Abstract
Objective: To evaluate the biomechanical performance of polyetheretherketone (PEEK) and titanium alloys in a base-type fixation system for mandibular defect reconstruction following partial resection due to tumors, trauma, or cancer, using finite element analysis. Methods: Finite element analysis was conducted to simulate a fixation system made from titanium alloys, including pure titanium (Ti), Ti6Al4V (TC4), and Ti35Nb7Zr5Ta (TiNb), as well as PEEK materials, including unmodified PEEK, glass fiber-reinforced PEEK (GFR-PEEK), and carbon fiber-reinforced PEEK (CFR-PEEK). The biomechanical performance of these materials was compared. Results: The PEEK-based fixation systems generated higher maximum stress on the mandible and fibula compared to the titanium alloy systems, particularly with the GFR-PEEK fixation system. When loaded in the anterior region, the maximum stress on the mandible exceeded its yield strength, indicating that both PEEK and GFR-PEEK are unsuitable for mandibular defect repair. CFR-PEEK, however, exhibited more favorable stress distribution, making it a better candidate for these applications. Conclusion: Prolonged low stress on the bone may result in resorption or degeneration. Among the materials analyzed, CFR-PEEK demonstrated the most stable performance, suggesting it as the optimal choice for mandibular defect repair in fixation systems.
Keywords: Mandibular reconstruction, finite element analysis, titanium alloy, polyetheretherketone, fixation system
Introduction
The mandible, as the only movable bone in the skull, plays a critical role in essential facial functions [1]. In clinical practice, partial mandibulectomy may be required due to conditions such as tumors, trauma, or cancer. To reconstruct mandibular defects following partial resection, initial fixation between the fibula and mandible is commonly achieved using fixation plates [2,3]. The materials selected for these implantable devices must meet specific criteria, including non-toxicity, excellent biocompatibility, and an elastic modulus similar to that of bone tissue.
Metals, alloys, and polymers are the primary materials used in fixation systems for mandibular defect reconstruction. Titanium and its alloys are particularly favored due to their elastic modulus, which closely approximates that of bone tissue, making them suitable for a wide range of applications supported by extensive research. For instance, Park et al. [4] investigated the stability of various titanium alloy fixation plates in mandibular defect reconstruction. Clinical studies by Sohmura et al. [5] have reported common complications such as inflammation, loosening, fractures, and bone resorption around implants. Vollmer et al. [6] also found that the most frequently encountered issue with titanium plates in mandibular reconstruction is loosening. Yoda et al. [7] analyzed the healing conditions of bone interfaces after titanium plate implantation, noting that discrepancies between implants and the surrounding bone tissue can significantly impact postoperative healing and reconstruction.
With advancements in the field, the integration of computer-aided design and manufacturing (CAD/CAM) into surgical treatment planning has become increasingly common [8]. Lee et al. [9] used CAD technologies to customize titanium plates for mandibular defect models in rabbits and conducted a comparative study on their impact on food intake and screw loosening. Their findings suggested that CAD/CAM-guided mandibular reconstruction can facilitate faster recovery.
Despite the widespread use of titanium and its alloys, the high incidence of complications associated with titanium plate implantation has prompted researchers to explore alternative reconstruction materials. Polyetheretherketone (PEEK), a polymeric material, has gained attention in orthopedics [10]. Kang et al. [11] investigated the biomechanical properties of PEEK reconstruction plates under various chewing conditions using finite element analysis, assessing their safety and stability. Although the elastic modulus of PEEK is closer to that of bone tissue compared to titanium alloys, its overall stiffness is relatively low. The mechanical properties of PEEK can be improved by incorporating rigid fibers [12-14].
The two most commonly used fiber-reinforced PEEK composite materials are glass fiber-reinforced polyetheretherketone (GFR-PEEK) and carbon fiber-reinforced polyetheretherketone (CFR-PEEK). Kurtz et al. [15] demonstrated that when the volume fraction of reinforcing fibers is approximately 30%, PEEK composite materials exhibit an elastic modulus that closely resembles that of human bone tissue.
Previously, we reported the design of a novel base-fixation system for mandibular defect repair, demonstrating its superiority over the traditional buccal-fixation system [16]. In this study, we aimed to further our research by determining the optimal material for this base-fixation system. Mandibular reconstruction was performed using the three-dimensional modeling software Mimics 21.0, and finite element analysis was conducted with Ansys 2019 R3 to evaluate six different fabrication materials and their effects on the fixation system under two loading conditions.
Materials and methods
Finite element model
A CT scan was conducted on the mandible of a healthy volunteer, who was informed of the study details and provided consent. The scan data were imported into Mimics 21.0 software for initial modeling. The cortical and cancellous bone structures of the mandible were segmented using threshold-based techniques. The model was then imported into Geomagic Wrap 2017 to complete the three-dimensional reconstruction. Similarly, a fibula model was created. Both the mandibular and fibula models were imported into Geomagic Design X, where Boolean operations were performed to generate the mandibular defect model.
A fixation system model was constructed using Solidworks 2020, a three-dimensional design software. This model was assembled with the mandibular defect model in Solidworks to create a comprehensive mandibular reconstruction model. Detailed schematics of the reconstructed model are provided in our previous report [16]. The completed model was then imported into Ansys 2019 R3 for finite element analysis and meshed using tetrahedral elements. Based on the study by Cheng et al. [17], fibula transplantation to the middle portion of the mandibular defect demonstrated superior biomechanical properties and facilitated subsequent prosthetic reconstruction. In the fixation system proposed in this study, the fibula was secured using two screws inserted into both the left and right sides of the mandibular segment.
Material parameters
The materials used for fabricating the fixation system were categorized into two groups: (1) titanium and its alloys, including pure titanium (Ti), Ti6Al4V (TC4), and Ti35Nb7Zr5Ta (TiNb, a low-elastic modulus alloy developed by Pérez et al. [18]); and (2) PEEK and its reinforced composites, including PEEK, glass fiber-reinforced PEEK (GFR-PEEK), and CFR-PEEK. The mandibular model was differentiated into two components: cortical bone and cancellous bone. The material parameters are summarized in Table 1 [18-22].
Table 1.
| Material | Elastic modulus (MPa) | Passion’s ratio |
|---|---|---|
| Cortical bone of mandible | 13700 | 0.30 |
| Cancellous bone of mandible | 7930 | 0.30 |
| Fibula | 13700 | 0.30 |
| Tia | 103000 | 0.30 |
| TC4b | 110000 | 0.34 |
| TiNbc | 43600 | 0.34 |
| PEEKd | 4000 | 0.36 |
| GFR-PEEKe | 12000 | 0.4 |
| CFR-PEEKf | 20000 | 0.4 |
Ti: pure titanium;
TC4: Ti6Al4V;
TiNb: Ti35Nb7Zr5Ta;
PEEK: polyetheretherketone;
GFR-PEEK: glass fiber-reinforced polyetheretherketone;
CFR-PEEK: carbon fiber-reinforced polyetheretherketone.
Loading and boundary conditions
Both condyles were fully constrained, and an occlusal load of 300 N was applied vertically to the posterior and anterior teeth on the healthy side, respectively [16,23].
Results
Overview of results
The finite element analysis results indicated that the titanium alloy group exhibited similar analytical outcomes across different material compositions. For illustration, the TiNb alloy is presented as a representative example. Likewise, within the PEEK group, various fabrication materials demonstrated comparable patterns in the finite element analysis results. Therefore, this article focuses on CFR-PEEK as a representative material. A summary of the simulation analysis results for both material groups is provided in Table 2.
Table 2.
Maximum stress and displacement of each part in the titanium alloy and PEEK groups
| Loading region | Material | Maximum stress (MPa) | Maximum displacement (mm) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Fixation plate | Fixation screw | Mandible | Fibula | Reconstruction model | ||
| Anterior tooth | TiNb | 105.07 | 149.69 | 120.32 | 65.63 | 2.53 |
| TC4 | 177.13 | 211.90 | 119.08 | 37.01 | 2.50 | |
| Ti | 175.97 | 145.91 | 100.71 | 75.26 | 2.54 | |
| PEEK | 43.63 | 36.52 | 130.35 | 155.58 | 2.55 | |
| GFR-PEEK | 85.05 | 62.07 | 143.19 | 127.09 | 2.54 | |
| CFR-PEEK | 94.36 | 124.75 | 120.70 | 71.11 | 2.53 | |
| Posterior tooth | TiNb | 52.09 | 75.87 | 76.21 | 35.62 | 1.53 |
| TC4 | 100.09 | 100.91 | 75.73 | 23.51 | 1.52 | |
| Ti | 111.05 | 88.50 | 69.58 | 38.47 | 1.67 | |
| PEEK | 19.46 | 20.54 | 76.95 | 92.18 | 1.54 | |
| GFR-PEEK | 36.80 | 34.67 | 77.13 | 80.42 | 1.54 | |
| CFR-PEEK | 40.69 | 60.03 | 78.06 | 38.95 | 1.53 | |
Stress analysis on fixation plates
Figure 1 shows the stress distribution diagram for the TiNb alloy fixation plate. The analysis reveals that under both loading conditions, the maximum equivalent stress occurs near the central screw hole, with elevated stress levels observed at the bottom of the fixation plate. Specifically, when the load is applied to the posterior tooth region, the stress value of the fixation plate is significantly lower than that when the load is applied to the anterior tooth region.
Figure 1.
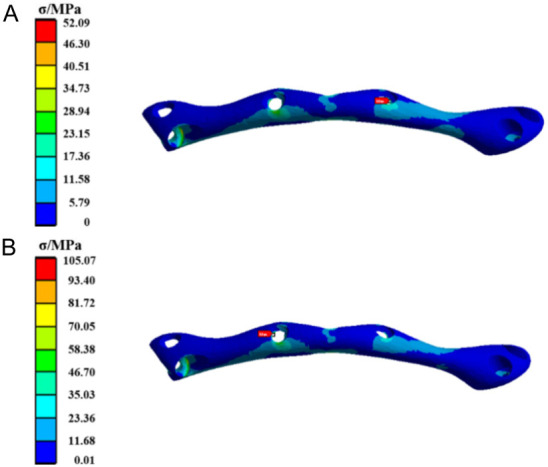
The stress cloud diagram of the Ti35Nb7Zr5Ta (TiNb) alloy fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Figure 2 illustrates the stress distribution for the base fixation plate made of CFR-PEEK. It is evident that under posterior tooth loading, the maximum equivalent stress on the fixation plate is concentrated at the screw hole on the middle-right side, with a stress value of 40.69 MPa. In contrast, under anterior tooth loading, the maximum equivalent stress shifts to the screw hole on the middle-left side, with a stress value increasing to 94.36 MPa.
Figure 2.
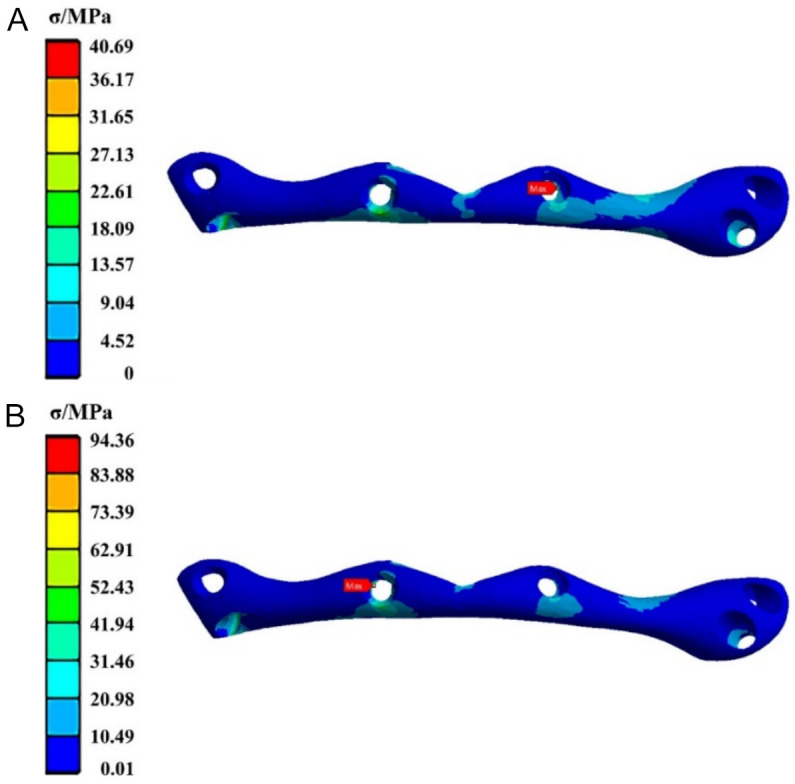
The stress cloud diagram of the carbon fiber-reinforced polyetheretherketone (CFR-PEEK) fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Stress analysis on fixation screws
The stress distribution for the TiNb alloy fixation screws is presented in Figure 3. The maximum equivalent stress is observed at the screw connecting the fibula to the right end of the mandible (the screw on the far left in the figure), which shows a higher overall stress level compared to the other screws. The maximum equivalent stress values under posterior and anterior tooth loading are 75.87 MPa and 149.69 MPa, respectively.
Figure 3.
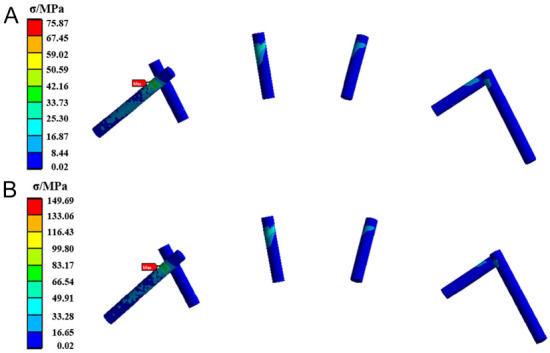
The stress cloud diagram of the TiNb alloy fixation screws when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Figure 4 displays the stress distribution of the CFR-PEEK fixation screws. Similar to the TiNb screws, under both loading conditions, the maximum equivalent stress occurs at the screw located at the junction of the distal end of the right mandible and fibula, specifically the leftmost screw in the figure. This screw exhibits elevated stress levels over a significant portion of its structure. The maximum equivalent stress values under posterior and anterior tooth loading are 60.03 MPa and 124.75 MPa, respectively.
Figure 4.
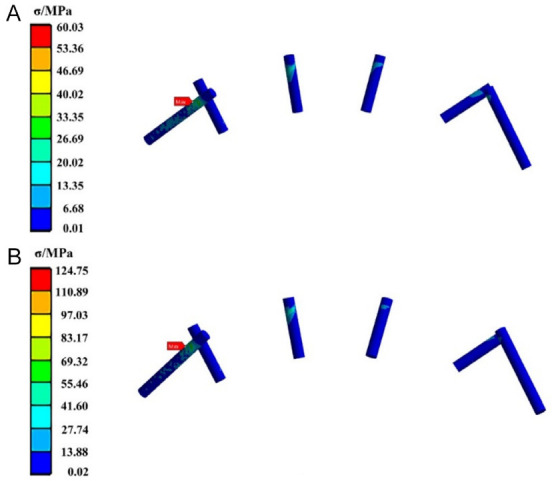
The stress cloud diagram of the CFR-PEEK fixation screws when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region. CFR-PEEK: carbon fiber-reinforced polyetheretherketone.
Stress analysis on mandibles
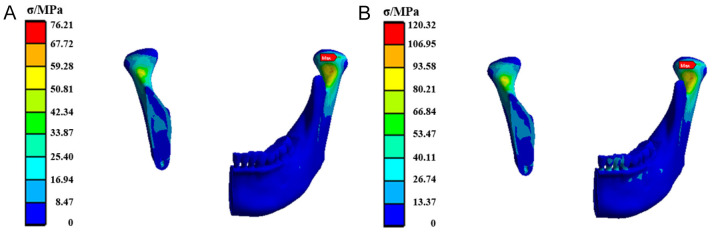
Figure 5 presents the stress distribution in the mandible stabilized by the TiNb fixation plate. The maximum equivalent stress was observed posterior to the right mandibular head, with elevated stress levels at both mandibular heads and condyloid processes. The maximum equivalent stress values under posterior and anterior tooth loading are 76.21 MPa and 120.32 MPa, respectively.
Figure 5.
The stress cloud diagram of the mandible fixed by the TiNb fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Figure 6 shows the stress distribution in the mandible reinforced with CFR-PEEK plates. The maximum equivalent stress under both posterior and anterior tooth loading is concentrated posterior to the right mandibular head, with recorded stress values of 78.06 MPa and 120.70 MPa, respectively.
Figure 6.
The stress cloud diagram of the mandible fixed by the CFR-PEEK fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region. CFR-PEEK: carbon fiber-reinforced polyetheretherketone.
Stress analysis on fibulas
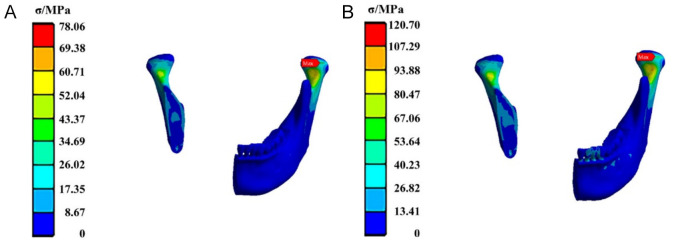
The stress distribution in the fibula is shown in Figure 7. Under both posterior and anterior tooth loading conditions, the maximum equivalent stress is observed on the lower left side of the fibula, with stress values of 35.62 MPa and 65.63 MPa, respectively.
Figure 7.
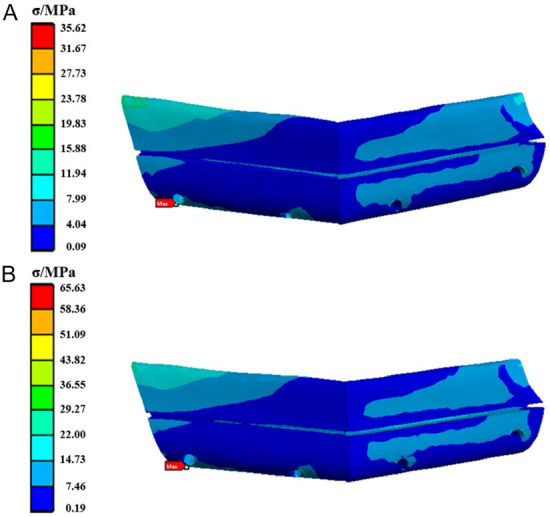
The stress cloud diagram of the fibula fixed by the TiNb fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Similarly, the stress distribution for the CFR-PEEK group is depicted in Figure 8. The maximum equivalent stress is also concentrated on the lower left side of the fibula, with values of 38.95 MPa under posterior loading and 71.11 MPa under anterior loading.
Figure 8.
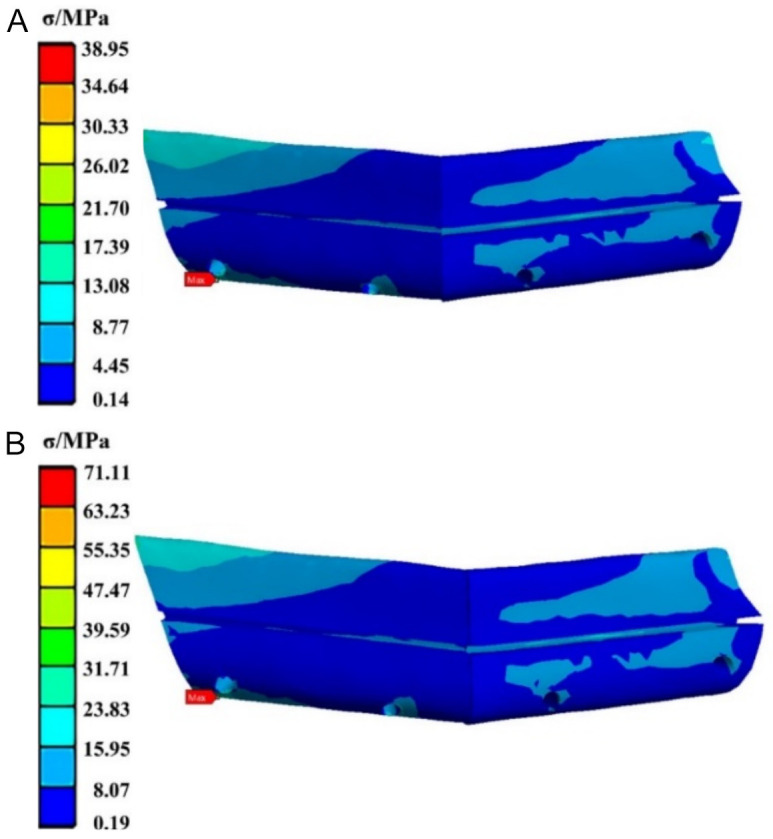
The stress cloud diagram of the fibula fixed by the CFR-PEEK fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region. CFR-PEEK: carbon fiber-reinforced polyetheretherketone.
Overall displacement of the mandibular reconstruction model
Figure 9 shows the overall displacement of the mandibular reconstruction model using TiNb. The maximum displacement occurs at the midpoint of the mandible, particularly at the chin, under both loading conditions. The displacement gradually decreases from the center of the mandible toward both lateral sides. The maximum displacement values for the reconstruction model under posterior and anterior tooth loading are 1.53 mm and 2.53 mm, respectively.
Figure 9.
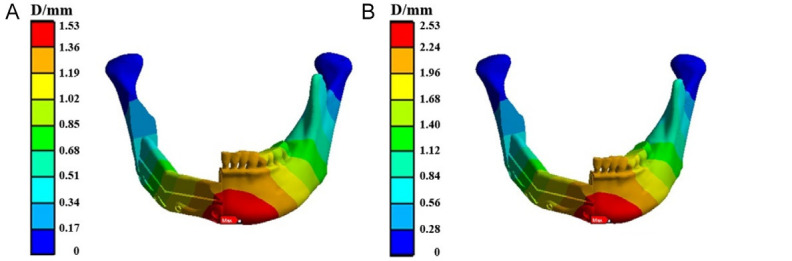
Overall displacement of the mandibular reconstruction model fixed by the TiNb fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region.
Figure 10 illustrates the overall displacement of the mandibular reconstruction model using CFR-PEEK. The displacement pattern in the CFR-PEEK group is similar to that of the titanium alloy group. The maximum displacement is observed at the mandibular symphysis, with values progressively decreasing toward the condyloid processes. The maximum displacement values under posterior and anterior tooth loading are 1.53 mm and 2.53 mm, respectively.
Figure 10.
Overall displacement of the mandibular reconstruction model fixed by the CFR-PEEK fixation plate when the load was applied to (A) the posterior tooth region and (B) the anterior tooth region. CFR-PEEK: carbon fiber-reinforced polyetheretherketone.
Summary of results
According to Pérez’s research [18], the yield strengths of Ti, TC4, and TiNb are 485 MPa, 960 MPa, and 680 MPa, respectively. The maximum equivalent stress observed in the fixation plates and screws of the titanium alloy group remains well within their respective yield strength limits. Additionally, the maximum equivalent stress values for both the mandible and fibula are below the bone yield strength of 140 MPa.
As shown in Table 2, the maximum stress values on the mandible for all cases are lower than the yield strength of 140 MPa, except for the GFR-PEEK group, which slightly exceeds the maximum stress value under anterior tooth loading. Regarding the maximum stress on the fibula, the PEEK group shows the highest values, followed by the GFR-PEEK group, while the CFR-PEEK group exhibits the lowest stress values on the fibula. Moreover, the maximum displacements across the three groups are nearly identical.
Discussion
Surgical resection of the mandible is often required due to trauma or tumors, necessitating the use of fixation systems for reconstruction. The materials used in these systems must possess not only excellent mechanical properties but also good biocompatibility.
Pure titanium and titanium alloys have a higher stiffness than bone tissue due to their high elastic modulus. Consequently, during load application in mandibular reconstruction models, the rigid structures tend to bear the majority of the load, leading to stress concentration primarily on the fixation plates and screws. The complete fixation of the bilateral condyles results in elevated stress levels in the condyle region, while stress is generally lower in other areas of the mandible. Among the titanium alloy groups, the TiNb group exhibits higher stress distribution on the fibula compared to the TC4 group. This can be attributed to TiNb’s elastic modulus, which is closer to that of bone tissue, thereby enhancing stress transmission efficiency compared to TC4.
The design of orthopedic implants must balance the need for sufficient mechanical strength with the goal of minimizing adverse effects on bone tissue post-implantation. The loading conditions significantly influence bone growth and resorption; specific levels of force can stimulate new bone growth, while prolonged periods of low stress or absence of force can lead to resorption or degeneration. Among the three materials in the titanium alloy group, TC4, which has the highest elastic modulus, bears more load during fixation, resulting in lower stress on the fibula. In contrast, Ti and TiNb alloys, with elastic moduli closer to that of bone tissue, provide greater mechanical stimulation to bone tissue during fixation. However, the elastic moduli of all titanium alloys are still significantly higher than that of bone, leading to the common complication known as stress shielding in orthopedic applications.
To address the stress shielding phenomenon, a key research focus in orthopedic implants is reducing the elastic modulus of implant materials to better match that of bone tissue. Pure titanium, with an elastic modulus closest to human bone, has been used as an oral implant material since the 1960s. Concurrently, medical titanium alloys have been developed alongside the use of pure titanium in implantology. Although the TC4 alloy used in this study is a traditional titanium alloy with certain limitations [24], it remains widely utilized and accepted in the medical field. To reduce complications following implantation, ongoing research is focused on developing low elastic modulus titanium alloys. Incorporating alloying elements, particularly β-phase stabilizers such as Nb, Zr, and Ta [25], is an effective strategy for lowering the elastic modulus while maintaining a non-toxic profile and excellent biocompatibility. Numerous studies have explored the application of low elastic modulus titanium alloys in orthopedics. For instance, Miura et al. demonstrated the high bone compatibility of Ti-25Nb-11Sn alloy rods implanted into rabbit femurs [26]. Additionally, Fukuda et al. [27] confirmed that the Ti-Zr-Nb-Ta alloy, after surface activation treatment, exhibits strong bone bonding capability.
PEEK and its composites have significant applications in artificial joints, trauma care, and oral implants. However, the inherent biological inertness of PEEK can hinder its integration with bone. Consequently, surface modifications to enhance its bioactivity have garnered considerable attention. Omrani et al. [28] explored the effects of various surface modification techniques on the adhesion and wettability of PEEK surfaces. Ma et al. [29] demonstrated that incorporating nanoscale active particles into PEEK composites can improve their surface activity.
Fiber-reinforced PEEK composites, developed from extensive research on PEEK, hold great promise for future applications. Notably, the elastic modulus of unmodified PEEK is lower than that of bone tissue, making it less suitable for load-bearing structures such as fixation plates. Stress analysis results support this, showing that the fibula in the PEEK model experiences the highest stress among the three PEEK materials examined, sometimes exceeding the bone’s yield strength.
In contrast, GFR-PEEK and CFR-PEEK, which incorporate rigid fiber structures, exhibit higher elastic moduli that more closely match that of bone tissue, thereby facilitating better stress transmission. While the elastic modulus of GFR-PEEK is slightly lower than that of bone, CFR-PEEK has a marginally higher modulus. Consequently, CFR-PEEK demonstrates the lowest maximum stress values on the mandible and fibula, making it a superior option for bearing occlusal loads, effectively transmitting stress, and minimizing potential damage to bone tissue.
As shown in Table 2, the maximum stress experienced by the mandible and fibula is generally higher in the PEEK group compared to the titanium alloy group. It is important to emphasize that, to prevent stress shielding, bone tissue must receive adequate mechanical stimulation. Thus, while the PEEK group offers some advantages over the titanium alloy group for mandibular defect repair, certain limitations must be noted. For fixation systems constructed from GFR-PEEK, the maximum stress on the mandible exceeds its yield strength under anterior tooth loading. Similarly, for systems made from PEEK, the maximum stress on the fibula also exceeds its yield strength under similar conditions. Therefore, neither material is suitable for mandibular defect repair. Overall, CFR-PEEK emerges as the most favorable material for current fixation systems in mandibular defect repair. However, it is crucial to recognize that this study involves model simplifications, such as uniform material properties, idealized boundary conditions, and the exclusion of individual variability, which may differ from real-world scenarios. These limitations could impact the performance of the fixation system; hence, further long-term clinical trials and observations are necessary for validation.
In summary, titanium and PEEK are two commonly used materials in orthopedic implants. Titanium, a metal with a high elastic modulus, often requires alloying to reduce its modulus. In contrast, PEEK, a polymer with a low elastic modulus, requires reinforcement to enhance its mechanical properties. Regardless of the approach, the objective remains to develop materials that more closely match the mechanical properties of human bone tissue, thereby minimizing complications and improving patient quality of life. Looking forward, the combined use of titanium alloys and polymers like PEEK could offer synergistic advantages, leading to the development of new fixation systems with superior mechanical properties and biocompatibility. Additionally, the use of digital technology and 3D printing can enable personalized design and manufacturing of mandibular defect repair fixation systems, tailored to individual patient conditions and mandibular morphology.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant Nos. 81901049 and 62373253), Shanghai Municipal Science and Technology Major Project (Grant No. 2021SHZDZX) and Shanghai Sailing Program (Grant No. 22YF1430500), PR China.
Disclosure of conflict of interest
None.
References
- 1.Steinbacher DM. Three-dimensional analysis and surgical planning in craniomaxillofacial surgery. J Oral Maxillofac Surg. 2015;73(Suppl):S40–S56. doi: 10.1016/j.joms.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Steffen C, Sellenschloh K, Polster V, Heyland M, Vollmer M, Morlock MM, Heiland M, Huber G, Rendenbach C. Biomechanical comparison of polylactide-based versus titanium miniplates in mandible reconstruction in vitro. J Stomatol Oral Maxillofac Surg. 2020;121:377–382. doi: 10.1016/j.jormas.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Zhang HQ, Huang ZX, Li SH, Zhang DM, Huang ZQ. Computer-assisted resection and reconstruction of bilateral osteoradionecrosis of the mandible using 2 separate flaps prepared from a single fibula. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:102–106. doi: 10.1016/j.oooo.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Park SM, Lee JW, Noh G. Which plate results in better stability after segmental mandibular resection and fibula free flap reconstruction? Biomechanical analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:380–389. doi: 10.1016/j.oooo.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 5.Sohmura T, Kusumoto N, Otani T, Yamada S, Wakabayashi K, Yatani H. CAD/CAM fabrication and clinical application of surgical template and bone model in oral implant surgery. Clin Oral Implants Res. 2009;20:87–93. doi: 10.1111/j.1600-0501.2008.01588.x. [DOI] [PubMed] [Google Scholar]
- 6.Vollmer D, Meyer U, Joos U, Vegh A, Piffkó J. Experimental and finite element study of a human mandible. J Craniomaxillofac Surg. 2000;28:91–96. doi: 10.1054/jcms.2000.0125. [DOI] [PubMed] [Google Scholar]
- 7.Yoda N, Zheng K, Chen J, Liao Z, Koyama S, Peck C, Swain M, Sasaki K, Li Q. Biomechanical analysis of bone remodeling following mandibular reconstruction using fibula free flap. Med Eng Phys. 2018;56:1–8. doi: 10.1016/j.medengphy.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Ciocca L, Mazzoni S, Fantini M, Persiani F, Marchetti C, Scotti R. CAD/CAM guided secondary mandibular reconstruction of a discontinuity defect after ablative cancer surgery. J Craniomaxillofac Surg. 2012;40:e511–e515. doi: 10.1016/j.jcms.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Kim HG, Ham MJ, Hong DGK, Kim SG, Rotaru H. Custom implant for reconstruction of mandibular continuity defect. J Oral Maxillofac Surg. 2018;76:1370–1376. doi: 10.1016/j.joms.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Li CS, Vannabouathong C, Sprague S, Bhandari M. The use of carbon-fiber-reinforced (CFR) PEEK material in orthopedic implants: a systematic review. Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:33–45. doi: 10.4137/CMAMD.S20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang J, Zhang J, Zheng J, Wang L, Li D, Liu S. 3D-printed PEEK implant for mandibular defects repair: a new method. J Mech Behav Biomed Mater. 2021;116:104335. doi: 10.1016/j.jmbbm.2021.104335. [DOI] [PubMed] [Google Scholar]
- 12.Di Maggio B, Sessa P, Mantelli P, Maniscalco P, Rivera F, Calori GM, Bisogno L, Scaravilli G, Caforio M. PEEK radiolucent plate for distal radius fractures: multicentre clinical results at 12 months follow up. Injury. 2017;48(Suppl 3):S34–S38. doi: 10.1016/S0020-1383(17)30655-1. [DOI] [PubMed] [Google Scholar]
- 13.Katthagen JC, Ellwein A, Lutz O, Voigt C, Lill H. Outcomes of proximal humeral fracture fixation with locked CFR-PEEK plating. Eur J Orthop Surg Traumatol. 2017;27:351–358. doi: 10.1007/s00590-016-1891-7. [DOI] [PubMed] [Google Scholar]
- 14.Schliemann B, Hartensuer R, Koch T, Theisen C, Raschke MJ, Kösters C, Weimann A. Treatment of proximal humerus fractures with a CFR-PEEK plate: 2-year results of a prospective study and comparison to fixation with a conventional locking plate. J Shoulder Elbow Surg. 2015;24:1282–1288. doi: 10.1016/j.jse.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui H, Gao L, Han J, Liu J. Biomechanical analysis of mandibular defect reconstruction based on a new base-fixation system. Comput Methods Biomech Biomed Engin. 2022;25:1618–1628. doi: 10.1080/10255842.2022.2029426. [DOI] [PubMed] [Google Scholar]
- 17.Cheng KJ, Liu YF, Wang JH, Jun JC, Jiang XF, Wang R, Baur DA. Biomechanical behavior of mandibles reconstructed with fibular grafts at different vertical positions using finite element method. J Plast Reconstr Aesthet Surg. 2019;72:281–289. doi: 10.1016/j.bjps.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Pérez DAG, Junior AMJ, Roche V, Lepretre JC, Afonso CRM, Travessa DN, Asato GH, Bolfarini C, Botta WJ. Severe plastic deformation and different surface treatments on the biocompatible Ti13Nb13Zr and Ti35Nb7Zr5Ta alloys: microstructural and phase evolutions, mechanical properties, and bioactivity analysis. J Alloys Compd. 2020;812:152116. [Google Scholar]
- 19.Lee WT, Koak JY, Lim YJ, Kim SK, Kwon HB, Kim MJ. Stress shielding and fatigue limits of poly-ether-ether-ketone dental implants. J Biomed Mater Res B Appl Biomater. 2012;100:1044–1052. doi: 10.1002/jbm.b.32669. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bujtár P, Simonovics J, Váradi K, Sándor GK, Avery C. The biomechanical aspects of reconstruction for segmental defects of the mandible: a finite element study to assess the optimization of plate and screw factors. J Craniomaxillofac Surg. 2014;42:855–862. doi: 10.1016/j.jcms.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Duggal I, Sidhu MS, Chawla A, Dabas A, Dhimole VK. Effects of miniplate-anchored Herbst appliance on skeletal, dental, and masticatory structures of the craniomandibular apparatus: a finite element study. Int Orthod. 2021;19:301–309. doi: 10.1016/j.ortho.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Nagasao T, Kaneko T, Tamaki T, Miyamoto J, Nakajima T. Adequate fixation of plates for stability during mandibular reconstruction. J Craniomaxillofac Surg. 2006;34:193–200. doi: 10.1016/j.jcms.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Frost HM. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004;74:3–15. doi: 10.1043/0003-3219(2004)074<0003:AUOBPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Yang C, Wang F, Zhao H, Qu S, Li X, Zhang W, Li Y. Biomedical TiNbZrTaSi alloys designed by d-electron alloy design theory. Mater Des. 2015;85:7–13. [Google Scholar]
- 26.Miura K, Yamada N, Hanada S, Jung TK, Itoi E. The bone tissue compatibility of a new Ti-Nb-Sn alloy with a low Young’s modulus. Acta Biomater. 2011;7:2320–2326. doi: 10.1016/j.actbio.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda A, Takemoto M, Saito T, Fujibayashi S, Neo M, Yamaguchi S, Kizuki T, Matsushita T, Niinomi M, Kokubo T, Nakamura T. Bone bonding bioactivity of Ti metal and Ti-Zr-Nb-Ta alloys with Ca ions incorporated on their surfaces by simple chemical and heat treatments. Acta Biomater. 2011;7:1379–1386. doi: 10.1016/j.actbio.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Omrani M, Hadjizadeh A, Milani A, Kim K. PEEK surface modification methods and effect of the laser method on surface properties. Biointerface Res Appl Chem. 2020;10:5132–5140. [Google Scholar]
- 29.Ma R, Guo D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J Orthop Surg Res. 2019;14:32. doi: 10.1186/s13018-019-1069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]