Abstract
Objective: To evaluate the effectiveness of real-time shear wave elastography (SWE) in quantitative evaluation of chronic kidney disease (CKD) in pediatric patients. Methods: SWE was conducted on 58 pediatric patients with CKD (CKD group) and 70 healthy volunteers (Control group). Computer-assisted quantitative analysis was utilized to determine the percentage of interstitial fibrosis (IF) in images from the CKD group, categorizing them into mild, moderate, and severe groups according to IF% values. The differences in Young’s modulus (YM) and estimated Glomerular Filtration Rate (eGFR) between the renal cortex and medulla in these groups were compared. Additionally, the relationships between YM and IF% as well as YM and eGFR, were analyzed. Results: The YM values in right lower pole cortex and medulla of the CKD group were significantly higher than those in the control group (all P < 0.05). Significant differences were observed in eGFR among mild, moderate, and severe CKD patients (F = 40.882). YM demonstrated a correlation with eGFR in both the renal cortex and medulla (r = -0.329, P = 0.012; r = 0.417, P = 0.001). YM values increased with the severity of renal interstitial fibrosis in a pronounced trend across groups (F = 109.962, F = 72.950, all P < 0.001). Additionally, YM correlated with IF% in both the renal cortex and medulla (r = 0.362, P = 0.006; r = 0.483, P < 0.001). The optimal cut-off value of renal cortex YM for distinguishing between CKD and control group was 4.05 kPa. Conclusion: SWE enables quantitative assessment of kidney YM values, revealing significantly higher values in children with CKD compared to healthy individuals. YM is correlated with the severity of renal interstitial fibrosis, thereby establishing SWE as a valuable non-invasive tool for quantitative evaluation of pediatric CKD.
Keywords: Real-time shear wave elastography, chronic kidney disease, pediatric, Young’s modulus
Introduction
Chronic kidney disease (CKD) is a progressive and incurable condition characterized by changes in renal structure and function due to multiple etiological factors [1]. CKD poses a significant global health burden, affecting approximately 10% of the adult population worldwide and resulting in an estimated 10,000 deaths and 20,000 life-years lost annually [2]. Projections indicate that by 2040, CKD will become the fifth leading cause of death worldwide [3]. The impact of CKD extends beyond adults, affecting pediatric populations as well [4]. The traditional diagnostic approach for CKD primarily relies on the measurement of glomerular filtration rate (GFR) and albuminuria [5], both of which have been validated as predictors of long-term health outcomes in adults [6]. However, diagnosing CKD in children is particularly challenging due to the natural developmental variations in GFR during childhood, complicating the application of a static diagnostic criterion. This complexity necessitates a more nuanced understanding and approach to the diagnosis of pediatric CKD [7]. The Schwartz formula, revised in 2009, is the most widely utilized estimated Glomerular Filtration Rate (eGFR) equation for diagnosing CKD in pediatric patients, based on serum creatinine levels [8]. However, the accuracy of this formula diminishes in individuals with GFR greater than 75 mL/min/1.73 m2 [9]. Additionally, the clinical manifestations of CKD in children vary widely, from subclinical conditions that may remain undetected to severe renal impairment, which can markedly reduce the quality of life and life expectancy for the child, while profoundly affecting family dynamics [10].
Renal fibrosis is a key pathological change associated with CKD in both adults and children, represents an unavoidable progression towards end-stage renal failure, with tubulointerstitial fibrosis being particularly prevalent [11]. Therefore, early detection and intervention of renal interstitial fibrosis are essential for preventing the progression of CKD. While renal biopsy remains the definitive diagnostic method for renal fibrosis [12], its invasive nature makes it unsuitable for routine monitoring or dynamic assessment of CKD in pediatric patients. The introduction and growing utilization of real-time shear wave elastography (SWE) technology provide a promising alternative for detecting renal fibrosis in pediatric CKD patients. SWE can qualitatively describe and quantitatively measure the elasticity of biological tissues. Research has demonstrated a correlation between SWE measurements and the presence of renal fibrosis, as confirmed by pathology examination [13]. Moreover, the thinner subcutaneous fat and muscle layers in children significantly enhance the feasibility and accuracy of SWE in detecting renal fibrosis.
In this study, we utilized SWE to assess and compare renal tissue in pediatric CKD patients to that in healthy controls. Additionally, we further examined the relationship between the degree of renal fibrosis, as determined by renal biopsy, and the quantitative SWE measurements. The objective is to evaluate the efficacy of SWE as a diagnostic tool for detecting renal interstitial fibrosis, offering a safer and more accessible method for monitoring disease progression and treatment response in pediatric CKD patients.
Materials and methods
Research subjects
Between January 2020 and December 2021, a retrospective cohort of 58 children diagnosed with chronic kidney disease (the CKD group) via renal biopsy at the Affiliated Children’s Hospital of Xiangya School of Medicine, Central South University (Hunan Children’s Hospital) was established. Inclusion criteria for CKD included: (i) Diagnosis of CKD according to K/DOQI criteria [14]; (ii) Positive findings on renal biopsy; and (iii) Identification of renal structural alterations using SWE within 2 days of admission. Exclusion criteria included: (i) Age ≥ 18 years; (ii) A history of chronic conditions, such as hypertension, diabetes, or coronary artery disease. Additionally, a control group of 70 healthy volunteers was included in this study. Patients in the CKD group underwent renal biopsy, periodic acid-Schiff (PAS) staining, and Masson staining procedures. Ethical approval for this study was granted by the Ethics Committee of the Affiliated Children’s Hospital of Xiangya School of Medicine, Central South University (Hunan Children’s Hospital).
Research methods
Calculation of eGFR
The eGFR was calculated using the Schwartz formula [8]: eGFR = K × Height/serum creatinine, where the K value was set at 40 for infants aged 0-18 months, 49 for girls aged 2-16 years and boys aged 2-13 years, and 62 for boys aged 14-16 years.
SWE testing
The Color Doppler ultrasound diagnostic device (Aixplore, SuperSonic Imagine) employed a convex array probe SC6-1 with a frequency range of 1 to 6 MHz. The control group underwent SWE examination of the lower pole of both kidneys, while the CKD group underwent SWE evaluation at the lower pole of the right kidney (the site of renal biopsy). SWE examinations for both groups were performed by the same two physicians. After urination, subjects reclined flat to expose the examination area. Upon inhalation, the children were instructed to hold their breath for 3-5 seconds. Once the image stabilized, the SWE detection mode was activated with the shear wave elasticity set between 0 and 180 kPa. The region of interest (ROI) square frame, measuring 2 mm in diameter, was strategically placed within the renal biopsy area at the lower pole of the right kidney. The ROI image was uniformly colored to ensure consistency throughout the frame. After stabilization, the images were captured, and Young’s modulus (YM) at the target site was determined using the quantitative measurement tool Q-box integrated into the Aixplore software. The YM value at the target site was assessed by performing three consecutive measurements, with the average value representing the test outcome. In instances of unclear demarcation between the renal cortex and medulla, the sampling site in the renal cortex was positioned within 7 mm beneath the renal capsule.
Histopathological examination and staining
The lower pole of the right kidney was selected as the puncture site with the assistance of the Hivision Preirus ultrasound guidance system. Initially, the skin at the puncture site was disinfected using iodophor and alcohol, followed by coverage with sterile drapes. Subsequently, local anesthesia was administered with 2% lidocaine, after which the puncture procedure was performed as guided by the display marker, allowing for automatic sample retrieval. Two to three tissue specimens were collected from each kidney and preserved in FAA fixative. The samples were subsequently embedded in paraffin, cut into sections of 2-3 microns in thickness, and subjected to periodic acid-Schiff (PAS) and Masson staining.
Assessment of renal interstitial fibrosis
The stained sections were scanned using a digital section scanner (NanoZoomer-XR, C12000-02). The scanned images were then processed with NDP.view2 software. Initially, the renal medulla region was extracted, magnified to 20×, and saved as JPEG format images. Subsequently, a C++ program was used to apply a color segmentation algorithm to analyze the Masson and PAS staining images, calculating the percentage of positive staining for each. The percentage of interstitial fibrosis (IF) was calculated using the formula: IF% = Masson staining positive pixel percentage - PAS staining positive pixel percentage. Based on the IF% value, children with CKD were classified into three categories: Mild (IF% < 25%), Moderate (IF%: 25-50%), and Severe (IF% > 50%).
Statistical analysis
Statistical analyses were conducted using SPSS version 27.0 software. Continuous data with normal distribution were presented as mean ± standard deviation. Independent sample t-tests were employed for between-group comparisons, while one-way analysis of variance was utilized for multi-group comparisons. The chi-square test was applied to analyze categorical data. The receiver operating characteristic (ROC) curve was employed to establish the diagnostic threshold and assess the area under the curve (AUC). Spearman’s rank correlation analysis was performed to examine the relationship between YM value and IF%. A p-value of less than 0.05 was deemed statistically significant.
Results
Comparison of baseline data between the CKD group and the control group
No significant differences were observed in age, sex, and body mass index between the CKD group and the control group (all P > 0.05; Table 1). All 58 patients with CKD were diagnosed with glomerular diseases with a detailed distribution of specific diseases presented in Table 2.
Table 1.
Comparison of baseline data between CKD group and control group
| Groups | n | Age (years) | BMI (kg/m2) | Gender (male/female) |
|---|---|---|---|---|
| Control group | 70 | 9.40 ± 2.23 | 20.64 ± 2.27 | 41/29 |
| CKD group | 58 | 9.55 ± 2.36 | 20.61 ± 2.25 | 35/23 |
| t-value/χ2-value | 0.373 | 0.098 | 0.041 | |
| P-value | 0.710 | 0.922 | 0.839 |
Data are presented as mean ± SD for continuous variables. BMI: body mass index; CKD: chronic kidney disease.
Table 2.
Disease distribution in CKD group
| CKD Group | n = 58, n (%) |
|---|---|
| IgA nephropathy | 16 (27.59) |
| Chronic glomerulonephritis | 10 (17.24) |
| Nephrotic syndrome | 10 (17.24) |
| Membranous nephropathy | 9 (15.52) |
| Lupus nephritis | 6 (10.34) |
| Purpura nephritis | 4 (6.90) |
| Psoriatic nephritis | 3 (5.17) |
CKD: chronic kidney disease.
Comparison of YM values between the CKD group and the control group
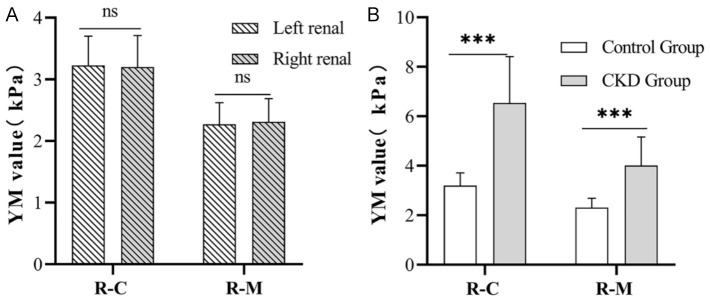
In the control group, the YM value of the renal cortex at the lower pole of the left kidney (3.23 ± 0.47 kPa) did not differ significantly from that at the lower pole of the right kidney (3.20 ± 0.51 KPa) (P = 0.669). Similarly, the YM value of the renal medulla at the lower pole of the left kidney (2.27 ± 0.35 kPa) did not differ significantly from that at the lower pole of the right kidney (2.31 ± 0.38 kPa), (P = 0.472; Figure 1A).
Figure 1.
Comparison of YM values. A. Comparison of YM values of renal cortex and medulla in the lower pole of both kidneys in the control group. B. Comparison of Young’s modulus values between CKD group and control group. R-C: renal cortex; R-M: renal medulla; YM: Young’s modulus; ns: non-significant (Left renal: R-C vs R-M, P = 0.669; Right renal: R-C vs R-M, P = 0.472), ***P < 0.001.
In the CKD group, the YM value of the renal cortex at the lower pole of the right kidney was significantly elevated (6.54 ± 1.87 kPa) compared to that in the control group (3.20 ± 0.51 kPa, P < 0.05). Additionally, the YM value of the renal medulla at the lower pole of the right kidney in the CKD group was significantly elevated (4.01 ± 1.15 kPa) compared to that in the control group (2.31 ± 0.38 kPa, P < 0.05; Figure 1B).
Comparison of YM values across various CKD groups
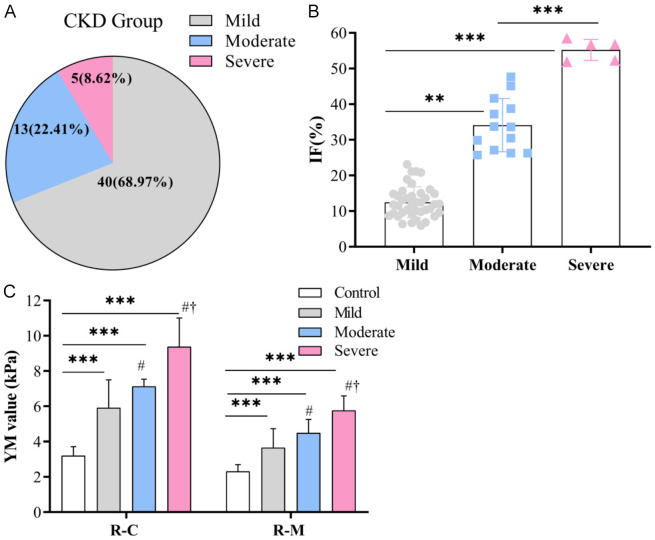
The distribution of interstitial fibrosis percentage (IF%) among the mild, moderate and severe CKD patients are shown in Figure 2A, revealing that the IF% in mild group was 12.49 ± 4.34%, moderate group was 34.12 ± 7.46%, and severe group was 55.24 ± 2.96%. Statistical difference was observed among the three groups (F = 210.393, P < 0.001; Figure 2B).
Figure 2.
Comparison of IF% and YM values among various groups. A. The distribution of CKD patients grouped according to IF%. B. Comparison of IF% among different groups. C. Comparison of YM values of renal cortex and medulla among different groups. R-C: renal cortex; R-M: renal medulla; IF: interstitial fibrosis; YM: Young’s modulus; **P < 0.01; ***P < 0.001; compared with Mild group, #P < 0.05 (R-C: Mild group vs Moderate group, P = 0.017; Mild group vs Severe group, P < 0.001; Moderate group vs Severe group, P = 0.008); compared with Moderate group, †P < 0.05 (R-M: Mild group vs Moderate group, P = 0.011; Mild group vs Severe group, P < 0.001; Moderate group vs Severe group, P = 0.020).
As shown in Figure 2C, the YM values for the renal cortex at the right kidney in the control, mild, moderate, and severe groups were 3.20 ± 0.51 kPa, 5.91 ± 1.59 kPa, 7.13 ± 0.41 kPa, and 9.38 ± 1.62 kPa, respectively. A significant difference was identified among these groups (F = 109.962, P < 0.001). The YM values for the medullar region at the right kidney in the control, mild, moderate, and severe groups were 2.31 ± 0.38 kPa, 3.65 ± 1.08 kPa, 4.49 ± 0.77 kPa, and 5.76 ± 0.83 kPa, respectively. Statistical difference was also noted among these groups (F = 72.950, P < 0.001).
No significant differences were observed in age, sex and body mass index among CKD patients across mild, moderate and severe groups (F = 0.934, F = 0.054, F = 3.458). However, significant differences were noted in eGFR, YM value of the renal cortex (R-C), and YM value of the renal medulla (R-M) among CKD patients across these groups (F = 40.882, F = 40.882, F = 23.291; Table 3).
Table 3.
Comparison of general data among mild, moderate and severe CKD groups
| Groups | n | Age (years) | BMI (kg/m2) | Gender (male/female) | eGFR (mL/min/1.73m2) | R-C YM (Kpa) | R-M YM (Kpa) |
|---|---|---|---|---|---|---|---|
| Mild group | 40 | 9.33 ± 2.16 | 17.59 ± 2.38 | 27/13 | 100.50 ± 16.59 | 5.91 ± 1.59 | 3.65 ± 1.08 |
| Moderate group | 13 | 9.77 ± 2.55 | 17.53 ± 1.78 | 5/8 | 72.73 ± 8.18 | 7.13 ± 0.41 | 4.49 ± 0.77 |
| Severe group | 5 | 10.80 ± 3.42 | 17.92 ± 2.62 | 3/2 | 47.07 ± 7.75 | 9.38 ± 1.62 | 5.76 ± 0.83 |
| F-value/χ2-value | 0.934 | 0.054 | 3.458 | 40.882 | 40.882 | 23.291 | |
| P-value | 0.399 | 0.947 | 0.178 | < 0.001 | < 0.001 | < 0.001 |
Data are presented as mean ± SD for continuous variables. BMI: body mass index; CKD: chronic kidney disease; R-C: renal cortex; R-M: renal medulla; YM: Young’s modulus; eGFR: estimated glomerular filtration rate.
The correlation between YM, IF%, and eGFR
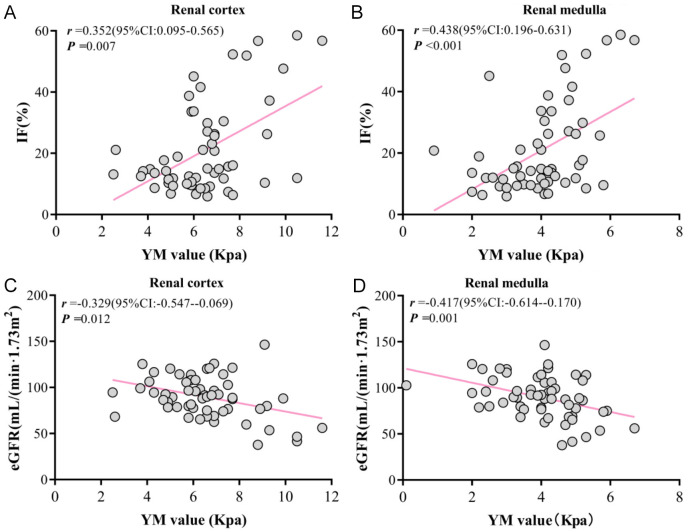
Spearman correlation analysis showed that YM values of both the renal cortex and renal medulla were positively correlated with IF% in CKD patients (r = 0.362, P = 0.006; r = 0.483, P < 0.001; Figure 3A, 3B), but negatively correlated with eGFR in CKD patients (r = -0.329, P = 0.012; r = 0.417, P = 0.001; Figure 3C, 3D).
Figure 3.
Correlation between YM value, IF%, and eGFR. A. Correlation between renal cortex YM value and IF%. B. Correlation between renal medulla YM value and IF%. C. Correlation between renal cortex YM value and eGFR. D. Correlation between renal medulla YM value and eGFR. IF: interstitial fibrosis; YM: Young’s modulus.
Diagnostic value of YM value
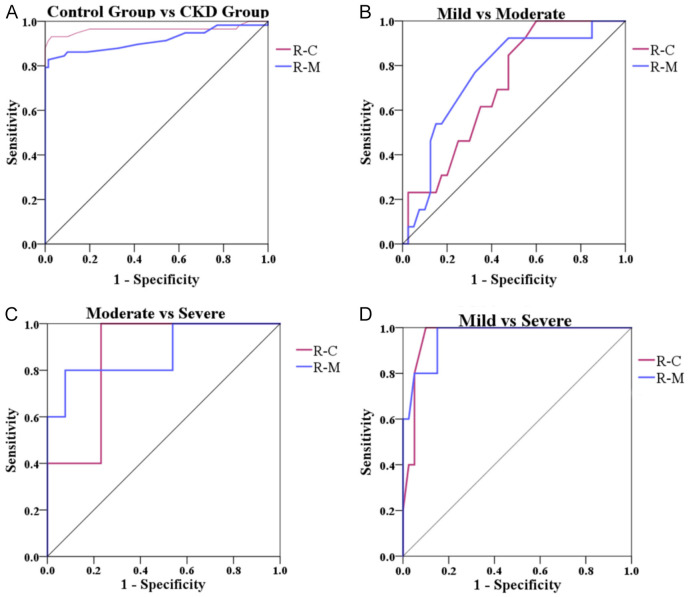
The AUC for the renal cortex YM value in distinguishing the pediatric CKD patients was 0.960 at a cut-off value of 4.05 kPa, with sensitivity and specificity values of 0.931 and 0.971, respectively. The AUC for distinguishing between patients with mild and moderate IF was 0.709 at a cut-off value of 5.75 kPa, with sensitivity and specificity of 1.000 and 0.400, respectively. The AUC for distinguishing between moderate and severe IF was 0.862 at a cut-off value of 7.50 kPa, with sensitivity and specificity of 1.000 and 0.769, respectively. The AUC for distinguishing between mild and severe IF was 0.963 at a cut-off value of 7.60 kPa, with sensitivity and specificity of 1.000 and 0.900, respectively.
The AUC of the renal medulla YM value in distinguishing the pediatric CKD patients was 0.915 at a cut-off value of 2.95 kPa, with sensitivity and specificity values of 0.828 and 0.986, respectively. The AUC for distinguishing between moderate and severe was 0.877 at a cut-off value of 5.25 kPa, with sensitivity and specificity values of 0.800 and 0.923, respectively. The AUC for distinguishing between mild and severe was 0.963 at a cut-off value of 4.50 kPa, with sensitivity and specificity values of 1.000 and 0.850, respectively (Table 4 and Figure 4).
Table 4.
The effect of YM value in distinguishing the CKD
| R-C YM value | Cut off (kPa) | Sensitivity | Specificity | AUC (95% CI) | P |
|
| |||||
| Control group vs CKD group | 4.05 | 0.931 | 0.971 | 0.964 (0.922-1.000) | < 0.001 |
| Mild vs Moderate | 5.75 | 1.000 | 0.400 | 0.709 (0.566-0.852) | 0.025 |
| Moderate vs Severe | 7.50 | 1.000 | 0.769 | 0.862 (0.685-1.000) | 0.021 |
| Mild vs Severe | 7.60 | 1.000 | 0.900 | 0.963 (0.909-1.000) | 0.001 |
|
| |||||
| R-M YM value | Cut off (kPa) | Sensitivity | Specificity | AUC (95% CI) | P |
|
| |||||
| Control group vs CKD group | 2.95 | 0.828 | 0.986 | 0.915 (0.857-0.973) | < 0.001 |
| Mild vs Moderate | 3.95 | 0.923 | 0.525 | 0.761 (0.615-0.906) | 0.005 |
| Moderate vs Severe | 5.25 | 0.800 | 0.923 | 0.877 (0.674-1.000) | 0.016 |
| Mild vs Severe | 5.42 | 1.000 | 0.850 | 0.963 (0.900-1.000) | 0.001 |
R-C: renal cortex; R-M: renal medulla; IF: interstitial fibrosis; CKD: chronic kidney disease; AUC: under the curve; YM: Young’s modulus.
Figure 4.
ROC curve of YM value of renal cortex and renal medulla in distinguishing disease severity. A. Distinguishing the control group and the CKD group; B. Distinguishing the Mild and Moderate groups; C. Distinguishing the Moderate and Severe groups; D. Distinguishing the Mild and Severe groups. R-C: renal cortex; R-M: renal medulla; IF: interstitial fibrosis; YM: Young’s modulus.
Discussion
In recent years, various quantitative and non-invasive techniques, such as real-time tissue elastography (RTE), transient elastography (TE), and real-time shear wave elastography (SWE), have been employed to evaluate renal stiffness. Among these techniques, SWE, a relatively new imaging technology, allows for quantitative assessment of tissue stiffness [15]. The correlation between the tissue hardness assessed by SWE and the actual tissue hardness has been well-documented in studies of liver disease [16], endometrial disease [17], and thyroid disease [18]. Similarly, SWE also exhibits notable potential for application in the assessment of renal disease [19,20]. Renal fibrosis is a prevalent feature of various CKDs, primarily manifests as glomerular sclerosis, tubular sclerosis, and renal interstitial fibrosis, ultimately progressing to chronic renal failure [21]. The extent of renal fibrosis is a crucial factor influencing the prognosis of kidney disease. Accurate evaluation of renal fibrosis is essential for timely intervention. Renal parenchymal fibrosis is considered a key determinant of renal function in CKD [22]. Recent studies have demonstrated that fibrosis significantly impacts tissue elasticity [23]. For example, during cirrhosis progression, normal liver tissue is progressively replaced by fibrous tissue, resulting in increased tissue hardness [24]. Similar pathological changes may also occur in the kidneys [25]. Research on fibrosis in renal transplantation has reported that SWE parameters of transplanted kidney are positively correlated with fibrosis [26,27]. In CKD, SWE results correlate with the degree of renal fibrosis and are considered useful for evaluating early renal injury [28].
Young’s modulus (YM) represents the ratio of stress to strain. According to Hooke’s law, within the material’s elastic limit, stress is directly proportional to strain, and its magnitude reflects tissue elasticity. A higher YM value reflects greater tissue hardness [29]. SWE measures the YM value of tissues, allowing for quantitative assessment of tissue elasticity under different physiological conditions. At present, the diagnosis of renal lesions largely depends on renal parenchymal biopsy, an invasive procedure unsuitable for frequent repetition and lacks real-time monitoring capability for dynamic changes in renal lesions [30]. Although traditional two-dimensional and three-dimensional ultrasound methods can detect changes in renal size and blood flow, they are insufficient for accurately assessing variations in renal stiffness [31]. SWE provides an alternative method to address these limitations.
In our study, we compared the YM values of the renal cortex and medulla between the control and CKD groups, revealing higher YM values in the renal cortex relative to the renal medulla in both groups. Similar findings have been reported in previous studies [32,33]. The renal parenchyma comprises the renal cortex and renal medulla. The renal cortex is highly vascularized and contains pink granules known as renal corpuscles, which are visible to the naked eye. CKD is characterized by an excessive accumulation of collagen fibers and fibronectin in the kidney, leading to glomerulosclerosis and tubulointerstitial fibrosis [34]. As a result, the damage to the renal cortex is more pronounced than that to the medulla, leading to a significant increase in the YM value. Moreover, vascular pressure affects tissue elasticity [35]. The renal cortex displays a higher degree of vascularization compared to the medulla, with predominant vascular resistance in this region [36]. Our study also observed a higher YM value in the CKD group compared to the control group. Liu et al. [19] found that the YM value of the right lower pole parenchyma in children with CKD (16.0 ± 4.7 kPa) was significantly higher than that in the control group (8.3 ± 2.4 kPa). Zhang et al. [37] showed that the YM value of the right lower pole parenchyma was higher in the CKD group (21.99 ± 11.87 kPa) compared to that in the control group (4.50 ± 0.59 kPa); however, notable discrepancies were observed when compared to our findings. This discrepancy might be due to Zhang et al.’s failure to differentiate between the YM values of the renal cortex and medulla. Reports indicate notable variability in the hardness measurements when different sizes of ROIs are used within the same patient cohorts [38]. Therefore, it is essential to distinguish between the renal cortex and medulla when assessing renal stiffness, as the use of larger ROIs may include both regions and average the tissue stiffness, potentially leading to inconsistent results [39]. Leong et al. [34] found that a smaller ROI of 2 mm may offer greater precision in evaluating renal stiffness compared to a larger ROI of 4 mm, especially in complexly compartmentalized organs such as the kidney. Therefore, our findings highlight the importance of evaluating renal stiffness by considering the differences between the renal cortex and medulla. Additionally, eGFR is a crucial index for assessing CKD in children. The severity of the disease correlates with a reduction in eGFR levels [40]. Our study further revealed a significant negative correlation between eGFR level and YM value in CKD patients, indicating that YM value could serve as a potential indicator for evaluating CKD.
Percutaneous renal biopsy (PRB) remains the most effective technique for evaluating renal fibrosis and other alterations. During PRB, Masson staining highlights collagen fibers in blue or green, delineating both the renal interstitial fibrosis and the basement membrane. In contrast, PAS staining colors the basement membrane purple [41]. Traditionally, the assessment of stained sections is depended on visual inspection [42], a method subject to low repeatability and is prone to evaluator subjectivity. Our study employed image processing software and programming tools to facilitate the evaluation of renal fibrosis. The findings indicated an increase in YM values of the renal cortex and medulla as fibrosis progressed, exceeding those of the control group. Spearman correlation analysis revealed a positive correlation between YM value and IF%. Simultaneously, ROC curve analysis was employed to evaluate the discriminative capability of YM in differentiating CKD and various IF% categories in our research. Among the different groups, the YM value of the renal cortex exhibited the highest AUC for distinguishing between healthy children and CKD patients, while demonstrating the lowest AUC in differentiating between mild and moderate cases.
A similar trend was observed for the YM value in renal medulla. Consistent with our findings, Hekimoglu et al. [43] utilized SWE to measure the YM value of renal cortex in CKD patients, and observed a gradual increase in YM values with advancing pathological grade. Leong et al. [34] reported that a renal cortex YM of 4.31 kPa effectively distinguished adult CKD cases, (AUC = 0.870, sensitivity = 0.803, and specificity = 0.795), corroborating our study’s findings.
However, several limitations are present in this study. First, the single-center, retrospective nature of the study may introduce potential selection bias. In addition, the small sample size limits the generalizability of our findings, highlighting the need for larger, multi-center studies to validate these results. Furthermore, as SWE examination may be influenced by factors such as renal depth and vascular perfusion, further research is needed to explore the impact of these variables on detection accuracy.
Conclusions
SWE technology enables quantitative assessment of YM value in the kidneys. YM values in renal cortex and medulla are elevated in children with CKD compared to healthy individuals and are correlated with the degree of renal interstitial fibrosis. SWE can serve as a non-invasive method for the quantitative evaluation of renal interstitial fibrosis in children with CKD.
Acknowledgements
This work was supported by Hunan Provincial Health Commission scientific research project (20200434).
Disclosure of conflict of interest
None.
References
- 1.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398:786–802. doi: 10.1016/S0140-6736(21)00519-5. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696–707. doi: 10.1038/s41581-022-00616-6. [DOI] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harada R, Hamasaki Y, Okuda Y, Hamada R, Ishikura K. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatr Nephrol. 2022;37:1215–29. doi: 10.1007/s00467-021-05145-1. [DOI] [PubMed] [Google Scholar]
- 5.Stevens PE, Levin A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, Massy Z, Wanner C, Anders HJ. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 7.Cirillo L, De Chiara L, Innocenti S, Errichiello C, Romagnani P, Becherucci F. Chronic kidney disease in children: an update. Clin Kidney J. 2023;16:1600–1611. doi: 10.1093/ckj/sfad097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao A, Cachat F, Faouzi M, Bardy D, Mosig D, Meyrat BJ, Girardin E, Chehade H. Comparison of the glomerular filtration rate in children by the new revised Schwartz formula and a new generalized formula. Kidney Int. 2013;83:524–530. doi: 10.1038/ki.2012.388. [DOI] [PubMed] [Google Scholar]
- 10.VanSickle JS, Warady BA. Chronic kidney disease in children. Pediatr Clin North Am. 2022;69:1239–1254. doi: 10.1016/j.pcl.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Rayego-Mateos S, Valdivielso JM. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin Ther Targets. 2020;24:655–670. doi: 10.1080/14728222.2020.1762173. [DOI] [PubMed] [Google Scholar]
- 12.Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8:129. doi: 10.1038/s41392-023-01379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Wang Y, Ying MTC, Su Z, Han X, Gunda ST. Association of renal elasticity evaluated by real-time shear wave elastography with renal fibrosis in patients with chronic kidney disease. Br J Radiol. 2024;97:392–398. doi: 10.1093/bjr/tqad030. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–266. [PubMed] [Google Scholar]
- 15.Cè M, Felisaz PF, Alì M, Re Sartò GV, Cellina M. Ultrasound elastography in chronic kidney disease: a systematic review and meta-analysis. J Med Ultrason (2001) 2023;50:381–415. doi: 10.1007/s10396-023-01304-z. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Wu L, Jin J, Wang J, Li S, Zeng J, Guo H, Zheng J, Chen S, Zheng R. Liver stiffness measured with two-dimensional shear-wave elastography is predictive of liver-related events in patients with chronic liver disease due to hepatis B viral infection. Radiology. 2020;295:353–360. doi: 10.1148/radiol.2020191481. [DOI] [PubMed] [Google Scholar]
- 17.Zhao HX, Du YY, Guo YJ, Zhou JH, Sun CQ, Wen XD, Wang J, Wang N, Yang Y, Yan XJ. Application value of real-time shear wave elastography in diagnosing the depth of infiltrating muscular layer of endometrial cancer. J Ultrasound Med. 2021;40:1851–61. doi: 10.1002/jum.15568. [DOI] [PubMed] [Google Scholar]
- 18.Hazem M, Al Jabr IK, AlYahya AA, Hassanein AG, Algahlan HA. Reliability of shear wave elastography in the evaluation of diffuse thyroid diseases in children and adolescents. Eur J Radiol. 2021;143:109942. doi: 10.1016/j.ejrad.2021.109942. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Wang Z. Diagnostic value of real-time shear wave elastography in children with chronic kidney disease. Clin Hemorheol Microcirc. 2021;77:287–293. doi: 10.3233/CH-200982. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Hou FL, Zhao C, Jiang CY, Li XM, Yu N. The role of real-time shear wave elastography in the diagnosis of idiopathic nephrotic syndrome and evaluation of the curative effect. Abdom Radiol (NY) 2020;45:2508–2517. doi: 10.1007/s00261-020-02460-3. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 22.Panizo S, Martínez-Arias L, Alonso-Montes C, Cannata P, Martín-Carro B, Fernández-Martín JL, Naves-Díaz M, Carrillo-López N, Cannata-Andía JB. Fibrosis in chronic kidney disease: pathogenesis and consequences. Int J Mol Sci. 2021;22:408. doi: 10.3390/ijms22010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyama T, Sugihara T, Takata T, Isomoto H. Renal ultrasound elastography: a review of the previous reports on chronic kidney diseases. Applied Sciences. 2021;11:9677. [Google Scholar]
- 24.Numao H, Shimaya K, Kakuta A, Shibutani K, Igarashi S, Hasui K, Hanabata N, Kanazawa K, Munakata M. The utility of two-dimensional real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2021;33:1400–7. doi: 10.1097/MEG.0000000000001887. [DOI] [PubMed] [Google Scholar]
- 25.Wang L. Applications of acoustic radiation force impulse quantification in chronic kidney disease: a review. Ultrasonography. 2016;35:302–8. doi: 10.14366/usg.16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma MK, Law HK, Tse KS, Chan KW, Chan GC, Yap DY, Mok MM, Kwan LP, Tang SC, Choy BY, Chan TM. Non-invasive assessment of kidney allograft fibrosis with shear wave elastography: a radiological-pathological correlation analysis. Int J Urol. 2018;25:450–455. doi: 10.1111/iju.13536. [DOI] [PubMed] [Google Scholar]
- 27.Yang JR, La Q, Ding XM, Song Y, Wang YY. Using real-time sound touch elastography to monitor changes in transplant kidney elasticity. Clin Radiol. 2020;75:963.e1–963.e6. doi: 10.1016/j.crad.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zhang F, Ma Y, Ju H, Zhang Y, Wang Y. The application of shear wave quantitative ultrasound elastography in chronic kidney disease. Technol Health Care. 2024;32:2951–2964. doi: 10.3233/THC-231270. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Li L, Xue HY. The impact of body position and exercise on the measurement of liver Young’s modulus by real-time shear wave elastography. Technol Health Care. 2022;30:445–54. doi: 10.3233/THC-213218. [DOI] [PubMed] [Google Scholar]
- 30.Bermejo S, García-Carro C, Soler MJ. Diabetes and renal disease-should we biopsy? Nephrol Dial Transplant. 2021;36:1384–6. doi: 10.1093/ndt/gfz248. [DOI] [PubMed] [Google Scholar]
- 31.Leong SS, Jalalonmuhali M, Md Shah MN, Ng KH, Vijayananthan A, Hisham R, Wong JH. Ultrasound shear wave elastography for the evaluation of renal pathological changes in adult patients. Br J Radiol. 2023;96:20220288. doi: 10.1259/bjr.20220288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gennisson JL, Grenier N, Combe C, Tanter M. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38:1559–67. doi: 10.1016/j.ultrasmedbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Li N, Xu T, Sun F, Li R, Gao Q, Chen L, Wen C. Effect of renal perfusion and structural heterogeneity on shear wave elastography of the kidney: an in vivo and ex vivo study. BMC Nephrol. 2017;18:265. doi: 10.1186/s12882-017-0679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong SS, Wong JHD, Md Shah MN, Vijayananthan A, Jalalonmuhali M, Ng KH. Shear wave elastography in the evaluation of renal parenchymal stiffness in patients with chronic kidney disease. Br J Radiol. 2018;91:20180235. doi: 10.1259/bjr.20180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano K, Ogata A, Tanaka K, Ide Y, Sankoda A, Kawakita C, Nishikawa M, Ohmori K, Kinomura M, Shimada N, Fukushima M. Acoustic radiation force impulse elastography of the kidneys: is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med. 2014;33:793–801. doi: 10.7863/ultra.33.5.793. [DOI] [PubMed] [Google Scholar]
- 36.Peride I, Rădulescu D, Niculae A, Ene V, Bratu OG, Checheriţă IA. Value of ultrasound elastography in the diagnosis of native kidney fibrosis. Med Ultrason. 2016;18:362–9. doi: 10.11152/mu.2013.2066.183.per. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Duan C, Duan X, Hu Y, Liu J, Chen W. Quantitative evaluation of real-time shear-wave elastography under deep learning in children with chronic kidney disease. Scientific Programming. 2022;2022:1–9. [Google Scholar]
- 38.Skerl K, Vinnicombe S, Giannotti E, Thomson K, Evans A. Influence of region of interest size and ultrasound lesion size on the performance of 2D shear wave elastography (SWE) in solid breast masses. Clin Radiol. 2015;70:1421–7. doi: 10.1016/j.crad.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Leong SS, Wong JHD, Rozalli FI, Yahya F, Tee YC, Yamin LSM, Razalli MM, Baharuddin H. 2D shear wave elastography for the assessment of quadriceps entheses-a methodological study. Skeletal Radiol. 2024;53:455–463. doi: 10.1007/s00256-023-04425-1. [DOI] [PubMed] [Google Scholar]
- 40.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22:176–86. doi: 10.1681/ASN.2009091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Wang Y, Gunda ST, Han X, Su Z, Ying MTC. Integrating shear wave elastography and estimated glomerular filtration rate to enhance diagnostic strategy for renal fibrosis assessment in chronic kidney disease. Quant Imaging Med Surg. 2024;14:1766–1777. doi: 10.21037/qims-23-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farris AB, Alpers CE. What is the best way to measure renal fibrosis?: A pathologist’s perspective. Kidney Int Suppl (2011) 2014;4:9–15. doi: 10.1038/kisup.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hekimoglu A, Tatar IG, Ergun O, Turan A, Aylı MD, Hekimoglu B. Shear wave sonoelastography findings of testicles in chronic kidney disease patients who undergo hemodialysis. Eurasian J Med. 2017;49:12–15. doi: 10.5152/eurasianjmed.2017.16173. [DOI] [PMC free article] [PubMed] [Google Scholar]