Abstract
Background: Fetal growth restriction, commonly referred to as small for gestational age (SGA) in academic contexts, is associated with increased mortality rates and significant health risks. Fetal development is influenced by a complex interplay of maternal factors, fetal characteristics, and placenta fiction. This meta-analysis explored the relationship between the prevalence of SGA infants and various maternal conditions, such as overall health, lifestyle choices, and underlying medical conditions. Methods: A comprehensive literature search on maternal factor and SGA was conducted in PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan Fang, and China Biology Medicine (CBM) (SinoMed) databases from 2000 to 2022. The Cochrane Collaboration tool was adopted to assess the quality of the selected literature. STATA 14.0 software was used to perform the statistical analysis and graphic presentation. Meta-analysis was registered with International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) (202410045). Results: In total, 15 studies with 41,446 infants identified as being SGA were included in this Meta-analysis. SGA occurrence was not associated with maternal age or multi-parameter, but was related to abnormal Body Mass Index (BMI) (RR=2.23, 95% CI [1.24, 4.00]). Smoking was strongly associated with SGA (RR=3.09, 95% CI [1.53, 6.23]), while drinking was not. SGA was negatively correlated with pregestational diabetes (RR=0.59, 95% CI [0.40, 0.88]) and pregnancy complications, including gestational diabetes (RR=0.74, 95% CI [0.56, 0.97]), hypertension (RR=2.84, 95% CI [1.88, 4.29]) and preeclampsia (RR=2.38, 95% CI [1.77, 3.20]). Conclusions: Maternal risk factors, including BMI, smoking, pregestational diabetes, gestational diabetes, gestational hypertension, and preeclampsia, are associated with SGA.
Keywords: Small for gestational age, meta-analysis, pregnant diseases, maternal factors
Introduction
Birth weight is a critical indicator of fetal development and newborn health [1-3]. Fetal growth restriction is associated with many adverse perinatal outcomes; however, its etiology and diagnosis remain subjects of debate [4,5]. The American College of Obstetricians and Gynecologists (ACOG) defines fetal growth restriction, commonly known as small for gestational age (SGA), as a condition in which a fetus’s weight is below the tenth percentile for the corresponding gestational age [6]. In 2010, about 32.4 million infants were born with SGA in low- and middle-income countries, with the prevalence of preterm SGA being 46.8% in Asia and 4.2% in Africa. Most of the infants with SGA were born in India, Pakistan, Nigeria and Bangladesh [7].
Infants with SGA often experience impaired organ development due to intrauterine growth retardation, which can manifest as neonatal respiratory distress syndrome, necrotizing enterocolitis, intracranial hemorrhage and other diseases [8-10]. Therefore, SGA is one of the leading causes of perinatal infant mortality [11-13]. Furthermore, newborns with SGA are prone to experience metabolic syndrome, cardiovascular disease, short stature, and other diseases in adulthood compared to non-SGA infants [14,15].
The causes of SGA are complex and not yet fully understood. Recent research suggests that a combination of maternal, fetal, placental, umbilical cord, and paternal factors may influence the occurrence of SGA. Maternal conditions are crucial for fetal growth and development. Factors such as age, height, body mass index (BMI), nutrient, race, income, educational level, parity, and history of spontaneous abortion or miscarriage, can significantly impact the health of both the fetus and infants. Advanced maternal age is a known risk factor for SGA. A systematic review revealed that the risk of intrauterine growth restriction was three times higher in women over the age of 35 [16,17]. Another study found that a low BMI would also increase the occurrence of SGA [18]. Voskamp et al. reported that women who have delivered an SGA infant are more likely to have subsequent SGA deliveries, and that women who were born as SGA themselves are likely to give birth to SGA infants [19,20]. Many studies have confirmed that the occurrence of SGA is closely related to certain maternal habits, such as smoking, alcohol consumption, and drug use. Both active smoking and exposure to secondhand smoke before or during pregnancy can increase the risk of SGA [21-23]. In addition, the incidence of SGA is associated with excessive alcohol consumption, while low to moderate alcohol intake does not appear to increase this risk [24,25]. Additionally, certain maternal health conditions can also elevate the risk of having an SGA infant. Expectant mothers who suffer from systemic illnesses like severe heart disease, chronic kidney issues, chronic hypertension, adrenal insufficiency, and antiphospholipid syndrome are at a heightened risk of giving birth to SGA babies [26-28]. Pregnancy complications, including gestational hypertension, diabetes, hypermesis gravidarum, placentae abruption, and preeclampsia, can also raise the risk of SGA [29-31].
Therefore, we conducted this meta-analysis to further verify the maternal factors influencing the incidence of SGA. The results from this analysis will provide suggestions for pregnant women to improve pregnancy outcomes and enhance the quality of their prenatal care.
Materials and methods
Search strategy
Following the PRISMA guidelines, a comprehensive literature search was conducted in the databases of Cochrane Library, PubMed, China National Knowledge Infrastructure (CNKI), Wan Fang and China Biology Medicine (CBM, SinoMed) from January 1, 2000, to October 1, 2022. We used the following search formulas: (((((((SGA) OR (fetal growth restriction)) OR (intrauterine growth retardation)) AND (mother)) OR (maternal)) AND (factor)) OR (risk)) AND (randomized controlled trial (RCT)). This Meta-analysis was registered at INPLASY (International Platform of Registered Systematic Review and Meta-Analysis Protocols, 202410045).
Selection criteria
Studies were eligible for inclusion based on the following criteria: (1) They included both SGA and non-SGA participant groups; (2) They were reported as randomized controlled trials (RCTs); (3) They provided adequate information necessary for conducting a meta-analysis; (4) They were published works with full-text access. Studies were excluded if they: (1) lacked a control group; (2) were animal studies, case reports, or reviews; (3) did not present relevant data; (4) were not accessible in full text; (5) involved duplicated data or research groups; or (6) were unrelated to the topic of interest.
Based on the inclusion and exclusion criteria, two authors (You Lu and Di Qie) independently reviewed all abstracts and articles to determine their eligibility for inclusion in this meta-analysis. In cases of disagreement, a third author (Jinhui Wu) was consulted to make the decision. All information extracted from the included articles were verified by all authors.
Data extraction
The information extracted from the selected studies included the first author’s name, year of publication, country of origin, sample size, and various maternal health factors. These factors encompassed maternal age and BMI, history of multiple pregnancies, as well as detrimental habits such as smoking and alcohol consumption. Additionally, any maternal health issues, including pregnancy-related conditions and associated complications, were documented. All information was recorded in a data collection form and validated by You Lu and Di Qie. In cases of disagreement, Fan Yang was consulted to assess the conflicting data and help achieve consensus. The quality of the studies included in the analysis was evaluated utilizing the Cochrane Collaboration risk of bias tool encompassing the aspects of sequence generation, allocation concealment, blinding of participants and healthcare professionals, blinding of outcome assessors, handling of incomplete data, selective outcome reporting, and other potential sources of bias. Based on these criteria, studies were categorized into three risk categories: ‘high risk’, ‘unclear risk’, and ‘low risk’ [32].
Statistical analysis
All statistical analyses were conducted with STATA 14.0 (College Station, Texas, USA). The analysis focused on dichotomous data, with the association between maternal factors and SGA expressed as relative risk (RR) with 95% confidence intervals (CI). An RR was deemed significant when CI did not include 1. Heterogeneity across the studies was assessed using the I2 statistic and p-values. I2 less than or equal to 50% or p greater than 0.1 indicated no significant heterogeneity, and a fixed-effect model was employed. In the presence of significant heterogeneity (I2 greater than 50% or p-value less than 0.1), a random-effects model was applied, and subgroup analyses were conducted to uncover potential sources of variance. Furthermore, in the presence of significant heterogeneity, a sensitivity analysis was conducted by sequentially excluding one study at a time to assess the robustness of the findings. To detect publication bias, Begg’s test and a funnel plot analysis were performed, with publication bias considered significant if the p-value was below 0.05.
Results
Literature search and evaluation
Initially, 4,711 potentially relevant articles were obtained according to the search strategy. Among these, 334 studies with full-text access were carefully screened for eligibility, and 15 studies were finally included in this meta-analysis (Figure 1). As shown in Table 1, this study involved a total of 601,495 infants, of whom 41,446 were considered to be SGA.
Figure 1.
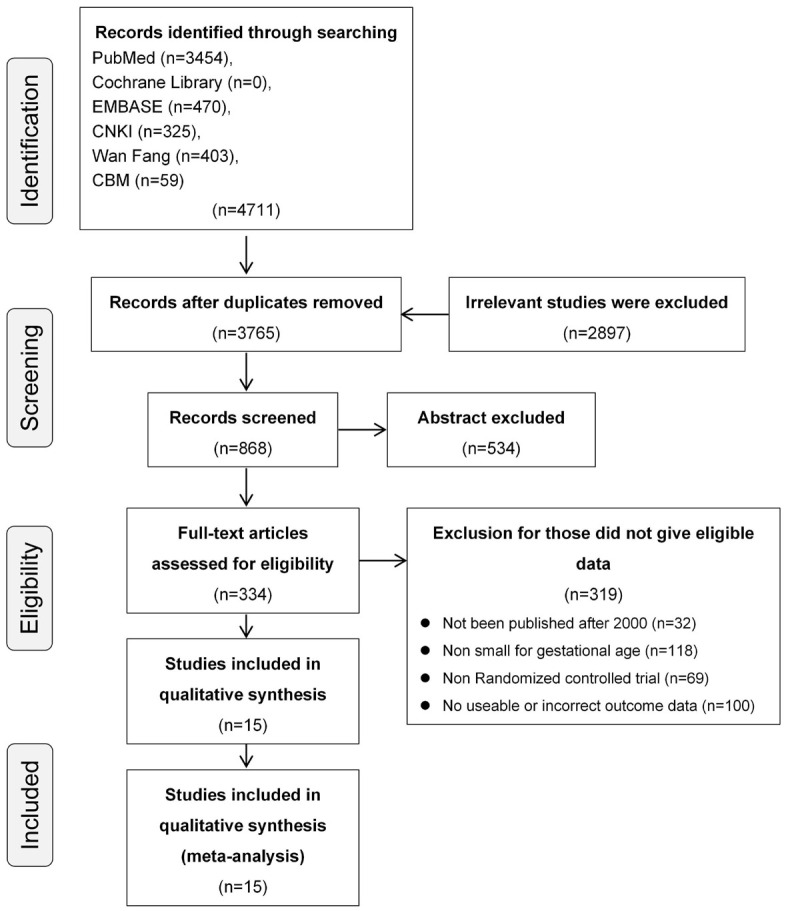
Flow diagram of literature selection.
Table 1.
Characteristics of the studies included
| Study | Year | Country | Sample size (SGA/AGA) | General conditions | Bad habits | Disease | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Age >35 (SGA/AGA) | Abnormal BMI (SGA/AGA) | Multipara (SGA/AGA) | Smoking (SGA/AGA) | Alcohol intake (SGA/AGA) | Prepregnancy (SGA/AGA) | Pregnancy complication (SGA/AGA) | ||||
| Iwama [45] | 2022 | Japan | 1126/15947 | 323/4298 | 582/7966 | - | 49/363 | 218/3072 | Diabetes, 4/59; SLE or APS, 4/27; CKD, 3/48; TD, 29/398 | HDP, 124/722; GD, 28/410 |
| Giuseppe [46] | 2021 | Italy | 10/90 | - | - | - | 2/10 | 1/18 | - | HDP, 2/3; GD, 2/8 |
| Souza [47] | 2020 | Brazil | 2481/20173 | - | 341/2644 | 1136/10898 | - | - | Hypertension, 65/454; Diabetes, 12/194 | Preeclampsia, 418/1878; GD, 127/1510 |
| Barquiel [48] | 2019 | Spain | 287/2390 | - | 90/807 | - | 62/496 | - | Hypertension, 13/48 | Preeclampsia, 3/21; HDP, 27/121 |
| Bartal [49] | 2019 | America | 426/1889 | 55/373 | 240/1103 | 197/1120 | 79/246 | - | Hypertension, 205/559 | GD, 20/232 |
| Galiano [50] | 2018 | Spain | 518/518 | - | - | - | 149/80 | 189/229 | - | Preeclampsia, 46/11 |
| Eskes [51] | 2017 | Netherlands | 162/465470 | 40/96819 | 37/133 | 81/254973 | 44/83 | - | Hypertension, 18/33506 | - |
| Fisher [52] | 2017 | America | 1045/10019 | 164/1349 | 418/4482 | - | 149/818 | - | Hypertension, 21/892 | GD, 47/448 |
| Leng [53] | 2016 | China | 164/1408 | - | - | 59/553 | - | - | - | - |
| Tul [54] | 2016 | Slovenija | 736/6928 | - | 125/1046 | - | - | - | Diabetes, 3/23; Hypertension, 27/110 | Preeclampsia, 178/518; GD, 23/310 |
| Milidou [55] | 2014 | Denmark | 6007/54149 | 3244/32002 | - | 2204/28753 | 2325/13050 | - | Diabetes, 6/162; Hypertension, 3/162; TD, 108/975 | Preeclampsia, 336/1462; GD, 36/650 |
| Zhang [56] | 2009 | China | 57/122 | - | - | 15/2 | - | - | TD, 10/3; Diabetes, 8/25; Hypertension, 4/1; Autoimmune disease, 2/0 | - |
| Rodrigues [57] | 2007 | Portugal | 342/3538 | 29/226 | - | 179/1714 | 55/293 | - | Chronic diseases, 62/456 | - |
| Tsukamoto [58] | 2007 | Japan | 250/2722 | 30/327 | 54/635 | 143/1277 | 59/433 | - | - | - |
| Chiaffarino [59] | 2006 | Italy | 555/1966 | 112/382 | - | - | 141/255 | 252/834 | - | HDP, 120/103 |
SGA: small for gestational age; AGA: average for gestational age; HDP: hypertensive disorders of pregnancy; GD: gestational diabetes; SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome; CKD: chronic kidney disease; TD: thyroid disease.
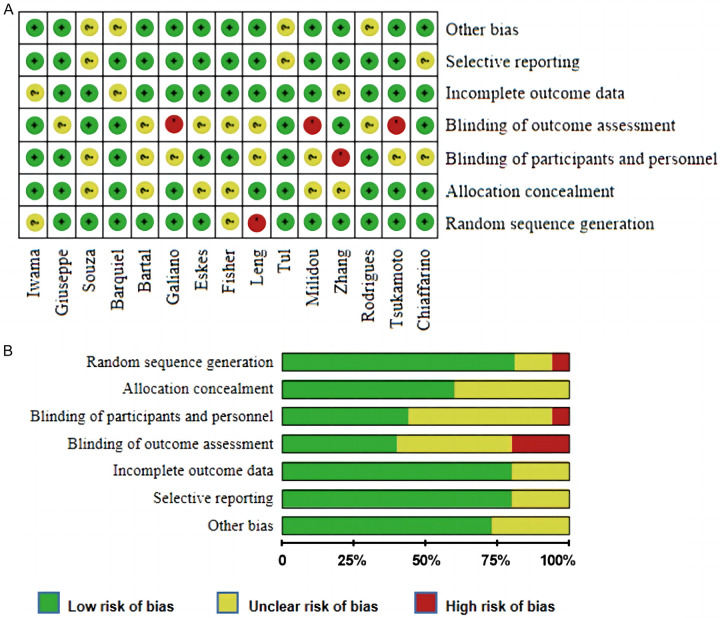
The quality of the included literature was assessed, as shown in Figure 2. Two studies were rated as ‘unclear risk’ for sequence generation, 1 for ‘high risk’ and 12 for ‘low risk’. For allocation concealment, nine studies were evaluated as ‘low risk’, while the remaining studies were assessed as ‘unclear’. Only one study was assessed as ‘high risk’ to blinding participants and personnel, while 3 were assessed as ‘high risk’ for blinding the outcome assessment. For incomplete outcome data and selective reporting, 12 articles were categorized as ‘low risk’.
Figure 2.
Quality and bias assessments. A. Risk of bias for each RCT; B. Risk of bias summary.
The relationship between general maternal conditions and SGA infants
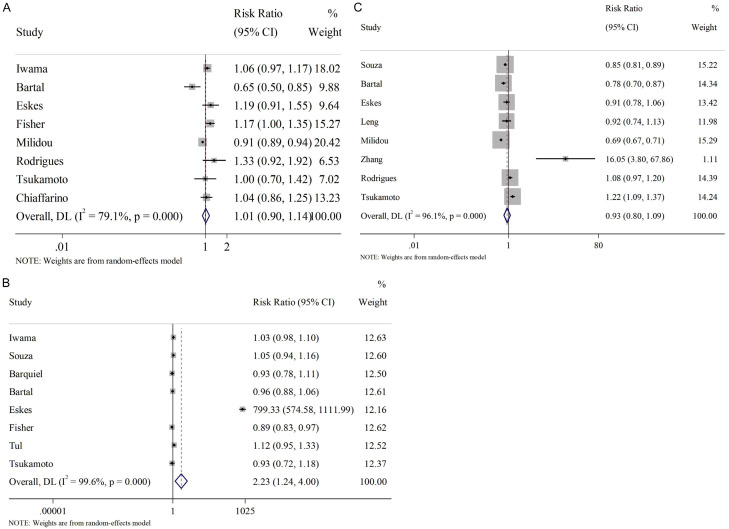
The influence of general maternal conditions, such as age, BMI, and number of pregnancies on the incidence of SGA infants was analyzed. Regarding age, a threshold of 35 years old was set. The results indicated that maternal age had little effect on SGA occurrence (RR=1.01, 95% CI [0.90, 1.14], Figure 3A), and no correlation was observed between multipara and SGA (RR=0.93, 95% CI [0.80, 1.09], Figure 3C). However, substantial heterogeneity was observed in these analyses, with I2 =79.1% and 96.1%, respectively. Therefore, a random-effect model was adopted. Conversely, as shown in Figure 3B, abnormal BMI appears to positively affect the occurrence of SGA (RR=2.23, 95% CI [1.24, 4.00]); while significant heterogeneity was also noted here (I2 =99.6%, P<0.001), warranting the use of random-effects model.
Figure 3.
Relationship between general maternal conditions and SGA infants. A. Age >35; B. Abnormal BMI; C. Multipara. SGA: small for gestational age.
The relationship between maternal habits and SGA infants
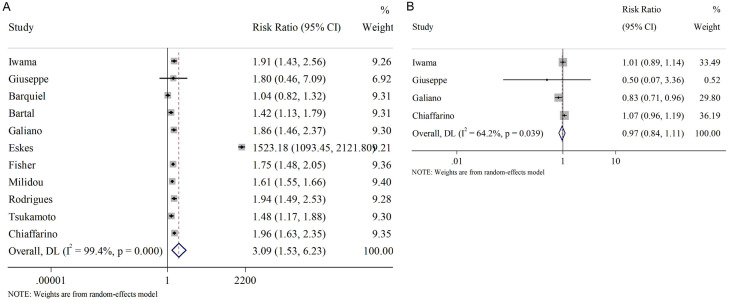
The association between unhealthy maternal habits and SGA were subsequently analyzed. As Figure 4 shows, a significant heterogeneity (random-effect model) was observed in both meta-analyses regarding smoking (Figure 4A) and alcohol intake (Figure 4B), with I2 =99.4% and 64.2%, respectively. The results indicated a positive association between smoking and SGA (RR=3.09, 95% CI [1.53, 6.23]), while no obvious correlation was found between SGA and alcohol intake (RR=0.97, 95% CI [0.84, 1.11]).
Figure 4.
Relationship between maternal bad habits and SGA infants. A. Smoking; B. Alcohol intake. SGA: small for gestational age.
The relationship between pre-maternal diseases and SGA infants
We also evaluated whether preexisting diseases, such as diabetes, hypertension, and thyroid disease, before pregnancy influence the occurrence of SGA. Initially, we considered all three pre-pregnancy conditions together and found no overall correlation between these diseases and SGA (RR=1.18, 95% CI [0.86, 1.61], Figure 5A).
Figure 5.
Relationship between pre-pregnancy diseases and SGA infants. A. Overall pre-pregnancy diseases; B. Subgroup analysis of pre-pregnancy diseases. SGA: small for gestational age.
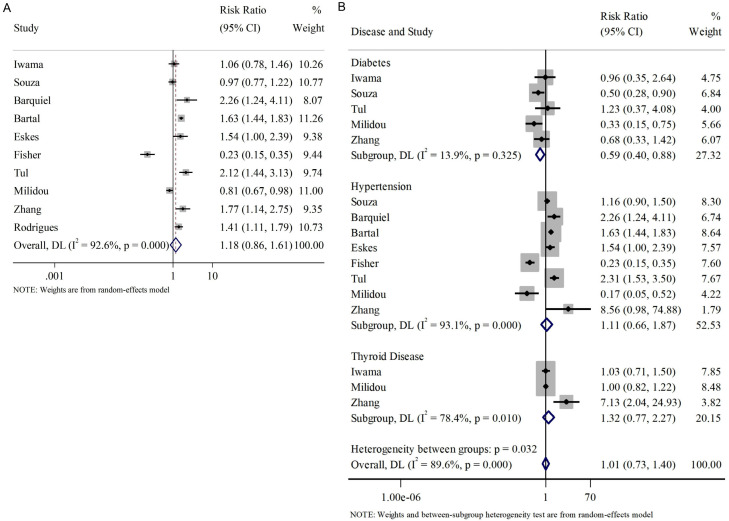
Subsequently, a subgroup analysis was performed to further investigate the association of each disease with SGA (Figure 5B). The results indicated that pre-maternal diabetes was negatively associated with SGA (RR=0.59, 95% CI [0.40, 0.88], fixed effect model). However, no significant relationship was observed between SGA and pre-maternal hypertension or thyroid disease (hypertension: RR=1.11, 95% CI [0.66, 1.87]; thyroid disease: RR=1.32, 95% CI [0.77, 2.27], random-effect models).
The relationship between pregnancy-related complications and SGA infants
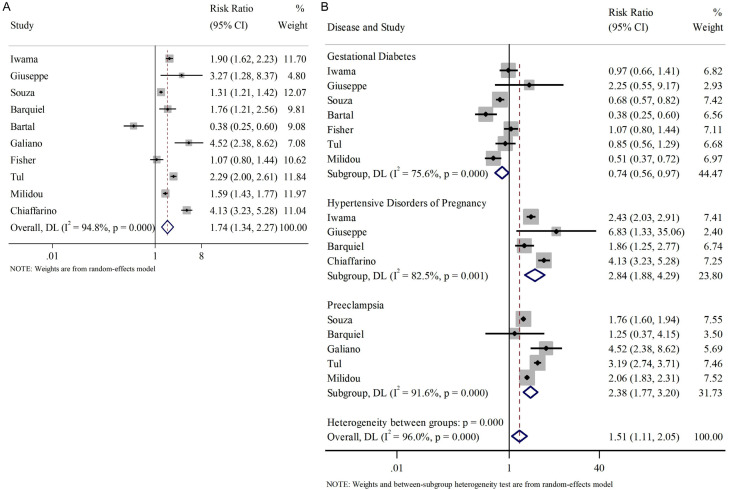
We also investigated the influence of pregnancy-induced complications on the occurrence of SGA. The complications studied here included gestational diabetes, gestational hypertension, and preeclampsia. Similarly, we analyzed these pregnancy-induced complications together and found an overall correlation with the incidence of SGA (RR=1.74, 95% CI [1.34, 2.27], Figure 6A).
Figure 6.
Relationship between pregnancy-related complications and SGA infants. A. Overall pregnancy-related complications; B. Subgroup analysis of pregnancy-related complications. SGA: small for gestational age.
Regarding the subgroup analysis, we found that gestational diabetes negatively affected the incidence of SGA (RR=0.74, 95% CI [0.56, 0.97], random effect model); while gestational hypertension and preeclampsia were positively associated with the incidence of SGA (RR=2.84, 95% CI [1.88, 4.29]; RR=2.38, 95% CI [1.77, 3.20], random effect models), as shown in Figure 6B.
Sensitivity analysis
Significant heterogeneity was observed in the meta-analysis concerning smoking. To determine the potential sources, a sensitivity analysis was performed, and the results are presented in Table 2. After excluding Wang et al.’s study [29], although some heterogeneity remained, the I2 decreased from 99.4% to 63.9%, and the positive relationship between smoking and SGA persisted (RR=1.61, 95% CI [1.56, 1.67]). This suggests that the heterogeneity was primarily driven by Eske’s and the results were robust.
Table 2.
Sensitivity analysis for smoking
| Excluded study | I2 | p | RR (95% CI) |
|---|---|---|---|
| Iwama [45] | 99.5% | <0.001 | 1.64 (1.59, 1.69) |
| Giuseppe [46] | 99.5% | <0.001 | 1.64 (1.59, 1.69) |
| Barquiel [48] | 99.5% | <0.001 | 1.66 (1.61, 1.72) |
| Bartal [49] | 99.5% | <0.001 | 1.65 (1.59, 1.70) |
| Galiano [50] | 99.5% | <0.001 | 1.64 (1.58, 1.69) |
| Eskes [51] | 63.9% | <0.001 | 1.61 (1.56, 1.67) |
| Fisher [52] | 99.5% | <0.001 | 1.64 (1.58, 1.69) |
| Milidou [55] | 99.4% | <0.001 | 1.77 (1.64, 1.91) |
| Rodrigues [57] | 99.5% | <0.001 | 1.64 (1.58, 1.69) |
| Tsukamoto [58] | 99.5% | <0.001 | 1.64 (1.59, 1.70) |
| Chiaffarino [59] | 99.5% | <0.001 | 1.63 (1.58, 1.68) |
Similarly, sensitivity analyses were also performed for the meta-analysis of pregnancy complications (Table 3). Notably, the I2 of studies reporting hypertension during pregnancy decreased from 94.8% to 35.5% after excluding Heaman’s study [37], while the positive association with SGA remained significant (RR=2.33, 95% CI [1.98, 2.74]). In contrast, for meta-analyses involving all pregnancy complications, gestational diabetes, and preeclampsia, the I2 value showed little change regardless of which study was excluded.
Table 3.
Sensitivity analysis for pregnancy complications
| Excluded study | All | Gestational Diabetes | Hypertensive disorders of pregnancy | Preeclampsia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| I2 | P | RR (95% CI) | I2 | p | RR (95% CI) | I2 | p | RR (95% CI) | I2 | p | RR (95% CI) | |
| Iwama [45] | 95.3% | <0.001 | 1.53 (1.45, 1.62) | 77.8% | <0.001 | 0.68 (0.60, 0.77) | 83.1% | 0.003 | 3.33 (2.71, 4.08) | - | - | - |
| Giuseppe [46] | 95.3% | <0.001 | 1.56 (1.49, 1.64) | 77.7% | <0.001 | 0.69 (0.62, 0.78) | 87.5% | <0.001 | 2.80 (2.45, 3.21) | - | - | - |
| Souza [47] | 93.9% | <0.001 | 1.76 (1.65, 1.88) | 79.7% | <0.001 | 0.71 (0.60, 0.83) | - | - | - | 88.1% | <0.001 | 2.39 (2.18, 2.62) |
| Barquiel [48] | 95.4% | <0.001 | 1.56 (1.48, 1.64) | - | - | - | 84.2% | 0.002 | 2.99 (2.59, 3.46) | 93.6% | <0.001 | 2.08 (1.94, 2.22) |
| Bartal [49] | 93.9% | <0.001 | 1.63 (1.55, 1.71) | 69.3% | 0.006 | 0.74 (0.65, 0.84) | - | - | - | - | - | - |
| Galiano [50] | 95.1% | <0.001 | 1.55 (1.47, 1.63) | - | - | - | - | - | - | 92.9% | <0.001 | 2.04 (1.91, 2.18) |
| Fisher [52] | 95.2% | <0.001 | 1.59 (1.51, 1.67) | 69.7% | 0.005 | 0.65 (0.57, 0.74) | - | - | - | - | - | - |
| Tul [54] | 94.4% | <0.001 | 1.49 (1.41, 1.58) | 79.5% | <0.001 | 0.69 (0.61, 0.78) | - | - | - | 74.2% | <0.001 | 1.92 (1.78, 2.07) |
| Milidou [55] | 95.4% | <0.001 | 1.56 (1.47, 1.65) | 75.4% | <0.001 | 0.74 (0.65, 0.84) | - | - | - | 93.7% | <0.001 | 2.08 (1.92, 2.26) |
| Chiaffarino [59] | 93.0% | <0.001 | 1.50 (1.42, 1.58) | - | - | - | 35.3% | 0.213 | 2.33 (1.98, 2.74) | - | - | - |
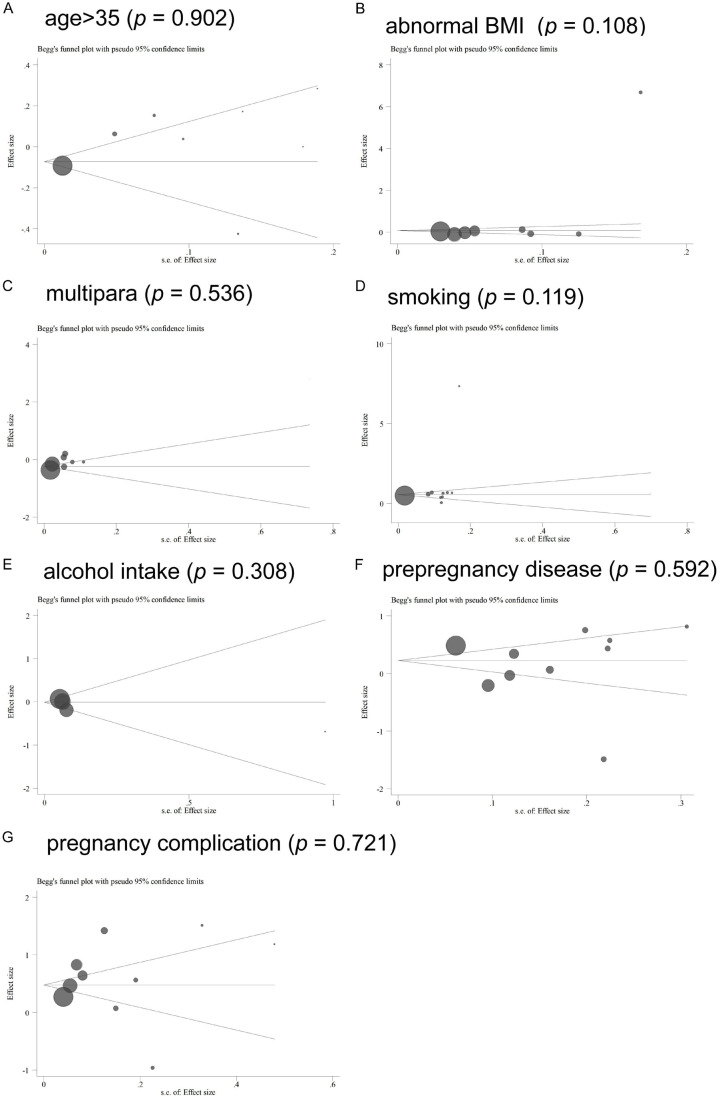
Publication bias
As shown in Figure 7, Begg’s test was adopted to assess possible publication bias. In general, the funnel plots appeared symmetrical in Figure 7A, 7C, 7E-G, but not in Figure 7B and 7D. According to the Begg’s test, no significant publication bias was found in all meta-analyses (age: P=0.902; BMI: P=0.108; multipara: P=0.536; smoking: P=0.119; alcohol intake: P=0.308; pre-maternal disease: P=0.592; pregnancy complication: P=0.108).
Figure 7.
Funnel plots of publication bias. A. Age >35; B. Abnormal BMI; C. Multipara; D. Smoking; E. Alcohol intake; F. Pre-pregnancy diseases; G. Pregnancy-related complications.
Discussion
Studies have shown that the perinatal mortality and prevalence of SGA are significantly higher than those of appropriate for gestational age infants (AGA). Additionally, SGA infants often exhibit lower brain development, resulting in inferior cognitive function and intellectual development in adulthood [33,34]. Understanding SGA risk factors is crucial for healthcare providers to identify high-risk pregnancies, implement perinatal health education targeting modifiable risk factors, prevent the occurrence of SGA, and reduce adverse effects. This meta-analysis primarily focuses on maternal factors.
The research findings on the influence of maternal age on SGA are inconsistent. A retrospective study analyzing the incidence of SGA in 1,393 pregnant women up to the age of 25 years concluded that there was no significant association between age and SGA [35]. Furthermore, Odibo et al. found that the incidence of SGA increased with age in pregnant women over 35 years [36]. This meta-analysis involved 8 studies investigating the association between age and SGA, and the result showed that maternal age over 35 years did not increase the risk of SGA. Heaman et al. found that pre-pregnancy BMI of less than 18 kg/m2 or gestational weight gain of less than 9.1 kg were associated with an increase in SGA [37]. The normal BMI range for women is between 18.5 and 24 kg/m2. In our study, we analyzed the correlation between abnormal BMI and SGA, demonstrating that abnormal BMI is positively related to the incidence of SGA (RR=2.23, 95% CI [1.24, 4.00]). Furthermore, we discovered that multipara is not associated with SGA occurrence.
Studies have shown that 18% of SGA cases are associated with maternal smoking [38], and our study also confirms this (RR=3.09, 95% CI [1.53, 6.23]). However, the correlation between alcohol intake and SGA in pregnant women remains controversial. While some studies believe that alcohol consumption during pregnancy is significantly associated with SGA [39,40], others have found no such link [41]. Our meta-analysis did not find any significant association between maternal alcohol intake and SGA.
Some pregnancy-related diseases are also closely related to SGA. Studies have found that gestational hypertension and preeclampsia are important factors contributing the occurrence of SGA [42,43]. Our study yielded similar results, showing that both gestational hypertension and preeclampsia were positively related to SGA, with RR=2.84, 95% CI [1.88, 4.29] and RR=2.38, 95% CI [1.77, 3.20], respectively. Furthermore, we found that both pre-pregnancy and gestational diabetes were negatively associated with SGA (pregestational: RR=0.59, 95% CI [0.40, 0.88]; gestational: RR=0.74, 95% CI [0.56, 0.97]). However, diabetes was shown to be related to large gestational age (LGA) [44].
Inevitably, this study has some limitations that shouldn’t be ignored. First, some analyses, such as those concerning alcohol intake and thyroid disease, were based on a limited number of studies. Second, the high heterogeneity observed in several analyses may have impacted the reliability of this meta-analysis. Third, publication bias could potentially affect the authenticity of the conclusions of this paper. Despite the these limitations, this study still holds value in highlighting key areas for pregnant women to focus on for optimal fetal development and health outcomes.
Conclusions
In conclusion, this meta-analysis provides insights into maternal risk factors, such as abnormal BMI, smoking, and pregnancy complications, which may increase the risk of SGA. These findings underscore the importance for pregnant women to focus on maintaining a healthy weight, quitting smoking, and preventing pregnancy-related complications to reduce the risk of SGA.
Acknowledgements
This research was supported by the Department of Science and Technology of Sichuan Province - Metagenomics and metabolomics study of the gut microbiota in Han children with autism spectrum disorder in Sichuan, China (No. 2021YFS0113).
Disclosure of conflict of interest
None.
References
- 1.Ardissino M, Morley AP, Slob EAW, Schuermans A, Rayes B, Raisi-Estabragh Z, de Marvao A, Burgess S, Rogne T, Honigberg MC, Ng FS. Birth weight influences cardiac structure, function, and disease risk: evidence of a causal association. Eur Heart J. 2024;45:443–454. doi: 10.1093/eurheartj/ehad631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horng HC, Lee WL, Wang PH. Maternal weight gain and birth weight. J Chin Med Assoc. 2021;84:741–742. doi: 10.1097/JCMA.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson PM. Birth weight and hypertension: nature or nurture? J Hypertens. 2023;41:909–911. doi: 10.1097/HJH.0000000000003443. [DOI] [PubMed] [Google Scholar]
- 4.Lees CC, Romero R, Stampalija T, Dall’Asta A, DeVore GA, Prefumo F, Frusca T, Visser GHA, Hobbins JC, Baschat AA, Bilardo CM, Galan HL, Campbell S, Maulik D, Figueras F, Lee W, Unterscheider J, Valensise H, Da Silva Costa F, Salomon LJ, Poon LC, Ferrazzi E, Mari G, Rizzo G, Kingdom JC, Kiserud T, Hecher K. Clinical opinion: the diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am J Obstet Gynecol. 2022;226:366–378. doi: 10.1016/j.ajog.2021.11.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, Berghella V, Nazareth A, Tahlak M, McIntyre HD, Da Silva Costa F, Kihara AB, Hadar E, McAuliffe F, Hanson M, Ma RC, Gooden R, Sheiner E, Kapur A, Divakar H, Ayres-de-Campos D, Hiersch L, Poon LC, Kingdom J, Romero R, Hod M. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. 2021;152(Suppl 1):3–57. doi: 10.1002/ijgo.13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fetal growth restriction: ACOG practice bulletin, number 227. Obstet Gynecol. 2021;137:e16–e28. doi: 10.1097/AOG.0000000000004251. [DOI] [PubMed] [Google Scholar]
- 7.Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LE, Christian P, Clarke SE, Ezzati M, Fawzi W, Gonzalez R, Huybregts L, Kariuki S, Kolsteren P, Lusingu J, Marchant T, Merialdi M, Mongkolchati A, Mullany LC, Ndirangu J, Newell ML, Nien JK, Osrin D, Roberfroid D, Rosen HE, Sania A, Silveira MF, Tielsch J, Vaidya A, Willey BA, Lawn JE, Black RE CHERG SGA-Preterm Birth Working Group. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson NJ, Lai MM, Starkman HE, Colditz PB, Wixey JA. Electroencephalographic studies in growth-restricted and small-for-gestational-age neonates. Pediatr Res. 2022;92:1527–1534. doi: 10.1038/s41390-022-01992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang KCW, James AL. Small for gestational age at term and adult lung function. Respirology. 2023;28:99–100. doi: 10.1111/resp.14414. [DOI] [PubMed] [Google Scholar]
- 10.Zhen L, Li DZ. Intrauterine growth restriction or small for gestational age? A simple question, but a complex answer. Am J Obstet Gynecol. 2023;229:570–571. doi: 10.1016/j.ajog.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Martinez S, Delgado JL, Paules C, Cavallaro A, De Paco C, Villar J, Papageorghiou A, Oros D. Clinical phenotypes for risk stratification in small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2022;59:490–496. doi: 10.1002/uog.23765. [DOI] [PubMed] [Google Scholar]
- 12.Meler E, Mazarico E, Peguero A, Gonzalez A, Martinez J, Boada D, Vellvé K, Arca G, Gómez-Roig MD, Gratacós E, Figueras F. Death and severe morbidity in isolated periviable small-for-gestational-age fetuses. BJOG. 2023;130:485–493. doi: 10.1111/1471-0528.17181. [DOI] [PubMed] [Google Scholar]
- 13.Pasquini L, Masini G, Cagninelli G, Polimeno T, Fratelli N, Fichera A, Prefumo F. Small-for-gestational-age fetus diagnosed in the second trimester: possible etiologies and short-term neonatal outcomes. Acta Obstet Gynecol Scand. 2023;102:1749–1755. doi: 10.1111/aogs.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzilai R, Bronshtein M, Steinberg M, Weiner Z, Gover A. Small for gestational age: the familial perspective. J Matern Fetal Neonatal Med. 2022;35:3840–3844. doi: 10.1080/14767058.2020.1841160. [DOI] [PubMed] [Google Scholar]
- 15.Molony CL, Hiscock R, Kaufman J, Keenan E, Hastie R, Brownfoot FC. Growth trajectory of preterm small-for-gestational-age neonates. J Matern Fetal Neonatal Med. 2022;35:8400–8406. doi: 10.1080/14767058.2021.1974835. [DOI] [PubMed] [Google Scholar]
- 16.Arya S, Mulla ZD, Plavsic SK. Outcomes of women delivering at very advanced maternal age. J Womens Health (Larchmt) 2018;27:1378–1384. doi: 10.1089/jwh.2018.7027. [DOI] [PubMed] [Google Scholar]
- 17.Gaudineau A. Prevalence, risk factors, maternal and fetal morbidity and mortality of intrauterine growth restriction and small-for-gestational age. J Gynecol Obstet Biol Reprod (Paris) 2013;42:895–910. doi: 10.1016/j.jgyn.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. 2015;28:1679–1686. doi: 10.3109/14767058.2014.964675. [DOI] [PubMed] [Google Scholar]
- 19.Voskamp BJ, Kazemier BM, Ravelli AC, Schaaf J, Mol BW, Pajkrt E. Recurrence of small-for-gestational-age pregnancy: analysis of first and subsequent singleton pregnancies in the Netherlands. Am J Obstet Gynecol. 2013;208:374, e371–376. doi: 10.1016/j.ajog.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Castrillio SM, Rankin KM, David RJ, Collins JW Jr. Small-for-gestational age and preterm birth across generations: a population-based study of Illinois births. Matern Child Health J. 2014;18:2456–2464. doi: 10.1007/s10995-014-1484-1. [DOI] [PubMed] [Google Scholar]
- 21.Shittu AAT, Kumar BP, Okafor U, Berkelhamer SK, Goniewicz ML, Wen X. Changes in e-cigarette and cigarette use during pregnancy and their association with small-for-gestational-age birth. Am J Obstet Gynecol. 2022;226:730.e1–730.e10. doi: 10.1016/j.ajog.2021.11.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Lee NL, Burstyn I. Exposure-response analysis of the association of maternal smoking and use of electronic cigarettes (vaping) in relation to preterm birth and small-for-gestational-age in a national US sample, 2016-2018. Glob Epidemiol. 2022;4:100079. doi: 10.1016/j.gloepi.2022.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor EJ, Doh P, Ziauddeen N, Godfrey KM, Berrington A, Alwan NA. Maternal smoking behaviour across the first two pregnancies and small for gestational age birth: analysis of the SLOPE (Studying Lifecourse Obesity PrEdictors) population-based cohort in the South of England. PLoS One. 2021;16:e0260134. doi: 10.1371/journal.pone.0260134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundsberg LS, Illuzzi JL, Belanger K, Triche EW, Bracken MB. Low-to-moderate prenatal alcohol consumption and the risk of selected birth outcomes: a prospective cohort study. Ann Epidemiol. 2015;25:46–54. e43. doi: 10.1016/j.annepidem.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louw KA. Substance use in pregnancy: the medical challenge. Obstet Med. 2018;11:54–66. doi: 10.1177/1753495X17750299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L SI, H B, Eo O, M CB. Maternal risk factors for small-for-gestational-age newborns in Mexico: analysis of a nationwide representative cohort. Front Public Health. 2021;9:707078. doi: 10.3389/fpubh.2021.707078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørbye IK, Haualand R, Wiull H, Letting AS, Langesaeter E, Estensen ME. Maternal beta-blocker dose and risk of small-for gestational-age in women with heart disease. Acta Obstet Gynecol Scand. 2022;101:794–802. doi: 10.1111/aogs.14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu S, Wang W, Li Q, Huang L, Chen X, Zhang X, Wang X, Han W, Hu X, Yang X, Hao L, Xiong G, Yang N. Association of maternal longitudinal hemoglobin with small for gestational age during pregnancy: a prospective cohort study. Nutrients. 2022;14:1403. doi: 10.3390/nu14071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LW, Lin HC, Tsai ML, Chang YT, Chang YC. Preterm birth and small for gestational age potentiate the association between maternal hypertensive pregnancy and childhood autism spectrum disorder. Sci Rep. 2023;13:9606. doi: 10.1038/s41598-023-36787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. doi: 10.1136/bmj-2021-067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giommi C, Lombό M, Montik N, Paolucci M, Notarstefano V, Delli Carpini G, Ciavattini A, Ragusa A, Maradonna F, Giorgini E, Carnevali O. Gestational diabetes mellitus and small-for-gestational-age: an insight into the placental molecular biomarkers. Int J Mol Sci. 2023;24:2240. doi: 10.3390/ijms24032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya-Kanamori L, Xu C, Hasan SS, Doi SA. Quality versus Risk-of-Bias assessment in clinical research. J Clin Epidemiol. 2021;129:172–175. doi: 10.1016/j.jclinepi.2020.09.044. [DOI] [PubMed] [Google Scholar]
- 33.Sadler L, Anderson N, Crengle S, McCowan L. Reduction in perinatal mortality among small for gestational age babies in New Zealand. Aust N Z J Obstet Gynaecol. 2021;61:505–512. doi: 10.1111/ajo.13299. [DOI] [PubMed] [Google Scholar]
- 34.Hogeveen M, Blom HJ, den Heijer M. Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr. 2012;95:130–136. doi: 10.3945/ajcn.111.016212. [DOI] [PubMed] [Google Scholar]
- 35.Stewart CP, Katz J, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr, Christian P. Preterm delivery but not intrauterine growth retardation is associated with young maternal age among primiparae in rural Nepal. Matern Child Nutr. 2007;3:174–185. doi: 10.1111/j.1740-8709.2007.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. Am J Perinatol. 2006;23:325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 37.Heaman M, Kingston D, Chalmers B, Sauve R, Lee L, Young D. Risk factors for preterm birth and small-for-gestational-age births among Canadian women. Paediatr Perinat Epidemiol. 2013;27:54–61. doi: 10.1111/ppe.12016. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JM, Clark PM, Robinson E, Becroft DM, Pattison NS, Glavish N, Pryor JE, Wild CJ, Rees K, Mitchell EA. Risk factors for small-for-gestational-age babies: the Auckland Birthweight Collaborative Study. J Paediatr Child Health. 2001;37:369–375. doi: 10.1046/j.1440-1754.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- 39.Loke YJ, Muggli E, Saffery R, Ryan J, Lewis S, Elliott EJ, Halliday J, Craig JM. Sex- and tissue-specific effects of binge-level prenatal alcohol consumption on DNA methylation at birth. Epigenomics. 2021;13:1921–1938. doi: 10.2217/epi-2021-0285. [DOI] [PubMed] [Google Scholar]
- 40.Cooper DL, Petherick ES, Wright J. The association between binge drinking and birth outcomes: results from the Born in Bradford cohort study. J Epidemiol Community Health. 2013;67:821–828. doi: 10.1136/jech-2012-202303. [DOI] [PubMed] [Google Scholar]
- 41.Weile LKK, Hegaard HK, Wu C, Tabor A, Wolf HT, Kesmodel US, Henriksen TB, Nohr EA. Alcohol intake in early pregnancy and spontaneous preterm birth: a cohort study. Alcohol Clin Exp Res. 2020;44:511–521. doi: 10.1111/acer.14257. [DOI] [PubMed] [Google Scholar]
- 42.Mishima S, Mitsui T, Tani K, Maki J, Eto E, Hayata K, Washio Y, Yoshimoto J, Tsukahara H, Masuyama H. Short stature in small-for-gestational-age offspring born to mothers with hypertensive disorders of pregnancy. Hypertens Pregnancy. 2023;42:2187623. doi: 10.1080/10641955.2023.2187623. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Wang X, Chen Z, Zhang F. Gestational hypertensive disease and small for gestational age infants in twin pregnancy: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2022;48:2677–2685. doi: 10.1111/jog.15401. [DOI] [PubMed] [Google Scholar]
- 44.McGrath RT, Glastras SJ, Hocking SL, Fulcher GR. Large-for-gestational-age neonates in type 1 diabetes and pregnancy: contribution of factors beyond hyperglycemia. Diabetes Care. 2018;41:1821–1828. doi: 10.2337/dc18-0551. [DOI] [PubMed] [Google Scholar]
- 45.Iwama N, Obara T, Ishikuro M, Murakami K, Ueno F, Noda A, Onuma T, Matsuzaki F, Hoshiai T, Saito M, Metoki H, Sugawara J, Yaegashi N, Kuriyama S. Risk scores for predicting small for gestational age infants in Japan: the TMM birthree cohort study. Sci Rep. 2022;12:8921. doi: 10.1038/s41598-022-12892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Giuseppe R, Bocchi M, Maffoni S, Del Bo E, Manzoni F, Cerbo RM, Porri D, Cena H. Mediterranean diet and lifestyle habits during pregnancy: is there an association with small for gestational age infants? An Italian single centre experience. Nutrients. 2021;13:1941. doi: 10.3390/nu13061941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza RT, Vieira MC, Esteves-Pereira AP, Domingues R, Moreira MEL, da Cunha Filho EV, Sandall J, Cecatti JG, do Carmo Leal M, Dias MAB, Pasupathy D. Risk stratification for small for gestational age for the Brazilian population: a secondary analysis of the Birth in Brazil study. Sci Rep. 2020;10:14725. doi: 10.1038/s41598-020-71252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barquiel B, Herranz L, Martínez-Sánchez N, Montes C, Hillman N, Bartha JL. Increased risk of neonatal complications or death among neonates born small for gestational age to mothers with gestational diabetes. Diabetes Res Clin Pract. 2020;159:107971. doi: 10.1016/j.diabres.2019.107971. [DOI] [PubMed] [Google Scholar]
- 49.Fishel Bartal M, Chen HY, Blackwell SC, Chauhan SP, Sibai BM. Neonatal morbidity in late preterm small for gestational age neonates. J Matern Fetal Neonatal Med. 2021;34:3208–3213. doi: 10.1080/14767058.2019.1680630. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Galiano JM, Amezcua-Prieto C, Salcedo-Bellido I, Olmedo-Requena R, Bueno-Cavanillas A, Delgado-Rodriguez M. Alcohol consumption during pregnancy and risk of small-for-gestational-age newborn. Women Birth. 2019;32:284–288. doi: 10.1016/j.wombi.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Eskes M, Waelput AJM, Scherjon SA, Bergman KA, Abu-Hanna A, Ravelli ACJ. Small for gestational age and perinatal mortality at term: an audit in a Dutch national cohort study. Eur J Obstet Gynecol Reprod Biol. 2017;215:62–67. doi: 10.1016/j.ejogrb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Fisher SC, Van Zutphen AR, Romitti PA, Browne ML National Birth Defects Prevention Study. Maternal hypertension, antihypertensive medication use, and small for gestational age births in the National Birth Defects Prevention Study, 1997-2011. Matern Child Health J. 2018;22:237–246. doi: 10.1007/s10995-017-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leng J, Hay J, Liu G, Zhang J, Wang J, Liu H, Yang X, Liu J. Small-for-gestational age and its association with maternal blood glucose, body mass index and stature: a perinatal cohort study among Chinese women. BMJ Open. 2016;6:e010984. doi: 10.1136/bmjopen-2015-010984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tul N, Lasic M, Bricelj K, Bregar AT, Verdenik I, Lucovnik M, Blickstein I. Outcome of small for gestational age preterm singletons: a population-based cohort study. J Perinat Med. 2016;44:941–944. doi: 10.1515/jpm-2015-0321. [DOI] [PubMed] [Google Scholar]
- 55.Milidou I, Søndergaard C, Jensen MS, Olsen J, Henriksen TB. Gestational age, small for gestational age, and infantile colic. Paediatr Perinat Epidemiol. 2014;28:138–145. doi: 10.1111/ppe.12095. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YL, Liu JT, Gao JS, Yang JQ, Bian XM. Influential and prognostic factors of small for gestational age infants. Chin Med J (Engl) 2009;122:386–389. [PubMed] [Google Scholar]
- 57.Rodrigues T, Barros H. Comparison of risk factors for small-for-gestational-age and preterm in a Portuguese cohort of newborns. Matern Child Health J. 2007;11:417–424. doi: 10.1007/s10995-007-0195-2. [DOI] [PubMed] [Google Scholar]
- 58.Tsukamoto H, Fukuoka H, Koyasu M, Nagai Y, Takimoto H. Risk factors for small for gestational age. Pediatr Int. 2007;49:985–990. doi: 10.1111/j.1442-200X.2007.02494.x. [DOI] [PubMed] [Google Scholar]
- 59.Chiaffarino F, Parazzini F, Chatenoud L, Ricci E, Sandretti F, Cipriani S, Caserta D, Fedele L. Alcohol drinking and risk of small for gestational age birth. Eur J Clin Nutr. 2006;60:1062–1066. doi: 10.1038/sj.ejcn.1602419. [DOI] [PubMed] [Google Scholar]