Abstract
Objective: To systematically evaluate the efficacy of traditional Chinese medicine (TCM) in the comprehensive post-resection treatment of primary liver cancer. Methods: Clinical studies on TCM in the comprehensive treatment of primary liver cancer post-resection were retrieved from PubMed, Embase, Cochrane and Google Scholar databases. The search encompassed the period from the inception of each database up to June 28, 2024. Two independent reviewers conducted literature screening based on pre-established inclusion and exclusion criteria, performed data extraction, and evaluated the risk of bias and the methodological quality of the included studies. A meta-analysis was conducted utilizing Review Manager 5.3 software. Results: A total of 12 studies involving 19,116 patients were included in this study. The meta-analysis revealed that TCM treatment following liver cancer resection significantly reduced the recurrence rate, mortality and incidence of adverse events (RR = 0.76, 95% CI 0.70-0.83, P < 0.00001; RR = 0.79, 95% CI 0.71-0.88, P < 0.0001; RR = 0.71, 95% CI 0.57-0.89, P = 0.003). Additionally, TCM notably increased the 1-, 3-, and 5-year overall survival rate (RR = 1.08, 95% CI 1.05-1.11, P < 0.00001; RR = 1.19, 95% CI 1.14-1.23, P < 0.00001; RR = 1.26, 95% CI 1.12-1.42, P < 0.0001), and extended the recurrence-free survival time and the 5-year overall survival time (HR = 0.72, 95% CI 0.65-0.81, P < 0.00001; HR = 0.73, 95% CI 0.61-0.87, P = 0.0004) in patients with primary liver cancer after resection. Conclusion: TCM has a beneficial impact on the comprehensive treatment of primary liver cancer post-resection. However, further validation through higher-quality clinical studies is warranted.
Keywords: Primary liver cancer, hepatectomy, traditional Chinese medicine, comprehensive treatment, meta-analysis
Introduction
Primary hepatic malignancies are the fifth most frequently occurring cancers, responsible for the second highest number of cancer fatalities in Asia [1]. It was reported that Asia accounted for a staggering 72.5% of global cases in 2002, with China specifically contributing to 30% of new cases [2,3]. In China, a shortage of organ donors has limited liver transplantation for early-stage primary liver cancer, making hepatectomy the favored alternative [4]. Resection of diseased liver tissue is an important way to treat a variety of benign and malignant liver diseases such as liver cancer and hepatic hemangioma, with over 300,000 hepatectomies performed worldwide each year [5]. However, the complexity of liver structure and function complicates resection procedures, with postoperative recurrence and metastasis significantly compromising the long-term outcomes of surgery. Enhancing survival rates of patients following resection remains a critical challenge. Traditional Chinese medicine (TCM) plays a prominent role in preventing postoperative recurrence and is applied flexibly based on an individual’s conditions through syndrome differentiation. TCM strategies such as Yiqi (vital energy), Shugan (soothing the liver), and Huoxue (activating blood circulation) help restore balance between Yin and Yang, enhancing the body’s anti-tumor capabilities and improving patient survival rates [6,7]. This study analyzed TCM’s efficacy in the comprehensive management of primary liver cancer following surgical resection, aiming to provide an objective and systematic assessment to strengthen the evidence base for clinical practice.
Data and methods
Literature retrieval strategy
Clinical studies on TCM in comprehensive treatment of primary liver cancer post-resection were systematically retrieved from PubMed, Embase, Cochrane and Google Scholar databases. The retrieval time started from the establishment of databases to June 28, 2024, using a combination of subject words and free words. The search terms include: ‘Chinese Medicine, Traditional’, ‘Zhong Yi Xue’, ‘Chinese herbal’, ‘Hepatectomy’, ‘Postoperative Period’, ‘Liver Cancer’, ‘Liver Neoplasms’, among others. The detailed search strategy is shown in Table 1.
Table 1.
Literature retrieval strategy
| No. | Retrieval Strategy |
|---|---|
| #1 | ((((((((“Medicine, Chinese Traditional”) OR (“Zhong Yi Xue”)) OR (“Traditional Medicine, Chinese”)) OR (“Chinese Traditional Medicine”)) OR (“Traditional Chinese Medicine”)) OR (“Chinese Medicine, Traditional”)) OR (“Traditional Tongue Diagnosis”)) OR (Herb)) OR (“Chinese herbal”) |
| #2 | ((((“Hepatectomy”) OR (Hepatectomies)) OR (resection)) OR (“Postoperative Period*”)) OR (“Period*, Postoperative”) |
| #3 | (((((((((((((((“Liver Neoplasms”) OR (“Hepatic Neoplasms”)) OR (“Hepatic Neoplasm”)) OR (“Neoplasm, Hepatic”)) OR (“Neoplasms, Hepatic”)) OR (“Neoplasms, Liver”)) OR (“Liver Neoplasm”)) OR (“Neoplasm, Liver”)) OR (“Cancer of Liver”)) OR (“Liver Cancer*”)) OR (“Cancer*, Liver”)) OR (“Hepatocellular Cancer*”)) OR (“Cancer of the Liver”)) OR (“Cancer*, Hepatocellular”)) OR (“Hepatic Cancer*”)) OR (“Cancer*, Hepatic”) |
| #4 | #1 AND #2 |
| #5 | #1 AND #3 |
Criteria for study inclusion
(1) Study categories: We included clinical studies publicly disseminated, such as randomized controlled trials (RCTs), cohort studies, and case-control studies. (2) Study subjects: Individuals who underwent surgery for primary liver cancer, without restriction to gender, nationality, or region. (3) Intervention measures: The experimental group received TCM treatment as part of comprehensive treatment after liver cancer resection, while the control group did not include TCM treatment in their post-operative treatment.
Criteria for study exclusion
(1) Duplicate publications; (2) Case reports, narrative reviews, theoretical discussions, and notable medical case compilations; (3) Review, Meta-analysis, animal experiments, and pharmacological experiments; (4) Conference papers, dissertations, achievements and withdrawal documents; (5) Studies lacking experimental data; (6) Inaccessibility of the full literature text.
Study selection and data collection process
The literature was independently reviewed by two researchers (Jiasong Wang and Yanli Wang) who were responsible for data extraction and verification. Discrepancies were resolved through discussions or consulting a third researcher (Qinming Yu). The initial literature screening involved reviewing titles and abstracts to exclude irrelevant studies. Subsequently, the full texts of the remaining articles were examined for inclusion. The data extracted encompassed: (1) Basic information about the study, such as research topic, first author, year of publication; (2) Interventions applied within the study; (3) Crucial aspects of the risk of bias assessment; (4) Outcome indicators and outcome measurement data.
Selection of outcome measures
The indicators that appear three times or more in the included literature were selected as the outcome indicators. (1) The recurrence rate, mortality and incidence of adverse events during the study period. (2) The recurrence-free survival (RFS) and overall survival (OS) of the patients. (3) The 1-, 3-, and 5-year OS rate. Relative risk (RR) was used as the effect index of recurrence rate, mortality, the incidence of adverse events, and 1-, 3-, and 5-year OS rate; Hazard Ratio (HR) was used as the effect index of RFS and OS, both of which were expressed as 95% CI.
Assessment of study quality
The potential biases within the selected studies were independently evaluated by the two researchers, with a cross-verification to ensure reliability. Any disagreement was solved by discussion or consulting a third researcher as needed. For randomized controlled trials, the Cochrane risk bias assessment tool [8] was utilized to appraise the risk of bias. Meanwhile, the Newcastle-Ottawa Scale (NOS) [9] served as the instrument for evaluating the methodological rigor of both cohort and case-control studies. The scale ranges from 0 to 9, with scores categorized into high quality (8-9 points), medium quality (5-7 points), and low quality (less than 5 points). The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system [10] was applied to rank the overall quality of evidence, considering five factors of limitations, inconsistency, non-directness, inaccuracy and publication bias. The evidence quality was divided into four grades: high, moderate, low and very low.
Statistical analysis
Statistical analyses were conducted using Review Manager 5.3 software. The included studies were subjected to chi-square tests for statistical significance, and heterogeneity was assessed quantitatively using the I2 statistic. A threshold of I2 ≤ 50% and a P ≥ 0.1 indicated no significant heterogeneity among the studies, justifying the use of a fixed-effects model for the analysis. Conversely, I2 > 50% and P < 0.1 suggested significant heterogeneity, prompting the use of a random-effects model. If clinical heterogeneity was identified, sensitivity analyses were conducted as appropriate.
Protocol registration
This study was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The study protocol was registered in PROSPERO (Registration number: CRD42024567508).
Results
Summary of literature search and characteristics of included studies
The initial search yielded a total of 1,645 articles. Following a rigorous screening process, 12 studies were ultimately selected for inclusion [11-22], comprising 5 RCTs [11-15], 5 cohort studies [16-20], and 2 case controls [21,22]. These studies collectively reported data from 19,116 patients. The process of literature screening and the outcomes are graphically represented in Figure 1, while the essential details of the included studies are presented in Table 2.
Figure 1.
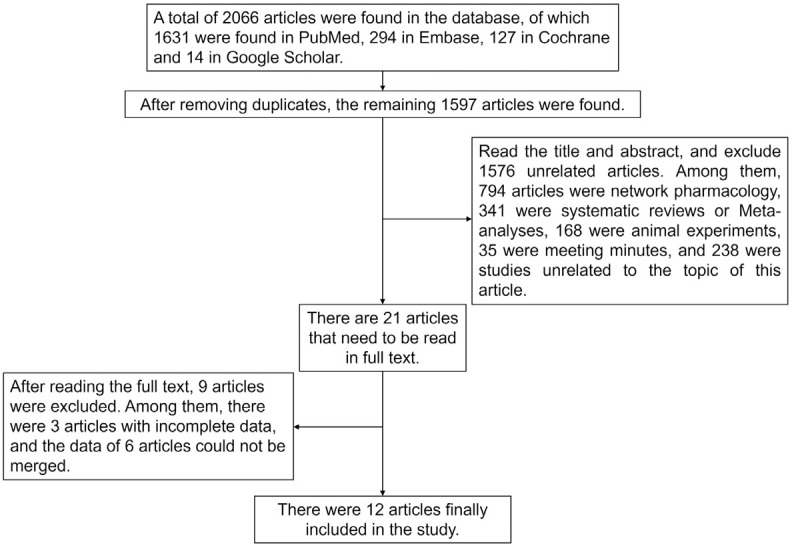
Flow chart of literature screening.
Table 2.
Basic information of included studies
| No. | Study | Country | Number of cases | Intervention | Outcome indicator | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| T | N | T | N | ||||
| 1 | Zhai et al. 2018 [11] | China | 180 | 184 | Cinobufacini Injection and Jiedu granule | TACE | ①②⑤⑥⑦ |
| 2 | Zhong et al. 2014 [12] | China | 60 | 60 | Jianpi Huayu Therapy | Conventional western medicine | ①⑤⑥⑦⑧ |
| 3 | Zhai et al. 2013 [13] | China | 180 | 184 | Cinobufacini Injection and Jiedu Granule | TACE | ①⑧ |
| 4 | Chen et al. 2018 [14] | China | 686 | 316 | Huaier granule | Conventional western medicine | ①②③④ |
| 5 | Luo et al. 2023 [15] | China | 405 | 706 | Huaier granule | Conventional western medicine | ①②③④⑤⑥⑦ |
| 6 | Sun et al. 2012 [16] | China | 122 | 172 | Compound RJH tablets | TAI or TAE | ⑦ |
| 7 | Liao et al. 2020 [17] | China | 1,339 | 13,390 | Adjunctive Chinese medicine treatment | Conventional western medicine | ⑤⑥⑦ |
| 8 | Luo et al. 2023 [18] | China | 80 | 80 | JPHYD | Conventional post-surgical management | ①②③④⑤⑥ |
| 9 | Yuan et al. 2023 [19] | China | 48 | 48 | Huqi formula and CPMs | Conventional western medicine | ⑤⑥⑦ |
| 10 | He et al. 2023 [20] | China | 249 | 370 | TCM decoction or granule | Conventional western medicine | ②⑤⑥⑦ |
| 11 | Cheng et al. 2021 [21] | China | 69 | 68 | Conventional western medicine and ESQJR | Conventional western medicine | ① |
| 12 | Chen et al. 2012 [22] | China | 60 | 60 | Jiedu Granules and Cinobufacini Injection | TACE | ⑤⑥⑦ |
Note: T: TCM group; N: non-TCM group; ①: recurrence rate; ②: mortality rate; ③: recurrence-free survival time; ④: 5-year overall survival time; ⑤: 1-year overall survival rate; ⑥: 3-year overall survival rate; ⑦: 5-year overall survival rate; ⑧: incidence of adverse events. TACE: hepatic arterial chemoembolization; RJH: Ruanjianhugan; TAI: transcatheter arterial infusion chemotherapy; TAE: arterial embolization; JPHYD: Jianpi Huayu decoction; CPMs: Chinese patent medicines; TCM: Traditional Chinese medicine; ESQJR: Erzhu Qinggan Jiedu Recipe.
Assessment of study quality
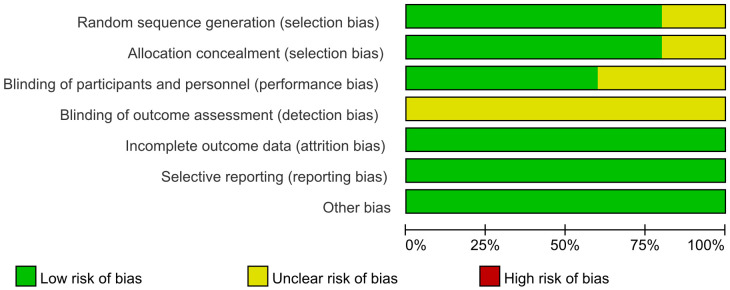
The risk of bias for the 5 RCTs [11-15] was evaluated using the ROB (Risk of Bias) scale as recommended by the Cochrane Handbook 5.1.0. The evaluation revealed that in the domain of randomization methods, four studies [11-14] employed appropriate randomization techniques and were classified as ‘low risk’. One study [15] failed to detail its randomization approach, resulting in an ‘unknown risk of bias’. Regarding allocation concealment, four studies [11-14] provided clear descriptions of their methods, earning a ‘low risk’ rating, while the remaining study [15] lacked such descriptions, leading to an ‘unknown risk of bias’. Regarding participant blinding, three studies [11,13,14] reported on blinding measures and were therefore rated as ‘low risk’. Conversely, two studies [12,15] did not report any participant blinding, resulting in an ‘unknown risk of bias’. In terms of blinding of outcome assessment, none of the five studies [11-15] provided relevant information, leading to a rating of ‘unclear risk of bias’. All five studies [11-15] were rated as ‘low risk’ concerning data integrity, selective reporting, and other potential biases. Visual representation of these assessments as shown in Figures 2, 3.
Figure 2.
Risk bias of included studies.
Figure 3.
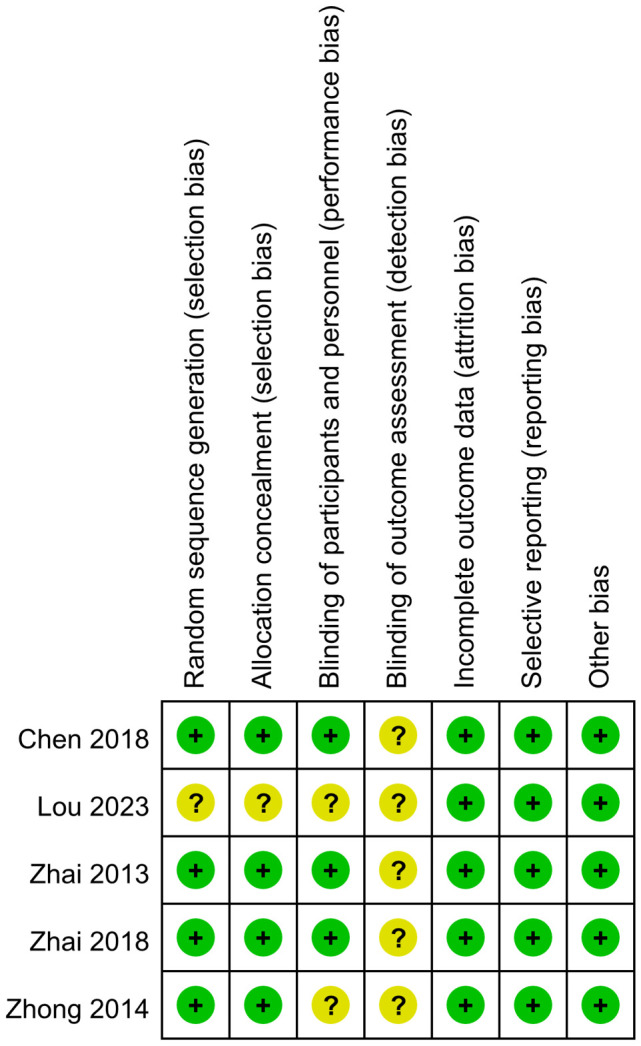
Overview of risk bias in included studies.
The methodological quality of the five cohort studies [16-20] and two case-control studies [21,22] was evaluated using the NOS. Within the cohort studies, three [16,17,20] were deemed to be of high quality, while the remaining two [18,19] were categorized as medium quality. For the case-control studies, both studies [21,22] were rated as medium quality.
Meta-analysis findings
Recurrence rate
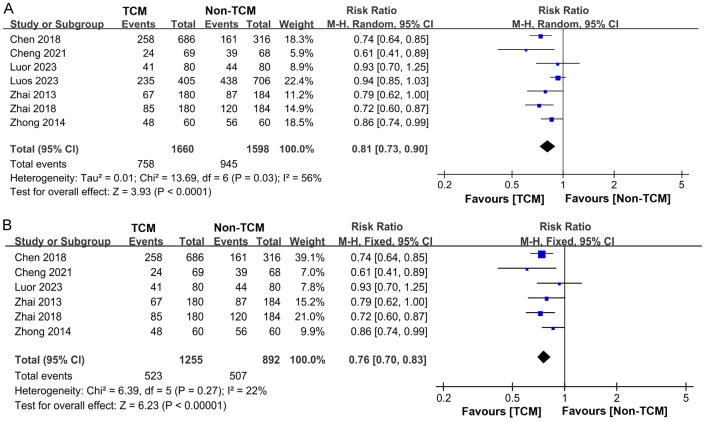
Our analysis incorporated data from 7 studies [11-15,18,21] on patient recurrence rates, encompassing a total of 3,258 patients. Of these, 1,660 were treated with TCM, while 1,598 received non-TCM treatments. Statistical heterogeneity was observed among the study groups (P = 0.03, I2 = 56%). Consequently, a random-effects model was employed for the meta-analysis. The results indicated that TCM treatment significantly lowered the recurrence rate in comparison to non-TCM treatment (RR = 0.81, 95% CI 0.73-0.90, P < 0.0001), as depicted in Figure 4A. A heterogeneity analysis revealed significant variability among the studies, prompting a sensitivity analysis of the included literature. Excluding the fifth study [15] resulted in a reduction of heterogeneity from I2 = 56% to I2 = 22%, as depicted in Figure 4B.
Figure 4.
Forest plot of Meta-analysis of recurrence rate. A. Forest plot of Meta-analysis of recurrence rate before excluding literature; B. Forest plot of Meta-analysis of recurrence rate after excluding literature. TCM: traditional Chinese medicine.
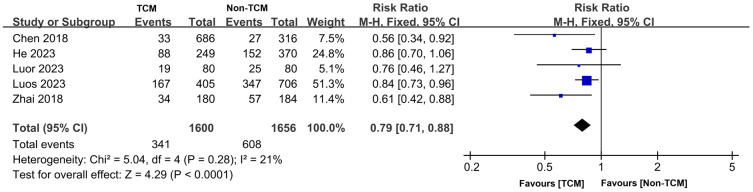
Mortality rate
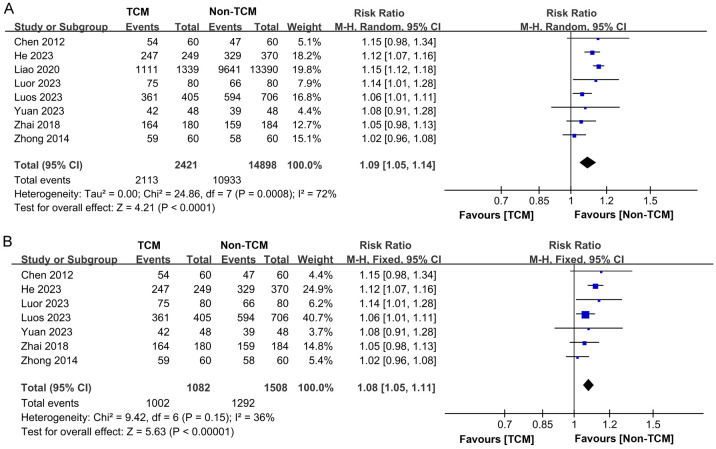
Our review included 5 studies [11,14,15,18,20] that reported on patient mortality rates, involving a total of 3,256 patients. The TCM group comprised 1,600 patients, while the non-TCM group included 1,656 patients. Good homogeneity was observed across the studies (P = 0.28, I2 = 21%), which justified the application of a fixed-effects model for the meta-analysis. The results demonstrated that the mortality rate among patients who received TCM treatment was significantly lower than that of the non-traditional Chinese medicine treatment group (RR = 0.79, 95% CI 0.71-0.88, P < 0.0001), as illustrated in Figure 5.
Figure 5.
Forest plot of Meta-analysis of mortality rate. TCM: traditional Chinese medicine.
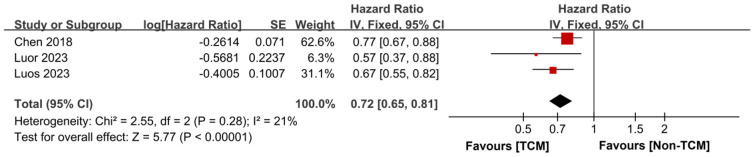
Recurrence-free survival time
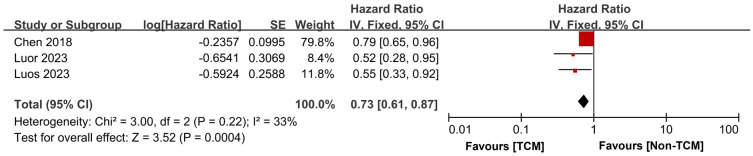
Our synthesis included 3 studies [14,15,18] that reported on the RFS of patients. These studies exhibited good homogeneity (P = 0.28, I2 = 21%), which allowed for the use of a fixed-effects model in the meta-analysis. The findings indicated that patients treated with TCM experienced an extended RFS time compared to those who did not receive TCM treatment (HR = 0.72, 95% CI 0.65-0.81, P < 0.00001), as depicted in Figure 6.
Figure 6.
Forest plot of Meta-analysis of recurrence-free survival time. TCM: traditional Chinese medicine; SE: standard error.
OS time and rate
Our analysis incorporated data from 3 studies [14,15,18] that assessed the OS of patients. These studies demonstrated satisfactory homogeneity (P = 0.22, I2 = 33%), justifying the application of a fixed-effects model for the meta-analysis. The findings revealed that the five-year OS time for patients who had TCM treatment was significantly extended compared to those received non-TCM treatment (HR = 0.73, 95% CI 0.61-0.87, P = 0.0004), as illustrated in Figure 7.
Figure 7.
Forest plot of Meta-analysis of 5-year overall survival time. TCM: traditional Chinese medicine; SE: standard error.
Eight studies [11,12,15,17-20,22] documented the one-year survival statistics for 17,319 participants. Among them, 2,421 received TCM intervention, while 14,898 did not. Statistical heterogeneity was observed across these studies (P = 0.0008, I2 = 72%), prompting the application of a random-effects model for the meta-analysis. The findings indicated that TCM group exhibited an enhanced one-year survival rate compare to the non-TCM group (RR = 1.09, 95% CI 1.05-1.14, P < 0.0001), as depicted in Figure 8A. A subsequent sensitivity analysis showed reduced heterogeneity from I2 = 72% to I2 = 36% after the exclusion of Liao’s study [17], as illustrated in Figure 8B.
Figure 8.
Forest plot of Meta-analysis of 1-year overall survival rate. A. Forest plot of Meta-analysis of 1-year overall survival rate before excluding literature; B. Forest plot of Meta-analysis of 1-year overall survival rate after excluding literature. TCM: traditional Chinese medicine.
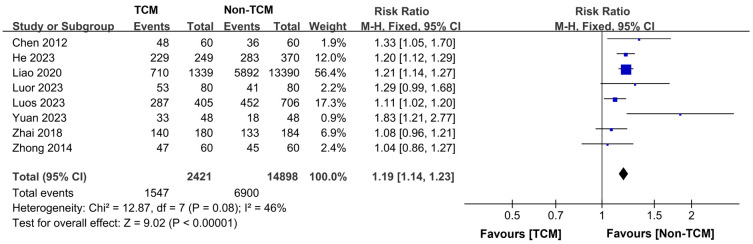
Eight studies [11,12,15,17-20,22] presented data on the three-year survival rates among 17,319 patients, with 2,421 receiving TCM treatment and 14,898 receiving non-TCM treatment. The heterogeneity across the studies was deemed satisfactory (P = 0.08, I2 = 46%), justifying using a fixed-effects model for the analysis. The findings indicated that patients who underwent TCM treatment had a significantly higher three-year survival rate than those not treated with TCM (RR = 1.19, 95% CI 1.14-1.23, P < 0.00001), as depicted in Figure 9.
Figure 9.
Forest plot of Meta-analysis of 3-year overall survival rate.
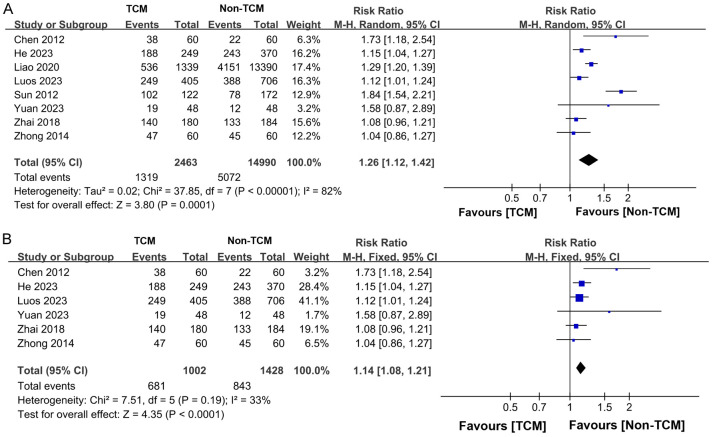
Eight studies [11,12,15-17,19,20,22] reported the five-year overall survival rate for a total of 17,453 patients, with 2,463 in the TCM group and 14,490 in the non-TCM group. Significant heterogeneity was observed among the study groups (P < 0.00001, I2 = 82%), necessitating the use of a random-effects model for the meta-analysis. The results indicated that the TCM treatment group exhibited a higher five-year OS rate compared to the non-TCM treatment group (RR = 1.26, 95% CI 1.12-1.42, P = 0.0001), as depicted in Figure 10A. A subsequent sensitivity analysis showed that excluding studies of Sun et al. [16] and Liao et al. [17] reduced heterogeneity from I2 = 82% to I2 = 33%, indicating these studies contributed significantly to the initial variability (Figure 10B).
Figure 10.
Forest plot of Meta-analysis of 5-year overall survival rate. A. Forest plot of Meta-analysis of 5-year overall survival rate before excluding literature; B. Forest plot of Meta-analysis of 5-year overall survival rate after excluding literature. TCM: traditional Chinese medicine.
Incidence of adverse events
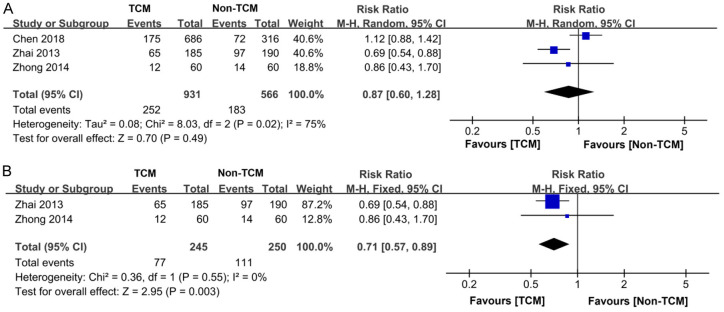
Three studies [12-14] documented the occurrence of adverse events among 1,497 participants, with 931 in the TCM treatment cohort and 566 in the non-TCM treatment cohort. Significant heterogeneity was observed across the studies (P = 0.02, I2 = 75%), necessitating the application of a random-effects model for the meta-analysis. The findings indicated that the TCM treatment group experienced a lower rate of adverse events compared to non-TCM treatment group. However, this difference did not reach statistical significance (RR = 0.87, 95% CI 0.60-1.28, P = 0.49), as depicted in Figure 11A. Subsequent sensitivity analysis revealed that removing the study of Chen et al. [14] resulted in a marked reduction in heterogeneity from I2 = 75% to I2 = 0%. Concurrently, this exclusion led to a significant decrease in the incidence of adverse events among patients receiving TCM treatment (P = 0.003), as illustrated in Figure 11B.
Figure 11.
Forest plot of Meta-analysis of incidence of adverse events. A. Forest plot of Meta-analysis of incidence of adverse events before excluding literature; B. Forest plot of Meta-analysis of incidence of adverse events after excluding literature. TCM: traditional Chinese medicine.
Publication bias analysis
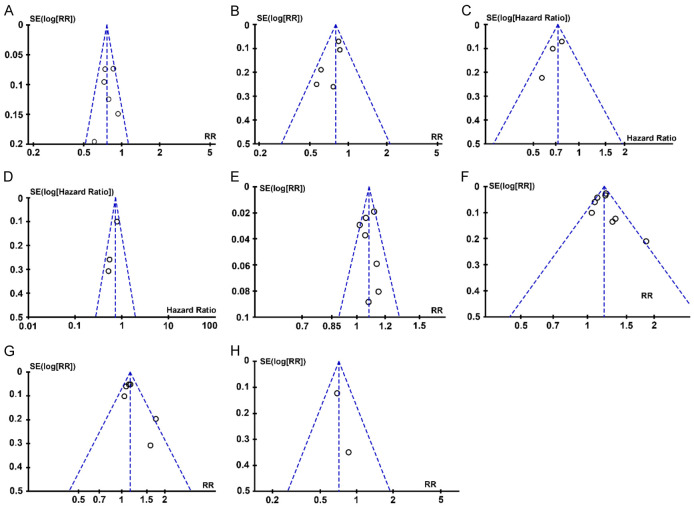
Examination of the funnel plots for the recurrence rate, mortality rate, incidence of adverse events, recurrence-free survival time, five-year overall survival time, and 1-, 3-, 5-year overall survival rates revealed a generally symmetrical distribution. Most data points fell within the confines of the funnel plot, suggesting an absence of significant publication bias. However, due to the small number of documents included in each indicator, potential for publication bias remains, as illustrated in Figure 12.
Figure 12.
Publication bias assessment using funnel plot. A. Funnel plot of recurrence rate; B. Funnel plot of mortality; C. Funnel plot of RFS; D. Funnel plot of 5-year OS; E. Funnel plot of 1-year OS rate; F. Funnel plot of 3-year OS rate; G. Funnel plot of 5-year OS rate; H. Funnel plot of incidence of adverse reactions. SE: standard error; RR: relative risk; RFS: recurrence free survival; OS: overall survival.
Quality evaluation of GRADE evidence
The study employed the GRADE system to assess the evidence quality of the relevant outcome indicators. Each indicator was evaluated for risk of bias, inconsistency, indirectness, imprecision, and publication bias. The findings indicated that of the five outcome indicators assessed, the recurrence rate and mortality demonstrated moderate evidence quality, while the overall survival rate, recurrence-free survival time, and overall survival time were characterized by low evidence quality.
Discussion
Radical operation and liver transplants are considered optimal for treating early-stage primary liver cancer. Yet, the scarcity of liver donors in China often leads to the reliance on hepatectomy as the primary treatment. Consequently, diminishing postoperative recurrence and enhancing survival rates are still major challenges in liver cancer management [23]. Postoperative supplementary treatments like interventional procedures, targeted drugs, and immunotherapies can help prevent tumor recurrence post-hepatectomy. However, some patients struggle with associated side effects. Additionally, the use of Western medicine post-resection can sometimes lead to complications such as nausea, fever, and exacerbation of liver function impairment, necessitating careful administration [24]. In clinical settings, fostering interdisciplinary collaboration is essential to align primary and supplementary treatment strategies for a holistic approach. Given its foundation in natural substances and its established efficacy and safety profile, TCM holds potential as a vital component of the post-hepatectomy comprehensive care regimen.
As modern medical science advances, an array of postoperative interventions have been developed to combat liver cancer, including transcatheter arterial chemoembolization (TACE), adoptive immunotherapy, and interferon therapy. Yet, the high recurrence rate, low overall survival, and suboptimal long-term outcomes persist in the management of primary liver cancer [25]. Moreover, the side effects of Western pharmaceuticals can be burdensome for some patients, potentially outweighing the benefits of treatment. While TCM literature does not explicitly name primary liver cancer, they reference similar conditions, such as hypochondriac pain and jaundice [26]. TCM’s diagnostic and therapeutic approach emphasizes syndrome differentiation and holistic treatment, viewing the body as an integrated system and tailoring treatments to individual needs, with a focus on prevention and the interception of disease progression [27,28]. TCM’s syndrome-based treatment has shown promise in enhancing survival rates for patients with primary liver cancer. Characterized by a multi-dimensional, multi-targeted, and layered therapeutic strategy, TCM can complement and amplify the efficacy of a comprehensive treatment plan. Herbal remedies, a prominent form of complementary and alternative medicine (CAM), are widely accepted in China for cancer therapy, aiding in symptom management, enhancement of life quality, and prevention of disease relapse. The impact of TCM on the prognosis of primary hepatic carcinoma (HCC) has garnered increasing interest. A randomized controlled trial with 291 hepatocellular carcinoma patients revealed that the integration of TCM with surgical treatment notably extended progression-free survival (PFS) and OS [29]. A retrospective analysis of 3,483 primary HCC cases [30] identified TCM as a significant factor contributing to 5-year survival rates. The mechanisms by which TCM impedes tumor growth and its efficacy in reducing recurrence have yielded promising outcomes. Consequently, TCM emerges as a promising intervention for the prevention of postoperative relapse in primary liver cancer.
This analysis included 12 clinical investigations: seven examined the rate of recurrence, five assessed mortality rates, three evaluated recurrence-free survival and the duration of 5-year OS, and eight scrutinized the 5-year survival rate, collectively encompassing 19,116 participants. The TCM interventions, including traditional herbal decoctions and proprietary Chinese medicines, significantly reduce recurrence and mortality rates as well as incidence of adverse events, while extending survival times in the post-resection management of primary liver cancer. Sensitivity analysis indicated that specific studies, such as those by Luo et al. [15], Sun et al. [16], and Liao et al. [17], influenced the observed heterogeneity in recurrence and survival rate evaluations. In TCM theory, primary liver cancer is linked to pathogenic influences and vital energy deficiencies. The underlying pathophysiology involves the dysfunction of the zang-fu organs, depletion of vital qi, stagnation of qi and blood, and the formation of phlegm dampness and toxins [31]. Post-surgery, loss of vital qi and remaining pathogenic factors create an environment conducive to recurrence, partly due to pro-angiogenic factors promoting new blood vessel formation in residual tumor tissue. Active TCM constituents, such as flavonoids, polysaccharides, alkaloids, saponins, volatile oils, terpenes, quinones, phenols, enzymes, and protein components, exert their anti-cancer effects by inhibiting tumor cell proliferation, blocking tumor cell cycle, suppressing tumor cell invasion and metastasis, and regulating various signaling pathways as well as vascular endothelial growth factor and reactive oxygen species [32,33]. Furthermore, TCM can enhance immune strength and improve overall quality of life, addressing multidrug resistance in liver cancer through various mechanisms like inhibiting membrane transporters, suppressing multiple drug resistance (MDR) genes, interfering anti-apoptotic pathways, and enhancing the tumor cell microenvironment [34]. Consequently, TCM can augment a patient’s resilience to surgical procedures, bolster the body’s immune capabilities, and ameliorate microcirculation. These actions contribute to an improved quality of life for patients and offer a distinct advantage in preventing the recurrence of liver cancer, aligning with the findings of this study. The role of TCM in the comprehensive management of liver cancer is becoming increasingly prominent.
Nonetheless, this study has several limitations: Firstly, the limited body of research on TCM post-resection complicates synthesis and analysis due to diverse intervention strategies. Secondly, many studies lack high-quality evidence, with random group allocation often reported without sufficient blinding methods. This overall suboptimal quality may undermine the reliability of the findings. Furthermore, there is insufficient data on adverse events, hindering a thorough safety assessment, and the included studies are predominantly from China, introducing potential regional bias.
In conclusion, the findings of this research suggest that integrating TCM into the comprehensive treatment for primary liver cancer can lead to decreased recurrence rates, mortality and adverse events, along with extended survival. We also call for global participation in TCM research and anticipate future high-quality clinical studies to provide stronger evidence supporting TCM’s benefits in managing primary liver cancer post-resection.
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029–2041. doi: 10.1111/liv.15251. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15:155–169. doi: 10.1007/s11684-021-0848-3. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364–377. doi: 10.1016/j.jhep.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Bai F, Huang Z, Luo J, Qiu Y, Huang S, Huang C, Liu T, Zhang H, Wang D. Bibliometric and visual analysis in the field of traditional Chinese medicine in cancer from 2002 to 2022. Front Pharmacol. 2023;14:1164425. doi: 10.3389/fphar.2023.1164425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang KY, Du SL, Wang QL, Zhang YF, Song HY. Traditional Chinese medicine targeting cancer stem cells as an alternative treatment for hepatocellular carcinoma. J Integr Med. 2020;18:196–202. doi: 10.1016/j.joim.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhai XF, Liu XL, Shen F, Fan J, Ling CQ. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: a randomized controlled study. Cancer. 2018;124:2161–2168. doi: 10.1002/cncr.30915. [DOI] [PubMed] [Google Scholar]
- 12.Zhong C, Li HD, Liu DY, Xu FB, Wu J, Lin XM, Guo RP. Clinical study of hepatectomy combined with Jianpi Huayu Therapy for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15:5951–7. doi: 10.7314/apjcp.2014.15.14.5951. [DOI] [PubMed] [Google Scholar]
- 13.Zhai XF, Chen Z, Li B, Shen F, Fan J, Zhou WP, Yang YK, Xu J, Qin X, Li LQ, Ling CQ. Traditional herbal medicine in preventing recurrence after resection of small hepatocellular carcinoma: a multicenter randomized controlled trial. J Integr Med. 2013;11:90–100. doi: 10.3736/jintegrmed2013021. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Shu C, Laurence AD, Chen Y, Peng BG, Zhen ZJ, Cai JQ, Ding YT, Li LQ, Zhang YB, Zheng QC, Xu GL, Li B, Zhou WP, Cai SW, Wang XY, Wen H, Peng XY, Zhang XW, Dai CL, Bie P, Xing BC, Fu ZR, Liu LX, Mu Y, Zhang L, Zhang QS, Jiang B, Qian HX, Wang YJ, Liu JF, Qin XH, Li Q, Yin P, Zhang ZW, Chen XP. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67:2006–2016. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Hu H. Huaier granule prolongs overall survival after curative resection of hepatocarcinoma carcinoma: a propensity score analysis. J Ethnopharmacol. 2023;301:115774. doi: 10.1016/j.jep.2022.115774. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Liang ST, Zhai XF, Lang QB, Zhou QH, Yue XQ, He J, Xu J, Zhu Y, Ling CQ. A traditional Chinese herbal medicine compound preparation versus interventional therapy after resection of small hepatocellular carcinoma: 22-year follow-up. J Tradit Chin Med. 2012;32:156–63. doi: 10.1016/s0254-6272(13)60005-9. [DOI] [PubMed] [Google Scholar]
- 17.Liao YP, Kung PT, Wang YH, Chu YR, Kao ST, Tsai WC. Effects and relative factors of adjunctive Chinese medicine therapy on survival of hepatocellular carcinoma patients: a retrospective cohort study in Taiwan. Integr Cancer Ther. 2020;19:1534735420915275. doi: 10.1177/1534735420915275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo R, Fang C, Chen C, Zhang Y, Yao R, Wang J, Shi H, Feng K, Hu M, Zhong C. Adjuvant therapy with Jianpi Huayu decoction improves overall and recurrence-free survival after hepatectomy for hepatocellular carcinoma: a retrospective propensity score-matching study. Front Pharmacol. 2023;14:1212116. doi: 10.3389/fphar.2023.1212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan H, Niu Q, Shang X, Zhang L, Li L, Yang H, Gou C, Li J, Li H, Li X. Impact of traditional Chinese medicine on prognosis of patients with hepatocellular carcinoma: a retrospective cohort study. Integr Cancer Ther. 2023;22:15347354231170536. doi: 10.1177/15347354231170536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He ZY, She WM. Retrospective cohort study on traditional Chinese medicine dialectical treatment improving survival rate of patients with hepatocellular carcinoma after radical resection. Chinese Hepatology. 2023;28:189–192. [Google Scholar]
- 21.Cheng Y, Ni S, Chen Y, Ling Q, Chen J. Erzhu Qinggan Jiedu Recipe improves the clinical outcome of hepatocellular cancer after surgical resection: a case-control retrospective study. Intern Med J. 2021;51:853–860. doi: 10.1111/imj.14844. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Chen HY, Lang QB, Li B, Zhai XF, Guo YY, Yue XQ, Ling CQ. Preventive effects of jiedu granules combined with cinobufacini injection versus transcatheter arterial chemoembolization in post-surgical patients with hepatocellular carcinoma: a case-control trial. Chin J Integr Med. 2012;18:339–44. doi: 10.1007/s11655-012-1083-1. [DOI] [PubMed] [Google Scholar]
- 23.Hao F, Li H, Li N, Li J, Wu H. Laparoscopic repeat hepatectomy versus conventional open repeat hepatectomy for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:960204. doi: 10.3389/fonc.2022.960204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li K, Xiao K, Zhu S, Wang Y, Wang W. Chinese herbal medicine for primary liver cancer therapy: perspectives and challenges. Front Pharmacol. 2022;13:889799. doi: 10.3389/fphar.2022.889799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu K, Wang C, Ma C, Zhou H, Li Y. The potential application of Chinese medicine in liver diseases: a new opportunity. Front Pharmacol. 2021;12:771459. doi: 10.3389/fphar.2021.771459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao X, Bu Y, Jia Q. Traditional Chinese Medicine as supportive care for the management of liver cancer: past, present, and future. Genes Dis. 2019;7:370–379. doi: 10.1016/j.gendis.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Wang L, Liu Y, Shao M, Yang W, Fu Y, Gao Q, Feng J, Xing Y, Xiang X. The influence of adjunctive traditional Chinese medicine therapy on survival in primary liver cancer: a real-world study based on electronic medical records. Front Pharmacol. 2023;14:1231933. doi: 10.3389/fphar.2023.1231933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Feng Y, Liu Y, Ye X, Ji X, Sun L, Gao F, Zhang Q, Li Y, Zhu B, Wang X. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine. 2021;87:153575. doi: 10.1016/j.phymed.2021.153575. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Li M, Wang X, Dang Z, Yu L, Wang X, Jiang Y, Yang Z. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine. 2019;62:152930. doi: 10.1016/j.phymed.2019.152930. [DOI] [PubMed] [Google Scholar]
- 31.She Y, Huang Q, Ye Z, Hu Y, Wu M, Qin K, Wei A, Yang X, Liu Y, Zhang C, Ye Q. The therapeutic principle of combined strengthening qi and eliminating pathogens in treating middle-advanced primary liver cancer: a systematic review and meta-analysis. Front Pharmacol. 2021;12:714287. doi: 10.3389/fphar.2021.714287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang RR, Shao MY, Fu Y, Zhao RX, Wang JW, Li M, Zhao YX, Shao FL. Network Meta-analysis of oral Chinese patent medicine for adjuvant treatment of primary liver cancer. Zhongguo Zhong Yao Za Zhi. 2021;46:2333–2343. doi: 10.19540/j.cnki.cjcmm.20200721.501. [DOI] [PubMed] [Google Scholar]
- 33.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou JY, Chen QL, Luo XC, Damdinjav D, Abdelmohsen UR, Li HY, Battulga T, Chen HB, Wang YQ, Zhang JY. Natural products reverse cancer multidrug resistance. Front Pharmacol. 2024;15:1348076. doi: 10.3389/fphar.2024.1348076. [DOI] [PMC free article] [PubMed] [Google Scholar]