Abstract
Objective: To evaluate the clinical efficacy of recombinant human interferon α-2b (rHuINF α-2b) gel combined with loop electrosurgical excision procedure (LEEP) conization in treating cervical intraepithelial neoplasia (CIN) with comorbid high-risk human papillomavirus (HR-HPV) infection. Methods: A retrospective analysis was conducted on the clinical data of 202 CIN patients with HR-HPV infection who were treated at Wuhan Yaxin General Hospital between July 2021 and February 2024. Among these patients, 106 received treatment with rHuINF α-2b gel combined with LEEP conization (study group), and the other 96 were treated with LEEP conization alone (control group). The two groups were compared in terms of efficacy on CIN, HPV clearance, vaginal bleeding duration, hospital stay and occurrence of adverse reactions. Patient prognosis within six months post-treatment was analyzed, and the risk factors affecting prognosis were analyzed through logistic regression. Results: The control group experienced notably longer vaginal bleeding duration and hospital stay than the study group (all P<0.0001). The study group showed a notably higher cure rate of CIN than the control group and presented a notably lower rate of persistent or residual CIN than the control group (P=0.0026). Six months after treatment, the total effective HPV clearance rate in the study group was greatly higher than that in the control group (P=0.0010). However, there was no insignificant difference between the two groups in the incidence of adverse reactions (P=0.4807). According to logistic regression analysis, age, grade of CIN, course of disease and treatment regimen were independent risk factors for patient prognosis. Conclusion: For CIN patients with comorbid high HPV risk infection, rHuINF α-2b gel combined with LEEP conization can effectively treat CIN, clear HPV, and shorten the duration of vaginal bleeding and hospitalization, without increasing adverse reactions. In addition, age, grade of CIN, course of disease and treatment regimen were independent risk factors for the prognosis of patients.
Keywords: Recombinant human interferon α-2b gel, LEEP conization, cervical intraepithelial neoplasia, HR-HPV infection, clinical efficacy
Introduction
Cervical intraepithelial neoplasia (CIN) is a non-invasive precancerous lesion frequently seen in clinical scenarios, resulting from pathological changes in the cervical epithelium [1,2]. The primary cause of cervical cancer and its precancerous lesions is persistent infection of high-risk human papilloma virus (HR-HPV) [3,4]. For patients with both CIN and HR-HPV infection, early and effective treatment can lower the risk of progression to cervical cancer and protect patient’s health and life [5].
Currently, surgery is the primary choice for the therapy of CIN. Among the available options, loop electrosurgical excision procedure (LEEP) conization offers favorable short-term and long-term outcomes, with advantages of small trauma, precise excision margins, simple operation, and minimal impact on cervical function, making it the preferred choice for CIN surgery [6,7]. However, due to the high prevalence of concurrent HPV infection in CIN patients, there is a significant risk of recurrence following surgery [8]. Therefore, adjunctive pharmacological treatments are often adopted alongside LEEP conization to lower the risk of postoperative infection [9]. Recombinant human interferon α-2b (rHuINF α-2b) gel is an interferon gel preparation that can rapidly dissolve in vaginal fornix and cervix, binding to interferon receptors on the surface of target cells to effectively control HPV replication [10,11]. Previous studies have confirmed the efficacy of rHuINF α-2b in the treatment of HPV infection. For instance, He et al. [12] demonstrated that rhIFNα-2b combined with Baofukang suppository is safe and effective in the treatment of cervical HPV infection. Sun et al. [13] found that lacidophilin vaginal capsules combined with rh-IFN-α2b has better clinical effects than rh-IFN-α2b alone in HPV-infected patients. However, there is limited clinical research on the combination of rhIFNα-2b and LEEP conization in CIN with comorbid HR-HPV infection.
Accordingly, this study explored the clinical efficacy of rHuINF α-2b gel combined with LEEP conization in the treatment of CIN with comorbid HR-HPV infection, with the purpose of providing reliable reference for the follow-up treatment of the disease. The innovation of the study lies in its focus on a combination therapy approach that has not been widely studied in clinical practice. Additionally, this study analyzed patient prognosis up to six months post-treatment, providing a longer-term evaluation of treatment outcomes.
Methods and materials
Sample information and screening
A retrospective analysis was conducted on the clinical data of 280 CIN patients with comorbid HR-HPV infection treated in the Wuhan Yaxin General Hospital between July 2021 and February 2024. Based on the following inclusion and exclusion criteria, 202 cases were screened out for subsequent analysis. This study was approved by the Medical Ethics Committee of the Wuhan Yaxin General Hospital.
Inclusion criteria: married women; patients diagnosed with CIN by pathological biopsy; patients with positive HR-HPV DNA test in cervical exfoliative cytology [14]; patients who had abstained from sexual activity for more than 3 months; patients without immediate fertility requirement; patients with complete clinical data.
Exclusion criteria: patients with vaginitis due to pathogens such as Neisseria gonorrhoeae, mycoplasma, chlamydia, Candida or trichomoniasis; patients with acute pelvic inflammation or visible condyloma acuminatum; patients with cervical or endometrial cancer; patients who had taken corticosteroids, antivirals or immunomodulators locally or systemically within 3 months prior to treatment; patients with known allergies to drugs used in this study. Among the enrolled patients, 106 patients treated with rHuINF α-2b gel combined with LEEP conization were assigned to the study group, and the other 96 patients treated with LEEP conization alone were assigned to the control group. The screening and grouping process is shown in Figure 1.
Figure 1.
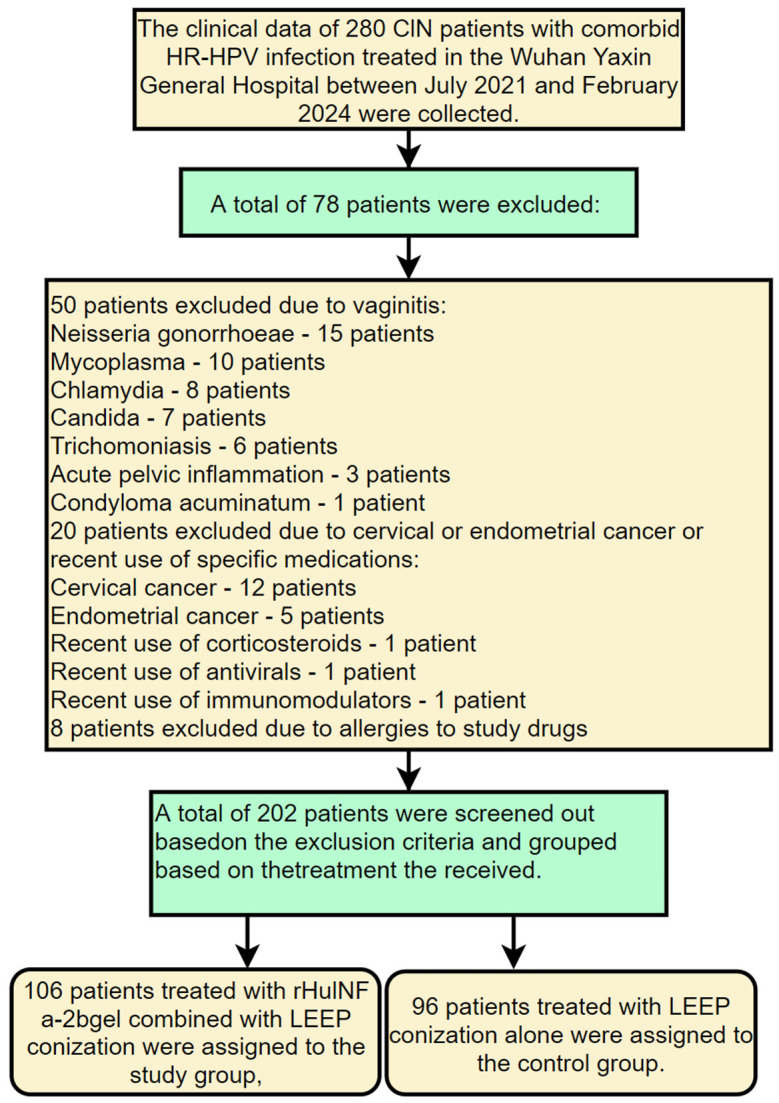
Flow chart of patient screening and grouping. CIN: cervical intraepithelial neoplasia; LEEP: loop electrosurgical excision procedure; rHuINF α-2b: recombinant human interferon α-2b.
Therapeutic regimen
Following consultation, all patients in both groups underwent cervical LEEP conization within 3-7 days after menstruation. The procedure was conducted as follows: patients received local anesthesia through lidocaine injection, followed by excision. First, a circular resection was performed to the normal tissue surrounding the lesion at a depth of 9 mm; Then, a circular resection was made around this area at a depth of 4-8 mm, with the depth adjusted appropriately based on the size and extent of the lesion; Finally, cotton balls with thread were placed in the vagina to stop bleeding. After application of moist exposed burn ointment, iodophor gauze was pressed and packed, and sensitive antibiotics were given for 5 days after operation.
The study group was additionally given rHuINF α-2b gel (Zhaoke Pharmaceutical (Hefei) Co., Ltd., specification: 5 g/piece, State Food and Drug Administration (SFDA) approval number: S20020079; approval number: 130425). Specifically, three days after LEEP conization, the iodophor gauze was removed, and SpecrHuINF α-2b gel was gently injected into the vagina with a thruster to cover the cervical surface, 1 g/time, once every other day, for 3 months except during menstruation. The treatment in the control group was the same as that of the study group except that the patients in the control group were not treated with rHuINF α-2b gel.
Outcome measures
Primary outcome measures
(1) Efficacy on CIN [15]: The efficacy of treatment on CIN was evaluated and compared between the two groups according to the following criteria: Cured: no presence of CIN at six months post-treatment; Persistent or residual lesions: presence of CIN at six months post-treatment. (2) Clearance of HPV [16]: HPV clearance was assessed and compared between the two groups according to the following criteria: Cured: all HR-HPV subtype tests were completely negative; Effective: some HR-HPV subtype tests were negative, but the result of 1 kind of HR-HPV subtype test or more was positive; Ineffective: none of the HR-HPV subtype tests turned negative or the number of positive subtypes increased. Total effective clearance rate = (the number of cured cases + effective cases)/total number of cases ×100%.
Secondary outcome measures
(1) Vaginal bleeding time and hospital stay: The duration of vaginal bleeding and length of hospital stay were compared between the two groups; (2) Factors impacting the prognosis: Patient prognosis was assessed within half a year after treatment, and logistic regression was used to analyze the risk factors affecting prognosis. A favorable prognosis was defined as effective CIN treatment with either complete clearance of HPV or an effective reduction in HPV presence. Conversely, an unfavorable prognosis was defined as either ineffective CIN treatment or persistence of HPV infection. (3) Adverse reactions: Adverse reactions during treatment, including infection, bleeding and cervical orifice contraction, were evaluated and compared between the two groups.
Statistical analyses
SPSS20.0 software package was used for statistical analysis of the collected data, and GraphPad 8 software package was used for data visualization. Measurement data following normal distribution were described by mean ± standard deviation (mean ± SD). Their inter-group comparison was conducted using the independent-samples t-test and their intra-group comparison was performed using the paired t-test. Enumeration data were described by rate (%), analyzed using the chi-square test (χ2). The risk factors affecting the patients’ prognosis were identified using logistic regression analysis. P<0.05 was indicative of statistical difference.
Results
Baseline data
Comparison of baseline data revealed no significant differences in age, body mass index (BMI), CIN grade, number of pregnancies and deliveries, course of disease, family history of cervical cancer, first occurrence of CIN and place of residence between the two groups (all P>0.05, Table 1).
Table 1.
Comparison of baseline data between the two groups
| Factors | Study group (n=106) | Control group (n=96) | χ2 | P value |
|---|---|---|---|---|
| Age | 1.0581 | 0.3037 | ||
| ≥45 years old | 65 | 52 | ||
| <45 years old | 41 | 44 | ||
| BMI | 0.1846 | 0.6674 | ||
| ≥23 kg/m2 | 54 | 46 | ||
| <23 kg/m2 | 52 | 50 | ||
| Grade of cervical intraepithelial neoplasia | 3.4111 | 0.0648 | ||
| Grade I-II | 81 | 62 | ||
| Grade III | 25 | 34 | ||
| Number of pregnancies | 0.9393 | 0.3325 | ||
| ≥2 times | 58 | 59 | ||
| <2 times | 48 | 37 | ||
| Number of deliveries | 0.1587 | 0.6903 | ||
| ≥2 times | 46 | 39 | ||
| <2 times | 60 | 57 | ||
| Course of disease | 1.7261 | 0.1889 | ||
| ≥28 d | 66 | 51 | ||
| <28 d | 40 | 45 | ||
| Family history of cervical cancer | 1.4151 | 0.2343 | ||
| Yes | 21 | 13 | ||
| No | 85 | 83 | ||
| First occurrence of cervical intraepithelial neoplasia | 2.4681 | 0.1162 | ||
| Yes | 80 | 81 | ||
| No | 26 | 15 | ||
| Place of residence | 0.2632 | 0.6079 | ||
| Urban areas | 35 | 35 | ||
| Rural areas | 71 | 61 |
BMI: body mass index.
Vaginal bleeding duration and hospital stay of the two groups
The study group experienced an average vaginal bleeding duration of 8.71 ± 1.78 days, while the control group experienced and average duration of 12.74 ± 1.93 days (P<0.0001). The average hospital stay for the study group was 4.95 days, significantly shorter than 7.07 days in the control group (P<0.0001) (Figure 2).
Figure 2.
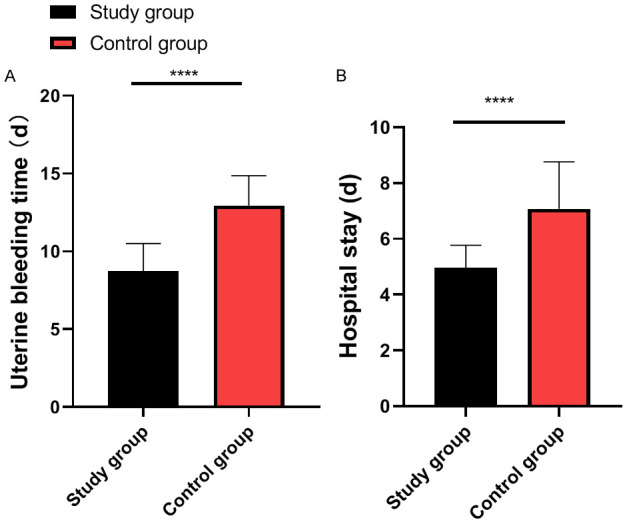
Comparison of vaginal bleeding duration and hospital stay between the two groups. A. Comparison of vaginal bleeding duration between the two groups (****P<0.0001). B. Comparison of hospital stay between the two groups (****P<0.0001).
Efficacy on CIN in the two groups
In the study groups, 97 patients were cured and 9 patients had persistent or residual CIN, while in the control groups, 73 patients were cured and 23 had persistent or residual CIN. Analysis of treatment efficacy on CIN in the two groups revealed a notably higher cure rate of CIN in the study group than that in the control group (P=0.0026, Figure 3).
Figure 3.
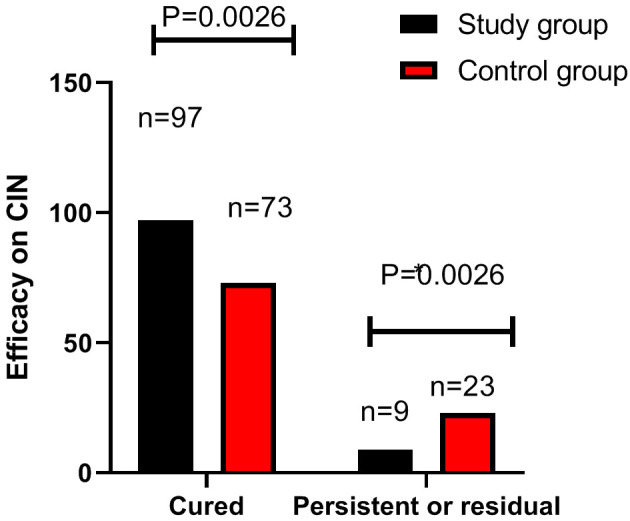
Comparison of treatment efficacy on CIN between the two groups. Note: CIN: cervical intraepithelial neoplasia.
Clearance of HPV in the two groups
In terms of HPV clearance, there were 57 cured patients, 42 effective cases and 7 ineffective cases in the study group, while the control group had 44 cured patients, 30 effective cases and 22 infective cases. Analysis of the HPV clearance rate revealed a notably higher total effective clearance rate in the study group than that in the control group (P=0.0010, Table 2).
Table 2.
Comparison of HPV clearance between the two groups [n (%)]
| Group | Cured | Effective | Ineffective | Overall response |
|---|---|---|---|---|
| Study group (n=106) | 57 (53.77) | 42 (39.62) | 7 (6.61) | 99 (90.83) |
| Control group (n=96) | 44 (45.83) | 30 (31.25) | 22 (22.92) | 74 (77.08) |
| χ2 | 1.2701 | 1.5391 | 10.901 | 10.901 |
| P value | 0.2597 | 0.2147 | 0.0010 | 0.0010 |
Incidence of adverse reactions in the two groups
Infection, bleeding and cervical orifice contraction occurred in 4, 3 and 2 patients, respectively, in the study group, and 4, 5, and 2 patients, respectively, in the control group. Statistical analysis revealed an insignificant difference between the two group in the total incidence of adverse reactions (P=0.4807, Table 3).
Table 3.
Comparison of incidence of adverse reactions between the two groups [n (%)]
| Group | Infection | Bleeding | Cervical orifice contraction | Total adverse reaction |
|---|---|---|---|---|
| Study group (n=106) | 4 (3.77) | 3 (2.83) | 2 (1.89) | 9 (8.49) |
| Control group (n=96) | 4 (4.17) | 5 (5.21) | 2 (2.08) | 11 (11.46) |
| χ2 | 0.0205 | 0.7491 | 0.0100 | 0.4974 |
| P value | 0.8862 | 0.3868 | 0.9202 | 0.4807 |
Analysis of related factors affecting prognosis
In this study, there were 131 patients with favorable prognosis (favorable prognosis group) and 71 patients with unfavorable prognosis (unfavorable prognosis group). Univariate analysis revealed that age, CIN grade, number of pregnancy, course of disease, and treatment regimen were associated with the patients’ prognosis (Table 4). These indicators with notable differences were assigned with values (Table 5) and subjected to multivariate analysis. Multivariate logistic regression analysis identified age (P=0.037, OR: 2.000; CI: 1.041-3.842), CIN grade (P=0.005; OR: 2.58; CI: 1.323-5.045), course of disease (P=0.001, OR: 3.019; CI: 1.556-5.858), and treatment regimen (P=0.021; OR: 2.098; CI: 1.121-3.927) as independent risk factors affecting the prognosis of patients (Table 6).
Table 4.
Univariate analysis of factors affecting patient prognosis
| Factors | Favorable prognosis group (n=131) | Unfavorable prognosis group (n=71) | χ2 | P value |
|---|---|---|---|---|
| Age | 5.5281 | 0.0187 | ||
| ≥45 years old | 68 | 49 | ||
| <45 years old | 63 | 22 | ||
| BMI | 2.3031 | 0.1291 | ||
| ≥23 kg/m2 | 70 | 30 | ||
| <23 kg/m2 | 61 | 41 | ||
| Grade of cervical intraepithelial neoplasia | 7.1701 | 0.0074 | ||
| Grade I-II | 101 | 42 | ||
| Grade III | 30 | 29 | ||
| Number of pregnancies | 31.751 | <0.0001 | ||
| ≥2 times | 57 | 60 | ||
| <2 times | 74 | 11 | ||
| Number of deliveries | 2.3391 | 0.1261 | ||
| ≥2 times | 50 | 35 | ||
| <2 times | 81 | 36 | ||
| Course of disease | 10.541 | 0.0012 | ||
| ≥28 d | 65 | 52 | ||
| <28 d | 66 | 19 | ||
| Family history of cervical cancer | 2.5441 | 0.1107 | ||
| Yes | 18 | 16 | ||
| No | 113 | 55 | ||
| First occurrence of cervical intraepithelial neoplasia | 0.3390 | 0.5604 | ||
| Yes | 106 | 55 | ||
| No | 25 | 16 | ||
| Place of residence | 2.7931 | 0.0947 | ||
| Urban areas | 40 | 30 | ||
| Rural areas | 91 | 41 | ||
| Therapeutic regimen | 4.5871 | 0.0322 | ||
| Recombinant human interferon α-2b gel + LEEP conization | 76 | 30 | ||
| LEEP conization | 55 | 41 |
BMI: body mass index; LEEP: loop electrosurgical excision procedure.
Table 5.
Assignment
| Factors | Assignment |
|---|---|
| Age | <45 years old =0, ≥45 years old =1 |
| Grade of cervical intraepithelial neoplasia | Grade I-II =0, Grade III =1 |
| Number of pregnancies | <2 times =0, ≥2 times =1 |
| Course of disease | <28 d =0, ≥28 d =1 |
| Therapeutic regimen | Recombinant human interferon α-2b gel + LEEP conization =0, LEEP conization =1 |
| Prognosis | Favorable prognosis =0, unfavorable prognosis =1 |
Table 6.
Multivariate logistic regression analysis of factors affecting patient prognosis
| Factors | B | S.E. | Wals | df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Age | 0.693 | 0.333 | 4.334 | 1 | 0.037 | 2.000 | 1.041 | 3.842 |
| Grade of cervical intraepithelial neoplasia | 0.949 | 0.341 | 7.732 | 1 | 0.005 | 2.584 | 1.323 | 5.045 |
| Number of pregnancies | 0.442 | 0.323 | 1.875 | 1 | 0.171 | 1.555 | 0.826 | 2.927 |
| Course of disease | 1.105 | 0.338 | 10.672 | 1 | 0.001 | 3.019 | 1.556 | 5.858 |
| Therapeutic regimen | 0.741 | 0.320 | 5.363 | 1 | 0.021 | 2.098 | 1.121 | 3.927 |
Discussion
Cervical intraepithelial neoplasia (CIN) is a common gynecological disease, classified as a cervical precancerous lesion. Without timely treatment, it can progress to cervical cancer, significantly impacting patients’ quality of life and reducing survival time [17-19]. Therapies for CIN with comorbid high-risk human papillomavirus (HR-HPV) infection is increasingly shifting toward more conservative surgical method [20,21]. LEEP conization is a frequently-seen surgical method in clinical practice [22]; however, LEEP conization alone may not completely remove all lesion tissues, potentially leaving residual CIN and HPV lesions that can lead to disease recurrence [23]. Recombinant human interferon rHu IFN-FN is a protein secreted by host cells in response to viral stimulation [24]. It can quickly infect the cervical and vaginal fornices, enters the bloodstream, and binds to interferon receptors on target cell surfaces. This action promotes cellular resistance to virus, inhibits HPV replication, enhances the phagocytosis of mononuclear phagocytes, and boosting the body’s immune response [13,25]. This study evaluated the clinical efficacy of rHuINF α-2b gel combined with LEEP conization in the treatment of CIN with comorbid HR-HPV infection.
Shorter vaginal bleeding time is an important factor for postoperative recovery and patient comfort [26]. The study demonstrated that patients who received the combination therapy experienced a notable reduction in the duration of vaginal bleeding compared to those who underwent LEEP conization alone. This finding suggests that additional application of rHuINF α-2b gel can promote faster healing and tissue regeneration, leading to a more favorable recovery period. Likewise, the study revealed a significant decrease in hospital stay for the study group, indicating that the combination therapy may facilitate a more efficient and accelerated recovery process, allowing for earlier discharge and reducing the overall healthcare burden. Shorter hospital stays not only benefit patient well-being but also have economic advantages by optimizing healthcare resource utilization [27]. The reductions in vaginal bleeding duration and hospital stay can be attributed to the immunomodulatory effects of rHuINF α-2b gel. Interferon α-2b has been shown to enhance the immune response, promote tissue healing, and inhibit viral replication [28,29]. The combination of rHuINF α-2b gel and LEEP conization may have synergistic effects on reducing inflammation, promoting wound healing, and suppressing viral activity, leading to faster recovery and improved clinical outcomes. In addition, this study found that the cure rate of CIN and the total effective clearance rate of HPV were significantly higher in the study group than in the control group six months after treatment. These results imply that rHuINF α-2b gel combined with LEEP conization is more effective than LEEP conization alone in treating CIN with comorbid HR-HPV infection. Zhang et al. [30] have found that thin LEEP can accelerate the clearance of HR-HPV infection, contributing to a rapid decline in HPV infection in the first year, suggesting that LEEP conization is effective in eliminating HR-HPV infection. These findings are clinically significant as achieving a high cure rate of CIN and effective clearance of HPV are essential goals in the management of patients with CIN and HR-HPV infection [31]. Successful treatment outcomes, such as CIN cure and HPV clearance, are associated with a reduced risk of disease progression, recurrence, and the development of cervical cancer [32]. Moreover, the combination of rHuINF α-2b gel with interferon receptor can inhibit HPV replication, control disease progression, reduce inflammatory factors, enhance immunity, and regulate the secretion of estrogen and progesterone. This improves the vaginal environment, accelerates the healing of cervical wounds, and relieves clinical symptoms [33]. In this study, the analysis of adverse reactions revealed that the study group showed an insignificantly lower incidence of adverse reactions than the control group, indicating that the addition of rHuINF α-2b gel did not increase the adverse reactions and may even help reduce the incidence.
At the end of the study, we identified age, grade of CIN, course of disease, and therapeutic regimen as independent risk factors for unfavorable prognosis through logistic regression analysis. Age is a commonly recognized factor in the development and progression of CIN [34]. Our study found that age significantly impacted the patient prognosis, highlighting its relevance in evaluating treatment outcomes and disease progression. The grade of CIN, as determined by histopathological examination, was identified as an independent risk factor affecting the prognosis of patients. This finding aligns with previous research indicating that higher grades of CIN, such as CIN 2 and CIN 3, are associated with an increased risk of disease persistence and progression to cervical cancer [35]. The number of pregnancies and the course of disease may reflect the impact of hormonal and physiological changes during pregnancy and the duration of disease on the progression and regression of CIN lesions [1]. Additionally, the therapeutic regimen was found to significantly affect patient prognosis, suggesting that the combination therapy may offer superior outcomes compared to LEEP conization alone.
Through retrospective analysis, this study confirmed the clinical efficacy of rHuINF α-2b gel combined with LEEP conization in the treatment of CIN with comorbid HR-HPV infection. However, the use of this combined treatment has been limited in clinical practice. Several factors may explain its restricted application in the past. Firstly, there is a lack of sufficient evidence demonstrating its safety, efficacy, and superiority over existing treatment options. Additionally, the absence of recommendations or inclusion in treatment guidelines by medical societies and organizations might have hindered its widespread acceptance. Furthermore, the cost and accessibility of rHuINF α-2b gel could pose barriers, particularly in resource-constrained settings. As research advances, treatment guidelines evolve, and awareness increases, the acceptance and utilization of this combined therapy are likely to expand.
The study has several limitations. First of all, the limited sample size may introduce some bias into the conclusion of the study, increasing the risk of false associations. This highlights the need for cautious interpretation of the results and further validation with larger sample sizes. In addition, this study does not provide data on the long-term prognosis of patients, so the effect of rHuINF α-2b gel combined with LEEP conization on the long-term outcomes of CIN patients with HR-HPV infection remains uncertain and requires further investigation. Therefore, we hope to conduct a more comprehensive analysis in the future to obtain more robust and effective results.
Conclusion
To sum up, for CIN patients with comorbid high HPV infection, rHuINF α-2b gel combined with LEEP conization is an effective treatment approach that can clear HPV, and shorten the duration of vaginal bleeding and hospitalization, without increasing adverse reactions. In addition, age, grade of CIN, course of disease, and therapeutic regimen can serve as independent risk factors for the prognosis of patients.
Disclosure of conflict of interest
None.
References
- 1.Curty G, de Carvalho PS, Soares MA. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int J Mol Sci. 2019;21:222. doi: 10.3390/ijms21010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mello V, Sundstrom RK. Cervical Intraepithelial Neoplasia. In: StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Renee Sundstrom declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024. [PubMed] [Google Scholar]
- 3.Zhou Y. Investigation of the clinical application value of HR-HPV DNA combined with liquid based cytology in colposcopy of cervical cancer. Contrast Media Mol Imaging. 2022;2022:5054507. doi: 10.1155/2022/5054507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Kusakabe M, Taguchi A, Sone K, Mori M, Osuga Y. Carcinogenesis and management of human papillomavirus-associated cervical cancer. Int J Clin Oncol. 2023;28:965–974. doi: 10.1007/s10147-023-02337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le D, Coriolan Ciceron A, Jeon MJ, Gonzalez LI, Jordan JA, Bordon J, Long B. Cervical cancer prevention and high-risk HPV self-sampling awareness and acceptability among women living with HIV: a qualitative investigation from the patients’ and providers’ perspectives. Curr Oncol. 2022;29:516–533. doi: 10.3390/curroncol29020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Jiang Y, Ding J, Xia L, Xu H. Clinical predictors of residual disease in hysterectomy following a loop electrosurgical excision procedure for cervical intraepithelial neoplasia grade 3. BMC Pregnancy Childbirth. 2022;22:971. doi: 10.1186/s12884-022-05281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez SI, Lutzkanin A. Management of cervical dysplasia using office loop electrosurgical excision procedure. Prim Care. 2021;48:583–595. doi: 10.1016/j.pop.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Ma MJ, Wang YN, Zhu JF, Wang XY, Jin YB, Liu XN, Wu SF, Yang YB. Characterization of HPV subtypes not covered by the nine-valent vaccine in patients with CIN 2-3 and cervical squamous cell carcinoma. Curr Probl Cancer. 2021;45:100761. doi: 10.1016/j.currproblcancer.2021.100761. [DOI] [PubMed] [Google Scholar]
- 9.Przybylski M, Pruski D, Millert-Kalinska S, Zmaczynski A, Baran R, Horbaczewska A, Jach R, Zaborowska L. Remission of HPV infection after LEEP-conization - a retrospective study. Ginekol Pol. 2022 doi: 10.5603/GP.a2021.0164. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Li R, Hu Y, Wu MZ, Chen K. Guttate psoriasis induced by interferon alfa-2b suppository treatment for high-grade cervical intraepithelial neoplasia. Dermatol Ther. 2022;35:e15834. doi: 10.1111/dth.15834. [DOI] [PubMed] [Google Scholar]
- 11.Misson DR, Abdalla DR, Borges AM, Shimba DS, Adad SJ, Michelin MA, Murta EF. Cytokine serum levels in patients with cervical intraepithelial neoplasia grade II-III treated with intralesional interferon-alpha 2b. Tumori. 2011;97:578–584. doi: 10.1177/030089161109700507. [DOI] [PubMed] [Google Scholar]
- 12.He C, Song C, Li M, Ma W, Sun S. Meta-analysis of the effect and safety of recombinant human interferon α-2b combined with Baofukang suppository in the treatment of HPV infection. Am J Transl Res. 2022;14:7632–7642. [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Xu J, Zhou H, You L, Zhu Y. Influence of lacidophilin vaginal capsules plus rh-IFN-α2b on efficacy, vaginal microecology, and safety of patients with HPV infection. Evid Based Complement Alternat Med. 2022;2022:3632053. doi: 10.1155/2022/3632053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tifaoui N, Maudelonde T, Combecal J, Vallo R, Doutre S, Didelot MN, Nagot N, Segondy M, Boulle N. High-risk HPV detection and associated cervical lesions in a population of French menopausal women. J Clin Virol. 2018;108:12–18. doi: 10.1016/j.jcv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim Khalil A, Zhang L, Muwonge R, Sauvaget C, Basu P. Efficacy and safety of therapeutic HPV vaccines to treat CIN 2/CIN 3 lesions: a systematic review and meta-analysis of phase II/III clinical trials. BMJ Open. 2023;13:e069616. doi: 10.1136/bmjopen-2022-069616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y, Li Q, Ke X, Zhang Y, Shen X, Wang W, Shi Q, Li C. Clearance of HR-HPV within one year after focused ultrasound or loop electrosurgical excision procedure in patients with HSIL under 30. Int J Hyperthermia. 2022;39:15–21. doi: 10.1080/02656736.2021.2010817. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Jiang Y, Liang Y, Wei L, Zhang W, Li L. Observation of the cervical microbiome in the progression of cervical intraepithelial neoplasia. BMC Cancer. 2022;22:362. doi: 10.1186/s12885-022-09452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempster-Rivett K, Innes CR, Simcock BJ, Harker D, Williman JA, Van Der Griend RA, Whitehead M, Hibma M, Lawton BA, Fitzgerald P, Dudley NM, Petrich S, Faherty J, Bergzoll C, Eva L, Sadler L, Pather S, Wrede CD, Sykes PH. Evaluation of guidelines for observational management of cervical intraepithelial neoplasia 2 in young women. Am J Obstet Gynecol. 2020;223:408.e1–408.e11. doi: 10.1016/j.ajog.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Wen TM, Xu XQ, Zhao XL, Pan CH, Feng YS, You TT, Gao M, Hu SY, Zhao FH. Efficacy and immunogenicity of AS04-HPV-16/18 vaccine in females with existing cervical HR-HPV infection at first vaccination: a pooled analysis of four large clinical trials worldwide. Int J Cancer. 2024;154:2075–2089. doi: 10.1002/ijc.34882. [DOI] [PubMed] [Google Scholar]
- 20.Sand FL, Frederiksen K, Kjaer SK. Risk of recurrent disease following conization of cervical intraepithelial neoplasia grade 3 according to post-conization HPV status and surgical margins. Gynecol Oncol. 2022;165:472–477. doi: 10.1016/j.ygyno.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Pruski D, Przybylski M, Millert-Kalinska S, Zmaczynski A, Jach R. Histopathological discrepancies between colposcopy-directed biopsy and LEEP-conization observed during SARS-CoV-2 pandemic. Ginekol Pol. 2023;94:12–18. doi: 10.5603/GP.a2022.0081. [DOI] [PubMed] [Google Scholar]
- 22.Ding T, Li L, Duan R, Chen Y, Yang B, Xi M. Risk factors analysis of recurrent disease after treatment with a loop electrosurgical excision procedure for high-grade cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2023;160:538–547. doi: 10.1002/ijgo.14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto V, Dellino M, Santarsiero CM, Cormio G, Loizzi V, Griseta V, Vimercati A, Cazzato G, Cascardi E, Cicinelli E. Ultrasound control of cervical regeneration after large loop excision of the transformation zone: results of an innovative measurement technique. Diagnostics (Basel) 2023;13:791. doi: 10.3390/diagnostics13040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong F, Wang Q, Wu GH, Liu WZ, Wang B, Chen YJ. Direct and indirect effects of IFN-α2b in malignancy treatment: not only an archer but also an arrow. Biomark Res. 2022;10:69. doi: 10.1186/s40364-022-00415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding W, Li X, Ji B, Wang Z. Functions of Traditional Chinese Medicine combined with recombinant human interferon α2b in cervical intraepithelial neoplasias patients. Evid Based Complement Alternat Med. 2021;2021:6881720. doi: 10.1155/2021/6881720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Lu Y, Liu H, Yang C. The effect of preoperative oral carbohydrate on the time to colostrum and amount of vaginal bleeding after elective cesarean section. J Obstet Gynaecol Res. 2022;48:2534–2540. doi: 10.1111/jog.15375. [DOI] [PubMed] [Google Scholar]
- 27.Asfari A, Borasino S, Mendoza E, Hock KM, Huskey JL, Rahman AKMF, Zaccagni H, Byrnes JW. Risk factors for long post-operative hospital stays after cardiopulmonary bypass surgery in full-term neonates. Cardiol Young. 2023;33:2487–2492. doi: 10.1017/S1047951123000379. [DOI] [PubMed] [Google Scholar]
- 28.Lewczuk N, Zdebik A, Bogusławska J. Interferon alpha 2a and 2b in ophthalmology: a review. J Interferon Cytokine Res. 2019;39:259–272. doi: 10.1089/jir.2018.0125. [DOI] [PubMed] [Google Scholar]
- 29.Ye YZ, Dou YL, Hao JH, Zhou L, Lin AW, Wang SN, Deng JK, Lei M, Luo RP, Liao YN, Chen Y, Long YY, Chen BQ, Yang Z, Gan L, Nong GM, Yan WL, Yu H. Efficacy and safety of interferon α-2b spray for herpangina in children: a randomized, controlled trial. Int J Infect Dis. 2021;107:62–68. doi: 10.1016/j.ijid.2021.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Gong X, Wu Q, Liu Y, Lao G, Xiao J, Yang L, Liu P, Ma C. The clearance of high-risk human papillomavirus is sooner after thin loop electrosurgical excision procedure (t-LEEP) J Invest Surg. 2019;32:560–565. doi: 10.1080/08941939.2018.1483449. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Xu Y, Zhang Z, Xiong Z, Wu D. 5-aminolevulinic acid-mediated photodynamic therapy effectively ameliorates HPV-infected cervical intraepithelial neoplasia. Am J Transl Res. 2022;14:2443–2451. [PMC free article] [PubMed] [Google Scholar]
- 32.Cang W, Gu L, Hong Z, Wu A, Di W, Qiu L. Effectiveness of photodynamic therapy with 5-aminolevulinic acid on HPV clearance in women without cervical lesions. Photodiagnosis Photodyn Ther. 2021;34:102293. doi: 10.1016/j.pdpdt.2021.102293. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen HDT, Le TM, Lee E, Lee D, Choi Y, Cho J, Park NJ, Chong GO, Seo I, Han HS. Relationship between human papillomavirus status and the cervicovaginal microbiome in cervical cancer. Microorganisms. 2023;11:1417. doi: 10.3390/microorganisms11061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannella L, Giorgi Rossi P, Delli Carpini G, Di Giuseppe J, Bogani G, Gardella B, Monti E, Liverani CA, Ghelardi A, Insinga S, Raspagliesi F, Spinillo A, Vercellini P, Roncella E, Ciavattini A. Age-related distribution of uncommon HPV genotypes in cervical intraepithelial neoplasia grade 3. Gynecol Oncol. 2021;161:741–747. doi: 10.1016/j.ygyno.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, Elliss-Brookes L, Sasieni P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–2092. doi: 10.1016/S0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]


