Abstract
1. Simultaneous patch clamp and fura-2 fluorescence measurements were used to study ATP-evoked membrane currents and intracellular [Ca2+] ([Ca2+]i) changes in rat megakaryocytes. 2. At negative potentials, under conditions that blocked K+ currents, 20 microM ATP activated a biphasic inward current and a concurrent biphasic increase in [Ca2+]i. The initial [Ca2+]i increase was due to Ca2+ influx whereas the delayed (1.70 +/- 0.13 s, mean +/- S.D.) increase was at least partly due to the release of internal Ca2+ stores. 3. The initial current was activated within 100 ms, inactivated within 1-4 s and was carried by both Na+ and Ca2+. 4. The delayed current was also transient and carried mainly by Na+ when Ca2+ buffering in the pipette was low. This Na+ conductance did not require an increase in [Ca2+]i for activation, but was triggered by inositol 1,4,5-trisphosphate (IP3), or a metabolite of IP3. 5. Buffering of [Ca2+]i changes with BAPTA revealed a third current activated by Ca2+ release from internal stores. This channel was selective for divalent cations with the permeability sequence Ca2+ >> Ba2+ > Mn2+, Mg2+. 6. Adenosine-5'-O-3-thiotriphosphate (ATP gamma S), like ATP, evoked all three influx currents, whereas ADP only stimulated Ca2+ release and the two currents associated with it. Increasing the external divalent cation concentration abolished the ATP-evoked Ca2+ release and delayed currents but not the initial transient current. 7. We conclude that rat megakaryocytes express two types of purinergic receptor. One type, activated by ATP, is closely coupled to a non-selective cation channel.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

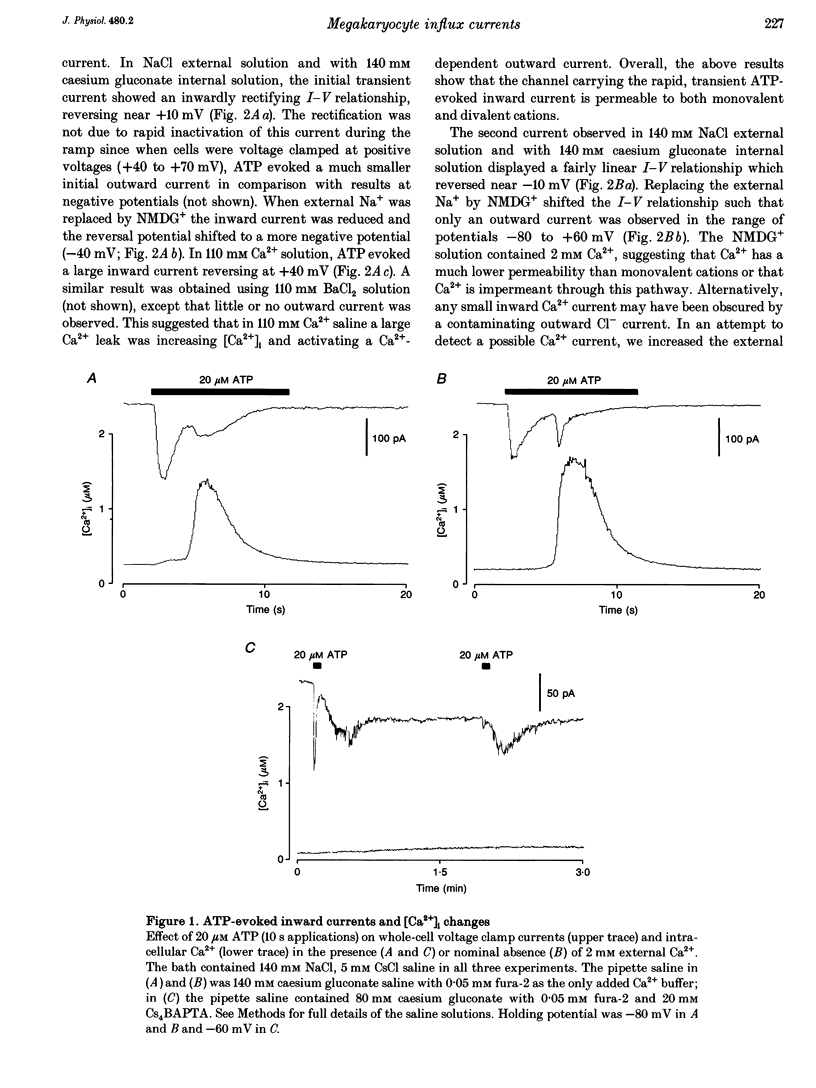
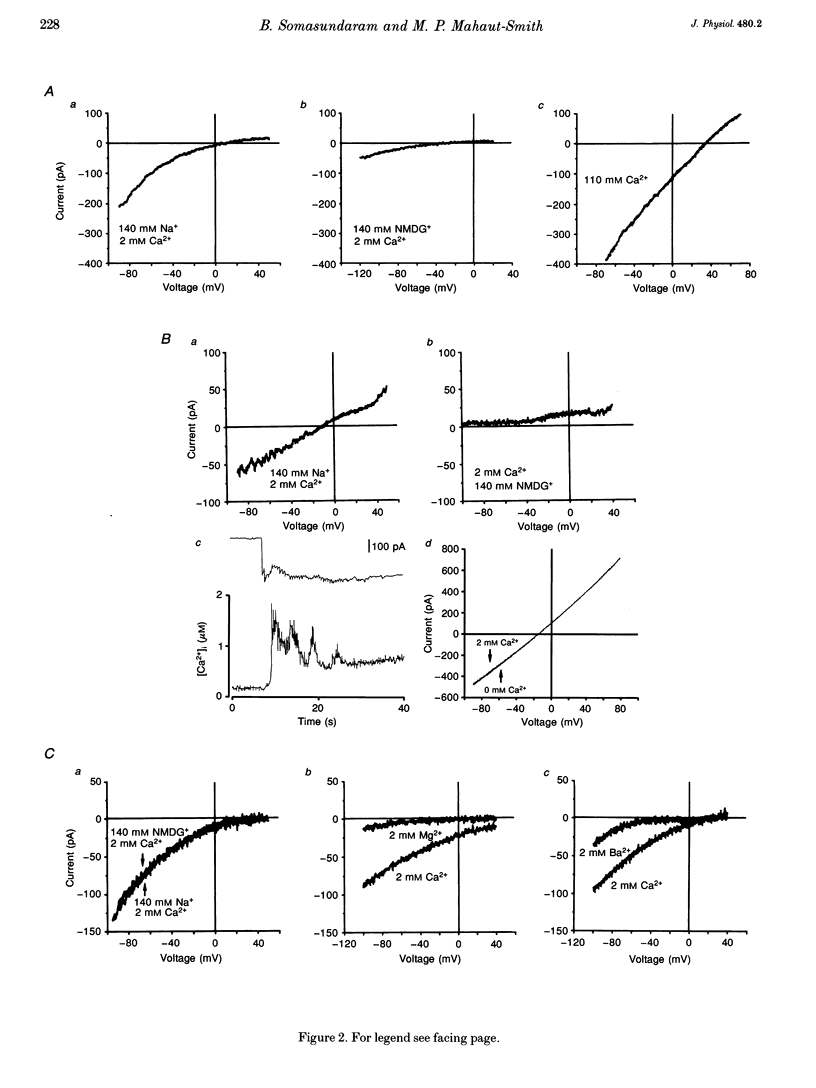

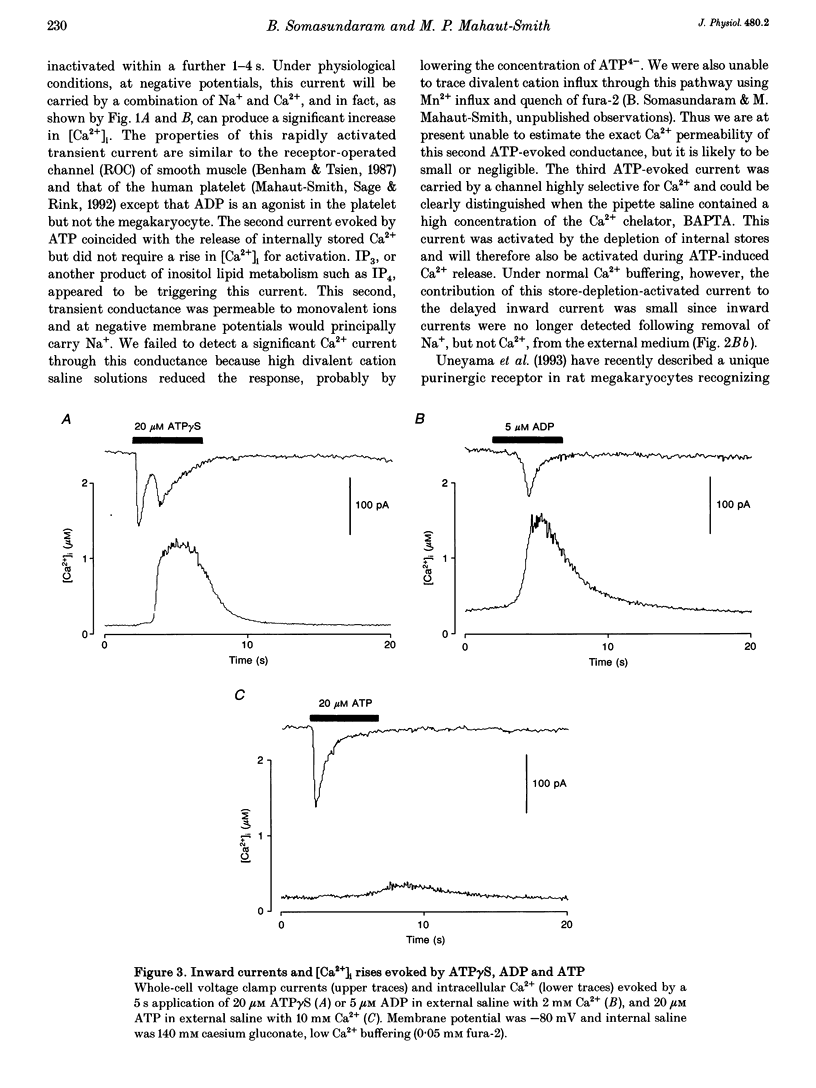

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hourani S. M., Hall D. A. Receptors for ADP on human blood platelets. Trends Pharmacol Sci. 1994 Apr;15(4):103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Kawa K. Voltage-gated calcium and potassium currents in megakaryocytes dissociated from guinea-pig bone marrow. J Physiol. 1990 Dec;431:187–206. doi: 10.1113/jphysiol.1990.sp018326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leven R. M., Mullikin W. H., Nachmias V. T. Role of sodium in ADP- and thrombin-induced megakaryocyte spreading. J Cell Biol. 1983 May;96(5):1234–1240. doi: 10.1083/jcb.96.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Sage S. O., Rink T. J. Rapid ADP-evoked currents in human platelets recorded with the nystatin permeabilized patch technique. J Biol Chem. 1992 Feb 15;267(5):3060–3065. [PubMed] [Google Scholar]
- Miller J. L. Characterization of the megakaryocyte secretory response: studies of continuously monitored release of endogenous ATP. Blood. 1983 May;61(5):967–972. [PubMed] [Google Scholar]
- Uneyama C., Uneyama H., Akaike N. Cytoplasmic Ca2+ oscillation in rat megakaryocytes evoked by a novel type of purinoceptor. J Physiol. 1993 Oct;470:731–749. doi: 10.1113/jphysiol.1993.sp019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Fay F. S. Intracellular calibration of the fluorescent calcium indicator Fura-2. Cell Calcium. 1990 Feb-Mar;11(2-3):75–83. doi: 10.1016/0143-4160(90)90061-x. [DOI] [PubMed] [Google Scholar]


