Abstract
Objective: To investigate the implications of serum hsa_circ_0007534 levels in ovarian cancer (OC) patients. Methods: A retrospective analysis was conducted on the clinical data of 102 OC patients, and 102 healthy controls (HCs) were included in this study. Serum levels of hsa_circ_0007534 were measured using qPCR. The relationship between circRNA levels and clinicopathologic characteristics was analyzed. Additionally, the diagnostic performances of serum hsa_circ_0007534 and CA-125 levels in OC patients were evaluated using Receiver Operating Characteristic (ROC) curve analyses. Results: OC patients had significantly elevated serum hsa_circ_0007534 levels compared to HCs, allowing a differentiation between OC patients and HCs. Higher serum hsa_circ_0007534 levels were associated with more aggressive disease features and poorer overall survival. Patients with elevated hsa_circ_0007534 levels tended to have worse clinical outcome compared to those with lower levels. Multivariate analyses indicated that high hsa_circ_0007534 levels were independently associated with poorer outcome. Furthermore, serum hsa_circ_0007534 levels effectively distinguished between chemoresistant and chemosensitive patient groups. Conclusions: Serum hsa_circ_0007534 levels may serve as valuable predictors of prognosis in OC patients.
Keywords: hsa_circ_0007534, prognostic biomarker, ovarian cancer
Introduction
Ovarian cancer (OC) is a highly aggressive gynecological tumor [1]. Despite significant advances in debulking surgeries, chemotherapy, and targeted treatment, long-term survival outcomes for OC patients remain unsatisfactory, and reliable prognostic biomarkers for effective management are still lacking [1-3]. Most OC patients are diagnosed at relatively advanced stages [4], emphasizing the urgent need for novel biomarkers for diagnostic and prognostic use.
Circular RNAs (circRNAs) are a class of noncoding RNAs that regulate target genes post-transcriptionally [5,6], influencing OC cell malignancy through miRNA/mRNA pathways [7]. For example, downregulation of circ-TFRC, can inhibit OC progression by the regulation of the miR-615-3p/insulin-like growth factor 2 (IGF2) axis [8], while circRHOBTB3 targets the phosphatidylinositol 3 kinase (PI3K)/AKT (protein kinase B) axis to prevent OC progression [9]. High-throughput sequencing and microarray techniques have highlighted the diagnostic and prognostic potential of specific circRNAs in various cancers [10,11], often revealing differential expression patterns linked to clinical characteristics. Several studies have confirmed the prognostic value of circRNAs across different cancer types, including OC [12]. For instance, hsa_circ_0120175 promotes OC development and is associated with poor prognosis [13], while upregulation of hsa_circRNA_102958 indicates poor outcomes and facilitates OC progression through the miR-1205/SH2 domain-containing protein 3A (SH2D3A) axis [14].
However, the role of circulating circRNAs in patient diagnosis and outcome prediction is not well understood. Upregulation of hsa_circ_0007534 has been reported in various cancers, including glioma, cervical cancer, colorectal cancer, osteosarcoma, non-small cell lung cancer, and breast cancer [15-20]. To date, no studies have explored the relevance of hsa_circ_0007534 in OC. This study investigates the diagnostic and prognostic value of serum hsa_circ_0007534 levels in a cohort of OC patients.
Materials and methods
Human samples
This retrospective study included 102 patients with ovarian cancer (OC), with blood and tissue samples collected between May 2018 and May 2023 at Huzhou Central Hospital. Serum samples from healthy controls were obtained from healthy volunteers at the same hospital. The study protocol was approved by the Ethics Committee of Huzhou Central Hospital (No. 202408003-02). All procedures adhered to the principles of the Helsinki Declaration, and blood processing was conducted uniformly following standard protocols. Inclusion criteria were a confirmed diagnosis of primary OC through pathologic examination and no prior immunotherapy. Exclusion criteria included age below 18 years, incomplete clinical data, concurrent malignancies in other areas, and concurrent autoimmune diseases.
Data collection
Data collected included age, histologic type, serum carbohydrate antigen 125 (CA-125) levels, tumor size, histologic grade, lymph node metastasis status, and International Federation of Gynecology and Obstetrics (FIGO) stage.
Real-time quantitative polymerase chain reaction (qPCR)
For RNA extraction, 200 μL of serum per donor was mixed with 800 μL of TRIzol (Invitrogen, CA, USA), following the manufacturer’s instructions. RNA purity was confirmed using a NanoDrop instrument (Thermo Fisher Scientific, MA, USA) with OD260/OD280 values between 1.8 and 2.0. cDNA was synthesized using a reverse transcription kit, and qPCR was conducted using a SYBR Green qPCR kit (TaKaRa, Shiga, Japan). Primers for the target circRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control are listed in Table 1. The 2-∆∆Ct method was used to quantify relative gene expression.
Table 1.
qPCR primer sequences
| Name | Sequence (5’-3’) |
|---|---|
| GAPDH Forward | TATGATGATATCAAGAGGGTAGT |
| GAPDH Reverse | TGTATCCAAACTCATTGTCATAC |
| hsa_circ_0007534 Forward | CTGGTGTGGTTCAGGAGGAA |
| hsa_circ_0007534 Reverse | ATGGAATTGCTGGCGAGTTG |
Statistical analyses
Measured data were expressed as mean ± standard deviation (SD) and compared using paired or unpaired t-tests. Counted data were expressed as case numbers and analyzed using chi-square tests. The diagnostic performance of serum CA-125 and hsa_circ_0007534 levels was evaluated using ROC curve analyses. Overall survival (OS) and progression-free survival (PFS) were assessed using the Kaplan-Meier method. Independent predictors of OS in OC patients were identified using multivariate Cox regression analysis. Statistical analyses were performed using SPSS 20.0 and GraphPad Prism 8.0, with P-values < 0.05 considered significant.
Results
OC patient characteristics
The participants had a median age of 52 years (range: 39-79), with 47 (46.1%) having FIGO stage I-II disease and 55 (53.9%) having FIGO stage III-IV disease. Histologically, 60 cases were classified as serous and 42 as other types. Tumor sizes were < 5 cm in 40 patients and ≥ 5 cm in 62 patients. Preoperative serum CA-125 levels were < 35 U/mL in 29 (28.4%) cases and ≥ 35 U/mL in 73 (71.6%) cases. Histologic grades included well-differentiated in 44 (43.1%) cases and moderately/poorly differentiated in 58 (56.9%) cases. Additionally, 38 (37.3%) patients were negative and 64 (62.7%) were positive for lymph node metastasis (Table 2).
Table 2.
Baseline OC information
| Characteristic | Numbers of Cases (%) |
|---|---|
| Age | |
| < 50 | 35 (34.3%) |
| > 50 | 67 (65.7%) |
| Histology | |
| Serous | 60 (58.8%) |
| Others | 42 (41.2%) |
| Serum CA-125 (U/mL) | |
| < 35 | 29 (28.4%) |
| > 35 | 73 (71.6%) |
| Tumor size | |
| < 5 cm | 40 (39.2%) |
| > 5 cm | 62 (60.8%) |
| Histological grade | |
| Well | 44 (43.1%) |
| Moderate/poor | 58 (56.9%) |
| Lymph node metastasis | |
| Negative | 38 (37.3%) |
| Positive | 64 (62.7%) |
| FIGO stage | |
| I/II | 47 (46.1%) |
| III/IV | 55 (53.9%) |
OC, Ovarian cancer; FIGO, Federation Internationale of Gynecology and Obstetrics.
Serum hsa_circ_0007534 was elevated in OC patients
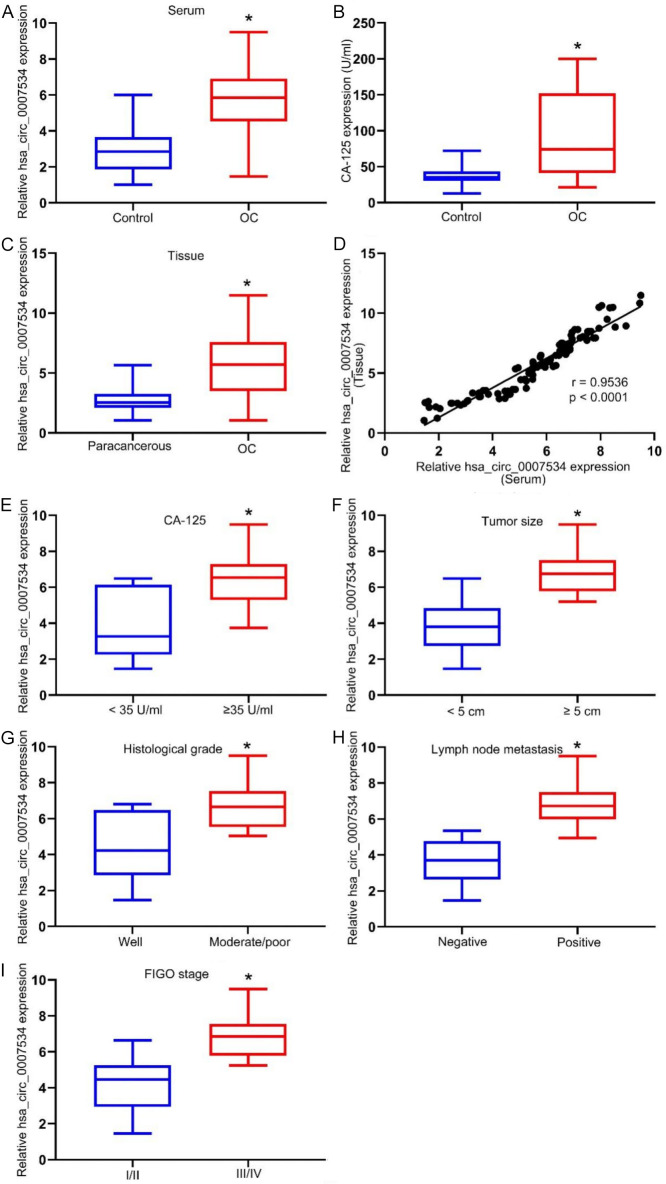
qPCR analysis revealed that hsa_circ_0007534 serum levels were significantly elevated in OC patients compared to HCs (Figure 1A), similar to the elevated levels of CA125 (Figure 1B). hsa_circ_0007534 expression was also significantly higher in OC tissues compared to adjacent non-cancerous tissues (Figure 1C), showing a strong positive correlation between tissue and serum expression levels (Figure 1D). Notably, elevated hsa_circ_0007534 expression was associated with high serum CA-125 levels (≥ 35 U/mL, Figure 1E), larger tumor size (≥ 5 cm, Figure 1F), poorer histologic grade (moderate/poor, Figure 1G), lymph node metastasis positivity (Figure 1H), and advanced FIGO stage (III/IV, Figure 1I).
Figure 1.
Ovarian cancer (OC) is characterized by increased serum hsa_circ_0007534. (A) Dramatic increases in serum hsa_circ_0007534 concentrations were evident in patients with OC compared to healthy controls. (B) Marked increases in CA-125 levels were observed in the serum of patients with OC compared to healthy controls. (C) Dramatic increases in hsa_circ_0007534 expression were evident in patients with OC tissues relative to paracancerous tissue subjects. (D) There were a significant positive correlation with hsa_circ_0007534 expression between OC tissues and serum. hsa_circ_0007534 expression were detected in CA-125 (E), tumor size (F), histologic grading (G), lymph node metastasis (H), and Federation Internationale of Gynecology and Obstetrics (FIGO) staging (I). *P < 0.05.
Association between serum hsa_circ_0007534 levels and clinicopathologic findings
Analysis of the relationship between serum hsa_circ_0007534 levels and clinicopathologic features of OC patients showed that higher levels of this circRNA were significantly associated with high serum CA-125 concentrations (P < 0.05), larger tumor size (P < 0.05), advanced FIGO stage (P < 0.05), poorer histological grade (P < 0.05), and lymph node metastasis (P < 0.05) (Table 3). However, serum hsa_circ_0007534 levels were not significantly associated with patient age (P = 0.835) or histologic subtype (P = 0.421).
Table 3.
Associations between serum hsa_circ_0007534 concentrations and clinicopathologic characteristics in patients with OC
| Characteristics | Case | hsa_circ_0007534 | p | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age | 0.835 | |||
| < 50 | 35 | 18 | 17 | |
| ≥ 50 | 67 | 33 | 34 | |
| Histology | 0.421 | |||
| Serous | 60 | 32 | 28 | |
| Others | 42 | 19 | 23 | |
| Serum CA-125 (U/mL) | < 0.05 | |||
| < 35 | 29 | 19 | 10 | |
| ≥ 35 | 73 | 32 | 41 | |
| Tumor size | < 0.05 | |||
| < 5 cm | 40 | 34 | 6 | |
| ≥ 5 cm | 62 | 17 | 45 | |
| Histological grade | < 0.05 | |||
| Well | 44 | 32 | 12 | |
| Moderate/poor | 58 | 19 | 39 | |
| Lymph node metastasis | < 0.05 | |||
| Negative | 38 | 31 | 7 | |
| Positive | 64 | 20 | 44 | |
| FIGO stage | < 0.05 | |||
| I/II | 47 | 36 | 11 | |
| III/IV | 55 | 15 | 40 | |
OC, Ovarian cancer; FIGO, Federation Internationale of Gynecology and Obstetrics.
Serum hsa_circ_0007534 was closely related to OC patient survival
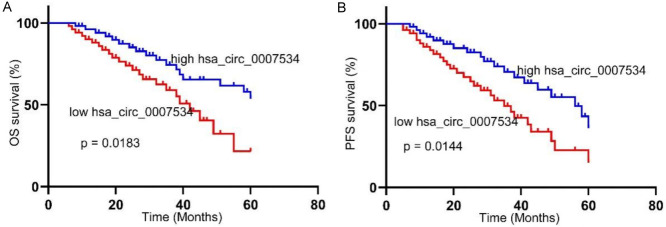
High serum hsa_circ_0007534 levels were significantly associated with poorer OS (P = 0.0183, Figure 2A) and PFS (P = 0.0144, Figure 2B) compared to low serum levels.
Figure 2.
Associations between serum levels of hsa_circ_0007534 and survival of OC patients. Patients with raised hsa_circ_0007534 in their sera had markedly worse OS (A) and PFS (B) relative to those with low levels of this circRNA. OC, Ovarian cancer; OS, overall survival; PFS, progression-free survival.
Diagnostic utility of serum hsa_circ_0007534 in OC
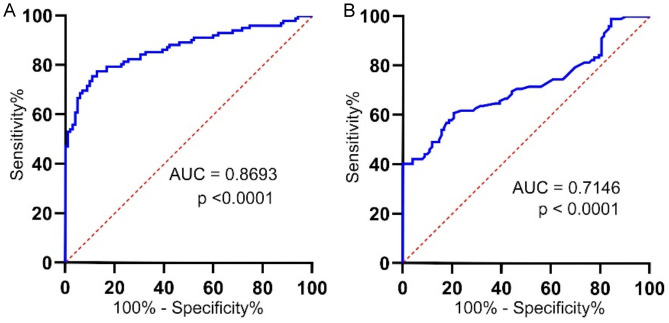
The diagnostic performance of serum hsa_circ_0007534 and CA-125 levels in OC patients was evaluated using ROC curve analyses. Serum hsa_circ_0007534 levels effectively distinguished OC patients from healthy controls, with an Area Under Curve (AUC) of 0.8693 (P < 0.0001) (Figure 3A), showing higher diagnostic accuracy than CA125 (Figure 3B). Multivariate Cox regression analysis identified tumor size (relative risk [RR] = 6.199, 95% confidence interval [CI] = 2.443-15.726, P = 0.000), histologic grade (RR = 3.486, 95% CI = 1.384-8.78, P = 0.008), and serum hsa_circ_0007534 levels (RR = 2.560, 95% CI = 1.074-6.101, P = 0.034) as independent predictors of OS in OC patients (Table 4).
Figure 3.
The diagnostic performance of serum hsa_circ_0007534 in OC. (A, B) The diagnostic accuracy of serum hsa_circ_0007534 (A) and CA-125 (B) levels were compared in patients with OC and healthy controls. OC, Ovarian cancer.
Table 4.
Multivariate analyses of prognostic factors in patients with OC
| Variable | Overall survival | ||
|---|---|---|---|
|
| |||
| RR | 95% CI | p | |
| Tumor size (< 5 cm vs. ≥ 5 cm) | 6.199 | 2.443-15.726 | 0.000 |
| Histological grade (moderate/poor vs. well) | 3.486 | 1.384-8.78 | 0.008 |
| Lymph node metastasis (negative vs. positive) | 0.371 | 0.126-1.097 | 0.073 |
| FIGO stage (III/IV vs. I/II) | 0.558 | 0.169-1.838 | 0.337 |
| hsa_circ_0007534 (low vs. high) | 2.560 | 1.074-6.101 | 0.034 |
OC, Ovarian cancer; FIGO, Federation Internationale of Gynecology and Obstetrics.
Serum hsa_circ_0007534 levels in OC treatment
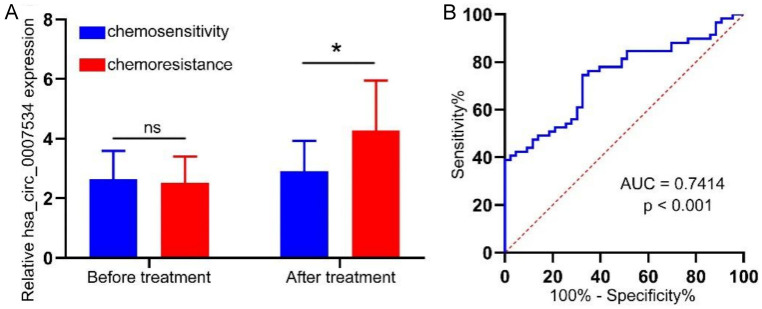
qPCR detection showed that serum hsa_circ_0007534 expression levels were higher in chemoresistant patients compared to chemosensitive patients (Figure 4A). ROC curve analyses further demonstrated that serum hsa_circ_0007534 levels could effectively differentiate between chemoresistant and chemosensitive groups (AUC = 0.7414, P < 0.0001, Figure 4B).
Figure 4.
The diagnostic performance of serum hsa_circ_0007534 in OC treatment. A. Serum hsa_circ_0007534 were detected by QPCR. B. The diagnostic accuracy of serum hsa_circ_0007534 levels were compared in patients with chemoresistance group and chemosensitivity group subjects. *P < 0.05. OC, Ovarian cancer.
Discussion
Ovarian cancer (OC) remains the deadliest and fifth most frequently diagnosed cancer among women [1]. High OCrelated mortality are primarily due to late-stage diagnosis, as the disease often lacks distinctive early-stage symptoms or reliable biomarkers [4]. While CA-125 is the most often used serum biomarker for assessing gynecologic malignancies, its limitations are well-documented. This study found that CA-125 expression was increased in OC patients compared to HCs, with CA-125 levels distinguishing between these groups. However, elevated CA-125 levels can also occur in various benign gynecologic and non-gynecologic conditions, such as pelvic inflammatory disease, endometriosis, pregnancy, and adenomyosis [21]. Thus, there remains a need to identify new biomarkers suitable for early-stage OC diagnosis and capable of differentiating between malignant tumors and benign ovarian masses. CircRNAs, due to their excellent stability, are promising candidates for extracellular biomarkers in this context.
CircRNAs can influence the onset and progression of OC and other cancers [22,23]. For example, circ_0015756 promotes OC progression through the miR-145-5p/Phosphoserine Aminotransferase 1 (PSAT1) axis [24], while circ_0072995 targets the miR-122-5p/Solute carrier family 1 member 5 (SLC1A5) axis to facilitate OC progression [25]. Conversely, hsa_circ_0000-144 inhibits OC progression by sequestering miR-610 and modulating ETS-domain protein (ELK3) expression [26]. In this study, we found that hsa_circ_0007534 expression was significantly higher in OC patients compared to HCs. Elevated serum levels of hsa_circ_0007534 were associated with high CA-125 concentrations, larger tumor size, advanced FIGO staging, poorer histologic grade, and lymph node metastases. Additionally, patients with elevated serum hsa_circ_0007534 had reduced OS, with serum hsa_circ_0007534 levels independently associated with OS.
Certain circRNAs have been proposed as biomarkers with diagnostic and prognostic value in OC, with their dysregulation correlating with clinicopathologic features in OC patients [27]. For instance, plasma circN4BP2L2 levels have been suggested as a novel diagnostic biomarker for epithelial OC [28], and circ-0001068 has similarly been proposed as a diagnostic marker for OC [29]. Given their accessibility, serum circRNAs are promising targets for diagnostic, prognostic, and therapeutic monitoring. Here, we evaluated serum hsa_circ_0007534 as a potential circulating biomarker for OC. Compared to HCs, OC patients exhibited increased circulating hsa_circ_0007534 levels, which effectively distinguished between OC patients and HCs (AUC = 0.8693). This diagnostic capability is superior to previously reported circ_0003972 (AUC = 0.724) [30] and circDENND4C (AUC = 0.675) [31]. Notably, serum hsa_circ_0007534 levels could also distinguish between chemoresistant and chemosensitive groups.
However, this study has limitations. First, the single-center study had a limited sample size, necessitating further research with a larger sample population. Second, the lack of investigation into the molecular mechanisms and functions of hsa_circ_000-7534 limits our understanding of its role.
In conclusion, this study is the first to report the clinical relevance of serum hsa_circ_0007534 concentrations in OC patients. Our findings indicate that OC patients exhibit significantly elevated circulating hsa_circ_0007534 levels compared to HCs. These elevated levels are closely associated with adverse clinicopathologic features and poorer overall prognostic outcomes. Thus, serum hsa_circ_0007534 may be a valuable diagnostic and/or prognostic biomarker for OC.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28:viii61–viii65. doi: 10.1093/annonc/mdx443. [DOI] [PubMed] [Google Scholar]
- 4.Lu KH. Screening for ovarian cancer in asymptomatic women. JAMA. 2018;319:557–558. doi: 10.1001/jama.2017.21894. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wang C, Sun H, Wang J, Liang Y, Wang Y, Wong G. The bioinformatics toolbox for circRNA discovery and analysis. Brief Bioinform. 2021;22:1706–1728. doi: 10.1093/bib/bbaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, He Y, Zhang Y. CircRNA in ocular neovascular diseases: fundamental mechanism and clinical potential. Pharmacol Res. 2023;197:106946. doi: 10.1016/j.phrs.2023.106946. [DOI] [PubMed] [Google Scholar]
- 7.Cammarata G, Barraco N, Giusti I, Gristina V, Dolo V, Taverna S. Extracellular vesicles-ceRNAs as ovarian cancer biomarkers: looking into circRNA-miRNA-mRNA code. Cancers (Basel) 2022;14:3404. doi: 10.3390/cancers14143404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Z, Duan C, Li X, Wang H, Li S, Zhou X, Miao Y. circ-TFRC downregulation suppresses ovarian cancer progression via miR-615-3p/IGF2 axis regulation. Cancer Cell Int. 2024;24:152. doi: 10.1186/s12935-024-03287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Liu Y, Chen H, Tan Y. Circular RNA circRHOBTB3 inhibits ovarian cancer progression through PI3K/AKT signaling pathway. Panminerva Med. 2024;66:36–46. doi: 10.23736/S0031-0808.20.03957-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, Zhang K, Tan S, Xin J, Yuan Q, Xu H, Xu X, Liang Q, Christiani DC, Wang M, Liu L, Du M. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol Cancer. 2021;20:13. doi: 10.1186/s12943-020-01298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei G, Zhu J, Hu HB, Liu JQ. Circular RNAs: promising biomarkers for cancer diagnosis and prognosis. Gene. 2021;771:145365. doi: 10.1016/j.gene.2020.145365. [DOI] [PubMed] [Google Scholar]
- 12.Sheng R, Li X, Wang Z, Wang X. Circular RNAs and their emerging roles as diagnostic and prognostic biomarkers in ovarian cancer. Cancer Lett. 2020;473:139–147. doi: 10.1016/j.canlet.2019.12.043. [DOI] [PubMed] [Google Scholar]
- 13.Xing T, Chen J, Ding J, Liu J, Ling S, Luo Y. CircRNA hsa_circ_0120175 promotes ovarian cancer tumorigenesis and predicts a poor prognosis. Discov Med. 2024;36:113–120. doi: 10.24976/Discov.Med.202436180.10. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Zhang H, Li P. Upregulation of hsa_circRNA_102958 indicates poor prognosis and promotes ovarian cancer progression through miR-1205/SH2D3A axis. Cancer Manag Res. 2020;12:4045–4053. doi: 10.2147/CMAR.S248560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li GF, Li L, Yao ZQ, Zhuang SJ. Hsa_circ_0007534/miR-761/ZIC5 regulatory loop modulates the proliferation and migration of glioma cells. Biochem Biophys Res Commun. 2018;499:765–771. doi: 10.1016/j.bbrc.2018.03.219. [DOI] [PubMed] [Google Scholar]
- 16.Rong X, Gao W, Yang X, Guo J. Downregulation of hsa_circ_0007534 restricts the proliferation and invasion of cervical cancer through regulating miR-498/BMI-1 signaling. Life Sci. 2019;235:116785. doi: 10.1016/j.lfs.2019.116785. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Yang S, Liu Y, Wang Y, Lin T, Li Y, Zhang R. Hsa_circ_0007534 as a blood-based marker for the diagnosis of colorectal cancer and its prognostic value. Int J Clin Exp Pathol. 2018;11:1399–1406. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Li J. Down-regulation of circular RNA hsa_circ_0007534 suppresses cell growth by regulating miR-219a-5p/SOX5 axis in osteosarcoma. J Bone Oncol. 2021;27:100349. doi: 10.1016/j.jbo.2021.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi Y, Zhang B, Wang J, Yao M. Upregulation of circular RNA hsa_circ_0007534 predicts unfavorable prognosis for NSCLC and exerts oncogenic properties in vitro and in vivo. Gene. 2018;676:79–85. doi: 10.1016/j.gene.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Song L, Xiao Y. Downregulation of hsa_circ_0007534 suppresses breast cancer cell proliferation and invasion by targeting miR-593/MUC19 signal pathway. Biochem Biophys Res Commun. 2018;503:2603–2610. doi: 10.1016/j.bbrc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem. 2011;44:884–888. doi: 10.1016/j.clinbiochem.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Qiu Q, Zhou Q, Li J, Yang J, Zheng C, Luo A, Li X, Zhang H, Cheng X, Lu W, Liu P, Lu B, Lu Y. circFBXO7/miR-96-5p/MTSS1 axis is an important regulator in the Wnt signaling pathway in ovarian cancer. Mol Cancer. 2022;21:137. doi: 10.1186/s12943-022-01611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Sun Y, Xu H, Shi Y, Shen R, Teng F, Xu J, Jia X. Circular RNA hsa_circ_0007444 inhibits ovarian cancer progression through miR-23a-3p/DICER1 axis. Acta Biochim Biophys Sin (Shanghai) 2023;55:574–586. doi: 10.3724/abbs.2023052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, Huang Q, Peng X, Yu S, Liu N. Circ_0015756 promotes ovarian cancer progression via the miR-145-5p/PSAT1 axis. Reprod Biol. 2022;22:100702. doi: 10.1016/j.repbio.2022.100702. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Luo Y, Li X. Circ_0072995 promotes ovarian cancer progression through regulating miR-122-5p/SLC1A5 axis. Biochem Genet. 2022;60:153–172. doi: 10.1007/s10528-021-10092-5. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Liu J, Yu L, Wu S, Qiu X. Circular RNA hsa_circ_0000144 aggravates ovarian Cancer progression by regulating ELK3 via sponging miR-610. J Ovarian Res. 2022;15:113. doi: 10.1186/s13048-022-01048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, He R, Yang Y, He Y, Shao K, Zhan L, Wei B. Circular RNAs: Novel biomarkers for cervical, ovarian and endometrial cancer (Review) Oncol Rep. 2020;44:1787–1798. doi: 10.3892/or.2020.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning L, Lang J, Wu L. Plasma circN4BP2L2 is a promising novel diagnostic biomarker for epithelial ovarian cancer. BMC Cancer. 2022;22:6. doi: 10.1186/s12885-021-09073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Yao Y, Jin M. Circ-0001068 is a novel biomarker for ovarian cancer and inducer of PD1 expression in T cells. Aging (Albany NY) 2020;12:19095–19106. doi: 10.18632/aging.103706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge L, Sun Y, Shi Y, Liu G, Teng F, Geng Z, Chen X, Xu H, Xu J, Jia X. Plasma circRNA microarray profiling identifies novel circRNA biomarkers for the diagnosis of ovarian cancer. J Ovarian Res. 2022;15:58. doi: 10.1186/s13048-022-00988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Yuan L, Li J, Liu Y, Wang H, Ren X. circDENND4C, a novel serum marker for epithelial ovarian cancer, acts as a tumor suppressor by downregulating miR-200b/c. Ann Med. 2023;55:908–919. doi: 10.1080/07853890.2023.2185289. [DOI] [PMC free article] [PubMed] [Google Scholar]