Abstract
Objective: To analyze the prognostic factors in patients with acute hypertensive intracerebral hemorrhage (HICH) undergoing minimally invasive puncture and drainage, providing scientific evidence to enhance clinical treatment strategies. Methods: A retrospective analysis was conducted on 350 patients with acute HICH treated at Gansu Provincial Hospital of Traditional Chinese Medicine and the First People’s Hospital of Lanzhou City from March 2017 to January 2024. Patients were divided into two groups based on surgical method: the control group (n = 211) received traditional craniotomy, while the observation group (n = 139) underwent minimally invasive puncture and drainage. Functional scores, inflammatory markers, clinical efficacy, surgical time, first hematoma clearance rate, and hospitalization duration were compared between the groups. Patients were classified into poor prognosis (Glasgow Outcome Scale (GOS) score < 3) and improved prognosis (GOS score ≥ 3) groups. Logistic regression analysis identified independent risk factors for poor prognosis and examined their interaction with patient outcomes. Results: Postoperative functional scores (National Institutes of Health Stroke Scale (NIHSS) score, GOS score, and Barthel Index) in the observation group were significantly better than those in the control group (all P < 0.001). Inflammatory markers (C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α)) were significantly lower post-treatment in the observation group compared to those in the control group (all P < 0.001). Multivariate logistic regression identified age (P = 0.003, OR = 0.573), time from onset to admission (P = 0.026, OR = 0.535), duration of hypertension (P = 0.006, OR = 1.766), and postoperative IL-6 levels (P = 0.048, OR = 1.870) as independent risk factors for poor prognosis. Prognosis was statistically associated with age (P = 0.040, OR = 0.978), time from onset to admission (P = 0.022, OR = 0.956), duration of hypertension (P = 0.022, OR = 1.085), and post-treatment IL-6 levels (P = 0.043, OR = 1.030). Conclusion: Minimally invasive puncture and drainage offer superior neurological recovery, reduced inflammatory response, and improved long-term prognosis compared to traditional craniotomy in the treatment of hypertensive intracerebral hemorrhage.
Keywords: Hypertensive intracerebral hemorrhage, minimally invasive surgery, neurological function score, global functional score, self-care ability assessment
Introduction
Hypertensive intracerebral hemorrhage (HICH) is a common and severe complication of hypertension, affecting approximately 4 million people worldwide each year [1]. In recent years, the incidence of hemorrhagic stroke has been rising, with an increasing number of cases occurring in younger populations in China [2]. HICH is primarily caused by damage to small cerebral arteries due to long-term hypertension, characterized by an acute onset, severe condition, and high mortality rate. When cerebral blood vessels rupture, a hematoma forms, compressing normal brain cells and significantly impacting the central nervous system [3]. Due to the rapid onset and progression of HICH, improving and controlling the patient’s prognosis are extremely challenging [4]. Various neurotoxic substances released from intracerebral hematomas, such as thrombin, erythrocyte degradation products, and liquefied substances, continuously damage the central nervous system and the blood-brain barrier, leading to severe brain edema, elevated intracranial pressure, and potentially brain herniation [5].
The primary treatment for cerebral hemorrhage involves hematoma evacuation combined with antioxidant drugs to alleviate oxidative stress, reducing both primary and secondary neural damage [6]. Therefore, rapidly evacuating hematomas, reducing intracranial pressure, and alleviating the hematoma mass effect are the ideal goals of surgical treatment for HICH. Craniotomy, a common surgical intervention for spontaneous intracerebral hemorrhage, can quickly reduce intracranial pressure [7]. However, compared to optimal medical management strategies, craniotomy has not demonstrated significant benefits, possibly due to longer surgical times, trauma to surrounding brain tissue, a high risk of blood loss, a high incidence of perioperative complications, and postoperative pathophysiological changes, reducing its effectiveness [8]. With advances in medical technology, more studies indicate that minimally invasive surgery offers unique advantages. When selecting surgical options for HICH patients in clinical practice, the most suitable method should be chosen based on the patient’s specific condition [9]. These minimally invasive techniques not only effectively evacuate hematomas but also reduce damage to surrounding brain tissue, improving treatment outcomes and patient prognosis. Multiple studies have confirmed the efficacy of minimally invasive surgery in treating HICH, but controversies remain regarding the specific prognostic factors for different patients [10]. Existing literature mainly focuses on improvements in surgical techniques and short-term efficacy evaluations, lacking systematic analysis of long-term prognostic factors [11-13].
This study aims to analyze the prognostic factors affecting patients with acute HICH undergoing minimally invasive puncture and drainage, providing more scientific evidence for clinical treatment. By investigating factors such as postoperative neurological function recovery, complication incidence, and survival rates, we aim to optimize surgical plans for HICH patients. The Glasgow Outcome Scale (GOS) is used to assess recovery and functional outcomes in patients after brain injury or stroke, ranging from 1 (death) to 5 (good recovery) [14]. Higher scores indicate better outcomes. In this study, the GOS score was used to classify patients into poor prognosis (GOS < 3) and improved prognosis (GOS ≥ 3) groups, aiding in the analysis of prognostic factors for acute HICH treated with minimally invasive puncture and drainage.
Materials and methods
Case source and patient grouping
We conducted a retrospective analysis on the clinical data of 350 patients with acute HICH treated in the Department of Neurosurgery at Gansu Provincial Hospital of Traditional Chinese Medicine and the First People’s Hospital of Lanzhou City from March 2017 to January 2024. The Medical Ethics Committee of Gansu Provincial Hospital of Traditional Chinese Medicine approved this study. Patients were assigned to a control group (n = 211), who underwent traditional craniotomy, and an observation group (n = 139), who received minimally invasive puncture and drainage, based on the surgical method chosen.
Inclusion and exclusion criteria
Inclusion criteria: (1) patients were at least 75 years old; (2) patients had a documented history of hypertension; (3) patients met the diagnostic criteria outlined in the “2018 Chinese Guidelines for Prevention and Treatment of Hypertension” [15]; (4) patients had a cranial CT scan confirming acute intracerebral hemorrhage [16]; (5) patients experienced their first cerebral hemorrhage within 24 hours of admission, with no prior symptoms of the disease present before admission; (6) patients required surgical intervention; (7) patients had complete case data.
Exclusion criteria: (1) severe cardiovascular, pulmonary, hepatic, or renal conditions; (2) tumors or other diseases with a life expectancy of less than 6 months; (3) brainstem hemorrhage or hematomas caused by vascular malformations; (4) coagulation disorders or other bleeding abnormalities; (5) long-term use of anticoagulants or previous thrombolytic therapy; (6) history of mental illness; and (7) brain infections or systemic infectious diseases.
Surgical methods
Control group: Patients underwent traditional craniotomy. After preoperative anesthesia, disinfection, and draping, a straight incision of approximately 6 cm was made at the maximum cross-section of the hematoma nearest the scalp. Sequential incisions were made through the skin, subcutaneous tissue, and galea aponeurotica. The periosteum was removed, a burr hole was drilled, and bone was removed to create a bone window approximately 3 cm in diameter. The dura mater was incised and suspended, and the hematoma’s location and depth were explored using a brain puncture needle. Partial evacuation of the hematoma was performed, followed by a cortical incision of approximately 2 cm under a microscope. The hematoma was repeatedly irrigated and evacuated, with thorough hemostasis performed. A drainage tube was placed, and the wound was sutured and dressed [17].
Observation group: Patients underwent minimally invasive puncture and drainage. A puncture point 1-2 cm below and behind the center of the hematoma was selected after preoperative anesthesia, disinfection, and draping. The coordinates and depth of the puncture point were calculated, and a straight incision of approximately 3-4 cm was made. The dura mater was exposed, and a disposable brain puncture cannula was used under CT guidance to reach the hematoma site. The needle core was removed, and a drainage tube was connected. Warm saline was used for irrigation, slowly aspirating 30-40% of the hematoma. Subsequently, 20,000 units of urokinase (Tianjin Biochemical Pharmaceutical Co., Ltd., National Drug Approval) and 3 ml of 0.9% saline were injected into the drainage tube. The tube was clamped for 3 hours before the needle was withdrawn, and the area was pressurized and dressed [18].
Both groups received routine postoperative symptomatic treatments, including hemostasis and anti-infection measures. During the acute phase, patients also underwent hyperbaric oxygen therapy and functional exercises for rehabilitation.
Clinical data collection
We collected baseline data and laboratory indicators from patients’ outpatient follow-up records, electronic medical records, and laboratory reports. Baseline data included age, sex, time from onset to admission, hemorrhage location, duration of hypertension, preoperative hypertension grade, intracerebral hemorrhage volume, history of diabetes, clinical efficacy, surgical time, first hematoma clearance rate, hospitalization time, National Institutes of Health Stroke Scale (NIHSS) score, GOS score, and Barthel index. Laboratory indicators (inflammatory markers) included C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Functional scores were assessed and evaluated both before and 6 months after treatment, while laboratory indicators were measured before and 2 weeks post-treatment.
Functional scores
NIHSS score: The NIHSS score [19] is used to assess and quantify the degree of neurological impairment in acute stroke patients. Scores range from 0 to 42, with higher scores indicating more severe neurological impairment.
GOS score: The GOS score [20] assesses overall recovery and functional outcomes in patients following brain injury or stroke. Scores range from 1 to 5, with higher scores indicating better recovery and functional outcomes.
Barthel index: The Barthel index [21] evaluates patients’ ability to perform daily living activities independently. Scores range from 0 to 100, with higher scores indicating greater self-care ability.
Laboratory indicator testing
Serum levels of CRP (CSB-E08617h), IL-6 (CSB-E04638h), and TNF-α (CSB-E09315h) were measured using enzyme-linked immunosorbent assay (ELISA) kits from Wuhan Huamei Biological Engineering Co., Ltd. Testing was conducted before and 2 weeks after treatment, following the kit instructions.
Outcome measurements
Primary outcomes
The primary outcomes included a comparison of functional scores and inflammatory markers between the observation and control groups. Patients were classified into poor prognosis (GOS score < 3) and improved prognosis (GOS score ≥ 3) groups based on their GOS scores. Logistic regression analysis was employed to identify risk factors for poor prognosis and to analyze the interaction between these factors and patient outcomes.
Secondary outcomes
Secondary outcomes included a comparison of baseline data between the observation and control groups. We also compared clinical efficacy, surgical time, first hematoma clearance rate, and hospitalization time between the two groups (Figure 1).
Figure 1.
Study flowchart. Note: GOS, Glasgow Outcome Scale; HICH, hypertensive intracerebral hemorrhage.
Statistical analysis
Data analysis was conducted using SPSS 26.00 software, with GraphPad Prism 9 software employed for grouping and summarizing images. Chi-square tests were used for comparisons between count data, while independent t-tests were used for inter-group comparisons and paired t-tests for intra-group comparisons of measures. Logistic regression analysis was performed using the stats package in R (4.3.2) to identify risk factors influencing poor prognosis. The RMSS package was used for prognostic and independent risk factor interaction analysis, and the ggplot2 package was used for visualization. Receiver operating characteristic (ROC) curve analysis was conducted using the pROC package to obtain cut-off values for patient ROC analysis. Statistical significance was set at P < 0.05.
Results
Baseline data
A comparison of the baseline data between the two groups revealed no significant differences in age, sex, time from onset to admission, hemorrhage location, duration of hypertension, preoperative hypertension grade, intracerebral hemorrhage volume, or history of diabetes (all P > 0.05, Table 1).
Table 1.
Comparison of baseline data between the observation and control groups
| Factors | Observation group (n = 139) | Control group (n = 211) | χ2 Value | P Value |
|---|---|---|---|---|
| Age (years) | ||||
| < 40 | 21 | 26 | 0.697 | 0.706 |
| 40-50 | 39 | 65 | ||
| > 50 | 79 | 120 | ||
| Sex | ||||
| Male | 86 | 114 | 2.104 | 0.147 |
| Female | 53 | 97 | ||
| Time from onset to admission | ||||
| < 12 h | 81 | 108 | 1.695 | 0.193 |
| ≥ 12 h | 58 | 103 | ||
| Hemorrhage location | ||||
| Basal ganglia | 82 | 131 | 0.904 | 0.636 |
| Lobar | 35 | 44 | ||
| Thalamus | 22 | 36 | ||
| Duration of hypertension (years) | ||||
| < 5 | 67 | 110 | 2.883 | 0.237 |
| 5-10 | 53 | 84 | ||
| > 10 | 19 | 17 | ||
| Preoperative hypertension grade | ||||
| I | 30 | 53 | 2.485 | 0.288 |
| II | 76 | 122 | ||
| III | 33 | 36 | ||
| Intracerebral hemorrhage volume (mL) | ||||
| < 60 | 53 | 74 | 0.339 | 0.560 |
| ≥ 60 | 86 | 137 | ||
| History of diabetes | ||||
| Yes | 18 | 32 | 0.336 | 0.562 |
| No | 121 | 179 |
Clinical efficacy after treatment
The evaluation of clinical efficacy after treatment showed that the effectiveness rate in the control group was significantly lower than that in the observation group, with statistical significance (P < 0.001, Table 2).
Table 2.
Comparison of clinical efficacy after treatment
| Group | Significant Effect | Effective | Ineffective |
|---|---|---|---|
| Observation (n = 139) | 84 | 38 | 17 |
| Control (n = 211) | 75 | 106 | 30 |
| χ2 | 22.531 | ||
| P Value | < 0.001 | ||
Comparison of surgical time, first hematoma clearance rate, and hospitalization time
Comparison of surgical time, first hematoma clearance rate, and hospitalization time between the two groups revealed that the control group had significantly longer surgical time [40.00 (35.00, 44.00) vs. 102.00 (90.00, 115.00)], a lower first hematoma clearance rate [44.87 (40.33, 50.45) vs. 72.37 (63.08, 79.19)], and a longer hospitalization time [15.00 (12.00, 18.00) vs. 22.00 (20.00, 25.50)] compared to the observation group (P < 0.001, Figure 2).
Figure 2.
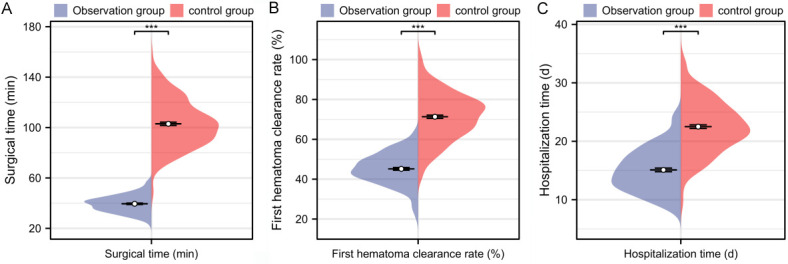
Comparison of surgical time, first hematoma clearance rate, and hospitalization time between the two groups. A. Comparison of surgical time during treatment between the two groups; B. Comparison of first hematoma clearance rate during treatment between the two groups; C. Comparison of hospitalization time after treatment between the two groups. Note: ***P < 0.001.
Changes in functional scores before and after treatment
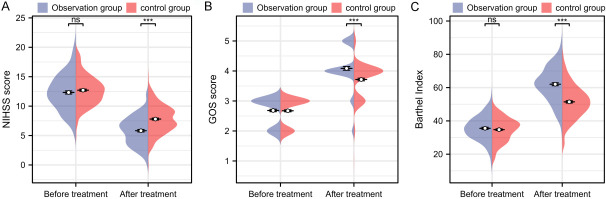
Before treatment, there were no significant differences in NIHSS score [13.00 (11.00, 14.00) vs. 12.00 (10.00, 14.00)], GOS score [3.00 (2.00, 3.00) vs. 3.00 (2.00, 3.00)], or Barthel index [34.74 ± 7.07 vs. 35.55 ± 7.38] between the two groups (all P > 0.05). However, after treatment, the observation group had a significantly lower NIHSS score [6.00 (4.00, 7.00) vs. 8.00 (6.00, 9.00)], higher GOS score [4.00 (4.00, 4.50) vs. 4.00 (3.00, 4.00)], and Barthel index [62.03 ± 10.30 vs. 51.45 ± 10.01] compared to the control group (all P < 0.001, Figure 3).
Figure 3.
Comparison of functional scores before and after treatment between the two groups. A. Comparison of NIHSS score before and after treatment between the two groups; B. Comparison of GOS score before and after treatment between the two groups; C. Comparison of Barthel index before and after treatment between the two groups. Note: nsP > 0.05, ***P < 0.001; NIHSS score, National Institutes of Health Stroke Scale score; GOS score, Glasgow Outcome Scale score.
Changes in inflammatory markers before and after treatment
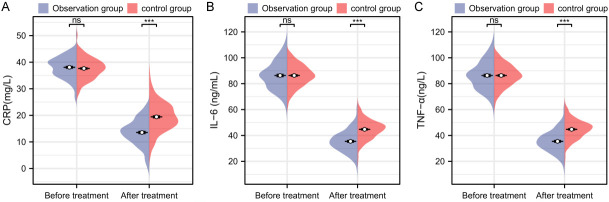
Before treatment, there were no significant differences in CRP [37.63 ± 3.91 vs. 38.08 ± 4.05], IL-6 [86.33 ± 8.25 vs. 86.33 ± 10.74], or TNF-α [289.06 ± 33.72 vs. 289.93 ± 37.49] between the two groups (P > 0.05). After treatment, the observation group had significantly lower levels of CRP [13.55 ± 4.96 vs. 19.43 ± 5.50], IL-6 [35.46 ± 8.19 vs. 44.73 ± 7.45], and TNF-α [146.88 ± 30.21 vs. 183.62 ± 29.75] compared to the control group (P < 0.001, Figure 4).
Figure 4.
Comparison of inflammatory markers before and after treatment between the two groups. A. Comparison of CRP before and after treatment between the two groups; B. Comparison of IL-6 before and after treatment between the two groups; C. Comparison of TNF-α before and after treatment between the two groups. Note: nsP > 0.05, ***P < 0.001; CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Comparison of clinical data between different prognostic groups
Based on the post-treatment GOS scores, patients were classified into the improved prognosis group (GOS score ≥ 3) and the poor prognosis group (GOS score < 3). Comparing clinical data between these groups revealed statistically significant differences in age, time from onset to admission, duration of hypertension, and treatment plan (P < 0.05, Table 3). Additionally, surgical time, first hematoma clearance rate, hospitalization time, post-treatment Barthel index, post-treatment CRP, and post-treatment IL-6 levels were significantly different between the two prognosis groups (P < 0.05, Table 3).
Table 3.
Comparison of clinical data between different prognostic groups
| Factors | Poor Prognosis (n = 79) | Improved Prognosis (n = 271) | Statistic | P Value |
|---|---|---|---|---|
| Age (years) | ||||
| < 40 | 16 | 35 | 9.733 | 0.008 |
| 40-50 | 32 | 76 | ||
| > 50 | 31 | 160 | ||
| Sex | ||||
| Male | 36 | 114 | 0.307 | 0.580 |
| Female | 43 | 157 | ||
| Time from onset to admission | ||||
| < 12 h | 46 | 115 | 6.141 | 0.013 |
| ≥ 12 h | 33 | 156 | ||
| Hemorrhage location | ||||
| Basal ganglia | 47 | 166 | 0.441 | 0.802 |
| Lobar | 17 | 62 | ||
| Thalamus | 15 | 43 | ||
| Duration of hypertension (years) | ||||
| < 5 | 29 | 148 | 7.877 | 0.019 |
| 5-10 | 40 | 97 | ||
| > 10 | 10 | 26 | ||
| Preoperative hypertension grade | ||||
| I | 20 | 63 | 0.709 | 0.701 |
| II | 46 | 152 | ||
| III | 13 | 56 | ||
| Intracerebral hemorrhage volume (mL) | ||||
| < 60 | 52 | 171 | 0.196 | 0.658 |
| ≥ 60 | 27 | 100 | ||
| History of diabetes | ||||
| Yes | 66 | 234 | 0.392 | 0.531 |
| No | 13 | 37 | ||
| Treatment plan | ||||
| Observation group | 17 | 122 | 14.109 | < 0.001 |
| Control group | 62 | 149 | ||
| Surgical time (min) | 93.00 (72.50, 108.50) | 81.00 (40.50, 104.00) | 2.740 | 0.006 |
| First hematoma clearance rate (%) | 66.73 (54.70, 75.91) | 57.30 (45.13, 74.35) | 2.530 | 0.011 |
| Hospitalization time (d) | 22.00 (17.50, 24.50) | 19.00 (15.00, 23.00) | 2.333 | 0.019 |
| After treatment NIHSS score | 7.00 (6.00, 10.00) | 7.00 (5.00, 9.00) | 1.355 | 0.172 |
| After treatment Barthel Index | 52.81 ± 12.41 | 56.48 ± 10.93 | -2.371 | 0.019 |
| After treatment CRP (mg/L) | 18.41 ± 5.84 | 16.71 ± 6.03 | 2.27 | 0.025 |
| After treatment IL-6 (ng/mL) | 43.07 ± 7.47 | 40.46 ± 9.30 | 2.577 | 0.011 |
| After treatment TNF-α (ng/L) | 173.25 ± 34.47 | 167.79 ± 34.98 | 1.236 | 0.219 |
Note: NIHSS score, National Institutes of Health Stroke Scale score; GOS, Glasgow Outcome Scale; CRP, C-reactive protein; IL-6, Interleukin-6; TNF-α, Tumor Necrosis Factor-alpha.
Logistic regression analysis of risk factors affecting prognosis
Based on the comparison of clinical data, indicators with significant differences were included in the logistic regression analysis. Surgical and laboratory indicators were dichotomized according to ROC analysis cut-off values for inclusion in the logistic regression (Figure 5; Table 4). Multivariate logistic regression analysis identified age (P = 0.003, OR = 0.573), time from onset to admission (P = 0.026, OR = 0.535), duration of hypertension (P = 0.006, OR = 1.766), and post-treatment IL-6 levels (P = 0.048, OR = 1.870) as significant risk factors affecting patient prognosis (Table 5).
Figure 5.
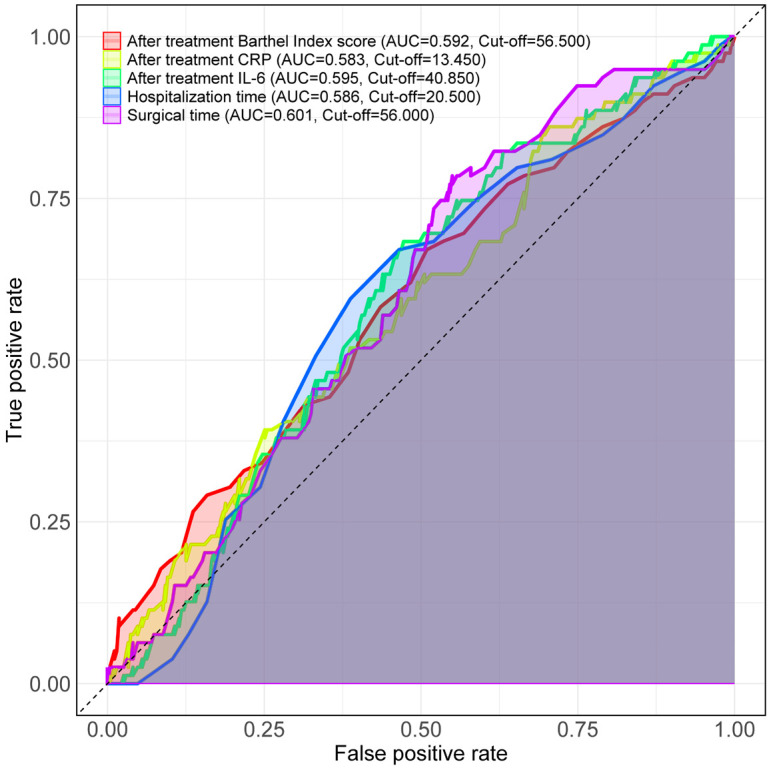
The best cut-off of the measurement data is determined. Note: CRP, C-reactive protein; IL-6, interleukin-6.
Table 4.
Assignment table
| Factors | Dichotomization |
|---|---|
| Age (years) | < 40 = 0, 40-50 = 1, > 50 = 2 |
| Time from onset to admission | < 12 h = 1, ≥ 12 h = 0 |
| Duration of hypertension (years) | < 5 = 0, 5-10 = 1, > 10 = 2 |
| Treatment plan | Observation group = 0, Control group = 1 |
| Surgical time (min) | ≤ 56 = 0, > 56 = 1 |
| First hematoma clearance rate (%) | ≤ 56.14 = 0, > 56.14 = 1 |
| Hospitalization time (d) | ≤ 20.5 = 0, > 20.5 = 1 |
| After treatment Barthel Index | ≤ 56.5 = 0, > 56.5 = 1 |
| After treatment CRP (mg/L) | ≤ 13.45 = 0, > 13.45 = 1 |
| After treatment IL-6 (ng/mL) | ≤ 40.85 = 0, > 40.85 = 1 |
| Prognosis | Improved = 0, Poor = 1 |
Note: CRP, C-reactive protein; IL-6, interleukin-6.
Table 5.
Multivariate logistics regression analysis
| Factors | Estimate | Std. Error | P Value | OR | 95% CI |
|---|---|---|---|---|---|
| Treatment plan | -0.219 | 1.353 | 0.871 | 0.803 | 0.053-11.761 |
| Age | -0.557 | 0.185 | 0.003 | 0.573 | 0.397-0.823 |
| Time from onset to admission | -0.625 | 0.280 | 0.026 | 0.535 | 0.307-0.924 |
| Duration of hypertension | 0.569 | 0.208 | 0.006 | 1.766 | 1.175-2.665 |
| Surgical time | 0.461 | 1.235 | 0.709 | 1.586 | 0.135-19.297 |
| First hematoma clearance rate | 0.189 | 0.431 | 0.661 | 1.207 | 0.523-2.856 |
| Hospitalization time | 0.542 | 0.340 | 0.111 | 1.720 | 0.891-3.395 |
| After treatment Barthel Index score | -0.164 | 0.315 | 0.602 | 0.849 | 0.455-1.57 |
| After treatment CRP | 0.590 | 0.398 | 0.138 | 1.804 | 0.848-4.083 |
| After treatment IL-6 | 0.626 | 0.317 | 0.048 | 1.870 | 1.012-3.519 |
Note: CRP, C-reactive protein; IL-6, interleukin-6.
Interaction between prognosis and independent risk factors
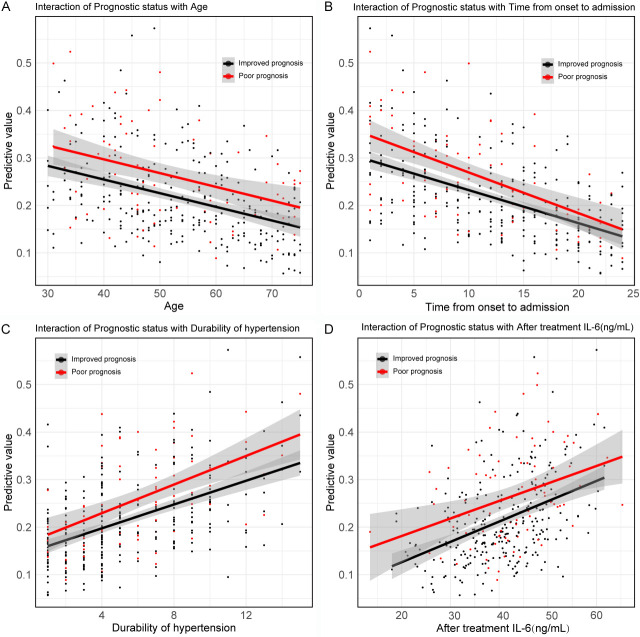
The analysis of interactions between prognosis and independent risk factors revealed statistical significance between prognosis and age (P = 0.040, OR = 0.978), time from onset to admission (P = 0.022, OR = 0.956), duration of hypertension (P = 0.022, OR = 1.085), and post-treatment IL-6 levels (P = 0.043, OR = 1.030) (Figure 6; Table 6).
Figure 6.
Interaction between independent risk factors and prognosis. A. Interaction between prognosis and age; B. Interaction between prognosis and time from onset to admission; C. Interaction between prognosis and duration of hypertension; D. Interaction between prognosis and after treatment IL-6. Note: IL-6, interleukin-6.
Table 6.
The mutual interaction relationship between the prognostic situation and the independent risk factors
| Factors | Estimate | Std. Error | z value | P Value |
|---|---|---|---|---|
| Age VS. Prognostic status | -0.022 | 0.011 | -2.049 | 0.040 |
| Time from onset to admission VS. Prognostic status | -0.045 | 0.020 | -2.286 | 0.022 |
| Durability of hypertension VS. Prognostic status | 0.081 | 0.036 | 2.288 | 0.022 |
| After treatment IL-6 (ng/mL) VS. Prognostic status | 0.030 | 0.015 | 2.025 | 0.043 |
Note: IL-6, Interleukin-6.
Discussion
HICH is a severe form of cerebral hemorrhage caused by hypertension, often resulting in basal ganglia hemorrhage due to small artery pathologies such as microaneurysm rupture [22]. Traditional craniotomy offers advantages over conventional medical methods, particularly in terms of precise hemostasis and safety [23]. However, it is also highly invasive and may cause more damage to brain tissue [24]. Compared to craniotomy, minimally invasive puncture and drainage have the benefit of being less traumatic. Each method has its own applicable scenarios and potential long-term effects, making the selection of an appropriate surgical strategy crucial for improving patient prognosis in clinical practice.
In this study, we compared the effects of minimally invasive puncture and drainage with traditional craniotomy in the treatment of HICH. The results showed that the observation group had significantly better outcomes on several key clinical indicators than the control group. Specifically, clinical efficacy was higher in the observation group, with a significantly greater effectiveness rate. Additionally, surgical and hospitalization times in the observation group were significantly shorter than those in the control group. The reduced invasiveness of minimally invasive surgery resulted in less intraoperative bleeding and fewer postoperative complications, accelerating patient recovery and thus shortening both surgical and hospitalization times [25,26]. Moreover, the smaller incisions and minimized tissue damage associated with minimally invasive surgery reduced postoperative pain and discomfort [27], enabling patients to begin rehabilitation and resume daily activities earlier. These advantages collectively highlight the significant benefits of minimally invasive surgery in treating acute HICH. For instance, Yan et al. [28] reported that patients undergoing minimally invasive surgery for HICH had significantly shorter surgical and hospitalization times compared to those undergoing traditional surgery. Similarly, Luan et al. [29] found that hospitalization time was shorter for HICH patients treated with minimally invasive surgery than for those treated with traditional surgery, consistent with our findings.
However, the observation group had a lower first hematoma clearance rate compared to the control group. This may be due to the smaller puncture point used in minimally invasive surgery, which can limit the complete removal of large or unevenly distributed hematomas. In contrast, traditional surgery provides direct access for more thorough hematoma removal, especially for large or complexly located hematomas [30]. Additionally, the texture and depth of the hematoma may affect the clearance effectiveness of minimally invasive surgery, particularly for hard or deep hematomas that are more challenging to manage thoroughly using minimally invasive methods.
Regarding functional recovery, the NIHSS score in the observation group was significantly lower, while the GOS score and Barthel index were significantly higher than those in the control group after treatment. This indicates that minimally invasive surgery is more effective in improving neurological function and daily self-care abilities. The reduced invasiveness of minimally invasive surgery leads to less postoperative pain and discomfort, reducing pressure on the central nervous system and inflammation, which favors the protection and recovery of neurological functions [31]. Furthermore, the precision of minimally invasive surgery minimizes damage to surrounding healthy brain tissue, aiding in preserving neurological functions and ultimately resulting in improved functional scores for patients. Previous studies by Huang et al. [32] found that HICH patients treated with minimally invasive surgery had significantly lower NIHSS scores after treatment compared to those receiving conservative treatment.
Additionally, post-treatment inflammatory markers CRP, IL-6, and TNF-α were significantly lower in the observation group compared to the control group, reflecting the reduced biological stress and inflammatory response associated with minimally invasive surgery. This reduction helps lower the risk of postoperative complications. Minimally invasive surgery employs small incisions and techniques that minimize tissue damage, reducing surgical trauma and intraoperative bleeding, thereby lowering the release of postoperative inflammatory mediators [33]. Furthermore, the shorter surgical time associated with minimally invasive surgery reduces the exposure time of tissues to the external environment, lowering the risk of infection and inflammation. For example, Xia et al. [34] reported that HICH patients treated with minimally invasive surgery showed more significant reductions in IL-6 and TNF-α compared to those receiving conservative treatment. These advantages collectively make minimally invasive surgery highly effective in reducing postoperative biological stress and inflammatory responses, significantly improving overall patient prognosis.
At the end of the study, we analyzed the risk factors affecting patient prognosis. Our results showed that age, time from onset to admission, duration of hypertension, and post-treatment IL-6 levels were significant risk factors influencing patient outcomes. Further interaction analysis revealed that as age increased, the probability of prognosis improvement significantly decreased. Additionally, longer times from onset to admission were associated with a lower likelihood of prognosis improvement. A longer duration of hypertension also corresponded to a reduced probability of prognosis improvement. Moreover, higher levels of post-treatment IL-6 were linked to a lower probability of prognostic improvement. This can be attributed to the fact that with increasing age, patients’ physical functions and the nervous system’s ability to recover decline, increasing the likelihood of chronic diseases and significantly reducing the chances of prognosis improvement [35]. Delays in admission result in increased cerebral hemorrhage volume and aggravated brain tissue damage, further diminishing the likelihood of prognosis improvement [36]. Long-term hypertension leads to pathological changes in cerebral blood vessels, heightening the risk and severity of cerebral hemorrhage, and the longer the duration of hypertension, the lower the probability of prognosis improvement [37]. Additionally, elevated post-treatment IL-6 levels indicate ongoing inflammatory responses and tissue damage, which inhibit neuronal repair and significantly reduce the probability of prognosis improvement [18]. These factors collectively influence patients’ recovery outcomes and long-term quality of life, emphasizing the need for early intervention and individualized management targeting these risk factors in clinical treatment to improve patient prognosis.
In our study, although minimally invasive puncture and drainage showed significant advantages over traditional craniotomy in postoperative functional scores and inflammatory markers, the treatment method did not achieve statistical significance in affecting patient prognosis in multivariate regression analysis. This may be due to individual patient differences, variations in surgical quality, postoperative management, and unadjusted confounding factors. Differences in patients’ specific conditions, hematoma location, and volume may lead to varying effects of the two surgical methods. Additionally, the success of surgery depends on the precision of surgical operations, postoperative management, and rehabilitation measures. The variability of these factors may obscure the impact of surgical methods on prognosis. Furthermore, the sample size and statistical power might have been insufficient to detect subtle effects of the treatment methods, and the severity of the condition could have influenced the choice of surgical method, introducing selection bias. Although minimally invasive surgery demonstrated advantages in postoperative recovery and inflammation control, the impact of the treatment method on prognosis did not reach statistical significance in multivariate regression analysis due to these factors.
The limitations of this study include its retrospective design, single-center nature, short follow-up period, and lack of a randomized controlled design. A retrospective analysis may introduce selection bias and information bias, potentially affecting the accuracy and completeness of the data. Since the data were collected from a single medical center, the external validity and generalizability of the results are limited. The short follow-up period may not fully capture the long-term prognosis and functional recovery of patients. Additionally, the absence of a randomized controlled design may leave unadjusted confounding factors, affecting the reliability of the results. Future studies should adopt prospective, multicenter, randomized controlled designs and extend the follow-up period to enhance the reliability and generalizability of the findings.
In conclusion, this study demonstrates that minimally invasive puncture and drainage offer significant advantages over traditional craniotomy in the treatment of acute HICH. Patients undergoing minimally invasive procedures experienced better neurological recovery, lower inflammatory responses, and an improved long-term prognosis. Key prognostic factors, including age, time from onset to admission, duration of hypertension, and postoperative IL-6 levels, were identified as independent risk factors affecting patient outcomes. Interaction analysis further highlighted the combined effects of these factors on prognosis, underscoring the importance of personalized treatment strategies. These findings support the adoption of minimally invasive techniques in clinical practice for managing HICH, optimize patient outcomes and enhance recovery.
Disclosure of conflict of interest
None.
References
- 1.Labib MA, Shah M, Kassam AB, Young R, Zucker L, Maioriello A, Britz G, Agbi C, Day JD, Gallia G, Kerr R, Pradilla G, Rovin R, Kulwin C, Bailes J. The safety and feasibility of image-guided brainpath-mediated transsulcul hematoma evacuation: a multicenter study. Neurosurgery. 2017;80:515–524. doi: 10.1227/NEU.0000000000001316. [DOI] [PubMed] [Google Scholar]
- 2.Wang YJ, Li ZX, Gu HQ, Zhai Y, Zhou Q, Jiang Y, Zhao XQ, Wang YL, Yang X, Wang CJ, Meng X, Li H, Liu LP, Jing J, Wu J, Xu AD, Dong Q, Wang D, Wang WZ, Ma XD, Zhao JZ China Stroke Statistics Writing Committee. China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2022;7:415–450. doi: 10.1136/svn-2021-001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerario MP, Merkler AE, Gialdini G, Parikh NS, Navi BB, Kamel H. Risk of stroke after the international classification of diseases-ninth revision discharge code diagnosis of hypertensive encephalopathy. Stroke. 2016;47:372–375. doi: 10.1161/STROKEAHA.115.011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Zhou L, Chen Y, Zhou H, Tan Y, Zhong W, Zhou Z. Prediction of short-term prognosis of patients with hypertensive intracerebral hemorrhage by radiomic-clinical nomogram. Front Neurol. 2023;14:1053846. doi: 10.3389/fneur.2023.1053846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan Y, Zou X, Wu J, Fu L, Huang W, Lin J, Luo F, Wang W. Neuroendoscopy surgery for hypertensive intracerebral hemorrhage with concurrent brain herniation: a retrospective study of comparison with craniotomy. Front Neurol. 2023;14:1238283. doi: 10.3389/fneur.2023.1238283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu Z, Tao C, Xiao A, Wu C, Fu M, Dong W, Liu M, Yu X, You C. Chinese multidisciplinary guideline for management of hypertensive intracerebral hemorrhage. Chin Med J (Engl) 2022;135:2269–2271. doi: 10.1097/CM9.0000000000001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Men D, Huang Z, Yin Y, Wu W, Li W, Liu H, Xu C. Advantages of small bone-window craniotomy under microscope combined postoperative intracranial pressure monitoring in the treatment of hypertensive intracerebral hemorrhage. J Craniofac Surg. 2021;32:e77–e80. doi: 10.1097/SCS.0000000000006986. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Wang H, Feng K, He B, Jia D. Neuroendoscopic-assisted versus mini-open craniotomy for hypertensive intracerebral hemorrhage: a retrospective analysis. BMC Surg. 2022;22:188. doi: 10.1186/s12893-022-01642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei JH, Tian YN, Zhang YZ, Wang XJ, Guo H, Mao JH. Short-term effect and long-term prognosis of neuroendoscopic minimally invasive surgery for hypertensive intracerebral hemorrhage. World J Clin Cases. 2021;9:8358–8365. doi: 10.12998/wjcc.v9.i28.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Cheng J, Zhou H, Deng C, Wang Z. Efficacy of minimally invasive surgery for the treatment of hypertensive intracerebral hemorrhage: a protocol of randomized controlled trial. Medicine (Baltimore) 2021;100:e24213. doi: 10.1097/MD.0000000000024213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Zhang L, Mao Y, Li Y, Wu G, Li Q. Regular-shaped hematomas predict a favorable outcome in patients with hypertensive intracerebral hemorrhage following stereotactic minimally invasive surgery. Neurocrit Care. 2021;34:259–270. doi: 10.1007/s12028-020-00996-2. [DOI] [PubMed] [Google Scholar]
- 12.Baker TS, Kalagara R, Hashmi A, Rodriguez B, Liu SH, Mobasseri H, Smith C, Rapoport B, Costa A, Kellner CP. Identifying predictors of initial surgical failure during minimally invasive endoscopic intracerebral hemorrhage evacuation. Biomedicines. 2024;12:508. doi: 10.3390/biomedicines12030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GQ, Li SQ, Huang YH, Zhang WW, Ruan WW, Qin JZ, Li Y, Yin WM, Li YJ, Ren ZJ, Zhu JQ, Ding YY, Peng JQ, Li PJ. Can minimally invasive puncture and drainage for hypertensive spontaneous Basal Ganglia intracerebral hemorrhage improve patient outcome: a prospective non-randomized comparative study. Mil Med Res. 2014;1:10. doi: 10.1186/2054-9369-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawnchhim AL, Mahajan C, Kapoor I, Sinha TP, Prabhakar H, Chaturvedi A. Comparison of Glasgow Coma Scale full outline of unresponsiveness and Glasgow Coma Scale: pupils score for predicting outcome in patients with traumatic brain injury. Indian J Crit Care Med. 2024;28:256–264. doi: 10.5005/jp-journals-10071-24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage 2019. Chin J Neurol. 2019;52:994–1005. [Google Scholar]
- 17.Gui C, Gao Y, Hu D, Yang X. Neuroendoscopic minimally invasive surgery and small bone window craniotomy hematoma clearance in the treatment of hypertensive cerebral hemorrhage. Pak J Med Sci. 2019;35:377–382. doi: 10.12669/pjms.35.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Zhang S. Analysis of the therapeutic effect and prognostic factors of 126 patients with hypertensive cerebral hemorrhage treated by soft-channel minimally invasive puncture and drainage. Front Surg. 2022;9:885580. doi: 10.3389/fsurg.2022.885580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CLH, Pokharkar Y, Venketasubramanian N CHIMES and CHIMES-E Investigators. Association between baseline NIHSS limb motor score and functional recovery after stroke: analysis based on a multicountry dataset. Cerebrovasc Dis. 2023;52:160–165. doi: 10.1159/000525984. [DOI] [PubMed] [Google Scholar]
- 20.Rezakhah A, Kobets AJ, Emami Sigaroudi F, Mahdkhah A, Barshan J, Gharajedaghi A, Naseri Alavi SA. Correlation between clinical findings at admission and Glasgow outcome scale score in children with traumatic brain injury. World Neurosurg. 2023;175:e1300–e1306. doi: 10.1016/j.wneu.2023.04.121. [DOI] [PubMed] [Google Scholar]
- 21.Uchinaka EI, Hanaki T, Morimoto M, Murakami Y, Tomoyuki M, Yamamoto M, Tokuyasu N, Sakamoto T, Hasegawa T, Fujiwara Y. The Barthel Index for predicting postoperative complications in elderly patients undergoing abdominal surgery: a prospective single-center study. In Vivo. 2022;36:2973–2980. doi: 10.21873/invivo.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand S, Choudhury SS, Pradhan S, Mulmuley MS. Normotensive state during acute phase of hypertensive intracerebral hemorrhage. J Neurosci Rural Pract. 2023;14:465–469. doi: 10.25259/JNRP_168_2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Chen X, Li F, Zheng X, Wang Q, Sun G, Zhang J, Xu B. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg. 2018;128:553–559. doi: 10.3171/2016.10.JNS161589. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Li Y, Zhang H, Gao H, Zhou X, Xu Y, Yan K, Wang X. Neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2020;134:477–488. doi: 10.1016/j.wneu.2019.10.115. [DOI] [PubMed] [Google Scholar]
- 25.Xiong J, Chen Y, Wang R, Hu S, Xu J, Mo X, Li X, Zhou Y, Guan C, Huang J, Su F. Minimally invasive puncture combined with a high frequency of urokinase therapy improves outcomes in patients with HICH. Neurotherapeutics. 2024;21:e00293. doi: 10.1016/j.neurot.2023.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu R, Qin H, Cai Z, Shi J, Cao J, Mao Y, Dong B. The clinical efficacy of electromagnetic navigation-guided hematoma puncture drainage in patients with hypertensive basal ganglia hemorrhage. World Neurosurg. 2018;118:e115–e122. doi: 10.1016/j.wneu.2018.06.137. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Yan Z, Jiang L, Chen S, Liu Y. The efficacy of stereotactic minimally invasive thrombolysis at different catheter positions in the treatment of small- and medium-volume basal ganglia hemorrhage (SMITDCP I): a randomized, controlled, and blinded endpoint phase 1 trial. Front Neurol. 2023;14:1131283. doi: 10.3389/fneur.2023.1131283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan YF, Ru DW, Du JR, Shen X, Wang ES, Yao HB. The clinical efficacy of neuronavigation-assisted minimally invasive operation on hypertensive basal ganglia hemorrhage. Eur Rev Med Pharmacol Sci. 2015;19:2614–2620. [PubMed] [Google Scholar]
- 29.Luan L, Li M, Sui H, Li G, Pan W. Efficacies of minimally invasive puncture and small bone window craniotomy for hypertensive intracerebral hemorrhage, evaluation of motor-evoked potentials and comparison of postoperative rehemorrhage between the two methods. Exp Ther Med. 2019;17:1256–1261. doi: 10.3892/etm.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Du L, Yu F. Clinical efficacy of minimally invasive puncture and drainage versus trepanation and drainage for chronic subdural hematoma: systematic review and meta-analysis. Medicine (Baltimore) 2023;102:e32860. doi: 10.1097/MD.0000000000032860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Zhang H, Zhang J, Luo M, Wang Q, Zhao Y, Gan Z, Xu B, Chen X MISICH study team. Minimally invasive surgeries for spontaneous hypertensive intracerebral hemorrhage (MISICH): a multicenter randomized controlled trial. BMC Med. 2024;22:244. doi: 10.1186/s12916-024-03468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Jiang L, Chen S, Li G, Pan W, Peng L, Yan Z. Comparison of the curative effect and prognosis of stereotactic drainage and conservative treatment for moderate and small basal ganglia haemorrhage. BMC Neurol. 2021;21:268. doi: 10.1186/s12883-021-02293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Mao Q, Qian Z, Zhu J, Qu Z, Wang C. Effect of mild hypothermia on expression of inflammatory factors in surrounding tissue after minimally invasive hematoma evacuation in the treatment of hypertensive intracerebral hemorrhage. Exp Ther Med. 2018;15:4906–4910. doi: 10.3892/etm.2018.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia L, Han Q, Ni XY, Chen B, Yang X, Chen Q, Cheng GL, Liu CF. Different techniques of minimally invasive craniopuncture for the treatment of hypertensive intracerebral hemorrhage. World Neurosurg. 2019;126:e888–e894. doi: 10.1016/j.wneu.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Gong X, Wang F, Li K, Li Y, Yan W. Nursing analysis of minimally invasive intracranial hematoma drainage for hypertensive cerebral hemorrhage. Minerva Surg. 2023 doi: 10.23736/S2724-5691.23.10107-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Tang W, Cheng L, Yang C, Wang T, Wang J, Miao Z, Zhao X, Fang X, Zhou Y. Early and delayed blood-brain barrier permeability predicts delayed cerebral ischemia and outcomes following aneurysmal subarachnoid hemorrhage. Eur Radiol. 2024;34:5287–5296. doi: 10.1007/s00330-023-10571-w. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Zeng H, Zhou H, Li J, Wang T, Guo Y, Cai L, Hu J, Zhang X, Chen G. Predicting the outcome of patients with aneurysmal subarachnoid hemorrhage: a machine-learning-guided scorecard. J Clin Med. 2023;12:7040. doi: 10.3390/jcm12227040. [DOI] [PMC free article] [PubMed] [Google Scholar]