Abstract
Objective: To evaluate the therapeutic effects of bisphosphonates (specifically zoledronic acid) combined with calcitriol on osteoporosis (OP) induced by endocrine therapy for breast cancer. Methods: A retrospective analysis was performed on the clinical data from 150 patients with OP induced by endocrine therapy for breast cancer, who were admitted to Yuebei People’s Hospital from May 2020 to March 2022. Patients were divided into two groups based on their treatment regimens: 78 patients received oral calcitriol alone (control group), and 72 patients received zoledronic acid combined with calcitriol (study group). Clinical efficacy, pain severity, bone metabolism, bone mineral density (BMD), quality of life, and medication safety were compared between the two groups. Results: After 12 months of treatment, the total effective rate was significantly higher in the study group compared to the control group (95.83% vs. 79.49%, P < 0.05). Visual analogue scale (VAS) scores decreased progressively at 3, 6, and 12 months in both groups, with the study group showing significantly lower scores (all P < 0.05). Serum alkaline phosphatase (ALP) levels and BMD of the hip, femoral neck, and lumbar spine significantly increased after 12 months in both groups. Scores on the Quality of Life Questionnaire of the European Foundation for Osteoporosis 41 (QUALEFFO-41) decreased significantly in both groups, with greater improvements seen in the study group (P < 0.05). Compared to the control group, the study group showed increased serum calcium and decreased serum phosphorus after 12 months (both P < 0.05). Fracture Risk Assessment Tool (FRAX) scores decreased at 12 and 24 months in both groups, with lower scores in the study group (both P < 0.05). No adverse events were observed in either group during the treatment period. Conclusion: The combination of bisphosphonates (zoledronic acid) and calcitriol is effective in treating OP induced by endocrine therapy for breast cancer. This combination therapy can regulate bone metabolism, enhance BMD, improve quality of life, and reduce fracture risk, demonstrating a favorable safety profile.
Keywords: Breast cancer, calcitriol, bisphosphonates, osteoporosis
Introduction
Breast cancer is a hormone-dependent tumor, with cell proliferation and differentiation regulated by various hormones, particularly estrogen. Estrogen stimulates the proliferation and differentiation of most breast cancer cells, playing a direct role in the onset and progression of the disease [1]. Endocrine therapy, which inhibits estrogen secretion, is widely used to alleviate symptoms and prolong survival in breast cancer patients, demonstrating proven efficacy [2,3]. However, endocrine therapy can disrupt bone metabolism, reduce bone mineral density (BMD), and lead to osteoporosis (OP), thereby adversely affecting treatment outcomes, reducing quality of life, and negatively impacting prognosis [4]. Consequently, effectively managing OP induced by endocrine therapy in breast cancer patients is crucial.
Calcitriol is commonly used in clinical practice to treat OP, as it promotes intestinal calcium absorption and regulates bone mineralization [5,6]. Despite its benefits, the treatment regimen requires further optimization. Recent studies indicate that bisphosphonates, known for inhibiting bone resorption and increasing bone mass, are effective in treating conditions like osteoporosis, osteoarthritis, hypercalcemia due to malignant bone metastases, and bone pain [7,8]. However, there is a lack of systematic clinical trials evaluating the combined use of bisphosphonates and calcitriol for treating OP induced by endocrine therapy in breast cancer patients. This study aims to systematically assess the efficacy of bisphosphonates (zoledronic acid) combined with calcitriol in treating OP caused by endocrine therapy for breast cancer, providing a reference for developing clinical treatment protocols. The study findings are summarized below.
Materials and methods
General information
A retrospective analysis was conducted on the clinical data from 150 patients with OP induced by endocrine therapy for breast cancer, who were admitted to Yuebei People’s Hospital between May 2020 and March 2022. Patients were grouped based on their treatment modalities: 78 patients received oral calcitriol and were designated as the control group, while 72 patients who received a combination of zoledronic acid and calcitriol and were designated as the study group. This study was approved by the Ethics Committee of Yuebei People’s Hospital.
Patient selection
Inclusion criteria: (1) Patients diagnosed with breast cancer and OP, where OP was induced by endocrine therapy such as tamoxifen or anastrozole [9,10]. (2) Patients with complete clinical data. (3) Patients with a survival time of more than 6 months. (4) Patients without tumor metastasis.
Exclusion criteria: (1) Patients with a prior history of OP. (2) Pregnant or lactating women. (3) Patients allergic to calcitriol, zoledronic acid, or other drugs used in this study. (4) Patients who used medications affecting bone metabolism within 6 months prior to enrollment. (5) Patients with other malignant tumors or severe organ dysfunction. (6) Patients with conditions affecting bone metabolism, such as hyperthyroidism. (7) Patients with immune system diseases or severe infectious diseases.
Methods
Control group: Patients were treated with calcitriol (Chengdu Tiantaishan Pharmaceutical Co., Ltd., Country Medicine Accurate Character Number: H20041946) at a dose of 0.5 μg, taken orally twice daily for 12 months.
Study group: Patients received a combination of calcitriol and bisphosphonate zoledronic acid (Sichuan Hairong Pharmaceutical Co., Ltd., Country Medicine Accurate Character Number: H20183098). Calcitriol administration was identical to that in the control group. Zoledronic acid was administered at a dose of 5 mg, dissolved in 250 ml of 0.9% NaCl solution, and given via intravenous infusion every 3 months for 12 months.
Observation indicators
Clinical efficacy [10]: Significant efficacy: Visual analogue scale (VAS) scores decreased by more than 70% compared to baseline, with a significant increase in BMD as shown by dual-energy X-ray absorptiometry (DEXA). Effective: VAS scores decreased by 30%-70% compared to baseline, without affecting daily activities or sleep, and DEXA showed no decrease in BMD. Ineffective: Did not meet the criteria for significant efficacy or effectiveness. The total effective rate is the sum of the significant efficacy and effective rates.
Pain severity: Pain was assessed using VAS scores before treatment and at 3, 6, and 12 months post-treatment. Scores range from 0 to 10, with higher scores indicating greater pain severity [11].
Bone metabolism [12]: Blood samples (3 ml fasting venous blood) were collected from both groups before and 12 months after treatment. Serum levels of calcium, phosphorus, and alkaline phosphatase (ALP) were measured using automated biochemical analyzers (Roche Diagnostic Products Ltd.).
BMD [13]: BMD levels at the hip, femoral neck, and lumbar spine were measured using DEXA before and 12 months after treatment.
Quality of life: The Quality of Life Questionnaire of the European Foundation for Osteoporosis 41 (QUALEFFO-41) was used to assess quality of life before and 12 months after treatment [14]. The questionnaire includes 7 dimensions, with scores ranging from 0 to 100; lower scores indicate better quality of life.
Fracture risk: The Fracture Risk Assessment Tool (FRAX) was used to calculate fracture risk coefficients before treatment and at 12 and 24 months post-treatment [15].
Medication safety: Adverse reactions were monitored and recorded for both groups.
Statistical methods
SPSS 23.0 software was used for statistical analysis. Categorical data (efficacy, medication safety) were expressed as n (%) and analyzed using the χ2 test. Continuous variables (VAS scores, bone metabolism indicators, BMD, QUALEFFO-41 scores) were expressed as mean ± standard deviation (x̅ ± sd) and analyzed using the t-test. Repeated measures ANOVA was used to analyze VAS scores over multiple time points. A P-value of < 0.05 was considered statistically significant.
Results
Comparison of general data
There were no statistically significant differences between the two groups regarding mean age, TNM stage, pathological type, or tumor site (all P > 0.05). See Table 1.
Table 1.
Comparison of baseline data between the two groups
| Group | Age (years) | TNM stage (stage I/II, n) | Pathologic type (invasive ductal carcinoma/mucinous carcinoma/invasive lobular carcinoma, n) | Tumor site (left/right, n) |
|---|---|---|---|---|
| Control group (n=78) | 58.84±5.37 | 35/43 | 55/8/15 | 40/38 |
| Study group (n=72) | 59.31±5.08 | 30/42 | 47/8/17 | 39/33 |
| χ2/t | 0.550 | 0.157 | 0.513 | 0.125 |
| P | 0.583 | 0.692 | 0.774 | 0.724 |
Comparison of clinical efficacy
After 12 months of treatment, the total effective rate in the study group was 95.83%, significantly higher than the 79.49% observed in the control group (P < 0.05). See Table 2.
Table 2.
Comparison of clinical efficacy between the two groups n (%)
| Group | Visible effectiveness | Effective | Ineffective | Total effectiveness |
|---|---|---|---|---|
| Control group (n=78) | 42 (53.85) | 20 (25.64) | 16 (20.51) | 62 (79.49) |
| Study group (n=72) | 49 (68.06) | 20 (27.78) | 3 (4.17) | 69 (95.83) |
| χ2 | 9.043 | |||
| P | 0.003 |
Comparison of pain severity
Before treatment, there was no significant difference in pain severity between the two groups (P > 0.05). However, VAS scores gradually decreased in both groups after 3, 6, and 12 months of treatment, with the study group showing significantly lower scores (all P < 0.05). See Figure 1.
Figure 1.
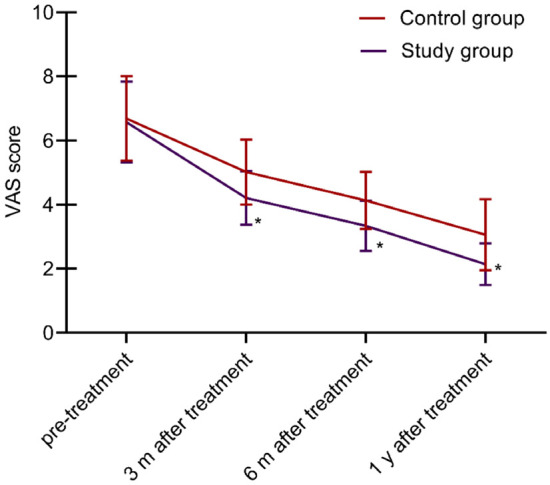
Comparison of pain levels between the two groups. This image shows a gradual decrease in VAS scores after 3, 6, and 12 months of treatment in both groups, with the study group showing lower scores. Note: VAS: visual analogue scale. Compared with Pre-treatment, *P < 0.05.
Comparison of bone metabolism indexes
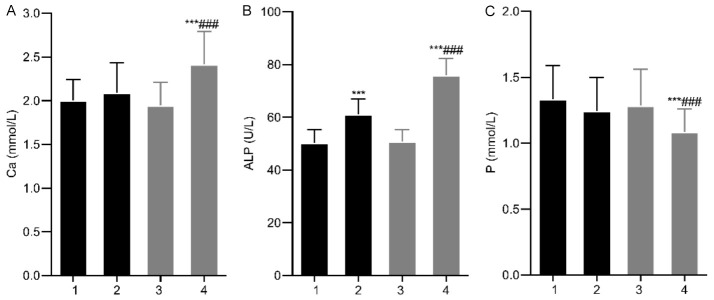
No significant differences in bone metabolism indexes were observed between the two groups before treatment (P > 0.05). After 12 months, ALP levels significantly increased in both groups, with higher levels in the study group (P < 0.05). The study group also showed a significant increase in calcium and a decrease in phosphorus compared to the control group (all P < 0.05). See Figure 2.
Figure 2.
Comparison of bone metabolism indexes between the two groups. A: Ca; B: ALP; C: P. Note: 1. Control group before treatment; 2. Control group after treatment; 3. Study group before treatment; 4. Study group after treatment. Compared with Pre-treatment, ***P < 0.001; Compared with the control group, ###P < 0.001. Ca, calcium; P, blood phosphorus; ALP, alkaline phosphatase.
Comparison of BMD at various sites
Before treatment, there were no significant differences in BMD at various sites between the two groups (all P > 0.05). After 12 months, BMD significantly increased at the hip, femoral neck, and lumbar spine in both groups, with the study group showing higher BMD levels (all P < 0.05). See Table 3.
Table 3.
Comparison of BMD at different locations between the two groups (x̅ ± Sd)
| Group | Hip | Femoral neck | Lumbar spine | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Pre-treatment | 12 m after treatment | Pre-treatment | 12 m after treatment | Pre-treatment | 12 m after treatment | |
| Control group (n=78) | 0.65±0.14 | 0.78±0.19* | 0.66±0.10 | 0.75±0.15* | 0.59±0.11 | 0.83±0.18* |
| Study group (n=72) | 0.68±0.16 | 0.92±0.22* | 0.64±0.08 | 0.91±0.17* | 0.60±0.13 | 0.95±0.20* |
| t | 1.224 | 4.180 | 1.345 | 6.122 | 0.510 | 3.867 |
| P | 0.223 | < 0.001 | 0.181 | < 0.001 | 0.611 | < 0.001 |
Note: Compared with Pre-treatment;
P < 0.05.
BMD, bone mineral density.
Comparison of quality of life
QUALEFFO-41 scores were not significantly different between the groups before treatment (P > 0.05). After 12 months, both groups showed significant reductions in QUALEFFO-41 scores, with the study group having lower scores (P < 0.05). See Table 4.
Table 4.
Comparison of quality of life between the two groups (x̅ ± Sd)
| Variable | Control group (n=78) | Study group (n=72) | ||
|---|---|---|---|---|
|
|
|
|||
| Pre-treatment | 12 m after treatment | Pre-treatment | 12 m after treatment | |
| Self-care ability | 7.52±1.78 | 5.11±1.21* | 7.69±1.83 | 3.45±1.13*,# |
| Somatic pain | 16.85±2.65 | 11.34±1.74* | 17.12±2.81 | 8.02±1.35*,# |
| Daily activities | 7.96±1.35 | 5.66±1.21* | 7.82±1.46 | 4.02±1.08*,# |
| Domestic work | 16.25±2.38 | 10.57±1.59* | 16.77±2.19 | 7.43±1.34*,# |
| Mental state | 15.39±2.34 | 8.45±1.97* | 15.71±2.50 | 6.32±1.31*,# |
| Social event | 18.15±2.65 | 13.05±1.76* | 17.89±2.39 | 9.12±1.34*,# |
| Health concept | 6.39±1.37 | 4.87±1.14* | 6.58±1.43 | 3.12±1.05*,# |
Note: Compared with Pre-treatment;
P < 0.05.
Compared with control group;
P < 0.05.
Comparison of fracture risk
There was no significant difference in FRAX fracture risk coefficients between the two groups before treatment (P > 0.05). Both groups experienced a gradual reduction in FRAX scores after 12 and 24 months of treatment, with significantly lower scores in the study group (both P < 0.05). See Table 5.
Table 5.
Comparison of fracture risk between the two groups (x̅ ± Sd, %)
| Group | Pre-treatment | 12 m after treatment | 24 m after treatment |
|---|---|---|---|
| Control group (n=78) | 3.85±0.56 | 3.35±0.47* | 3.05±0.32* |
| Study group (n=72) | 3.81±0.61 | 2.84±0.42* | 2.24±0.28* |
| t | 0.419 | 6.986 | 16.440 |
| P | 0.676 | < 0.001 | < 0.001 |
Note: Compared with Pre-treatment;
P < 0.05.
Comparison of medication safety
No adverse effects were observed in either group during the treatment period.
Discussion
Breast cancer remains one of the most prevalent tumors in women, with an increasing incidence and a trend towards affecting younger patients. With advancements in medical technology, endocrine therapy has become a primary treatment modality for breast cancer. This therapy controls estrogen levels, inhibits hormone aromatization, reduces estrogen receptor levels, or blocks their expression, effectively alleviating symptoms [16]. The commonly used drugs in endocrine therapy are aromatase inhibitors and estrogen receptor antagonists, which reduces estrogen secretion, alters the tumor cell environment, and inhibits tumor growth. However, reduced estrogen levels also decrease osteoblast activity and increase osteoclast resorption, leading to bone loss, secondary OP and a higher risk of fractures [17,18]. While calcitriol is widely used in OP treatment due to its ability to promote calcium absorption and regulate bone mineralization, its efficacy as a monotherapy is limited [19]. Therefore, identifying effective strategies to improve clinical outcomes for osteoporosis induced by endocrine therapy for breast cancer is crucial.
Bisphosphonates, such as zoledronic acid and alendronate sodium, have become first-line treatments for OP due to their ability to inhibit bone resorption and increase bone mass [20]. This study evaluated the effects of combining bisphosphonates (zoledronic acid) with calcitriol in treating OP induced by endocrine therapy for breast cancer. Results indicated that, compared to the control group, the study group showed higher overall clinical efficacy, improved QUALEFFO-41 scores, and lower VAS scores at 3, 6, and 12 months of treatment. Wang et al. found that bisphosphonates combined with calcitriol enhanced immune function, corrected bone metabolism abnormalities, and increased BMD in patients with OP induced by endocrine therapy for breast cancer [21]. Their study highlighted the efficacy of ibandronate, which aligns with the findings of our study using zoledronic acid, although their research involved alendronate and ibandronate [21].
Our study demonstrates that combining zoledronic acid with calcitriol further enhances treatment efficacy, alleviates pain, and improves quality of life for patients. This can be attributed to the following reasons: Calcitriol, a vitamin D analog, promotes the intestinal absorption of calcium and phosphorus, facilitating bone metabolism, accelerating mineralization, increasing BMD, and reducing bone pain [22]. Zoledronic acid targets osteoclasts, inhibiting their activity and inducing apoptosis. By binding to bone, it reduces osteoclast-mediated bone resorption and destruction of mineralized bone and cartilage [23]. Additionally, zoledronic acid inhibits the release of tumor-stimulating factors, further hindering osteoclast activity, reducing calcium release from bones, and effectively increasing bone mass [24]. The combination of these two drugs may exert a synergistic effect through their distinct mechanisms, enhancing overall efficacy. However, further studies are needed to compare the efficacy of different bisphosphonates specifically.
The clinical findings indicate that bone metabolism disorders are integral to the pathogenesis of OP. Bone metabolism is regulated through the coordinated actions of osteoblasts, which are responsible for bone formation, and osteoclasts, which mediate bone resorption. Under normal conditions, these processes are balanced. However, when osteoclast activity surpasses that of osteoblasts, bone loss occurs, leading to OP. Bone metabolism indicators are effective in reflecting these changes [25]. Calcium and phosphorus are key indicators of bone metabolism. Calcium deficiency significantly contributes to bone loss, while phosphorus imbalance can be seen in various metabolic bone diseases. ALP, primarily produced by osteoblasts, has protease activity and plays a direct role in the mineralization process of bones.
The results of this study show that after 12 months of treatment, the bone metabolism indicators in the study group were significantly better than those in the control group, with higher BMD at the hip, femoral neck, and lumbar spine. This suggests that the combination of bisphosphonates (zoledronic acid) and calcitriol effectively improves bone metabolism and increases BMD. This improvement is attributed to zoledronic acid’s ability to inhibit osteoclast activity, induce osteoclast apoptosis, and suppress bone resorption, thereby enhancing bone metabolism and increasing BMD [26,27]. Additionally, this study demonstrated that FRAX fracture risk scores decreased gradually over 12 and 24 months of treatment, with the study group showing significantly lower scores. These findings suggest that the combination of zoledronic acid and calcitriol may help reduce the risk of fractures in patients, likely due to zoledronic acid’s effectiveness in increasing BMD and enhancing bone strength.
However, this study has certain limitations, including a relatively small sample size, reliance on a single data source, and the use of basic observation indicators. Future research should expand the study size to allow for more comprehensive exploration.
In summary, the combination of bisphosphonates (zoledronic acid) and calcitriol for treating OP induced by endocrine therapy for breast cancer has proven to be an effective treatment option. It regulates bone metabolism, increases BMD, improves quality of life, reduces fracture risk, and demonstrates good medication safety.
Disclosure of conflict of interest
None.
References
- 1.Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, Huang CS, Huober J, Jaliffe GG, Cicin I, Tolaney SM, Goetz MP, Rugo HS, Senkus E, Testa L, Del Mastro L, Shimizu C, Wei R, Shahir A, Munoz M, San Antonio B, André V, Harbeck N, Martin M monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidard FC, Kaklamani VG, Neven P, Streich G, Montero AJ, Forget F, Mouret-Reynier MA, Sohn JH, Taylor D, Harnden KK, Khong H, Kocsis J, Dalenc F, Dillon PM, Babu S, Waters S, Deleu I, García Sáenz JA, Bria E, Cazzaniga M, Lu J, Aftimos P, Cortés J, Liu S, Tonini G, Laurent D, Habboubi N, Conlan MG, Bardia A. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 2022;40:3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laws A, Punglia RS. Endocrine therapy for primary and secondary prevention after diagnosis of high-risk breast lesions or preinvasive breast cancer. J. Clin. Oncol. 2023;41:3092–3099. doi: 10.1200/JCO.23.00455. [DOI] [PubMed] [Google Scholar]
- 4.Yang S, Wang N, Wang J, Lix LM, Leslie WD, Yuan B. Association between prior cancer diagnosis and osteoporosis: a matched case-control study. Arch Osteoporos. 2022;17:112. doi: 10.1007/s11657-022-01152-3. [DOI] [PubMed] [Google Scholar]
- 5.Huang JF, Tan QC, Bai H, Wang J, Bergman M, Wu Z. Bone mineral density, osteopenia and osteoporosis among US adults with cancer. QJM. 2022;115:653–660. doi: 10.1093/qjmed/hcac015. [DOI] [PubMed] [Google Scholar]
- 6.Jing W, Dai Y, Zhu J, Deng S. Clinical efficacy and safety evaluation of calcitriol combined with bisphosphonates in the therapy of postmenopausal osteoporosis: based on a retrospective cohort study. Biomed Res Int. 2022;2022:2711938. doi: 10.1155/2022/2711938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Albert SG, Wood E. Meta-analysis of clinical fracture risk reduction of antiosteoporosis drugs: direct and indirect comparisons and meta-regressions. Endocr Pract. 2021;27:1082–1092. doi: 10.1016/j.eprac.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Han HW, Jeon NM, Lee JM, Kim JH. Post-pregnancy osteoporosis-related multiple vertebral fractures associated with post-partum thyroiditis: a CARE-compliant case report. Medicine (Baltimore) 2021;100:e27615. doi: 10.1097/MD.0000000000027615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Society of Breast Cancer China Anti-Cancer Association. Guidelines for breast cancer diagnosis and treatment by China Anti-cancer Association (2019 edition) Chin Oncol. 2019;29:609–679. [Google Scholar]
- 10.Osteoporosis and Bone Mineral Disease Branch of Chinese Medical Association. Guidelines for the diagnosis and management of primary osteoporosis (2017) Chin J Osteoporosis. 2019;25:281–309. [Google Scholar]
- 11.Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Xie L, Xu J, Lin X, Ye J, Shao R, Yao X. Changes in alkaline phosphatase, calcium, C-reactive protein, D-dimer, phosphorus and hemoglobin in elderly osteoporotic hip fracture patients. Ann Palliat Med. 2021;10:1079–1088. doi: 10.21037/apm-20-218. [DOI] [PubMed] [Google Scholar]
- 13.Syed Z, Khan A. Bone densitometry: applications and limitations. J Obstet Gynaecol Can. 2002;24:476–84. doi: 10.1016/s1701-2163(16)31095-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee YK, Kim HJ, Park JW, Won S, Hwang JS, Ha YC, Koo KH. Transcultural adaptation and psychometric properties of the Korean version of the quality of life questionnaire of the European foundation for osteoporosis (QUALEFFO-41) Arch Osteoporos. 2019;14:96. doi: 10.1007/s11657-019-0647-5. [DOI] [PubMed] [Google Scholar]
- 15.Vandenput L, Johansson H, McCloskey EV, Liu E, Åkesson KE, Anderson FA, Azagra R, Bager CL, Beaudart C, Bischoff-Ferrari HA, Biver E, Bruyère O, Cauley JA, Center JR, Chapurlat R, Christiansen C, Cooper C, Crandall CJ, Cummings SR, da Silva JAP, Dawson-Hughes B, Diez-Perez A, Dufour AB, Eisman JA, Elders PJM, Ferrari S, Fujita Y, Fujiwara S, Glüer CC, Goldshtein I, Goltzman D, Gudnason V, Hall J, Hans D, Hoff M, Hollick RJ, Huisman M, Iki M, Ish-Shalom S, Jones G, Karlsson MK, Khosla S, Kiel DP, Koh WP, Koromani F, Kotowicz MA, Kröger H, Kwok T, Lamy O, Langhammer A, Larijani B, Lippuner K, Mellström D, Merlijn T, Nordström A, Nordström P, O’Neill TW, Obermayer-Pietsch B, Ohlsson C, Orwoll ES, Pasco JA, Rivadeneira F, Schei B, Schott AM, Shiroma EJ, Siggeirsdottir K, Simonsick EM, Sornay-Rendu E, Sund R, Swart KMA, Szulc P, Tamaki J, Torgerson DJ, van Schoor NM, van Staa TP, Vila J, Wareham NJ, Wright NC, Yoshimura N, Zillikens MC, Zwart M, Harvey NC, Lorentzon M, Leslie WD, Kanis JA. Update of the fracture risk prediction tool FRAX: a systematic review of potential cohorts and analysis plan. Osteoporos Int. 2022;33:2103–2136. doi: 10.1007/s00198-022-06435-6. [DOI] [PubMed] [Google Scholar]
- 16.Bardia A, Aftimos P, Bihani T, Anderson-Villaluz AT, Jung J, Conlan MG, Kaklamani VG. EMERALD: phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019;15:3209–3218. doi: 10.2217/fon-2019-0370. [DOI] [PubMed] [Google Scholar]
- 17.Mbese Z, Aderibigbe BA. Bisphosphonate-based conjugates and derivatives as potential therapeutic agents in osteoporosis, bone cancer and metastatic bone cancer. Int J Mol Sci. 2021;22:6869. doi: 10.3390/ijms22136869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Gómez I, Gray SR, Ho FK, Petermann-Rocha F, Welsh P, Cleland J, Iliodromiti S, Ara I, Pell J, Sattar N, Ferguson LD, Celis-Morales C. Osteoporosis and its association with cardiovascular disease, respiratory disease, and cancer: findings from the UK biobank prospective cohort study. Mayo Clin Proc. 2022;97:110–121. doi: 10.1016/j.mayocp.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 19.BioMed Research International. Retracted: clinical efficacy and safety evaluation of calcitriol combined with bisphosphonates in the therapy of postmenopausal osteoporosis: based on a retrospective cohort study. Biomed Res Int. 2023;2023:9781281. doi: 10.1155/2023/9781281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolopoulos K, Moshi MR, Stringer D, Ma N, Jenal M, Vreugdenburg T. The clinical effectiveness of denosumab (Prolia®) in patients with hormone-sensitive cancer receiving endocrine therapy, compared to bisphosphonates, selective estrogen receptor modulators (SERM), and placebo: a systematic review and network meta-analysis. Arch Osteoporos. 2023;18:18. doi: 10.1007/s11657-023-01211-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Sun XD, Fan AL. Effect of bisphosphonates combined with calcitriol on bone mineral density of patients with osteoporosis due to endocrine therapy for breast cancer. J Clin Med Pract. 2021;25:85–88. 92. [Google Scholar]
- 22.Zhao F, Guo Z, Kwok LY, Zhao Z, Wang K, Li Y, Sun Z, Zhao J, Zhang H. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr. 2023;62:965–976. doi: 10.1007/s00394-022-03042-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Tan Y, Huang W, Luo H, Pan B, Wu S. Cardiovascular safety of zoledronic acid in the treatment of primary osteoporosis: a meta-analysis and systematic review. Semin Arthritis Rheum. 2024;64:152304. doi: 10.1016/j.semarthrit.2023.152304. [DOI] [PubMed] [Google Scholar]
- 24.Dito G, Lugaresi M, Degradi C, Guabello G, Longhi M, Corbetta S. Efficacy of switching from teriparatide to zoledronic acid or denosumab on bone mineral density and biochemical markers of bone turnover in older patients with severe osteoporosis: a real-life study. Endocrine. 2023;82:181–189. doi: 10.1007/s12020-023-03431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryhänen EM, Koski AM, Löyttyniemi E, Välimäki MJ, Kiviniemi U, Schalin-Jäntti C. Postoperative zoledronic acid for osteoporosis in primary hyperparathyroidism: a randomized placebo-controlled study. Eur J Endocrinol. 2021;185:515–524. doi: 10.1530/EJE-21-0322. [DOI] [PubMed] [Google Scholar]
- 26.Wang WY, Chen LH, Ma WJ, You RX. Drug efficacy and safety of denosumab, teriparatide, zoledronic acid, and ibandronic acid for the treatment of postmenopausal osteoporosis: a network meta-analysis of randomized controlled trials. Eur Rev Med Pharmacol Sci. 2023;27:8253–8268. doi: 10.26355/eurrev_202309_33586. [DOI] [PubMed] [Google Scholar]
- 27.Ward LM, Choudhury A, Alos N, Cabral DA, Rodd C, Sbrocchi AM, Taback S, Padidela R, Shaw NJ, Hosszu E, Kostik M, Alexeeva E, Thandrayen K, Shenouda N, Jaremko JL, Sunkara G, Sayyed S, Aftring RP, Munns CF. Zoledronic acid vs placebo in pediatric glucocorticoid-induced osteoporosis: a randomized, double-blind, phase 3 trial. J Clin Endocrinol Metab. 2021;106:e5222–e5235. doi: 10.1210/clinem/dgab458. [DOI] [PubMed] [Google Scholar]