Abstract
Objective: To identify the influencing factors of dry eyes after cataract surgery and construct a prediction model to provide a reference for ophthalmologists in assessing the risk of postoperative dry eyes. Methods: A retrospective study was conducted from January 2023 to April 2024, involving 219 patients (219 eyes) who underwent phacoemulsification with intraocular lens implantation at the Department of Ophthalmology, Ninth People’s Hospital of Suzhou. Patients were divided into two groups based on the presence or absence of dry eyes at 2 weeks postoperatively. Data from both groups were analyzed to determine the influencing factors of dry eyes after cataract surgery. A nomogram prediction model was constructed using R software. The model’s discrimination was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC), and model calibration was assessed using the Hosmer-Lemeshow (H-L) goodness-of-fit test and the Bootstrap method (self-sampling technique). Decision curve analysis was employed to evaluate the clinical utility of the model. Results: Among the 219 cataract patients, 53 (24.20%) developed dry eyes during the 2-week follow-up period. Multivariate logistic regression analysis identified smoking (OR = 1.809, P = 0.037), diabetes mellitus (OR = 3.248, P = 0.002), elevated IL-6 (OR = 3.019, P = 0.016), a high Hospital Anxiety and Depression Scale (HADS) score (OR = 2.147, P = 0.029), and longer surgical incision length (OR = 2.995, P = 0.014) as significant risk factors for postoperative dry eye. The AUC of the nomogram model was 0.857 (95% CI: 0.803-0.913), and the H-L goodness-of-fit test showed no statistical significance (χ2 = 4.472, P = 0.812), indicating good discrimination and calibration of the model. The average absolute error between predicted and actual probabilities after 1000 Bootstrap iterations was 0.021. Decision curve analysis demonstrated that the net benefit of the model was higher than the two extreme scenarios. Conclusion: Postoperative dry eyes in cataract patients is associated with smoking, diabetes, elevated IL-6, high HADS scores, and longer incision lengths. The nomogram model demonstrates good predictive capability for assessing the risk of dry eyes after cataract surgery.
Keywords: Cataract, postoperative, dry eyes, influencing factors, prediction model
Introduction
Cataracts are characterized by lens opacity and blurred vision [1]. If not treated effectively and in a timely manner, cataracts can progress, eventually leading to blindness as the condition worsens [2]. Surgery is the most effective treatment for cataracts, with phacoemulsification combined with artificial lens implantation being particularly advantageous due to minimal injury and fast recovery. However, some patients experience dry eye symptoms postoperatively, such as foreign body sensation, dryness, swelling, redness, discomfort, and fluctuating vision [3]. Research indicates that severe dry eye symptoms can lead to corneal squamous metaplasia, corneal epithelial cell apoptosis, reduced visual quality, and visual deterioration [4], which can affect daily life and work.
Identifying the risk factors for dry eyes after cataract surgery and developing a prediction model can help stratify patients based on individual risk, enabling the implementation of preventive measures to minimize postoperative dry eye. However, reliable tools for assessing the risk of dry eyes following cataract surgery are lacking. This study aims to analyze the factors influencing dry eye syndrome after cataract surgery and to construct a risk prediction model presented as a nomogram. The model objectively displays each variable, the corresponding score for each variable, and the predicted incidence of dry eyes. This tool will provide ophthalmologists with a reference for early risk assessment and help guide preventive measures for postoperative dry eyes.
Materials and methods
Research subjects
A retrospective study was conducted between January 2023 and April 2024, involving 219 patients (219 eyes) who underwent phacoemulsification combined with intraocular lens implantation at the Department of Ophthalmology, Ninth People’s Hospital of Suzhou. Patients were divided into two groups: the dry eye group and the non-dry eye group, based on the presence or absence of dry eyes 2 weeks postoperatively.
Inclusion criteria: (1) All patients met the clinical diagnostic criteria for cataracts [5]; (2) First diagnosis and treatment; (3) Complete clinical information and follow-up data.
Exclusion criteria: (1) History of glaucoma or conjunctivitis; (2) Preoperative eye infection; (3) High myopia; (4) Presence of systemic connective tissue or autoimmune diseases; (5) History of ocular trauma within 6 months before surgery; (6) History of eye surgery within 6 months before the current procedure.
This study was approved by the Ethics Committee of Suzhou Ninth People’s Hospital.
Research methods
Surgical methods
All surgeries were performed by the same chief surgeon. Thirty minutes before surgery, compound tropicamide eye drops were applied to dilate the pupils. After standard anesthesia, a tunnel-type corneal incision was made, followed by an auxiliary incision at the corneal margin using anterior chamber puncture. Viscoelastic agent was injected into the anterior chamber to increase its depth. If the agent’s expansion was insufficient, an iris restorer and a crystalline lens positioning hook were used to pull the pupil through the corneal incision. In cases of inadequate pupil dilation, low-concentration adrenaline was injected into the anterior chamber, or the iris was pushed peripherally. Circular capsulotomy was performed, followed by hydrodissection and phacoemulsification to remove the crystalline lens. After removing the lens cortex, viscoelastic agent was reintroduced, and an artificial intraocular lens was implanted. The viscoelastic agent was then removed, ensuring no leakage at the incision site. Postoperative antibiotics were administered to prevent infection.
Data collection
To ensure the temporal relationship between predictor variables and outcomes, relevant patient data were collected before the occurrence of dry eye. Data included age, gender, education level, place of residence, body mass index (BMI), smoking status, underlying conditions (e.g., hypertension, diabetes), history of keratoconjunctival diseases, pterygium, meibomian gland dysfunction, lens nucleus hardness (grade I-V), preoperative biochemical markers [fibrinogen (FIB), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)], preoperative psychological status, surgical incision length, and presence of postoperative infections.
Preoperative psychological status was evaluated using the Hospital Anxiety and Depression Scale (HADS), which consists of two subscales for anxiety and depression, with a total of 14 items. The scale is suitable for rapid assessment before outpatient surgery. The total score ranges from 0 to 21 points, with higher scores indicating more severe anxiety and depression [6].
Diagnosis of dry eye
Diagnostic criteria followed relevant guidelines [7]. Dry eye syndrome was diagnosed if the patient met one of the following subjective symptoms postoperatively: dry eyes, fatigue, foreign body sensation, discomfort, burning, redness, or fluctuating vision, combined with an Ocular Surface Disease Index (OSDI) score ≥ 13 points. Additionally, objective measures included: (1) Tear film break-up time (FBUT) ≤ 5 seconds; (2) Schirmer I test (SIT) ≤ 5 mm/5 min; (3) Sodium fluorescein staining score ≥ 5 points. After excluding other ocular conditions, dry eye syndrome was diagnosed if any subjective symptom was present along with one or more objective criteria.
Statistical analysis
SPSS 27.0 software was used for analysis. Continuous data were expressed as mean ± standard deviation (SD), and comparisons between groups were performed using the t-test. Categorical data were expressed as numbers and percentages [n (%)], and comparisons were made using the χ2 test. Multivariate logistic regression was employed to identify factors influencing dry eye syndrome after cataract surgery, with P < 0.05 considered statistically significant. A nomogram prediction model was constructed using R 3.4.3 software, and the area under the receiver operating characteristic (ROC) curve (AUC) was calculated to assess the model’s discrimination. The Hosmer-Lemeshow (H-L) goodness-of-fit test and Bootstrap method were used for internal validation of model fit and calibration. Decision curve analysis was used to evaluate the clinical net benefit of the model.
Results
Analysis of the incidence and general information of dry eye syndrome in cataract patients after surgery
Among the 219 patients (219 eyes) included in the study, 53 patients developed dry eye syndrome during the 2-week follow-up period (Dry eye group), with an incidence rate of 24.20% (53/219). The remaining 166 patients did not develop dry eye syndrome (No-Dry eye group). A comparison of general information such as age, BMI, gender, education level, and place of residence between the two groups showed no statistically significant differences (P > 0.05), as presented in Table 1.
Table 1.
General information comparison between the dry eye group and non-dry eye group
| General Information | Dry eye group (n = 53) | No-dry eye group (n = 166) | t/χ2 | P value |
|---|---|---|---|---|
| Age (years) | 59.39±7.95 | 58.11±7.49 | 1.067 | 0.287 |
| BMI (kg/m2) | 34 (64.15) | 131 (78.92) | 0.604 | 0.546 |
| Gender | 1.941 | 0.164 | ||
| Male | 21 (39.62) | 84 (50.60) | ||
| Female | 32 (60.38) | 82 (49.40) | ||
| Degree of education | 0.044 | 0.834 | ||
| Junior high school and below | 18 (33.96) | 59 (35.54) | ||
| High school and above | 35 (66.04) | 107 (64.46) | ||
| Place of residence | 0.059 | 0.807 | ||
| Cities and towns | 29 (54.72) | 94 (56.63) | ||
| Rural area | 24 (45.28) | 72 (43.37) |
Note: BMI: body mass index.
Univariate analysis of postoperative dry eye syndrome in cataract patients
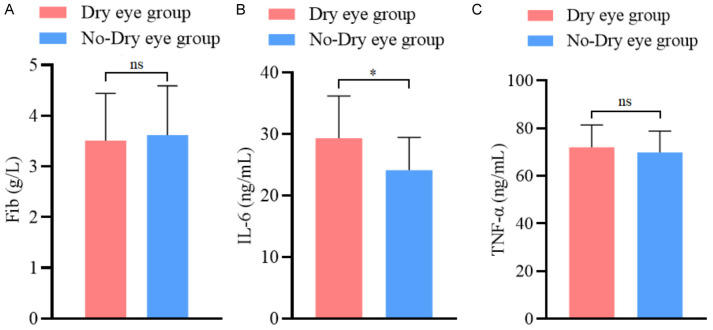
Univariate analysis revealed that smoking, diabetes, and meibomian gland dysfunction were significantly different between the dry eye and non-dry eye groups (all P < 0.05), as shown in Table 2. No significant difference was found in preoperative fibrinogen (FIB) and tumor necrosis factor-α (TNF-α) levels between the groups (both P > 0.05). However, the preoperative IL-6 levels in the dry eye group were significantly higher than in the non-dry eye group (P < 0.05), as shown in Figure 1. Additionally, the preoperative HADS scores and the length of the surgical incision were significantly higher in the dry eye group compared to the non-dry eye group (P < 0.05), as illustrated in Figure 2.
Table 2.
Univariate analysis of postoperative dry eye syndrome in cataract patients
| Characteristics | Dry eye group (n = 53) | No-dry eye group (n = 166) | χ2 | P value |
|---|---|---|---|---|
| Smoking | 4.714 | 0.030 | ||
| Yes | 19 (35.85) | 35 (21.08) | ||
| No | 34 (64.15) | 131 (78.92) | ||
| Combined hypertension | 0.542 | 0.462 | ||
| Yes | 5 (9.43) | 22 (13.25) | ||
| No | 48 (90.57) | 144 (86.75) | ||
| Combined diabetes | 9.096 | 0.003 | ||
| Yes | 18 (33.96) | 25 (15.06) | ||
| No | 35 (66.04) | 141 (84.94) | ||
| History of conjunctival diseases | 2.065 | 0.151 | ||
| Yes | 24 (45.28) | 57 (34.34) | ||
| No | 29 (54.72) | 109 (65.66) | ||
| Pterygium | 1.151 | 0.283 | ||
| Yes | 16 (30.19) | 38 (22.89) | ||
| No | 37 (69.81) | 128 (77.11) | ||
| Meibomian gland dysfunction | 4.409 | 0.036 | ||
| Yes | 21 (39.62) | 41 (24.70) | ||
| No | 32 (60.38) | 125 (75.30) | ||
| Hardness of lens nucleus (grade) | 2.826 | 0.093 | ||
| I-II | 23 (43.40) | 94 (56.63) | ||
| III-IV | 30 (56.60) | 72 (43.37) | 6.068 | < 0.001 |
| Is there any infection after surgery | 0.325 | 0.569 | ||
| Yes | 4 (7.55) | 9 (5.42) | ||
| No | 49 (92.45) | 157 (94.58) |
Figure 1.
Comparison of preoperative blood indicators between the dry eye group and non-dry eye group. A: Comparison of fibrinogen levels between the dry eye group and non-dry eye group; B: Comparison of interleukin-6 (IL-6) levels between the dry eye group and non-dry eye group; C: Comparison of tumor necrosis factor - α (TNF-α) levels between the dry eye group and non-dry eye group. Note: ns indicates P > 0.05; * Indicating P < 0.05.
Figure 2.
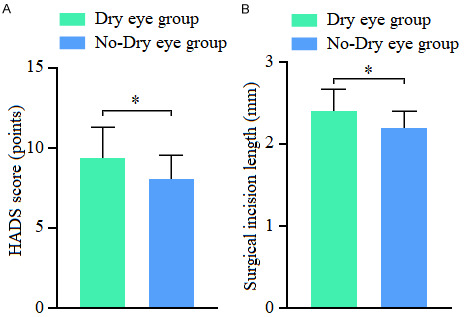
Comparison of preoperative HADS scores and surgical incision length between the dry eye group and non-dry eye group. A: Comparison of preoperative Hospital Anxiety and Depression Scale (HADS) scores between the dry eye group and non-dry eye group; B: Comparison of surgical incision length between the dry eye group and non-dry eye group. Note: * Indicating P < 0.05.
Multivariate logistic regression analysis on the occurrence of dry eye syndrome in cataract patients after surgery
Using the occurrence of postoperative dry eye syndrome (0 = no; 1 = yes) as the dependent variable, variables with P < 0.05 in the univariate analysis (Table 1) were selected as independent variables. The assigned values are shown in Table 3. Multivariate logistic regression analysis indicated that smoking, diabetes mellitus, elevated IL-6 levels, higher HADS scores, and longer surgical incisions were significant risk factors for developing dry eyes after cataract surgery (all P < 0.05), as presented in Table 4.
Table 3.
Variable assignment description
| Variable | Description of valuation |
|---|---|
| Smoking | 0 = No, 1 = Yes |
| Combined diabetes | 0 = No, 1 = Yes |
| Meibomian gland dysfunction | 0 = No, 1 = Yes |
| Preoperative IL-6 | continuous variable |
| Preoperational HADS score | continuous variable |
| Surgical incision length | continuous variable |
Note: IL-6: interleukin-6; HADS: Hospital Anxiety and Depression Scale.
Table 4.
Results of multivariate logistic analysis
| Variable | β | SE | Wald χ2 | P | OR (95% CI) |
|---|---|---|---|---|---|
| Smoking | 0.593 | 0.284 | 4.359 | 0.037 | 1.809 (1.037-2.319) |
| Combined diabetes | 1.178 | 0.381 | 9.560 | 0.002 | 3.248 (1.539-6.855) |
| Meibomian gland dysfunction | 0.239 | 0.152 | 2.472 | 0.115 | 1.270 (0.943-1.711) |
| High expression of IL-6 | 1.105 | 0.459 | 5.796 | 0.016 | 3.019 (1.229-7.419) |
| HADS score was high | 0.764 | 0.350 | 4.765 | 0.029 | 2.147 (1.081-4.263) |
| Long surgical incision length | 1.097 | 0.446 | 6.049 | 0.014 | 2.995 (1.249-7.178) |
Note: IL-6: interleukin-6; HADS: Hospital Anxiety and Depression Scale.
Construction of nomogram model for risk of dry eye after cataract surgery
Based on the regression coefficients and constant term from the multivariate analysis, a prediction model was developed using the following formula:
Z = -10.698 + 0.593 × smoking (0 = no, 1 = yes) + 1.178 × combined diabetes (0 = no, 1 = yes) + 0.239 × meibomian gland dysfunction (0 = no, 1 = yes) + 1.105 × preoperative IL-6 index (actual value) + 0.764 × preoperative HADS score (actual value) + 1.097 × surgical incision length (actual value).
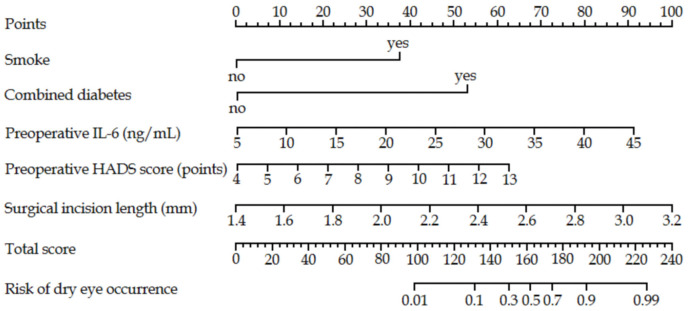
This logistic regression model was visualized as a nomogram using R software. Each factor’s value was represented by a corresponding score through graphic line segments. The total score was calculated by summing the individual scores, which was then converted into a predicted probability of developing dry eyes after cataract surgery, as shown in Figure 3.
Figure 3.
Nomogram prediction model of dry eye risk in cataract patients. Note: IL-6: interleukin-6; HADS: Hospital Anxiety and Depression Scale.
Effectiveness analysis of nomogram prediction model for the risk of dry eyes after cataract surgery
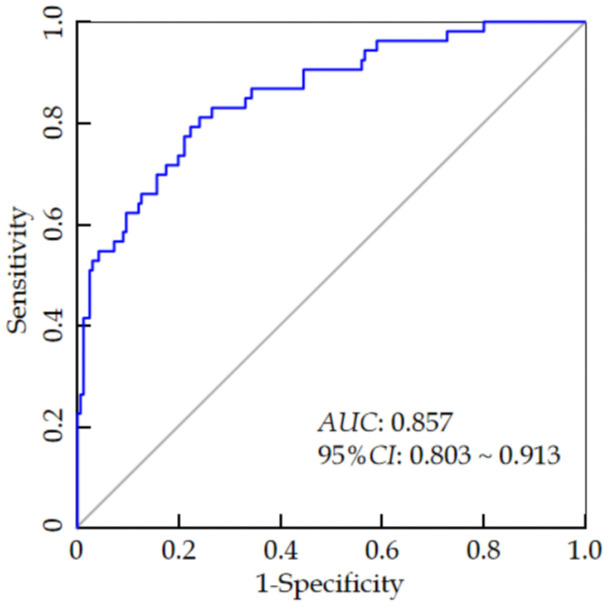
The ROC curve analysis revealed that the AUC value of the nomogram model for predicting the risk of dry eyes after cataract surgery was 0.857 (95% CI: 0.803-0.913), with a sensitivity of 0.821 and a specificity of 0.795 (see Figure 4). The H-L goodness-of-fit test showed no significant difference between the predicted probabilities and the actual outcomes (χ2 = 4.472, P = 0.812), indicating that the model does not exhibit overfitting. After 1000 internal sampling verifications using the Bootstrap method, the average absolute error between the predicted probability and the actual probability was 0.021, demonstrating good consistency between the model’s predictions and the actual incidence of postoperative dry eye syndrome (see Figure 5). Decision curve analysis indicated that the net benefit of using the model to predict postoperative dry eyes in cataract patients was higher than the two extreme curves, suggesting that the model has clinical utility (see Figure 6).
Figure 4.
ROC curve analysis of the postoperative dry eye prediction model in cataract patients.
Figure 5.
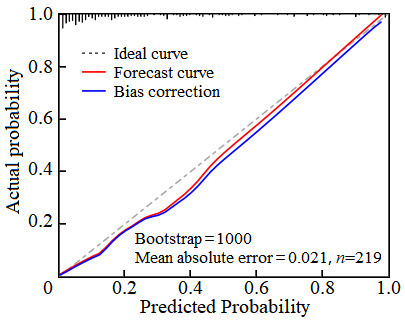
Analysis of the calibration curve for the postoperative dry eye prediction model in cataract patients.
Figure 6.
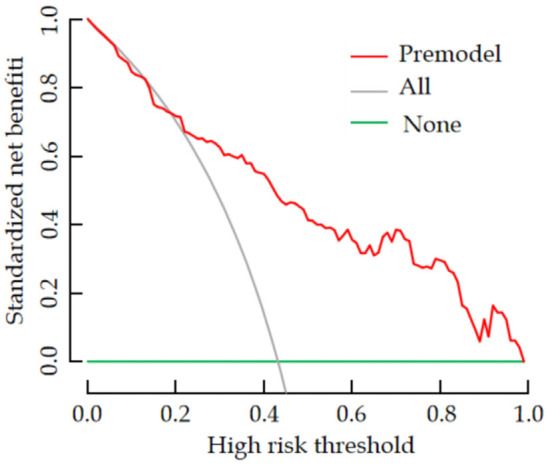
Analysis of the decision curve for the prediction model for postoperative dry eye in cataract patients.
Discussion
Severe cataracts can lead to visual impairment and blindness [8,9]. Phacoemulsification is the primary clinical treatment for cataracts, significantly improving patients’ symptoms. However, dry eye syndrome frequently occurs after surgery [10]. Dry eyes are characterized by tear film instability and ocular surface damage caused by abnormalities in tear quality, quantity, or dynamics, which can result in eye discomfort and reduced quality of life. Early identification of patients at risk for postoperative dry eye syndrome is crucial for implementing targeted interventions to reduce or prevent its harmful effects.
As the eyes are exposed directly to the environment, smoking was found to be a contributing factor to postoperative dry eye in cataract patients, consistent with previous literature [11]. This may be due to the smoke exposure causing lipid peroxidation in the outer layer of the corneal tear film [12], leading to tear film instability, reduced lipid layer thickness, and accelerated tear evaporation, all of which contribute to dry eye syndrome. Additionally, smoking can stimulate the release of inflammatory factors from inflammatory cells [13], causing an inflammatory response in the meibomian glands, leading to meibomian gland dysfunction and exacerbating the development of dry eye syndrome.
Dysfunction of the tear film is widely recognized as the underlying pathological mechanism of dry eyes. Studies have shown that high blood sugar not only directly impairs tear film function but also reduces tear secretion. It can further activate inflammatory responses and oxidative stress, promoting the development of dry eye syndrome [14]. This study found that diabetes was a significant factor influencing the occurrence of dry eyes after cataract surgery, consistent with findings in other research [15]. Long-term hyperglycemia in diabetic patients likely disrupts the proliferation of conjunctival goblet cells, inhibiting the secretion of collagen and tears [16]. This results in insufficient tear production, rapid evaporation, and tear film instability, which ultimately leads to dry eye syndrome.
Cataract surgery is an invasive procedure, and during the operation, the eye may produce peroxidase, which can combine with substances like oxygen free radicals, inducing an inflammatory response and increasing the secretion of inflammatory cytokines. High preoperative inflammation levels can exacerbate these effects. Mechanical damage during surgery may further stimulate inflammatory cytokines to respond to tissue injury. IL-6 is one of the commonly detected inflammatory cytokines in clinical practice, promoting lymphocyte proliferation and neutrophil activation, thereby inducing inflammatory reactions. Narayanan et al. [17] found a correlation between IL-6 expression levels and the occurrence of dry eye, aligning with the findings of this study. Specifically, preoperative high IL-6 levels were a contributing factor to postoperative dry eye in cataract patients. Elevated IL-6 may activate the p38 MAPK signaling pathway, which mediates inflammation through IL-6 [18]. This can impair corneal cell proliferation and tissue repair, prolong the exposure of corneal nerve endings, alter corneal epithelial morphology and density [19], and ultimately inducing dry eye syndrome.
It has been well-established that most cataract surgery patients experience anxiety and depression before surgery [20]. Research has shown that anxiety and depression can increase the risk of postoperative dry eyes in cataract patients by stimulating inflammatory responses [21], which is consistent with the findings of this study. Anxiety and depression may stimulate the release of large amounts of diuretics and adrenocorticotropins in the hippocampus, leading to local microcirculatory disorders and ischemia-hypoxia reactions. This alters inflammatory factor levels in the body [22], impairs cell proliferation and tissue repair, and increases the risk of dry eyes.
Phacoemulsification cataract extraction is the most widely used and effective method for treating cataracts. Common surgical incisions include scleral tunnel incisions, corneoscleral margin tunnel incisions, and clear corneal incisions. However, regardless of the incision type, it impacts the corneal nerve fibers, reducing corneal sensitivity and blinking frequency, which in turn decreases meibomian gland secretion and accelerates tear evaporation. Studies have shown that once corneal nerves are damaged, the repair process is slow [23]. This study found that longer surgical incisions are a contributing factor to postoperative dry eyes. A larger incision may cause greater damage to corneal nerves, slowing their repair. This not only reduces corneal sensitivity but also destabilizes the tear film, thereby increasing the risk of dry eyes.
There are multiple factors influencing the occurrence of postoperative dry eyes in cataract patients, each with distinct mechanisms, and some factors may interact. Therefore, it is essential to integrate these factors into a prediction model to assess the potential risk of dry eyes in patients after surgery. In this study, a nomogram prediction model was constructed by selecting five key influencing factors for dry eyes after cataract surgery. The analysis revealed an AUC value of 0.857 for the prediction model, indicating its prediction accuracy. The H-L goodness-of-fit test showed no overfitting, suggesting good consistency between the predicted risk and the actual incidence of postoperative dry eyes. Furthermore, decision curve analysis indicated that the model’s predicted net benefit was higher than the extreme curves, confirming its clinical utility.
Medical staff can utilize the nomogram model to assess the risk of postoperative dry eyes and implement targeted preventive measures based on risk stratification. For instance: ① Preoperative health education: Patients and their families should be informed that smoking increases the risk of postoperative dry eyes. Quitting smoking can effectively reduce this risk. Patients should be encouraged to quit smoking, and those with difficulty quitting may benefit from psychological counseling and behavioral interventions such as mandatory smoking cessation, potentially in collaboration with smoking cessation clinics. Family members should also support and supervise the patient to reduce the risk of postoperative dry eye. ② Diabetes management: Patients with diabetes should receive appropriate hypoglycemic therapy to maintain normal blood sugar levels. They should be advised to continue blood sugar control postoperatively to minimize the risk of dry eye. ③ Inflammation management: For patients with elevated preoperative inflammatory markers, it is important to first address the inflammation. Postoperative monitoring of inflammatory factors should be conducted to observe changes due to surgical stress. Patients with high levels of postoperative inflammation should be encouraged to consume fiber-rich foods, including vegetables, beans, and fruits, which are rich in vitamins and trace elements that enhance anti-inflammatory properties, thereby reducing the risk of dry eyes. ④ Psychological counseling: Providing appropriate counseling before and after surgery can help alleviate negative emotions such as anxiety and depression, which are linked to an increased risk of dry eye syndrome. ⑤ Surgical technique: Smaller surgical incisions should be used to minimize the risk of postoperative dry eyes associated with incision size.
In conclusion, the risk of dry eyes after cataract surgery is associated with smoking, diabetes, elevated IL-6 levels, high HADS scores, and long surgical incisions. The nomogram prediction model developed in this study has predictive value and can guide clinical evaluations on the risk of postoperative dry eyes in cataract patients, helping to implement preventive measures. However, uncontrollable factors such as climate, living environment, and the interaction of other diseases may also affect the risk of postoperative dry eye. Additionally, this study only analyzed patients undergoing cataract surgery at a single center, limiting the generalizability of the model. Future multicenter, large-scale studies are needed to provide a more reliable risk screening model for medical personnel.
Disclosure of conflict of interest
None.
References
- 1.Huang Q, Huang Y, Luo Q, Fan W. Ocular biometric characteristics of cataract patients in western China. BMC Ophthalmol. 2018;18:99. doi: 10.1186/s12886-018-0770-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutu C, Fukuoka H, Afshari NA. Mechanisms and management of dry eye in cataract surgery patients. Curr Opin Ophthalmol. 2016;27:24–30. doi: 10.1097/ICU.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 3.Sajnani R, Raia S, Gibbons A, Chang V, Karp CL, Sarantopoulos CD, Levitt RC, Galor A. Epidemiology of persistent postsurgical pain manifesting as dry eye-like symptoms after cataract surgery. Cornea. 2018;37:1535–1541. doi: 10.1097/ICO.0000000000001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr CE, Gupta PK, Farid M, Beckman KA, Chan CC, Yeu E, Gomes JAP, Ayers BD, Berdahl JP, Holland EJ, Kim T, Mah FS ASCRS Cornea Clinical Committee. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45:669–684. doi: 10.1016/j.jcrs.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Rajavi Z, Javadi MA, Daftarian N, Safi S, Nejat F, Shirvani A, Ahmadieh H, Shahraz S, Ziaei H, Moein H, Motlagh BF, Feizi S, Foroutan A, Hashemi H, Hashemian SJ, Jabbarvand M, Jafarinasab MR, Karimian F, Mohammad-Rabei H, Mohammadpour M, Nassiri N, Panahi-Bazaz M, Rohani MR, Sedaghat MR, Sheibani K. Customized clinical practice guidelines for management of adult cataract in Iran. J Ophthalmic Vis Res. 2015;10:445–460. doi: 10.4103/2008-322X.176913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X, Chen Z, Jin L, Gao W, Qu B, Zuo Y, Liu R, Yu M. Rasch analysis of the hospital anxiety and depression scale among Chinese cataract patients. PLoS One. 2017;12:e0185287. doi: 10.1371/journal.pone.0185287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donthineni PR, Deshmukh R, Ramamurthy C, Sangwan VS, Mehta JS, Basu S. Management of cataract in dry eye disease: preferred practice pattern guidelines. Indian J Ophthalmol. 2023;71:1364–1372. doi: 10.4103/IJO.IJO_2807_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gali HE, Sella R, Afshari NA. Cataract grading systems: a review of past and present. Curr Opin Ophthalmol. 2019;30:13–18. doi: 10.1097/ICU.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 9.Lim JC, Caballero Arredondo M, Braakhuis AJ, Donaldson PJ. Vitamin C and the lens: new insights into delaying the onset of cataract. Nutrients. 2020;12:3142. doi: 10.3390/nu12103142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labetoulle M, Rousseau A, Baudouin C. Management of dry eye disease to optimize cataract surgery outcomes: two tables for a daily clinical practice. J Fr Ophtalmol. 2019;42:907–912. doi: 10.1016/j.jfo.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Zhang W, Zhu XY, Suo T, Fan XQ, Fu Y. Smoking and the risk of dry eye: a meta-analysis. Int J Ophthalmol. 2016;9:1480–1486. doi: 10.18240/ijo.2016.10.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Zhang G, Nian S, Lv Y, Shao Y, Qiao N, Liang R, Huang L, Luo A. Dry eye induced by exposure to cigarette smoke pollution: an in vivo and in vitro study. Free Radic Biol Med. 2020;153:187–201. doi: 10.1016/j.freeradbiomed.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Commun. 2004;318:32–37. doi: 10.1016/j.bbrc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Wan L, Zhou QJ, Xie LX. Research progress on the pathogenesis of diabetes-related dry eye. Zhonghua Yan Ke Za Zhi. 2022;58:1099–1105. doi: 10.3760/cma.j.cn112142-20220503-00227. [DOI] [PubMed] [Google Scholar]
- 15.Doan S, Zagorski Z, Palmares J, Yagmur M, Kaercher T, Benitez-Del-Castillo JM, Van Dooren B, Jonckheere P, Jensen PK, Maychuk DY, Bezdetko P. Eyelid disorders in ophthalmology practice: results from a large international epidemiological study in eleven countries. Ophthalmol Ther. 2020;9:597–608. doi: 10.1007/s40123-020-00268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu JH, Li L, Tian L, Zhang XY, Thomas R, Sun XG. Epithelial changes with corneal punctate epitheliopathy in type 2 diabetes mellitus and their correlation with time to healing. BMC Ophthalmol. 2018;18:1. doi: 10.1186/s12886-017-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan S, Corrales RM, Farley W, McDermott AM, Pflugfelder SC. Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye. Cornea. 2008;27:811–817. doi: 10.1097/ICO.0b013e31816bf46c. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Zou J, He L, Zhang Y. Dry eye management in a Sjogren’s syndrome mouse model by inhibition of p38-MAPK pathway. Diagn Pathol. 2014;9:5. doi: 10.1186/1746-1596-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recchioni A, Siso-Fuertes I, Hartwig A, Hamid A, Shortt AJ, Morris R, Vaswani S, Dermott J, Cervino A, Wolffsohn JS, O’Donnell C. Short-term impact of FS-LASIK and SMILE on dry eye metrics and corneal nerve morphology. Cornea. 2020;39:851–857. doi: 10.1097/ICO.0000000000002312. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Du Z, Lai C, Seth I, Wang Y, Huang Y, Fang Y, Liao H, Hu Y, Yu H, Zhang X. The association between cataract surgery and mental health in older adults: a review. Int J Surg. 2024;110:2300–2312. doi: 10.1097/JS9.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed KJ, Pilling JD, Ahmed K, Buchan J. Effect of a patient-information video on the preoperative anxiety levels of cataract surgery patients. J Cataract Refract Surg. 2019;45:475–479. doi: 10.1016/j.jcrs.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Socea SD, Abualhasan H, Magen O, Zayit-Soudry S, Blumenthal EZ, Duvdevan N, Mimouni M. Preoperative anxiety levels and pain during cataract surgery. Curr Eye Res. 2020;45:471–476. doi: 10.1080/02713683.2019.1666996. [DOI] [PubMed] [Google Scholar]
- 23.Stepp MA, Pal-Ghosh S, Tadvalkar G, Williams A, Pflugfelder SC, de Paiva CS. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp Eye Res. 2018;169:91–98. doi: 10.1016/j.exer.2018.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]