Abstract
Objectives: To evaluate the prognostic value of body composition-related imaging parameters in assessing Crohn’s disease (CD) severity and biological responses in Chinese patients. Methods: We retrospectively analyzed electronic medical records and Computed tomography (CT) images from 117 CD patients, including 90 with sarcopenia and 27 without. We calculated subcutaneous fat area (SFA), visceral fat area, skeletal muscle area (SMA), mesenteric fat index (MFI), skeletal muscle index (SMI), and muscle attenuation (MA). CD Activity Index (CDAI) score and Simple Endoscopic Score for CD (SES-CD) were used to evaluate inflammation and biologic efficacy. Correlation and comparative analyses were performed to determine associations between imaging parameters and clinical data. Receiver operating characteristic curve analysis evaluated the predictive performance of combined body composition indicators. Results: Sarcopenia was associated with higher CDAI scores and lower body mass index, albumin, and hemoglobin levels but was not associated with SES-CD or rates of clinical/endoscopic remission or response to biologic therapy. SMI was inversely correlated with CDAI score and SES-CD and positively correlated with albumin and hemoglobin. Endoscopy responders had higher SMA, MFI, SMI, and MA than non-responders. SES-CD improvement was positively correlated with MFI and MA and negatively correlated with SFA. The combined analysis of SMI, MFI, and MA yielded an area under the curve of 0.743 for predicting endoscopic response to biologic therapies in CD patients. Conclusions: SMI may indicate CD severity, while MFI and MA could predict biologic response. Integrating multiple body composition parameters enhances treatment outcome evaluation, suggesting their potential utility in CD assessment.
Keywords: Crohn’s disease, computed tomography, body composition, biologic efficacy, prognosis
Introduction
Crohn’s disease (CD) is a chronic, idiopathic inflammatory bowel disease (IBD) that can affect any segment of the gastrointestinal tract, causing chronic diarrhea, malabsorption, anorexia, and weight loss. Additionally, up to 52% of CD patients experience sarcopenia, a condition associated with reduced skeletal muscle mass and function that was originally described as an age-related phenomenon but is now recognized as a consequence of various chronic diseases that cause cachexia and wasting [1,2]. However, the assessment of sarcopenia in CD patients is not straightforward, as it requires specific tools and scales that are often not accessible or practical in routine clinical settings.
Computed tomography (CT) is a reliable and reproducible technique for measuring body composition, including abdominal fat and muscle tissue, by quantifying their attenuation values. The third lumbar spine (L3) level has been validated as a representative site for whole-body composition and as a prognostic indicator in other clinical scenarios, such as cancer and obesity [3,4].
Although CT can be used for assessment of muscle loss in CD patients, the relationship between sarcopenia, as measured by CT, and CD-associated inflammation and disease outcome remains unclear, with various studies reporting inconsistent results. Some have found that sarcopenia is linked to higher rates of post-operative complications, loss of response to anti-tumor necrosis factor (TNF) therapeutic agents, and need for bowel resection in CD patients [5,6]. However, other studies have reported that sarcopenia is not significantly correlated with risks of surgery, hospitalization, or poor response to immunosuppressive therapy and has no predictive value for medical treatment and bowel resection in CD patients [7,8]. Of note, a recent meta-analysis suggested that sarcopenia may be a negative prognostic factor in IBD patients, but the definition of sarcopenia varies considerably across studies and regions [9-12].
Hence, the prognostic value and assessment of disease severity in sarcopenia, as evaluated through CT imaging in CD patients, remain ambiguous. This study aims to bridge this knowledge gap by conducting a retrospective analysis to ascertain whether CT-derived body composition parameters can accurately evaluate disease severity and predict the response to biologic therapy in CD patients. This innovative approach holds the potential to provide invaluable clinical insights, significantly enhancing the management of CD patients through more precise condition assessment and customized therapeutic strategies.
Materials and methods
Study design and patients
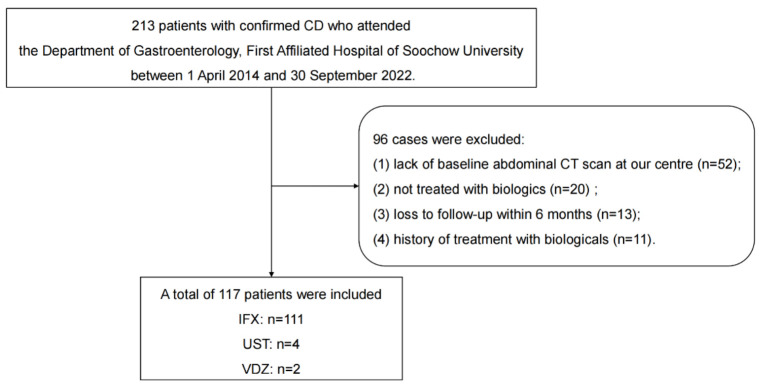
This study used a retrospective cohort study design and was approved by the Ethics Committee of First Affiliated Hospital of Soochow University (approval number: 2023-220). All patient records were de-identified before analysis. Using the electronic medical record system, we assessed 213 patients with CD who were treated at the Gastroenterology Department, First Affiliated Hospital of Soochow University, from April 2014 to September 2022. Ultimately, 117 CD patients who initiated biologic therapy during this time period were included in this study (Figure 1). The inclusion criteria were as follows: 1) CD diagnosis based on the World Gastroenterology Organization practice guidelines [13]; 2) treatment with the biologics infliximab (IFX), adalimumab (ADA), ustekinumab (UST), or vedolizumab (VDZ); and 3) a baseline abdominal CT scan at our center within 3 months prior to biologic initiation. We excluded patients 1) <16 years of age, 2) without baseline CT scan at our center, 3) who did not receive biologic therapy, 4) lost to follow-up within 6 months, or 5) with prior biologic exposure.
Figure 1.
Flow chart showing patient selection criteria and study design. A total of 213 patients with CD who were treated at our hospital during the study period were identified in our medical records. In total, 117 patients were included in this study. Abbreviations: CD, Crohn’s disease; CT, computed tomography; IFX, infliximab; UST, Ustekinumab; VDZ, vedolizumab.
Data collection
Clinical and laboratory assessment
We collected patient demographics, clinical history, laboratory results, and biologic agents administered from electronic medical records. Variables included age, sex, height, weight, body mass index (BMI), CD-related abdominal surgery (e.g., colectomy, ileo-colon resections, segmental colectomies), anal fistulas, serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin (ALB), and hemoglobin (Hb). Some patients switched to a different biologic therapy due to loss of response or intolerance. Disease activity was evaluated based on CD Activity Index (CDAI) score at baseline and week 48, and endoscopic lesions were assessed by Simple Endoscopic Score for CD (SES-CD) at baseline and 9-15 months after starting biologics. The following criteria [14,15] were used for patient assessment: 1) clinical remission, CDAI scores <150; 2) clinical response, CDAI score decrease of ≥100 or clinical remission at week 48; 3) endoscopic response, an SES-CD decrease of >50%; 4) endoscopic remission, SES-CD ≤2 and no ulceration. All endoscopic assessments were performed by a blinded endoscopist experienced in IBD, ensuring the evaluations were conducted without external influence. Patients with incomplete endoscopic examinations or missing CDAI scores were excluded from the analysis.
CT-based body composition analysis
Baseline CT data for all patients were obtained from the electronic medical record and analyzed using the 3D Slicer program [16]. We measured skeletal muscle area (SMA), visceral fat area (VFA), and subcutaneous fat area (SFA) in cm2 at the L3 level using a semi-automated threshold-based module and manually corrected regions of interest. Skeletal muscle index (SMI) was calculated by dividing SMA by height squared, and mesenteric fat index (MFI) was calculated by dividing VFA by SFA. We also measured muscle attenuation (MA) as the mean CT value of skeletal muscle. If patients had unilateral hydropic muscle due to phlegmon or abscess, we doubled the muscle area of the opposite side. Two radiologists with 3 and 4 years of abdominal radiology experience performed CT measurements, and these were reviewed and adjusted, if necessary, by an expert with 15 years of experience. We defined sarcopenia using the L3-SMI cut-offs of <44.77 cm2/m2 for males and <32.50 cm2/m2 for females based on findings from a Chinese multicenter study [17].
Outcome measures and statistical analysis
We assessed the relationship between sarcopenia and inflammation in CD patients by comparing baseline clinical data in the sarcopenia vs. non-sarcopenia groups. To determine whether CT-derived body composition parameters can be used to assess CD severity, we performed correlation analyses evaluating the relationships between baseline body composition-related imaging parameters and baseline clinical parameters. We further assessed the relationship between sarcopenia and outcome in CD patients by comparing the therapeutic efficacy of biologics in the sarcopenia vs. non-sarcopenia groups. To explore whether CT-derived body composition parameters can be used to predict response to biologic therapy in CD patients, we compared baseline body composition-related imaging parameters in the endoscopic remission, endoscopic non-remission, endoscopic response, and non-endoscopic response groups and performed correlation analyses evaluating the relationships between baseline body composition-related imaging parameters and post-treatment changes. To evaluate the predictive performance of the combined body composition indicators, we used receiver operating characteristic curve analysis. The area under the curve (AUC) was calculated to determine the effectiveness of these indicators in predicting clinical outcomes.
SPSS 26 (IBM, NY, USA) was used for all statistical analysis. Normally distributed data were expressed as mean ± standard deviation and compared by one-way analysis of variance. Non-normally distributed data were expressed as median (interquartile range) and compared by the Mann-Whitney U test. We compared proportions using the chi-squared test and assessed the correlations between imaging and clinical variables by calculating Pearson’s or Spearman’s correlation coefficients. In all cases, a two-tailed P-value of .05 was considered statistically significant.
Results
Patient characteristics
We identified 213 patients in our medical records who were treated for CD at our institution during the study period, 117 of whom met the inclusion criteria and were enrolled in this study (Figure 1). Most patients were male (74.4%); 18 (15.4%) had undergone prior gastrointestinal surgery, and 25 (21.4%) had perianal disease. IFX was the most common biologic agent administered to study patients (94.9%), followed by UST (3.4%) and VDZ (1.7%). Based on the published sarcopenia definition [17], we divided study patients into sarcopenia (n=90) and non-sarcopenia (n=27) groups. The sarcopenia group had higher disease activity and lower BMI, ALB, and Hb than the non-sarcopenia group at baseline. There were no significant differences in history of CD-related surgery, perianal disease, ESR, CRP, or SES-CD between the groups (Table 1).
Table 1.
Comparison of baseline clinical parameters in CD patients with and without sarcopenia
| CD patients | P-value# | |||
|---|---|---|---|---|
|
| ||||
| Total patients (n=117) | Sarcopenia (n=90) | Non-sarcopenia (n=27) | ||
| Demographic characteristics | ||||
| Age, years, median (IQR) | 32.00 (13.50) | 32.00 (13.00) | 33.00 (14.00) | .455 |
| Gender (female/male) | 30/87 | 20/70 | 10/17 | .122 |
| BMI, kg/m2, median (IQR) | 19.14 (3.52) | 18.33 (2.80) | 20.57 (3.12) | <.001* |
| Previous history | ||||
| CD-related surgery, n (%) | 18 (15.38) | 15 (16.67) | 3 (11.11) | .761 |
| Perianal disease, n (%) | 25 (21.37) | 21 (23.33) | 4 (14.81) | .430 |
| Biochemical indices | ||||
| ESR, mm/h, median (IQR) | 20.00 (32.00) | 21.00 (40.00) | 18.00 (28.00) | .153 |
| CRP, mg/l, median (IQR) | 13.90 (34.75) | 13.79 (36.32) | 15.00 (23.03) | .357 |
| ALB, g/l, mean ± SD | 36.67±6.42 | 35.75±5.93 | 39.72±7.14 | .004* |
| Hb, g/l, mean ± SD | 120.08±23.28 | 117.33±22.43 | 129.22±24.16 | .019* |
| Disease activity indices | ||||
| CDAI, mean ± SD | 247.01±104.16 | 263.78±100.79 | 191.12±97.05 | .001* |
| Endoscopy | ||||
| SES-CD, median (IQR) | 11.00 (9.00) | 11.00 (9.00) | 11.00 (10.00) | .718 |
| Baseline body composition-related imaging parameters | ||||
| SFA, cm2, median (IQR) | 63.48 (67.04) | 54.03 (67.54) | 78.32 (77.40) | .002* |
| VFA, cm2, median (IQR) | 55.56 (73.63) | 53.77 (66.69) | 75.79 (72.62) | .103 |
| SMA, cm2, mean ± SD | 108.05±24.40 | 102.96±20.51 | 125.00±28.80 | <.001* |
| MFI, median (IQR) | 0.99 (.72) | 1.07 (.72) | 0.73 (.26) | .006* |
| SMI, cm2/m2, mean ± SD | 37.13±6.99 | 35.00±5.40 | 44.23±7.09 | <.001* |
| MA, HU, mean ± SD | 49.63±4.91 | 49.49±4.66 | 50.13±5.74 | .550 |
P-value, sarcopenia vs. non-sarcopenia.
P<.05.
Abbreviations: ALB, albumin; BMI, body mass index; CD, Crohn’s disease; CDAI, Crohn’s disease activity Index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; IQR, interquartile range; MA, muscle attenuation; MFI, mesenteric fat index; SD, standard deviation; SES-CD, Simple endoscopic score for Crohn’s disease; SFA, subcutaneous fat area; SMA, skeletal muscle area; SMI, skeletal muscle index; VFA, visceral fat area.
Correlations between baseline body composition-related imaging parameters and clinical parameters
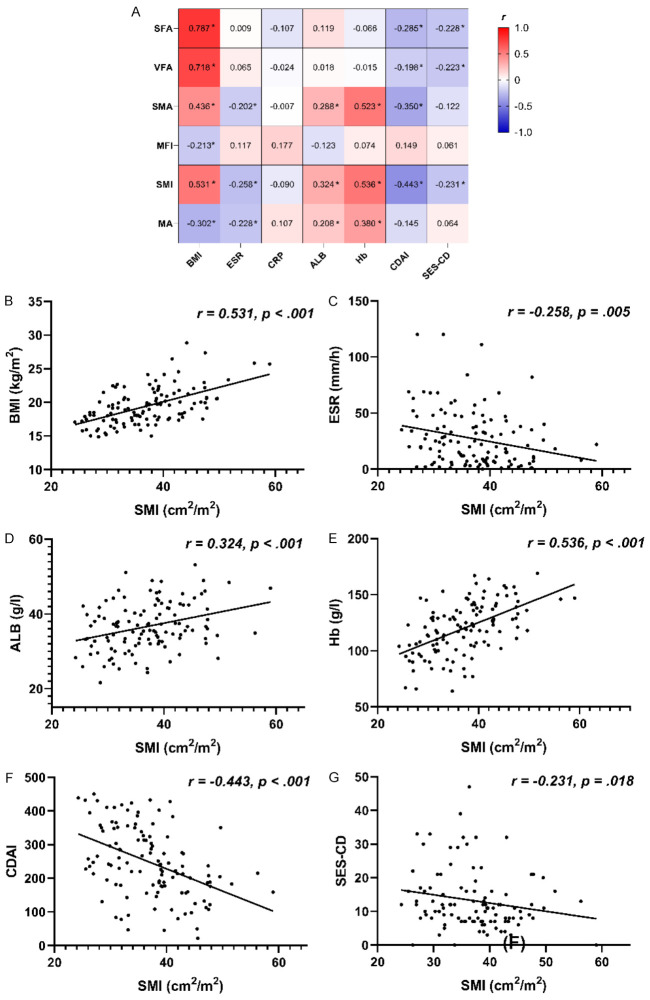
We next assessed the correlations between baseline body composition-related imaging parameters and clinical parameters by calculating Pearson’s or Spearman’s correlation coefficients. From these analyses, we found that SFA, VFA, SMA, and SMI were positively correlated with BMI, whereas MFI and MA were negatively correlated with BMI. In addition, SMA, SMI, and MA were negatively correlated with ESR but positively correlated with ALB and Hb. Our data further show that SFA, VFA, and SMI were negatively correlated with both CDAI score and SES-CD, with SMA also showing a negative correlation with CDAI score (all P<.05) (Figure 2).
Figure 2.
Correlation analysis assessing the relationships between baseline body composition-related imaging parameters and baseline clinical parameters. (A) Correlation coefficients between baseline body composition-related imaging parameters and baseline clinical parameters; (B) Correlation plots comparing SMI and baseline BMI in all 117 patients; (C) SMI and baseline ESR in all 117 patients; (D) SMI and baseline ALB in all 117 patients; (E) SMI and baseline Hb in all 117 patients; (F) SMI and baseline CDAI in all 117 patients; and (G) SMI and baseline SES-CD in 105 patients. *P<.05. Abbreviations: ALB, albumin; BMI, body mass index; CDAI, Crohn’s disease activity Index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; MA, muscle attenuation; MFI, mesenteric fat index; SES-CD, Simple endoscopic score for Crohn’s disease; SFA, subcutaneous fat area; SMA, skeletal muscle area; SMI, skeletal muscle index; VFA, visceral fat area.
Effects of biologics on clinical and endoscopic outcomes in the sarcopenia and non-sarcopenia groups
(1) Clinical remission and response: Of the 117 CD patients included in our study, 55 (47.0%) had CDAI score data available after approximately 48 weeks of biologic treatment for assessment of clinical remission and response (44 with sarcopenia and 11 without sarcopenia). We found that the rates of both clinical remission and response were comparable between the sarcopenia and non-sarcopenia groups (remission: 68.2% vs. 72.7%, P=.77, respectively; response: 75.0% vs. 72.7%, P=.88, respectively) (Figure 3).
Figure 3.
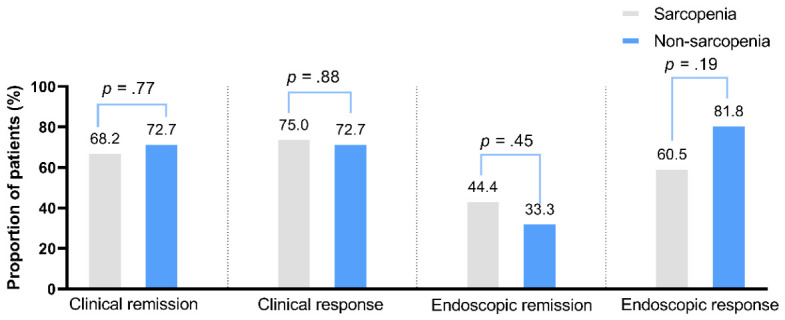
Biologic therapeutic efficacy was similar in the sarcopenia and non-sarcopenia groups. From left to right, graphs show the proportions of sarcopenia (gray bars) and non-sarcopenia patients (blue bars) that achieved clinical remission (68.2% vs. 72.7%, P=.77), clinical response (75.0% vs. 72.7%, P=.88), endoscopic remission (44.4% vs. 33.3%, P=.45), and endoscopic response (60.5% vs. 81.8%, P=.19). Rates for all clinical and endoscopic outcomes were similar in the two groups.
(2) Endoscopic remission and response: Among the 117 patients in our study, 60 (51.3%) underwent endoscopy after approximately 1 year of biologic treatment (45 with sarcopenia and 15 without sarcopenia). Of these, 54 had both pre- and post-treatment endoscopic data for endoscopic response analysis. Results show that the rates of endoscopic remission and response did not differ significantly between the sarcopenia and non-sarcopenia groups (remission: 44.4% vs. 33.3%, P=.45, respectively; response: 60.5% vs. 81.8%, P=.19, respectively) (Figure 3).
Relationships between baseline body composition-related imaging parameters and endoscopic outcomes in CD
Among the 60 patients who completed endoscopy, 25 achieved endoscopic remission. There were no significant differences in the baseline body composition-related imaging parameters between the endoscopic remission and non-remission groups (Table 2). Among the 54 patients who had both pre- and post-treatment endoscopic data, 35 achieved endoscopic response. Of note, we found that the endoscopic response group had higher baseline SMA, MFI, SMI, and MA than the non-response group (all P<.05) (Table 3).
Table 2.
Comparison of baseline body composition-related imaging parameters of CD patients in the endoscopic remission vs. endoscopic non-remission groups
| Baseline body composition-related imaging parameters | Endoscopic remission | P-value# | |
|---|---|---|---|
|
| |||
| Yes (n=25) | No (n=35) | ||
| SFA, cm2, mean ± SD | 65.74±43.26 | 83.70±51.45 | .160 |
| VFA, cm2, median (IQR) | 65.50 (58.40) | 58.90 (79.71) | .356 |
| SMA, cm2, mean ± SD | 105.55±19.34 | 102.79±29.39 | .683 |
| MFI, median (IQR) | 1.03 (.80) | 0.96 (.69) | .333 |
| SMI, cm2/m2, mean ± SD | 35.85±6.55 | 35.75±7.41 | .954 |
| MA, HU, mean ± SD | 50.02±4.33 | 48.60±4.27 | .214 |
P-value, endoscopic remission vs. endoscopic non-remission.
Abbreviations: MA, muscle attenuation; MFI, mesenteric fat index; SFA, subcutaneous fat area; SMA, skeletal muscle area; SMI, skeletal muscle index; VFA, visceral fat area.
Table 3.
Comparison of baseline body composition-related imaging parameters of CD patients in the endoscopic response vs. non-endoscopic response groups
| Baseline body composition-related imaging parameters | Endoscopic response | P-value# | |
|---|---|---|---|
|
| |||
| Yes (n=35) | No (n=19) | ||
| SFA, cm2, mean ± SD | 70.51±48.72 | 83.94±45.78 | .328 |
| VFA, cm2, median (IQR) | 65.50 (53.43) | 58.85 (85.10) | .935 |
| SMA, cm2, mean ± SD | 109.07±23.69 | 93.18±25.18 | .025* |
| MFI, median (IQR) | 1.09 (.82) | 0.92 (.49) | .047* |
| SMI, cm2/m2, mean ± SD | 36.90±6.75 | 32.93±5.84 | .036* |
| MA, HU, mean ± SD | 50.25±4.17 | 47.54±4.14 | .026* |
P-value, endoscopic response vs. endoscopic non-response.
P<.05.
Abbreviations: MA, muscle attenuation; MFI, mesenteric fat index; SFA, subcutaneous fat area; SMA, skeletal muscle area; SMI, skeletal muscle index; VFA, visceral fat area.
Relationship between baseline body composition-related imaging parameters and changes in BMI, CDAI score, and SES-CD after treatment
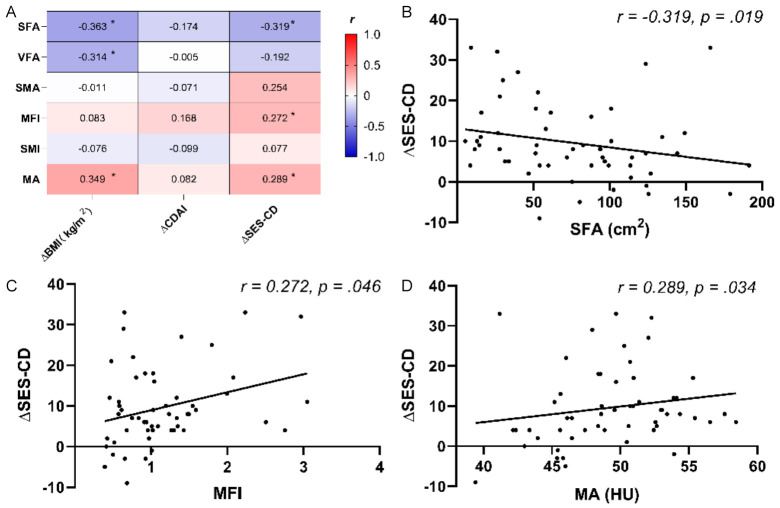
We assessed the correlations between baseline body composition-related imaging parameters and changes in BMI, CDAI score, and SES-CD before and after treatment. Our results showed that SFA and VFA were negatively correlated with BMI increases, whereas MA was positively correlated with BMI increases. In addition, MFI, and MA were positively correlated with SES-CD decreases, and SFA was negatively correlated with SES-CD decreases (Figure 4).
Figure 4.
Correlation analysis assessing the relationships between baseline body composition-related imaging parameters and post-treatment changes. (A) Correlation coefficients between baseline body composition-related imaging parameters and changes in BMI, CDAI score, and SES-CD before and after treatment; (B) Correlation plots comparing SFA and SES-CD changes in 54 patients; (C) MFI and SES-CD changes in 54 patients; and (D) MA and SES-CD changes in 54 patients. *P<.05. Abbreviations: BMI, body mass index; CDAI, Crohn’s disease activity Index; MA, muscle attenuation; MFI, mesenteric fat index; SES-CD, Simple endoscopic score for Crohn’s disease; SFA, subcutaneous fat area; SMA, skeletal muscle area; SMI, skeletal muscle index; VFA, visceral fat area.
Predictive value of combined body composition parameters for CD outcomes
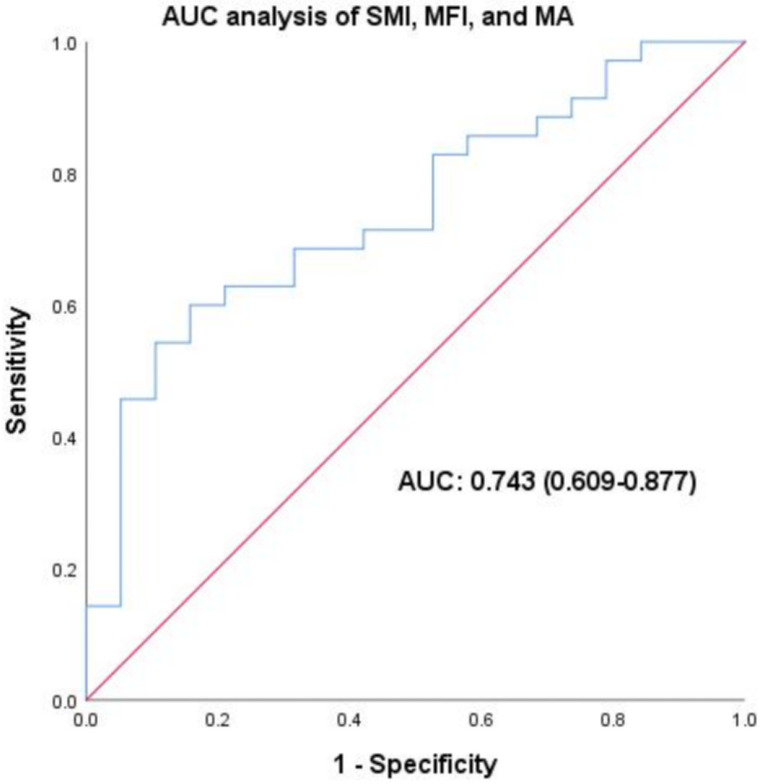
Lastly, we assessed the predictive ability of combined SMI, MFI, and MA for endoscopic response to biologic therapies. The analysis yielded an AUC value of 0.743 (95% CI: 0.609-0.877, P<.01), indicating a significant predictive capability for endoscopic response (Figure 5).
Figure 5.
Predictive value of combined body composition parameters for endoscopic response: AUC Analysis of SMI, MFI, and MA. The analysis yielded an AUC value of 0.743 (95% CI: 0.609-0.877, P<.01), indicating significant predictive capability for endoscopic response. Abbreviations: AUC, area under the curve; SMI, skeletal muscle index; MFI, mesenteric fat index; MA, muscle attenuation.
Discussion
In this retrospective cohort study, we investigated how CT-derived body composition parameters relate to inflammation and biologic response in CD patients, using CDAI score and SES-CD as measures of disease activity. Of note, we found that sarcopenia was linked to malnutrition and higher CDAI score but not to SES-CD or biologic response. SMI, a component of sarcopenia, also showed an inverse correlation with both CDAI score and SES-CD, indicating that it may be leveraged as potential as a marker of disease severity. Furthermore, we identified several body composition parameters, including SMI, SMA, MA, and MFI, that predicted endoscopic response to biologic therapies in CD patients. These results suggest that body composition assessment by CT may offer valuable insights for evaluating CD activity and prognosis, with potential utility for guiding biologic therapy.
Our finding that sarcopenia did not predict biologic response contrasts with some previous reports that associated sarcopenia with primary non-response or loss of response to anti-TNF therapy in CD patients [18,19]. However, these studies used different definitions of sarcopenia and biologic response. Here, given our study population, we used sarcopenia diagnostic criteria [17] derived from a multicenter population in China, which considered the influence of ethnic and dietary differences on the definition of sarcopenia. We further note that our criteria for defining biological response were based on endoscopic assessment, which is more reliable and objective than biochemical or clinical remission. Moreover, unlike in the prior study [18], we evaluated the long-term efficacy of biologics rather than the short-term induction phase. Therefore, our results may better reflect the sustained impact of body composition on biologic response in CD patients.
Previous investigations have found that SMI is related to inflammatory and nutritional markers, such as CRP, ESR, BMI, ALB, and Hb [7,20]. However, to our knowledge, this is the first study to report an association between SMI and both CDAI score and SES-CD in CD patients. We posit that SMI may decrease in active CD due to reduced nutrient intake, impaired absorption, increased energy expenditure, and chronic inflammation-induced muscle catabolism. Conversely, a high SMI may indicate a better nutritional and inflammatory status and lower disease burden [21-23]. In contrast to our findings, Barajas Ordonez F. et al. did not observe a significant difference in body composition parameters between CD patients with inflammatory vs. complicated disease [24]. However, in another study assessing the same population, this group detected a significant difference in body composition parameters between the mild-to-moderate and severe disease groups [25]. These inconsistent results may result from the different criteria used to define disease severity in prior studies. To avoid this confounding factor, we performed correlation analysis to assess the relationship between body composition parameters and clinical parameters rather than comparing body composition parameters across different disease-severity categories.
When considering the efficacy of biologics for CD treatment, a low SMI may also impair immune function and wound healing of CD patients, leading to an increased risk of complications and surgery [26]. Ando K. et al. were the first to investigate the association between altered body composition and long-term outcomes over 5 years after anti-TNF therapy in anti-TNF-naïve CD patients [6]. Results from this study showed that the 5-year cumulative secondary failure-free and bowel resection-free rates were significantly lower in patients with low SMI than in those with high SMI [6]. However, few studies have assessed the relationship between baseline skeletal muscle status and endoscopic outcomes in CD patients treated with biologic agents. Here, we found that endoscopically responsive patients had higher SMI at baseline than non-responsive patients, suggesting that SMI may be not only a measure of disease severity but also a predictor of endoscopic response to biologics.
In addition to SMI, our study identified SMA and MA, both of which reflect skeletal muscle mass, as predictors of endoscopic response to biologics in CD patients-findings with potential implications for the pathophysiology and treatment of CD. SMA reflects overall muscle area, whereas MA represents myosteatosis, a component of sarcopenic obesity characterized by fat infiltration into skeletal muscle, leading to impaired muscle quality and function [27]. Previous studies have reported that myosteatosis is associated with worse outcomes after IBD surgery [28], but ours is the first study to note its relevance for endoscopic response to biologics in CD patients, showing that higher MA and SMA are associated with improved endoscopic response. These findings suggest a potential interaction between skeletal muscle status, chronic inflammation, and the therapeutic effect of biologics in CD. For example, active CD can cause reduced oral intake and nutrient loss due to mucosal inflammation and diarrhea. In parallel, chronic CD-associated inflammation can suppress insulin-like growth factor 1 level via the production of TNF-α and interleukin 6 and increase myostatin, a myokine produced by skeletal muscle. Critically, both myostatin and proinflammatory cytokines, such as TNF-α, promote muscle catabolism and inhibit muscle synthesis [1]. Intriguingly, a recent animal study showed that irisin, another myokine, ameliorated experimental colitis and decreased colonic TNF-α levels [29]. Thus, skeletal muscle status may be a novel therapeutic target and a predictive marker for anti-TNF therapy efficacy in CD.
We further measured MFI as an indicator of visceral fat mass, which is thought to promote CD pathogenesis and adversely affect prognosis. However, we found that higher MFI predicted better endoscopic response to biologics in CD patients. This observation contradicts most prior studies in which visceral fat was linked to worse outcomes in CD [3,6,30-39]. Several factors may account for this discrepancy. First, our results may reflect fat consumption rather than the pathogenic role of mesenteric fat in patients with severe or longstanding CD, as suggested previously by Thiberge C. et al. [40]. Previous studies have mainly focused on the proinflammatory effects of mesenteric fat in CD. However, our study has demonstrated that MFI is higher in CD patients with complicated disease behavior. This finding is consistent with previous research, indicating that a higher proportion of visceral fat in total fat mass is correlated with increased levels of proinflammatory cytokines and disease activity [22,41-43]. Second, the mean VFA and BMI of our CD patients were much lower than those of Western CD patients in previous studies, reflecting racial and dietary differences between the East and West [30-34]. Anti-TNF drug clearance has been shown to vary with body weight extremes in IBD patients, implying that optimal visceral fat volume may help maintain adequate anti-TNF drug concentrations in CD patients [44]. Third, most previous studies have examined the impact of visceral fat on post-operative complications and recurrence in CD, reporting inconsistent results [30,34-36]. Additionally, the few prior studies [3,6,37,38] assessing the non-surgical prognosis of CD patients had notable limitations, such as including only children [37] or only female CD patients in remission [38]. In contrast, our study population was more representative of the clinical reality, as we enrolled active CD patients who received biologics for the first time. Moreover, unlike the studies by Bamba S. et al. [3] and Ando K. et al. [6], which evaluated the associations between body composition parameters and long-term drug efficacy (i.e., bowel resection rate), we aimed to investigate the link between body composition parameters and endoscopic efficacy of biologics. Finally, we note that a Chinese study [39] reported an inverse relationship between VFA and mucosal healing in biologically naive CD patients treated with IFX induction therapy, which contrasts with our data showing an inverse correlation between VFA and disease activity. There are two possible explanations for this discrepancy. First, the prior study was conducted at a leading surgical center for IBD in China, where enteral nutrition therapy is widely used. Consequently, most patients (96.91%) received enteral nutrition before biological therapy, resulting in lower BMI but higher SFA values than were recorded for subjects in our study. Critically, subcutaneous fat has been reported to have metabolic and immunological properties different from those of visceral fat and to produce substances that can improve systemic glucose metabolism [45]. Therefore, the effect of VFA on biological efficacy may have been confounded by higher patient SFA in the earlier study. Second, this study measured endoscopic mucosal healing after three IFX infusions, which reflects short-term biologic efficacy. In contrast, we measured endoscopic response after a longer follow-up period, thus assessing the long-term efficacy of biologic therapies. Overall, our findings suggest the need to re-evaluate the role of adipocytes in CD pathophysiology toward the goal of identifying new prognostic biomarkers and therapeutic targets.
In addition to the primary analyses, we conducted combined analyses of SMI, MFI, and MA to assess their effectiveness in predicting clinical outcomes. The results indicate a reasonably strong predictive capability for endoscopic response to biologic therapies. This finding demonstrates that these body composition parameters, when combined, can be valuable in forecasting treatment outcomes. The inclusion of these combined metrics underscores the importance of a multifaceted approach to evaluating treatment efficacy, providing a more comprehensive understanding of patient responses to biologic therapies. Future research should continue to explore and validate these combined measures to further refine and improve their predictive accuracy in clinical settings.
Several limitations of this study should be noted. Firstly, the retrospective single-center design may introduce selection bias and limit the generalizability of the findings to broader populations. Secondly, the relatively small sample size, particularly among patients receiving non-anti-TNF biologics, restricts the ability to make robust comparisons between different biologic therapies. Additionally, although our follow-up period is longer than in some previous studies, it may still be insufficient to fully capture the long-term effects of biologic therapies on body composition and disease outcomes in CD patients. Data for SES-CD and CDAI after biological treatment is notably missing for some patients, which may limit the generalizability of the findings and introduce selection bias. Lastly, while the AUC value of 0.743 for endoscopic response indicates moderate predictive ability, it also highlights some limitations. This value suggests that SMI, MFI, and MA alone may not fully capture the complexity of CD and response to biologic therapies. The moderate performance could be due to disease heterogeneity and unaccounted confounding factors. These limitations underscore the need for larger, multicenter, prospective studies to validate and further investigate our findings. Despite these limitations, the study’s strengths-including the use of objective endoscopic assessments and new insights into the predictive value of body composition parameters such as SMI, MFI, and MA for forecasting endoscopic response to biologic therapies in CD-underscore its significant contribution to the field.
In summary, we found that sarcopenia was prevalent among CD patients and associated with lower BMI, lower ALB levels, lower Hb levels, and higher disease activity, as measured by CDAI score but not by SES-CD. Our results further show that baseline SMI was inversely correlated with both CDAI score and SES-CD, suggesting SMI may reflect the severity of inflammation and mucosal damage in CD. Furthermore, although we found that sarcopenia did not affect the response to biologics, higher baseline values of SMI, SMA, MA, and MFI were predictive of endoscopic response, and baseline MA and MFI were linked to improved endoscopic scores after biologic therapy. Combining SMI, MFI, and MA offers moderate predictive value for CD outcomes. Collectively, these findings indicate that body composition-related imaging parameters may be useful tools for evaluating the severity and the response to treatment in CD patients.
Acknowledgements
The study was supported by the General Program of Suzhou Medical Association (Grant No. 2022YX-M01) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20240439).
Disclosure of conflict of interest
None.
References
- 1.Dhaliwal A, Quinlan JI, Overthrow K, Greig C, Lord JM, Armstrong MJ, Cooper SC. Sarcopenia in inflammatory bowel disease: a narrative overview. Nutrients. 2021;13:656. doi: 10.3390/nu13020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan E, McNicholas D, Creavin B, Kelly ME, Walsh T, Beddy D. Sarcopenia and inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2019;25:67–73. doi: 10.1093/ibd/izy212. [DOI] [PubMed] [Google Scholar]
- 3.Bamba S, Inatomi O, Takahashi K, Morita Y, Imai T, Ohno M, Kurihara M, Takebayashi K, Kojima M, Iida H, Tani M, Sasaki M. Assessment of body composition from CT images at the level of the third lumbar vertebra in inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:1435–1442. doi: 10.1093/ibd/izaa306. [DOI] [PubMed] [Google Scholar]
- 4.Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. 2019;137:117–124. doi: 10.1016/j.radonc.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Yu D, Hong L, Zhang T, Liu H, Fan R, Wang L, Zhong J, Wang Z. Prevalence of sarcopenia and its effect on postoperative complications in patients with Crohn’s disease. Gastroenterol Res Pract. 2021;2021:3267201. doi: 10.1155/2021/3267201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando K, Uehara K, Sugiyama Y, Kobayashi Y, Murakami Y, Sato H, Kunogi T, Sasaki T, Takahashi K, Ueno N, Kashima S, Moriichi K, Tanabe H, Okumura T, Fujiya M. Correlation among body composition parameters and long-term outcomes in Crohn’s disease after anti-TNF therapy. Front Nutr. 2022;9:765209. doi: 10.3389/fnut.2022.765209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Yoon H, Oh DJ, Lee JM, Choi YJ, Shin CM, Park YS, Kim N, Lee DH, Kim JS. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn’s disease. Intest Res. 2020;18:79–84. doi: 10.5217/ir.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam K, Lee JY, Ko Y, Kim KW, Lee HS, Hong SW, Park JH, Hwang SW, Yang DH, Ye BD, Byoun JS, Myung SJ, Yang SK, Park SH. Impact of sarcopenia on clinical course of inflammatory bowel disease in Korea. Dig Dis Sci. 2023;68:2165–2179. doi: 10.1007/s10620-023-07838-z. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Feng W, Xu M, Wu C, Yang H, Wang Y, Gan H. Sarcopenia and treatment failure in inflammatory bowel disease: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2024;116:68–76. doi: 10.17235/reed.2023.9808/2023. [DOI] [PubMed] [Google Scholar]
- 10.Faye AS, Khan T, Cautha S, Kochar B. Sarcopenia in inflammatory bowel diseases: reviewing past work to pave the path for the future. Curr Treat Options Gastroenterol. 2022;20:250–260. doi: 10.1007/s11938-022-00389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T, Kuzuya M, Lee JSW, Lee SY, Lee WJ, Lee Y, Liang CK, Lim JY, Lim WS, Peng LN, Sugimoto K, Tanaka T, Won CW, Yamada M, Zhang T, Akishita M, Arai H. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein CN, Fried M, Krabshuis JH, Cohen H, Eliakim R, Fedail S, Gearry R, Goh KL, Hamid S, Khan AG, LeMair AW, Malfertheiner, Ouyang Q, Rey JF, Sood A, Steinwurz F, Thomsen OO, Thomson A, Watermeyer G. World gastroenterology organization practice guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112–124. doi: 10.1002/ibd.21048. [DOI] [PubMed] [Google Scholar]
- 14.Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 15.Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160:1570–1583. doi: 10.1053/j.gastro.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, Zhang LY, Tan W, Shi PM, Yu H, Zhang CQ, Xie WF. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948–1958. doi: 10.1002/jcsm.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Tang H, Lin T, Wang J, Cui W, Xie C, Wang Z, Chen Y, Chen X. Sarcopenia assessed by computed tomography or magnetic resonance imaging is associated with the loss of response to biologic therapies in adult patients with Crohn’s disease. Clin Transl Sci. 2023;16:2209–2221. doi: 10.1111/cts.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding NS, Malietzis G, Lung PFC, Penez L, Yip WM, Gabe S, Jenkins JT, Hart A. The body composition profile is associated with response to anti-TNF therapy in Crohn’s disease and may offer an alternative dosing paradigm. Aliment Pharmacol Ther. 2017;46:883–891. doi: 10.1111/apt.14293. [DOI] [PubMed] [Google Scholar]
- 20.Choi EJ, Baek DH, Lee HS, Song GA, Kim TO, Park YE, Lee CM Busan Ulsan Gyeongnam Intestinal Study Group Society (BIGS) Lee JH. The effect of biological agent on body composition in patients with Crohn’s disease. BMC Gastroenterol. 2023;23:100. doi: 10.1186/s12876-023-02742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Kim KW, Ko Y, Oh CH, Kim BH, Park SJ, You MW. Serial changes in body composition and the association with disease activity during treatment in patients with Crohn’s disease. Diagnostics (Basel) 2022;12:2804. doi: 10.3390/diagnostics12112804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labarthe G, Dolores M, Verdalle-Cazes M, Charpentier C, Roullee P, Dacher JN, Savoye G, Savoye-Collet C. Magnetic resonance imaging assessment of body composition parameters in Crohn’s disease. Dig Liver Dis. 2020;52:878–884. doi: 10.1016/j.dld.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Singh A, Midha V, Mahajan R, Verma S, Kakkar C, Grover J, Singh D, Kaur R, Masih A, Bansal N, Wall C, Sood A. Evaluation of nutritional characteristics reveals similar prevalence of malnutrition in patients with ulcerative colitis and Crohn’s disease. Dig Dis Sci. 2023;68:580–595. doi: 10.1007/s10620-022-07652-z. [DOI] [PubMed] [Google Scholar]
- 24.Barajas Ordonez F, Melekh B, Rodríguez-Feria P, Melekh O, Thormann M, Damm R, Omari J, Pech M, Surov A. Body composition predictors of complicated Crohn’s disease. Dig Dis. 2023;41:589–599. doi: 10.1159/000529426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barajas Ordonez F, Melekh B, Rodríguez-Feria P, Damm R, Thormann M, March C, Omari J, Pech M, Surov A. Parameters of body composition and creeping fat are associated with activity of Crohn’s disease. Magn Reson Imaging. 2023;98:1–6. doi: 10.1016/j.mri.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Nagayoshi K, Mizuuchi Y, Zhang J, Hisano K, Tamura K, Sada M, Nakata K, Ohuchida K, Nakamura M. Strong impact of sarcopenic state defined by skeletal muscle mass index on postoperative complication of Crohn’s disease patients. Surg Open Sci. 2023;15:54–59. doi: 10.1016/j.sopen.2023.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74:1671–1678. doi: 10.1093/gerona/glz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly M, Driever D, Ryan ÉJ, Elliott JA, Finnegan J, McNamara D, Murphy I, Conlon KC, Neary PC, Kavanagh DO, O’Riordan JM. Obesity, sarcopenia and myosteatosis: impact on clinical outcomes in the operative management of Crohn’s disease. Inflamm Bowel Dis. 2023 doi: 10.1093/ibd/izad225. izad225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan SA, Metzger CE, Bloomfield SA, Zawieja DC. Inflammation-induced lymphatic architecture and bone turnover changes are ameliorated by irisin treatment in chronic inflammatory bowel disease. FASEB J. 2018;32:4848–4861. doi: 10.1096/fj.201800178R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connelly TM, Juza RM, Sangster W, Sehgal R, Tappouni RF, Messaris E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn’s disease patients. Dig Surg. 2014;31:219–224. doi: 10.1159/000365359. [DOI] [PubMed] [Google Scholar]
- 31.Grillot J, D’Engremont C, Parmentier AL, Lakkis Z, Piton G, Cazaux D, Gay C, De Billy M, Koch S, Borot S, Vuitton L. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin Nutr. 2020;39:3024–3030. doi: 10.1016/j.clnu.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Bryant RV, Schultz CG, Ooi S, Goess C, Costello SP, Vincent AD, Schoeman S, Lim A, Bartholomeusz FD, Travis SPL, Andrews JM. Visceral adipose tissue is associated with stricturing Crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm Bowel Dis. 2019;25:592–600. doi: 10.1093/ibd/izy278. [DOI] [PubMed] [Google Scholar]
- 33.Lim Z, Welman CJ, Raymond W, Thin L. The effect of adiposity on anti-tumor necrosis factor-alpha levels and loss of response in Crohn’s disease patients. Clin Transl Gastroenterol. 2020;11:e00233. doi: 10.14309/ctg.0000000000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Z, Wu XR, Remer EM, Lian L, Stocchi L, Li Y, McCullough A, Remzi FH, Shen B. Association between high visceral fat area and postoperative complications in patients with Crohn’s disease following primary surgery. Colorectal Dis. 2016;18:163–172. doi: 10.1111/codi.13128. [DOI] [PubMed] [Google Scholar]
- 35.Holt DQ, Moore GT, Strauss BJ, Hamilton AL, De Cruz P, Kamm MA. Visceral adiposity predicts post-operative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2017;45:1255–1264. doi: 10.1111/apt.14018. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Zhu W, Gong J, Zhang W, Gu L, Guo Z, Cao L, Shen B, Li N, Li J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis. 2015;17:225–234. doi: 10.1111/codi.12798. [DOI] [PubMed] [Google Scholar]
- 37.Uko V, Vortia E, Achkar JP, Karakas P, Fiocchi C, Worley S, Kay MH. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:2286–2291. doi: 10.1097/MIB.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 38.Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, Haas V. Visceral adipose tissue in patients with Crohn’s disease correlates with disease activity, inflammatory markers, and outcome. Inflamm Bowel Dis. 2015;21:2590–2597. doi: 10.1097/MIB.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 39.Shen W, Cao L, Li Y, Cai X, Ge Y, Zhu W. Visceral fat is associated with mucosal healing of infliximab treatment in Crohn’s disease. Dis Colon Rectum. 2018;61:706–712. doi: 10.1097/DCR.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 40.Thiberge C, Charpentier C, Gillibert A, Modzelewski R, Dacher JN, Savoye G, Savoye-Collet C. Lower subcutaneous or visceral adiposity assessed by abdominal computed tomography could predict adverse outcome in patients with Crohn’s disease. J Crohns Colitis. 2018;12:1429–1437. doi: 10.1093/ecco-jcc/jjy124. [DOI] [PubMed] [Google Scholar]
- 41.Erhayiem B, Dhingsa R, Hawkey CJ, Subramanian V. Ratio of visceral to subcutaneous fat area is a biomarker of complicated Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:684–687. e681. doi: 10.1016/j.cgh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 45.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]