Abstract
Objectives: Bisphenol A (BPA) is a representative environmental estrogen that is known to impair the function of the female reproductive system by affecting steroid hormones. However, not much research has been done on bisphenol S (BPS), which is an analogue of BPA and has been found in humans. Methods: We retrospectively studied 152 female volunteers at childbearing age who came to the Shandong Provincial Reproductive Medicine Center between January 2018 and January 2023. Anti-Müllerian hormone (AMH), follicle-stimulating hormone (FSH) and estradiol (E2) were used as indicators of their ovarian reserve function. Enzyme-linked immunosorbent assay (ELISA) was used to quantify the concentration of Bisabolol S in the serum of the enrolled volunteers. Linear regression models and logistic regression models were used to assess the relationship between urinary Bisphenol S levels and ovarian reserve indicators and decrease ovarian reserve (DOR) indicators, respectively. Restricted cubic spline (RCS) models were further used to explore potential non-linear associations. Results: The results showed that serum Bisabolol S levels were negatively correlated with AMH and E2 levels and positively correlated with FSH levels. After the volunteers were grouped and analysed according to the DOR diagnostic criteria,the serum BPA content in the DOR group was significantly higher than that in the non-DOR group (P < 0.05). Compared with the non-DOR group, the levels of anti-Müller test tube hormone (AMH) and estrogen (E2) were lower in the DOR group and the level of FSH was higher (P < 0.05). The serum BPS content in the DOR group was negatively correlated with AMH and E2. The concentration of FSH in women in the DOR group increased when the serum BPS content was also increased. Conclusions: Serum BPS is a potential risk factor for ovarian reserve function in women of reproductive age.
Keywords: Bisphenol S, reproductive, ovarian function
Introduction
Currently, approximately 186 million people worldwide suffer from infertility, the majority of whom are women of reproductive age in developing countries [1]. In China, between 1980 and 2012, the prevalence of infertility among newly married couples was 12.5% at one year and 6.6 at two years [2]. The etiology of infertility is complex and may include socio-demographic risk factors such as age, lower education, and employment, as well as clinical risk factors such as irregular menstrual cycles, low menstrual flow, and miscarriage [3]. Therefore, exploring the risk factors for infertility has become a hot research topic.
Compounds in the environment that can affect normal hormone synthesis, secretion, transport, and metabolism in organisms are called environmental endocrine-disrupting compounds (EDCs). The substances that can play an estrogen-like role in these environmental endocrine disruptors are called environmental estrogens. In the 1930s, researchers discovered that environmental estrogen can produce estrogen-like activity in the human body [4]. It can affect the secretion of sex hormones in the body through competitive binding of estrogen receptors and disrupt the homeostasis of the body. Bisphenol A (BPA, 2,2-bishydroxyphenylpropane) is one of the representative environmental estrogens (EEs) [5]. As an important chemical material, BPA has an annual output of more than 3 million tons [6]. BPA has a wide range of uses and is often widely added to daily necessities such as food packaging, stomatology materials, baby bottles, eyeglass materials, and medical devices [7]. When using inferior plastic containers or heating plastic containers at high temperatures, BPA is also released and absorbed by the body through the intestine in the digestive system. A cohort study of more than 5,000 people in Canada showed that more than 90% of the enrolled volunteers had detectable BPA in their urine [8]. In 2013, the US Centers for Disease Control and Prevention also detected BPA components in most of the samples it collected [9]. In recent years, researchers have successively detected BPA in human serum, plasma, milk and other body fluids [10]. Human epidemiological studies have shown that excessive intake of BPA may be related to the occurrence of breast cancer and prostate cancer. Moreover, daily exposure to BPA is also related to adverse pregnancy outcomes, such as maternal abortion, offspring birth defects, and developmental delay [11]. Animal experiments have found that exposure to environmental estrogen (BPA) can impair the reproductive system functions of experimental animals. For example, BPA can cause dysplasia of the sex organs (testicular defect, testicular weight loss) [12] and decrease of sperm quality (less sperm, weak sperm, etc.) [13] and sexual ability (low mating success rate [14], erectile dysfunction) [15], and sexual psychological changes (lag in sexual behavior) [16]. As a result, BPA has been banned in many countries, leading to the increasing use of its alternatives such as bisphenol F (BPF) and bisphenol S (BPS).
BPS is an organic synthetic compound with two sulfone phenol groups and the chemical formula (CH3)2C(C6H4SO2)2 [17]. Bisphenol S (BPS), an analogue of Bisphenol A, is considered as a safer substitute and is utilized in a diverse range of applications, such as food packaging, maternal and child products, personal care items, epoxy resins and currency bills. Recent studies have found that BPS is widely found in surface water [18], food [19], and house dust [20]. Therefore, the human body, mainly through the continuous intake of BPS from contaminated food, drinking water and living environment, the detection and levels of BPS in human serum, umbilical cord serum, urine and breast milk are increasing. Study also indicates potential associations between Bisphenol S exposure and serum uric acid levels, hyperuricemia and prevalence of gout in US adults [21], Bisphenol S exposure causes atopic dermatitis in pregnant women through DNA methylation [17], Bisphenol S was reported to impair ovarian and adipocyte function in experimental pigs [22]. In terms of human reproductive safety, the ovarian toxicity of Bisphenol S is similar to or greater than that of Bisphenol A due to its structural similarity to Bisphenol A [23]. However, there are no epidemiological studies that have investigated the relationship between Bisphenol S exposure and female fertility (ovarian reserve function).
Ovarian reserve function (OR), also known as ovarian reserve, refers to the potential of the ovary to produce the number of follicles and the quality of oocytes, which indirectly reflects the function of the ovary, and is an important indicator for assessing female fertility [24]. Currently, the main clinical indicators for assessing ovarian reserve are: follicle stimulating hormone (FSH), luteinising hormone (LH), antimullerian hormone (AMH), estradiol (E2), and the number of eggs in the ovary [25]. Among them, AMH is secreted by the granulosa cells of the ovarian antral sinus follicle and small sinus follicle, which is an important regulator of follicular growth and development and can be used to reflect follicle number. While FSH is a hormone produced by the anterior pituitary gland and plays an important role in follicle development and maturation. LH can synergistically promote follicular maturation and secretion of estrogen and progesterone. The FSH/LH ratio reflects the sign of ovarian age. Estradiol (E2), the most active estrogen, is produced by ovarian cells (granulosa cells and follicular membrane cells), and is therefore also used as a marker of ovarian reserve function [26].
The incidence of decrease ovarian reserve (DOR) is about 10% in the female population and is mainly influenced by age, ovarian surgery, pelvic radiotherapy or chemotherapy, smoking, infections, decreased blood supply to the ovaries, genes, and immune system abnormalities [27]. Studies have found that 10% of female infertility is attributed to DOR, and patients with DOR can progress to premature ovarian failure after 1-6 years without clinical intervention [28,29]. Although in vitro fertilization-embryo transfer (IVF-ET) has achieved significant results in the treatment of female infertility, clinical observations have found that patients with DOR have a lower pregnancy rate than non-DOR patients [30]. The rates of miscarriage and euploidy were higher than those of non-DOR patients. DOR has seriously affected female reproductive safety and eugenics. The living environment, family inheritance and various exogenous injuries may cause women to have DOR-related symptoms [31], but the specific cause of the disease is still unclear. Recent studies have found that [32] BPA can damage the functions of trophoblast cells and granulosa cells in the female reproductive system, but there is still no clinical evidence of environmental estrogen damage to the female reproductive system leading to female DOR. This study aims to explore the relationship between BPS and female DOR by detecting female serum BPS concentrations and collecting reproductive indicators so as to explore the associations between BPS exposure and ovarian reserve.
Methods
Study participants and data collection
We retrospectively studied 152 female volunteers of childbearing age attending the Shandong Provincial Reproductive Medicine Centre between January 2018 and January 2023. During the study period, the lifestyle, residential address, and occupation of female volunteers remained unchanged.
All female volunteers signed an informed consent form and filled out a questionnaire, and the personal privacy of the volunteers was strictly protected. The study was approved by the Medical Ethics Committee of Jinan Central Hospital in Shandong Province (KYLL2016219). Demographic and medical information collected through questionnaires or medical records included age, body mass index (BMI), living status, occupation, and smoking status.
Inclusion criteria
(1) Volunteers who have not used sex hormone drugs in the last 3 months; (2) Volunteers who do not have endocrine diseases such as hyperprolactinemia, polycystic ovary syndrome, endometriosis, thyroid or adrenal gland; (3) Volunteers who have no history of ovarian surgery; (4) Criteria for patients with DOR: ① Serum AMH < 1.1 ng/ml; ② FSH > 10 mIU/ml; ③ E2 < 20 pg/ml or > 80 pg/ml and AFC ≤ 5, which can be diagnosed if one of the above 3 is met. The definition of normal ovarian reserve function is as follows: ① AMH > 2.0 ng/ml; ② Basic FSH < 10 mIU/ml; ③ 20 pg/ml ≤ basic E2 ≤ 80 pg/ml and basic AFC > 5.
Serum specimen collection and testing
(1) Six milliliters of fasting blood was collected from the volunteers after menstruation, incubated at room temperature for 30 min, and stored in a refrigerator at 4°C for 4 h. The serum (approximately 3 ml) was withdrawn from the centrifuge tube, transferred into a 10-ml centrifuge tube, and centrifuged at 3000 g for 15 min at 4°C. After centrifugation, the supernatant was removed and transferred to a sterile centrifuge tube. Serum was stored in a -80°C ultralow temperature refrigerator.
(2) Follicle stimulating hormone (FSH), luteinising hormone (LH), antimyelinising hormone (AMH), and oestradiol (E2) in the sera of the volunteers were provided by the laboratory of the Department of Laboratory Medicine, Jinan Central Hospital, Jinan City, Shandong Province, and the above data were recorded with the volunteers’ consent. Serum BPS levels were detected using an ELISA kit (Amyjet Scientific) according to the instructions.
Statistical analysis
SPSS 21.0 software was used for statistical analysis. The measurement data with a normal distribution were analyzed using a t test, and the results are expressed as the mean and standard deviation. The mean is used to describe the central trend, the quartile method is used to describe the discrete trend, and the correlation analysis uses Spearman rank correlation. The distribution status of SPA was determined by calculating the mean, variance and deviation of SPA levels. The state of distribution of the data was determined by determining whether the data conformed to a normal distribution through the normality test method (Kolmogorov-Smirnov test). P < 0.05 means the difference is statistically significant.
Result
Characteristics of the volunteers
From January 2018 to January 2023, according to the screening criteria, a total of 152 volunteers’ demographic data and biological samples were collected (Table 1). The age range of the 152 volunteers was 19-47 years old, with an average age of 31.33 ± 5.716 years old. The average value of the BMI index was 23.95 ± 4.236 kg/m2. Most of the patients were from urban areas (85, 55.9%), and most of the volunteers had no history of smoking (137, 90.1%) and no occupational BPS exposure (133, 87.5%). The median values for the indicator of ovarian reserve were 6.39 mIU/mL for FSH, 1.22 ng/mL for AMH, and 20.07 pg/mL for E2, respectively.
Table 1.
Volunteers’ demographic characteristics
| Characteristics | Median | Mean | Range | Total (%) |
|---|---|---|---|---|
| Age (Years) | 30 | 31.33 ± 5.716 | 19-47 | |
| BMI (kg/m2) | 23.011 | 23.95 ± 4.236 | 10.9113-35.261 | |
| Residential Address | ||||
| Urban | 85 (55.9%) | |||
| Rural | 67 (44.1%) | |||
| Education | ||||
| High school and less | 94 (61.8%) | |||
| College | 38 (25%) | |||
| Above college | 20 (13.2%) | |||
| Smoking history | ||||
| Yes | 15 (9.9%) | |||
| No | 137 (90.1%) | |||
| Passive smoking | ||||
| Yes | 102 (67.1%) | |||
| No | 50 (32.9%) | |||
| Occupational exposure | ||||
| Yes | 19 (12.5%) | |||
| No | 133 (87.5%) | |||
| Plastic barreled water | ||||
| Yes | 87 (57.2%) | |||
| No | 65 (42.8%) | |||
| Usage of plastic cup | ||||
| Yes | 95 (62.5%) | |||
| No | 57 (37.5%) | |||
| AMH levels (ng/mL) | 1.22 | 2.134 ± 2.656 | 0.08-15.28 | |
| FSH levels (mIU/mL) | 6.39 | 8.70 ± 7.30 | 0.19-44.84 | |
| E2 levels (pg/mL) | 20.07 | 28.43 ± 39.92 | 0.02-338.30 |
E2, Oestradiol; AMH, Anti-Müllerian hormone; FSH, Follicle-stimulating hormone.
Distribution of bisphenol S concentrations
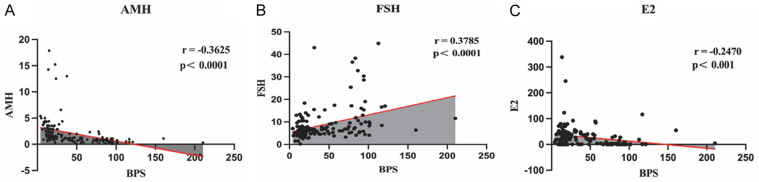
Correlation analysis modelling explored the relationship between blood Bisphenol S concentrations (Table 2) and ovarian reserve parameters (AMH, FSH and E2). The results showed that serum BPS concentration was negatively correlated with AMH and E2 levels, and positively correlated with FSH levels (Figure 1). BPS may be an important influence on ovarian reserve function.
Table 2.
Distribution of serum bisphenol S (BPS) levels (n=152)
| Geometric mean | Mean | 25th centile | 50th centile | 75th centile | |
|---|---|---|---|---|---|
| BPS (ng/mL) | 30.04 | 42.48 ± 36.09 | 14.87 | 24.67 | 73.17 |
Figure 1.
Correlation analysis modelling explored the relationship between blood Bisphenol S (BPS) concentrations and ovarian reserve parameters. A. Serum Bisphenol S (BPS) concentration was negatively correlated with Anti-Müllerian hormone (AMH). B. Serum Bisphenol S (BPS) concentration was negatively correlated with Follicle-stimulating hormone (FSH). C. Serum Bisphenol S (BPS) concentration was positively correlated with Oestradiol (E2).
DOR patient characteristics
According to the inclusion criteria, of the 152 female volunteers, there were 78 female volunteers with reduced ovarian function (DOR group) and 74 female volunteers with nondecreased ovarian function (non-DOR group). The average age of female volunteers in the DOR group was 32.051 ± 5.711 years, and the average age of females in the non-DOR group was 30.310 ± 5.586 years. The average BMI index of volunteers in the DOR group was 24.117 ± 4.251 kg/m2, and the average BMI index of the control group was 23.774 ± 4.242 kg/m2. There were significantly more volunteers in the DOR group with a smoking history and occupational exposure than in the non-DOR group (Table 3).
Table 3.
DOR patients’ characteristics
| Characteristics | DOR Group (n=78) | Non-DOR Group (n=72) | P |
|---|---|---|---|
| Age (Years) | 32.051 ± 5.711 | 30.310 ± 5.586 | P=0.059 |
| BMI (kg/m2) | 24.117 ± 4.251 | 23.774 ± 4.242 | P=0.619 |
| Residential Address | P=0.038 | ||
| Urban | 51 | 36 | |
| Rural | 28 | 39 | |
| Smoking | P < 0.05 | ||
| Yes | 11 | 5 | |
| No | 67 | 67 | |
| Occupational exposure | P < 0.05 | ||
| Yes | 13 | 7 | |
| No | 65 | 65 |
DOR, decrease ovarian reserve.
Serum BPS concentration and reproductive hormone levels
This study measured the concentration of female serum BPS in the DOR group and the non-DOR group (Table 4). The average BPS of the DOR group was 60.133 ± 37.823 µg/ml, and the average BPA of the non-DOR group was 23.878 ± 22.402 µg/ml. The serum BPS level of volunteers in the DOR group was significantly higher than that in the non-DOR group (P < 0.05).
Table 4.
Serum Bisphenol S (BPS) concentration and reproductive hormone levels
| DOR Group (n=78) | Non-DOR Group (n=72) | P | |
|---|---|---|---|
| Bisphenol S (BPS) (µg /ml) | 60.133 ± 37.823 | 23.878 ± 22.402 | P=0.006 |
| Luteinising hormone (LH) (mIU/ml) | 6.879 ± 3.912 | 8.002 ± 5.088 | P=0.131 |
| Oestradiol (E2) (pg/ml) | 20.967 ± 26.412 | 36.291 ± 49.379 | P=0.020 |
| Anti-Müllerian hormone (AMH) (ng/ml) | 1.066 ± 0.869 | 3.264 ± 3.358 | P < 0.001 |
| Follicle-stimulating hormone (FSH) (mIU/ml) | 10.606 ± 8.569 | 6.697 ± 4.989 | P=0.001 |
| FSH/LH | 2.108 ± 2.274 | 1.052 ± 0.704 | P < 0.001 |
DOR, decrease ovarian reserve.
The average LH was 6.879 ± 3.912 mIU/ml (DOR group) and 8.002 ± 5.088 mIU/ml (non-DOR group). The average value of E2 was 20.967 ± 26.412 pg/ml (DOR group) and 36.291 ± 49.379 pg/ml (non-DOR group). The average values of AMH were 1.066 ± 0.869 ng/ml (DOR group) and 3.264 ± 3.358 ng/ml (non-DOR group). The average value of FSH was 10.606 ± 8.56 mIU/ml (DOR group) and 6.697 ± 4.989 (non-DOR group). FSH/LH was 2.108 ± 2.274 (DOR group) and 1.052 ± 0.704 (non-DOR group). The values of AMH and E2 in patients with DOR were significantly lower than those of non-DOR volunteers (P < 0.05), and the values of FSH and FSH/LH were significantly higher than those of non-DOR volunteers (P < 0.05).
There was no correlation between the serum BPS concentration of women in the DOR group and various demographic characteristics, such as age and BMI. Regarding the reproductive hormone index, the AMH and E2 values of patients in the DOR group decreased with increasing serum BPS, and the FSH of the patients in the DOR group increased with increasing serum BPS (P < 0.05). There was no correlation between serum BPS and LH (Table 4).
Discussion
Reproductive health is essential for the continuation of the human race. Reproductive health includes the health of the reproductive system, reproductive function, and all physical, mental, and social aspects involved in the reproductive process [33]. Reproductive health is affected by both the internal and external environment. Organisms often show high sensitivity to specific hormones during development. Hormones will change cell growth and differentiation by changing gene expression and epigenetic levels to maintain homeostasis within an environment [34]. As a kind of environmental endocrine-disrupting compound, bisphenol affects various functions of the reproductive system by interfering with endogenous estrogen synthesis and metabolism. Women’s body fat is generally higher than men’s, and the compound’s metabolic rate, renal clearance and excretion rate are lower than those in men, so women are more likely to accumulate bisphenol in their bodies. Therefore, female reproductive health is more vulnerable to threats. As a result, bisphenols, represented by Bisphenol A, have been banned in many countries. Alternatives such as bisphenol F (BPF) and bisphenol S (BPS), which are structurally similar to bisphenol A, have become the mainstay of use. Although Bisphenol S has been detected in human urine and serum by several assays, there is no clinical evidence of the effects of BPS on the human reproductive system. As an important indicator of female fertility, ovarian reserve function can directly reflect the functional level of the reproductive system in women of reproductive age. Therefore, this study is the first epidemiological cohort study on the effects of BPS on the female reproductive system from the aspect of ovarian reserve function in women of reproductive age.
In this study, we found that the serum AMH concentration of volunteers decreased while the level of BPS increaseD. After dividing the volunteers into the DOR group and the normal group according to the ovarian reserve function index, the results showed that the serum BPS concentration in the DOR group was also negatively correlated with the AMH level. AMH, as a member of the transforming factor superfamily, is derived from granulosa cells of the preovarian sinus follicles and small sinus follicles and is involved in the recruitment of dominant follicles and the formation of follicles. Since the AMH level does not fluctuate significantly during the female menstrual cycle, it can be used to reflect ovarian follicle function [35]. Meczekalski [36] et al. also found that the AMH level is an effective indicator to assess the outcome of female fertility and assisted reproductive pregnancy. The AMH level can accurately predict and evaluate the female reproductive lifespan.
Based on the above cohort data and references, we believe that BPS is an important risk factor for the decline of ovarian function, which may play a negative role by affecting AMH hormone levels and thus follicle growth and maturation in the ovary. Although there is no research report on the mechanism of action of BPS after entering the human body, it has been reported that Bisphenol A, which is similar to BPS, may reduce the number of sinus follicles through a feedback mechanism affecting the hypothalamo-pituitary-ovarian axis, thereby reducing the secretion of AMH [37]. And bisphenols are able to act on the P13K-AKT pathway in the mouse ovary, inducing follicular atresia and accelerating follicular pool depletion. It has also been reported that bisphenols can reduce the number of sinus follicles in human female ovaries, accelerate the apoptosis of granulosa cells, and affect the normal synthesis and secretion of AMH [38,39]. The above research evidence from BPA will provide important research clues for our subsequent mechanism research work.
Animal experiments have shown that [29] exposure to BPA can cause granulosa cells to stagnate in the G2 to M phase, resulting in a decrease in their activity, while affecting the synthesis and secretion of E2 hormones by inhibiting the synthesis of related enzymes. Some researchers [40] also found that BPA can reduce the expression of P450arom by affecting the PPAR-γ pathway, resulting in a reduction in E2 synthesis. Animal studies have found that BPA can lower the expression of E2 in dysfunctional ovaries, which can lead to ovarian insufficiency [41,42]. Our study conclued similar results. Serum BPS levels were negatively correlated with E2 levels, and elevated BPS was able to reduce E2 expression. Compared with the control group, volunteers in the DOR group had lower serum E2 levels. There was a negative correlation between serum BPS concentration and E2 in the DOR group.
In clinical practice, women with premature ovarian failure or reduced ovarian reserve function often experience positive feedback from reduced E2 levels, further reducing serum E2 levels. At the same time, lowo E2 level will increase the basal FSH level due to the feedback effect, which in turn affects the emergence of the LH peaks, leading to granulosa cell atrophy and follicle size reduction [43,44]. In this study, we also found the crosstalk effect on female ovarian hormones production due to elevated BPS. The results showed a positive correlation between level of serum FSH and BPS expression in volunteers. Serum FSH levels were higher in the DOR patients than in the normal group, and serum FSH levels increased with the increase of BPS concentration, and serum LH levels were lower in the DOR group than in the normal group.
In conclusion, our data show that serum Bisphenol S level is an important risk factor for ovarian function in women of reproductive age. Bisphenol S may affect the synthesis and secretion of important ovarian hormones such as AMH, E2, FSH and LH, and regulate the growth and maturation of follicles, thus impairing the ovarian reserve function.
While our clinical cohort data reveals the impairment of female fertility by BPS, it is important to note that our study has some limitations. Firstly, due to the limited number of cases, we were unable to stratify the information of the enrolled volunteers to obtain more key conclusions about the factors of BPS intake and the change curves of ovarian function indexes. The absence of long-term follow-up records of the volunteers prevented further studies on the effects of BPS intake on fertility outcomes in women of reproductive age. Additionally, we need to further reveal the crosstalk mechanism of AMH, FSH, LH, and E2 caused by BPS exposure in the laboratory through animal models.
Disclosure of conflict of interest
None.
References
- 1.Toljan K, Briggs FBS. Male sexual and reproductive health in multiple sclerosis: a scoping review. J Neurol. 2024;271:2169–2181. doi: 10.1007/s00415-024-12250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HK, Kim TJ. Current status and future prospects of stem cell therapy for infertile patients with premature ovarian insufficiency. Biomolecules. 2024;14:242. doi: 10.3390/biom14020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laven JSE, Louwers YV. Can we predict menopause and premature ovarian insufficiency? Fertil Steril. 2024;121:737–741. doi: 10.1016/j.fertnstert.2024.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Ashby J, Odum J, Paton D, Lefevre PA, Beresford N, Sumpter JP. Re-evaluation of the first synthetic estrogen, 1-keto-1,2,3, 4-tetrahydrophenanthrene, and bisphenol A, using both the ovariectomised rat model used in 1933 and additional assays. Toxicol Lett. 2000;115:231–238. doi: 10.1016/s0378-4274(00)00198-3. [DOI] [PubMed] [Google Scholar]
- 5.Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW. Global assessment of bisphenol A in the environment: review and analysis of its occurrence and bioaccumulation. Dose Response. 2015;13:1559325815598308. doi: 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 8.Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010;21:7–18. [PubMed] [Google Scholar]
- 9.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sajiki J, Takahashi K, Yonekubo J. Sensitive method for the determination of bisphenol-A in serum using two systems of high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;736:255–261. doi: 10.1016/s0378-4347(99)00471-5. [DOI] [PubMed] [Google Scholar]
- 11.Schonfelder G, Friedrich K, Paul M, Chahoud I. Developmental effects of prenatal exposure to bisphenol a on the uterus of rat offspring. Neoplasia. 2004;6:584–594. doi: 10.1593/neo.04217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salian S, Doshi T, Vanage G. Neonatal exposure of male rats to bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265:56–67. doi: 10.1016/j.tox.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T, Taketan Y. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15:71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hiyasat AS, Darmani H, Elbetieha AM. Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci. 2002;110:163–167. doi: 10.1034/j.1600-0722.2002.11201.x. [DOI] [PubMed] [Google Scholar]
- 15.Moon DG, Sung DJ, Kim YS, Cheon J, Kim JJ. Bisphenol A inhibits penile erection via alteration of histology in the rabbit. Int J Impot Res. 2001;13:309–316. doi: 10.1038/sj.ijir.3900734. [DOI] [PubMed] [Google Scholar]
- 16.Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(Suppl 3):409–414. doi: 10.1289/ehp.02110s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Yu SY, Choo JH, Kim J, Ahn K, Hwang SY. Epigenetic methylation changes in pregnant women: bisphenol exposure and atopic dermatitis. Int J Mol Sci. 2024;25:1579. doi: 10.3390/ijms25031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y, Xia W, Yang S, Pan X, He Z, Kannan K. Spatial distribution of bisphenol S in surface water and human serum from Yangtze River watershed, China: implications for exposure through drinking water. Chemosphere. 2018;199:595–602. doi: 10.1016/j.chemosphere.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Cao XL, Zhou S, Popovic S, Dabeka R. Bisphenol S in individual and composite meat and meat products and implication for its sources. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2022;39:572–579. doi: 10.1080/19440049.2021.2023765. [DOI] [PubMed] [Google Scholar]
- 20.Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol. 2012;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- 21.Jiang S, Wang Y, Wang Z, Xu Y, Li X, Sun M, Li B. Bisphenol A and its alternatives bisphenol S and F exposure with serum uric acid levels, hyperuricemia, and gout prevalence among US adults: a nationally representative cross-sectional study. BMC Public Health. 2024;24:370. doi: 10.1186/s12889-024-17883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berni M, Gigante P, Bussolati S, Grasselli F, Grolli S, Ramoni R, Basini G. Bisphenol S, a Bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domest Anim Endocrinol. 2019;66:48–56. doi: 10.1016/j.domaniend.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Shin SH, Kim SM, Jung SE, Shin BJ, Ahn JS, Lim KT, Kim DH, Lee K, Ryu BY. Bisphenol analogs downregulate the self-renewal potential of spermatogonial stem cells. World J Mens Health. 2024 doi: 10.5534/wjmh.230166. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall MA, Garcia de Mattos Barbosa M, Hanby N, Cai MM, Brunette M, Pavlidis DI, Arrowsmith P, Tan AQ, Cascalho M, Shikanov A. Effect of donor age on endocrine function of and immune response to ovarian grafts. Int J Mol Sci. 2024;25:3431. doi: 10.3390/ijms25063431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Zhang J, Xin X, Jin Y, Zhu Y, Zhang H, Fan R, Ye Y, Li D. Efficacy of natural products on premature ovarian failure: a systematic review and meta-analysis of preclinical studies. J Ovarian Res. 2024;17:46. doi: 10.1186/s13048-024-01369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Bree BE, Jorissen LM, Pattinaja DAPM, Bons JAP, Spaanderman MEA, Valkenburg O, van Golde RJT. No evidence for a diminished ovarian reserve among patients with hypertensive disorders of pregnancy: a case control study. J Ovarian Res. 2024;17:5. doi: 10.1186/s13048-023-01333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner L, van der Schouw YT, de Kat AC. A systematic review of the association between modifiable lifestyle factors and circulating anti-Müllerian hormone. Hum Reprod Update. 2024;30:262–308. doi: 10.1093/humupd/dmae004. [DOI] [PubMed] [Google Scholar]
- 28.Gurtcheff SE, Klein NA. Diminished ovarian reserve and infertility. Clin Obstet Gynecol. 2011;54:666–674. doi: 10.1097/GRF.0b013e3182353c65. [DOI] [PubMed] [Google Scholar]
- 29.Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Cory Slechta D, Thompson C, Hanson M. Developmental origins of health and disease: integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Jiang W, Liao X, Sun Y, Chen X, Zheng B. Effect of diminished ovarian reserve on the outcome of fresh embryo transfer in IVF/ICSI cycles among young women: a retrospective cohort study. BMC Womens Health. 2024;24:230. doi: 10.1186/s12905-024-03039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Zhang L, Liu Y, Li J, Xu X, Niu W, Xu J, Sun B, Guo Y. Higher chromosomal aberration frequency in products of conception from women older than 32 years old with diminished ovarian reserve undergoing IVF/ICSI. Aging (Albany NY) 2021;13:10128–10140. doi: 10.18632/aging.202772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZY, Lu J, Zhang YZ, Zhang M, Liu T, Qu XL. Effect of bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int J Clin Exp Pathol. 2015;8:14355–14364. [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen EM, Alderman MH 3rd, Taylor HS. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016;30:3194–3201. doi: 10.1096/fj.201500089R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gamez JM, Penalba R, Cardoso N, Bernasconi PS, Carbone S, Ponzo O, Pandolfi M, Scacchi P, Reynoso R. Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats: effects on early folliculogenesis. Environ Toxicol Pharmacol. 2015;39:9–15. doi: 10.1016/j.etap.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Claes AN, Ball BA. Biological functions and clinical applications of anti-mullerian hormone in stallions and mares. Vet Clin North Am Equine Pract. 2016;32:451–464. doi: 10.1016/j.cveq.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y, Qu X, Ming Z, Yao Y, Zhang Y. The correlation between exposure to BPA and the decrease of the ovarian reserve. Int J Clin Exp Pathol. 2018;11:3375–3382. [PMC free article] [PubMed] [Google Scholar]
- 38.Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod Toxicol. 2013;42:224–231. doi: 10.1016/j.reprotox.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007-2016) Fertil Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knez J, Kranvogl R, Breznik BP, Voncina E, Vlaisavljevic V. Are urinary bisphenol A levels in men related to semen quality and embryo development after medically assisted reproduction? Fertil Steril. 2014;101:215–221. e215. doi: 10.1016/j.fertnstert.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011;25:1084–1093. doi: 10.1016/j.bbi.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Yang L, Wang S, Zhang Z, Yu Y, Wang M, Cromie M, Gao W, Wang SL. The classic EDCs, phthalate esters and organochlorines, in relation to abnormal sperm quality: a systematic review with meta-analysis. Sci Rep. 2016;6:19982. doi: 10.1038/srep19982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bae T, Hallis SP, Kwak MK. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med. 2024;56:501–514. doi: 10.1038/s12276-024-01180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenners A, Grambach J, Koss J, Maass N, Jonat W, Schmutzler A, Mundhenke C. Reduced ovarian reserve in young early breast cancer patients: preliminary data from a prospective cohort trial. BMC Cancer. 2017;17:632. doi: 10.1186/s12885-017-3593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]