] [Google Scholar]
- 3.Pishori T, Furia FF, Manji K. A cross-sectional study of clinical features of bacterial meningitis among neonates presumed to have sepsis in a tertiary hospital, Dar es Salaam, Tanzania. Pan Afr Med J. 2023;46:123. doi: 10.11604/pamj.2023.46.123.32787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, Park MH, Oh DK, Lim CM Korean Sepsis Alliance (KSA) investigators. Polymicrobial bloodstream infections per se do not increase mortality compared to monomicrobial bloodstream infections in sepsis patients: a Korean nationwide sepsis cohort study. BMC Infect Dis. 2024;24:285. doi: 10.1186/s12879-024-09130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Zhang L, Gao D, Wang J, Li Y, Sun N. Comparison of metagenomic next-generation sequencing and blood culture for diagnosis of bloodstream infections. Front Cell Infect Microbiol. 2024;14:1338861. doi: 10.3389/fcimb.2024.1338861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abafogi AT, Lee J, Kim J, Lee SW, Jang S, Park S. Automated sepsis detection with vancomycin- and allantoin-polydopamine magnetic nanoparticles. Sci Rep. 2024;14:3693. doi: 10.1038/s41598-024-54236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Wu Y, Qi S, Shao H, Feng M, Xing L, Liu H, Gao Y, Zhu Z, Zhang S, Du Y, Lu Y, Yang J, Chen P, Sun T. Polymyxin B therapy based on therapeutic drug monitoring in carbapenem-resistant organisms sepsis: the PMB-CROS randomized clinical trial. Crit Care. 2023;27:232. doi: 10.1186/s13054-023-04522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes MZR, Braga DQ, Pinheiro DOBP, Verduc RCAS, Dos Reis LV, de Lima EM, Lourenço ND, Cid PA, Beck DS, Pinheiro LHZ, Tonhá JPS, de Sousa LS, Dias MLS, da Silva Machado AA, Castro MM, Dutra VPR, de Mello LS, da Silva MC, Tozo TM, Mathuiy YR, de Abreu Rosas LLP, Barros PCM, da Silva JO, da Silva PP, Bandeira CS, de Sant Anna Reis Di Chiara Salgado SM, de Oliveira Alves MZ, Santos RQ, Marques JA, Rodrigues CAS, Dos Santos Gomes Junior SC Nucleus of Hospital Research Study Collaborators. Predictive score for carbapenem-resistant gram-negative bacilli sepsis: single-center prospective cohort study. Antibiotics (Basel) 2022;12:21. doi: 10.3390/antibiotics12010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballı FN, Ekinci PB, Kurtaran M, Kara E, Dizman GT, Sönmezer MÇ, Hayran M, Demirkan K, Metan G. Battle of polymyxin induced nephrotoxicity: polymyxin B versus colistin. Int J Antimicrob Agents. 2024;63:107035. doi: 10.1016/j.ijantimicag.2023.107035. [DOI] [PubMed] [Google Scholar]
- 10.Scudeller L, Righi E, Chiamenti M, Bragantini D, Menchinelli G, Cattaneo P, Giske CG, Lodise T, Sanguinetti M, Piddock LJV, Franceschi F, Ellis S, Carrara E, Savoldi A, Tacconelli E. Systematic review and meta-analysis of in vitro efficacy of antibiotic combination therapy against carbapenem-resistant gram-negative bacilli. Int J Antimicrob Agents. 2021;57:106344. doi: 10.1016/j.ijantimicag.2021.106344. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Zhu HH, Li GH, Qi TT, Ye LJ, Teng XQ, Qu Q, He GF, Qu J. A comparative study of the microbiological efficacy of polymyxin B on different carbapenem-resistant gram-negative bacteria infections. Front Med (Lausanne) 2021;8:620885. doi: 10.3389/fmed.2021.620885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Yan L, Wang C, Peng M. Clinical analysis of sepsis with extensively drug resistant Gram-negative bacteria in intensive care unit treated with polymyxin B-based combination therapy. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:150–154. doi: 10.3760/cma.j.cn121430-20200108-00028. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Han WH, Im HJ, Kim JH. Effects of early initiation of polymyxin B hemoperfusion therapy in patients with cancer with refractory septic shock. J Clin Med. 2024;13:1009. doi: 10.3390/jcm13041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Ding H, Wang ZY, Zhang K. Research progress on microcirculatory disorders in septic shock: a narrative review. Medicine (Baltimore) 2024;103:e37273. doi: 10.1097/MD.0000000000037273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baraldi E, Lindahl O, Savic M, Findlay D, Årdal C. Antibiotic pipeline coordinators. J Law Med Ethics. 2018;46:25–31. doi: 10.1177/1073110518782912. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Qi S, Duan X, Han B, Zhang S, Liu S, Wang H, Zhang H, Sun T. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study. J Transl Med. 2021;19:431. doi: 10.1186/s12967-021-03111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Patil N, Li F, Wang Z, Zhan H, Schmidt D, Thompson P, Guo Y, Landersdorfer CB, Shen HH, Peleg AY, Li J, Song J. PmxPred: a data-driven approach for the identification of active polymyxin analogues against gram-negative bacteria. Comput Biol Med. 2024;168:107681. doi: 10.1016/j.compbiomed.2023.107681. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Sun L, Qiao H, Sun Z, Wang P, Xie C, Hu X, Nie T, Yang X, Li G, Zhang Y, Wang X, Li Z, Jiang J, Li C, You X. Polymyxin resistance caused by large-scale genomic inversion due to IS26 intramolecular translocation in Klebsiella pneumoniae. Acta Pharm Sin B. 2023;13:3678–3693. doi: 10.1016/j.apsb.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Niu J, Chen G, Xu L. Treatment of carbapenem-resistant multidrug-resistant gram-negative bacilli with intracerebroventricular injection of polymyxin B: a retrospective study. Infect Drug Resist. 2022;15:7653–7666. doi: 10.2147/IDR.S392818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nwabor OF, Chukamnerd A, Terbtothakun P, Nwabor LC, Surachat K, Roytrakul S, Voravuthikunchai SP, Chusri S. Synergistic effects of polymyxin and vancomycin combinations on carbapenem- and polymyxin-resistant Klebsiella pneumoniae and their molecular characteristics. Microbiol Spectr. 2023;11:e0119923. doi: 10.1128/spectrum.01199-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui R, Zhang J, Liu X, Hu C, Zhou F, Zhang M, Wang X, Zou Q, Huang W. Dronedarone enhances the antibacterial activity of polymyxin B and inhibits the quorum sensing system by interacting with LuxS. ACS Infect Dis. 2024;10:961–970. doi: 10.1021/acsinfecdis.3c00591. [DOI] [PubMed] [Google Scholar]
- 24.Barth PO, Volpato FCZ, Moreira NK, Wink PL, de Souza ÂC, Barth AL. Evaluation of a rapid susceptibility test of polymyxin B by MALDI-TOF. Front Microbiol. 2022;13:1075650. doi: 10.3389/fmicb.2022.1075650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray M, Manjunath A, Halami PM. Prevalence of polymyxin resistance through the food chain, the global crisis. J Antibiot (Tokyo) 2022;75:185–198. doi: 10.1038/s41429-022-00502-0. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Ding L, Yang Y, Han R, Yin D, Wu S, Zhu D, Hu F. Multicenter antimicrobial resistance surveillance of clinical isolates from Major Hospitals - China, 2022. China CDC Wkly. 2023;5:1155–1160. doi: 10.46234/ccdcw2023.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi R, Fu Y, Gan Y, Wu D, Zhou S, Huang M. Use of polymyxin B with different administration methods in the critically ill patients with ventilation associated pneumonia: a single-center experience. Front Pharmacol. 2023;14:1222044. doi: 10.3389/fphar.2023.1222044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyagin I, Stepanov N, Presnov D, Trifonov A, Efremenko E. Self-assembling enzymatic nanocomplexes with polypeptides and low-weight organic compounds: preparation, characterization, and application of new antibacterials. Int J Mol Sci. 2023;24:1831. doi: 10.3390/ijms24031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idowu T, Ammeter D, Rossong H, Zhanel GG, Schweizer F. Homodimeric tobramycin adjuvant repurposes novobiocin as an effective antibacterial agent against gram-negative bacteria. J Med Chem. 2019;62:9103–9115. doi: 10.1021/acs.jmedchem.9b00876. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji BT, Landersdorfer CB, Lenhard JR, Cheah SE, Thamlikitkul V, Rao GG, Holden PN, Forrest A, Bulitta JB, Nation RL, Li J. Paradoxical effect of polymyxin B: high drug exposure amplifies resistance in acinetobacter baumannii. Antimicrob Agents Chemother. 2016;60:3913–3920. doi: 10.1128/AAC.02831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol Rev. 2021;73:679–728. doi: 10.1124/pharmrev.120.000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ardebili A, Izanloo A, Rastegar M. Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: is it superior to polymyxin monotherapy? Expert Rev Anti Infect Ther. 2023;21:387–429. doi: 10.1080/14787210.2023.2184346. [DOI] [PubMed] [Google Scholar]
- 33.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J REIPI/ESGBIS/INCREMENT Investigators. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 34.Liang Q, Huang M, Xu Z. Early use of polymyxin B reduces the mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Braz J Infect Dis. 2019;23:60–65. doi: 10.1016/j.bjid.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velkov T, Thompson PE, Nation RL, Li J. Structure--activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu SQ, Zhan R. Clinical observation of neutropenia patients with hematonosis treated with polymyxin B sulfate. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30:1596–1600. doi: 10.19746/j.cnki.issn.1009-2137.2022.05.046. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Cheng Y, Chen B, Chen Y, Huang Y, Zhang B, Que W, Liu M, Zhang H, Qiu H. Population pharmacokinetics of polymyxin B in patients with liver dysfunction. Br J Clin Pharmacol. 2023;89:3561–3572. doi: 10.1111/bcp.15855. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS. International consensus guidelines for the optimal use of the polymyxins: endorsed by the american college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP) Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]