Abstract
Objective: To explore the effectiveness of the Caprini model in preventing Deep venous thrombosis (DVT) in patients with nephrotic syndrome (NS), in order to provide a reference for reducing poor prognosis in patients with NS. Methods: A retrospective study was conducted on 150 cases of NS patients treated at Zibo Central Hospital from January 2021 to January 2023. Their clinical data were collected from the medical record management system of the hospital. The patients were divided into two groups: an observation group (75 patients) who received Caprini risk evaluation at admission and designed interventions based on their risk level, and a routine group (75 patients) who received conventional interventions. Clinical data, including the occurrence of DVT, renal function, inflammation, complications and satisfaction with the work of medical staff, were compared between the evaluation and routine groups. Results: The incidence of DVT in the observation group (6.67%) was significantly lower than that in the Routine group (17.33%) (P < 0.05). Three days after surgery, the difference in thigh circumference increased in both groups. However over time, the difference decreased, with the observation group consistently showing lower values (P < 0.05). On the fifth day after surgery, the D-D level was lower, and PT level higher in the observation group compared to the Routine group (both P < 0.05). Before intervention, 24 h urinary protein, BUN, SCr, WBC, Hs-CRP and IL-6 levels were similar between the two groups (all P > 0.05). After intervention, renal function and inflammation improved in both groups, with greater improvement observed in the observation group (P < 0.05). Additionally, the incidence of complications (except DVT) in the observation group (8.00%) was lower than in the Routine group (21.33%) (P < 0.05). Patients in the observation group also reported higher satisfaction with nursing and treatment. Conclusion: The Caprini score is an effective tool for guiding predictive care in NS patients, significantly reducing the risk of DVT. Additionally, implementing risk-based interventions according to the Caprini score enhances renal function, decreases the incidence of complications, and improves patient satisfaction with nursing and treatment.
Keywords: Caprini risk assessment model, nephrotic syndrome, deep vein thrombosis, renal function
Introduction
Nephrotic syndrome (NS) is primarily characterized by macroproteinuria, hypoalbuminemia, hyperlipidemia and edema, with the first two being essential for diagnosing NS [1,2]. If NS is not treated promptly and effectively, it can progress to renal failure or even uremia, resulting in increased mortality [3,4]. As kidney damage caused by NS is irreversible, untreated NS patients often experience complications such as infection, thromboembolism, acute renal failure, and metabolic disorders related to protein and fat [5,6]. Thromboembolism is a common complication of NS, including arterial thromboembolism and venous thromboembolic events (VTE). Among these, VTE is more prevalent in NS patients, with deep venous thrombosis (DVT) being the most common type. DVT can cause limb swelling and pain, greatly impacting patients’ quality of life [7]. If left untreated, DVT can progress to pulmonary embolism, which is fatal.
DVT has a high rate of missed diagnosis, making its early detection and prevention in nephrotic syndrome (NS) patients essential. A reliable and convenient assessment tool is needed to quickly identify high-risk individuals. The Caprini model is a VTE prediction tool that incorporates a wide range of risk factors, including patient-specific, surgical, and treatment-related factors, each assigned a specific score [8,9]. According to the total Caprini score of patients, medical staff can assess the risk and implement appropriate preventive measures. The Caprini model has been applied in surgical and cancer patients, demonstrating good predictive performance [10,11]. However, while the Caprini model is also employed to evaluate thrombosis risk in various contexts, its application in NS patients, who present with diverse pathological types and symptoms, has not been fully validated.
This study assessed the effectiveness of the Caprini risk level assessment in NS patients, aiming to clarify the value of the Caprini model in preventing DVT in NS patients. The innovation of this study lies in the application of the Caprini risk assessment model to the NS patient population, with the goal of evaluating its effectiveness in predicting DVT risk. We hope to provide more precise guidance for the clinical management of NS patients, thereby improving their prognosis and quality of life.
Material and methods
Research design and subjects
The clinical data of 150 patients with NS admitted to Zibo Central Hospital from January 2021 to January 2023 were retrospectively collected. The patients included 75 who underwent Caprini risk evaluation at admission and received designed interventions based on their risk level, and 75 who received routine interventions. For patients in the routine group, Caprini score was determined based on collected clinical data.
Inclusion criteria: (1) Age > 18 years old; (2) Diagnosed with NS [12]; (3) Availability of complete clinical data. Exclusion criteria: (1) Presence of malignant tumors; (2) Presence of atrial fibrillation; (3) Presence of mental illness. This study was approved by the Ethics Committee of Zibo Central Hospital.
Data collection
The following data were collected from the medical records of the patients: gender, age, body mass index (BMI), smoking history, alcohol consumption, disease duration, surgery history, leg swelling, varicose veins, bedridden status, drug treatment, sepsis, lung disease, heart disease, gastrointestinal disease, abortion history (for females), pregnancy (for females), malignancy, family history of VTE, coagulation factor mutations, lupus anticoagulant, anticardiolipin antibody, serum homocysteine, platelet count, 24-hour urinary protein, blood urea nitrogen (BUN), serum creatinine (Scr), white blood cell count (WBC), high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), history of stroke, multiple trauma, joint replacement, acute spinal cord injury, perioperative difference in lower limb circumference, perioperative coagulation function, whether Caprini risk assessment has been performed, and the corresponding assessment grade. Additionally, patient satisfaction with their care and treatment was assessed using a self-made questionnaire administered by staff at the time of discharge. The questionnaire provided four options to reflect the patients’ satisfaction with the medical staff’s performance: “very satisfied”, “basic satisfaction”, “less satisfied” and “not satisfied”.
All investigators in this study strictly adhered to standardized protocols, having undergone unified training before data collection. The case data were entered and reviewed by two independent individuals to minimize data entry errors. Each variable was assigned a reasonable data range to further reduce errors.
Caprini risk assessment [13-15]
(1) One point was assigned for each of the following factors: Age 41-60 years old; BMI > 25 kg/m2; Surgery lasting less than 45 minutes; History of major surgery within the past month; Leg swelling; Varicose veins; Bedridden; Hormone therapy or oral contraceptive use; Sepsis within the past month; Severe lung disease, including abnormal lung function or pneumonia; Acute myocardial infarction; Congestive heart failure within the past month; History of inflammatory bowel disease; Unexplained miscarriage, pregnancy or postpartum. (2) Two points were assigned for each of the following factors: Age: 61-74 years old; Illness lasting more than 72 hours; Arthroscopic surgery; Major open surgery lasting longer than 45 minutes; Chest/laparoscopy lasting longer than 45 minutes; Plaster fixation; Malignant tumor; Central venous access. (3) Three points were given for each of the following factors: History of venous thrombosis; Family history of VTE; Age > 75 years; Mutation of clotting factor V Leiden (FVL); Mutation of prothrombin gene G20210A; Positive lupus anticoagulant; Positive anti-cardiolipin antibody; Elevated serum homocysteine; Heparin-induced thrombocytopenia; Other congenital or acquired thrombosis tendencies. (4) Five points were given for each of the following factors: Stroke within the past month; Multiple trauma within the past month; Elective joint replacement; Hip, pelvic or lower limb fracture; Acute spinal cord injury. A total score of 0-1 is classified as low risk, 2 as medium risk, 3-4 as high risk, and ≥ 5 as extreme risk.
Statistical analysis
SPSS 25.0 software was used for data analysis. The measurement data that conformed to a normal distribution was represented in the form of (x̅ ± s). The t test was used for the comparison of single time point between the two groups, and the repeated measures analysis of variance was used for the comparison of multiple time points between the two groups. Counting data were expressed as n (%), chi-square test was used for comparison between the two groups. Rank sum test was used when the data were hierarchical. P < 0.05 was considered with statistical significance.
Results
Baseline data
As shown in Table 1, baseline data such as gender, age, BMI, NS course, smoking history and alcohol consumption were comparable between the observation and routine groups (all P > 0.05).
Table 1.
Baseline data of the two groups
| Data | Observation group (n=75) | Routine group (n=75) | t/χ2 | P |
|---|---|---|---|---|
| Gender | 0.244 | 0.621 | ||
| Male | 41 (48.24) | 44 (51.76) | ||
| Female | 34 (52.31) | 31 (47.69) | ||
| Age (years) | 57.46±6.37 | 56.19±6.85 | 1.176 | 0.242 |
| BMI (kg/m2) | 22.37±1.08 | 22.62±1.22 | 1.329 | 0.186 |
| Disease (years) | 4.31±1.71 | 4.57±1.65 | 0.948 | 0.345 |
| Smoking history | 0.440 | 0.507 | ||
| Yes | 33 (53.23) | 29 (46.77) | ||
| No | 42 (47.73) | 46 (52.27) | ||
| Alcohol Consumption | 0.429 | 0.513 | ||
| Yes | 38 (47.50) | 42 (52.50) | ||
| No | 37 (52.86) | 33 (47.14) | ||
| Operation history | 0.312 | 0.577 | ||
| Yes | 21 (53.85) | 18 (46.15) | ||
| No | 54 (48.65) | 57 (51.35) |
Note: BMI: Body Mass Index.
Caprini risk score and grading of patients
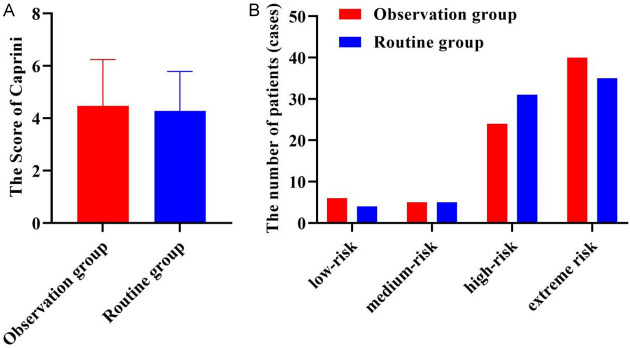
As shown in Figure 1, the Caprini score and grade distribution were comparable between the two groups (both P > 0.05).
Figure 1.
The Caprini risk score (A) and grading (B) of patients.
DVT formation in NS patients
As shown in Figure 2, the incidence of DVT in patients of the observation group (6.67%) was significantly lower than that in patients of routine group (17.33%), (P < 0.05). This suggests that adjusting care or prevention programs according to Caprini evaluation results can effectively reduce the risk of DVT in NS patients.
Figure 2.
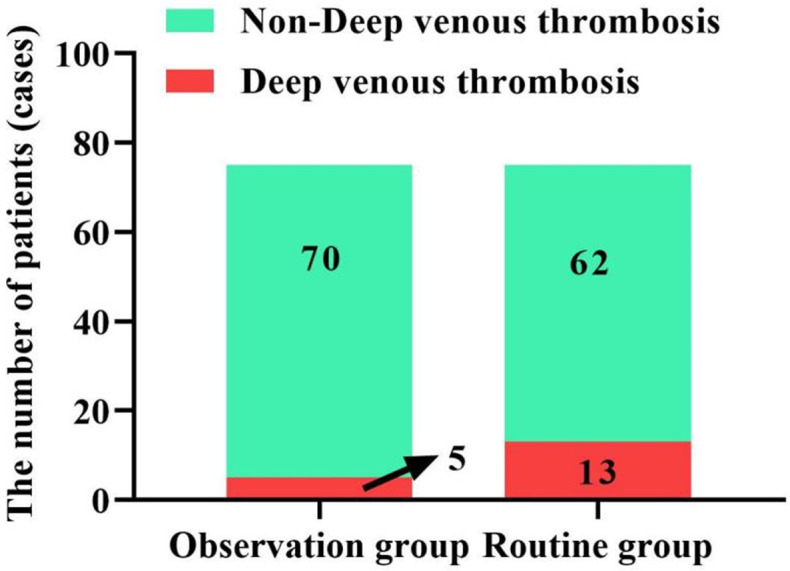
DVT formation in NS patients. DVT: Deep venous thrombosis; NS: nephrotic syndrome.
Comparison of thigh circumference difference between two groups of patients
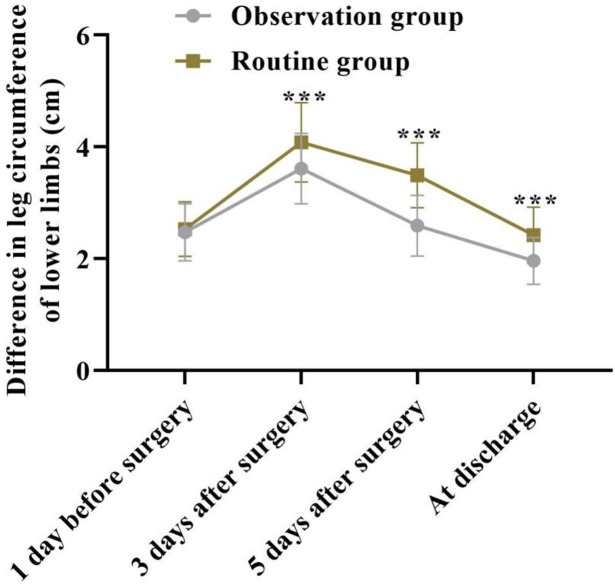
The differences in thigh circumference between the two groups at multiple time points were compared, revealing significant overall changes over time between the groups (F=14.380, P < 0.001). As shown in Figure 3, the difference in thigh circumference between the two groups was similar on the day before surgery (P > 0.05). However, three days after surgery, the difference in thigh circumference increased in both groups compared to the pre-surgery measurements. Over time, the difference in thigh circumference decreased in both groups, but the observation group consistently had lower values than the routine group at all subsequent time points (P < 0.05).
Figure 3.
Comparison of the difference in thigh circumference between the two groups of patients. ***P < 0.001.
Comparison of coagulation function between two groups of patients
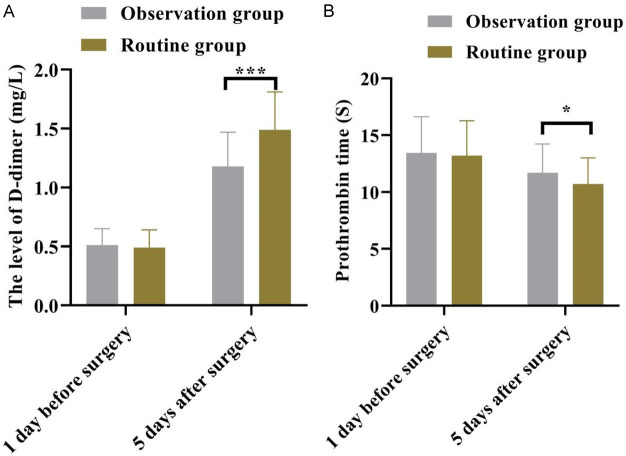
As shown in Figure 4, one day before surgery, the D-D (D-dimer) and prothrombin time (PT) levels were similar in both groups (all P > 0.05). However, on the fifth day after surgery, the D-D level was lower while the PT level was higher in the observation group than those in the routine group (all P < 0.05). This suggests that the observation group had better coagulation function compared to the routine group.
Figure 4.
The changes of coagulation function in the two groups were compared. The level of (A) D-dimer, (B) Prothrombin time. *P < 0.05, ***P < 0.001.
Comparison of kidney function between the two groups
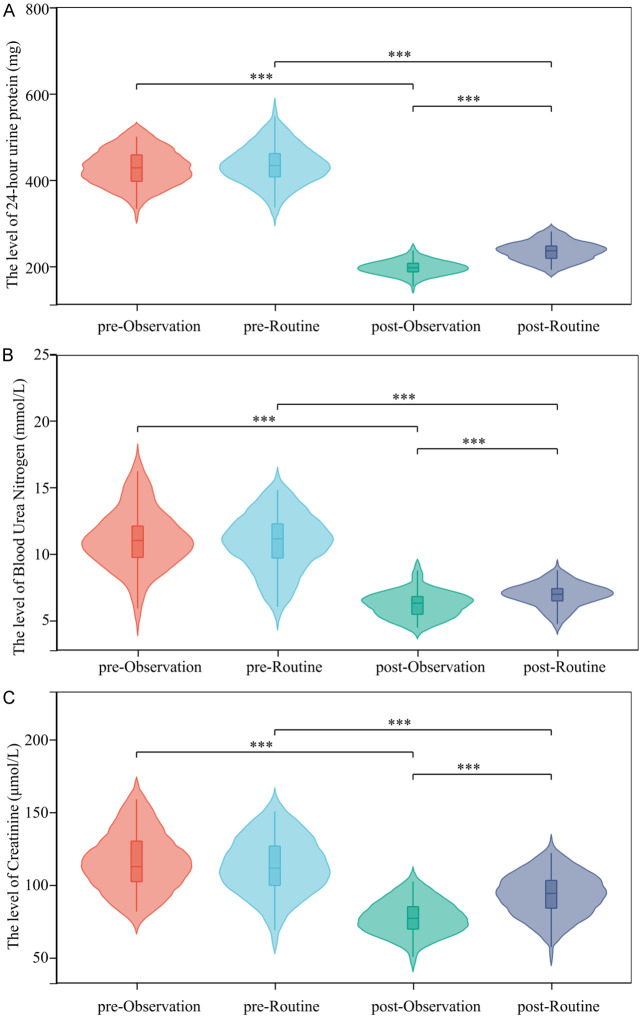
As shown in Figure 5, before intervention, 24 h urinary protein, BUN and Scr levels were similar in the two groups (all P > 0.05). After intervention, patients in the observation group showed a higher degree of improvement in kidney function compared to the routine group (all P < 0.05).
Figure 5.
Changes in renal function related indicators of patients. The level of (A) 24-hour urine protein, (B) Blood Urea Nitrogen, (C) Creatinine. ***P < 0.001.
Comparison of inflammatory status between the two groups
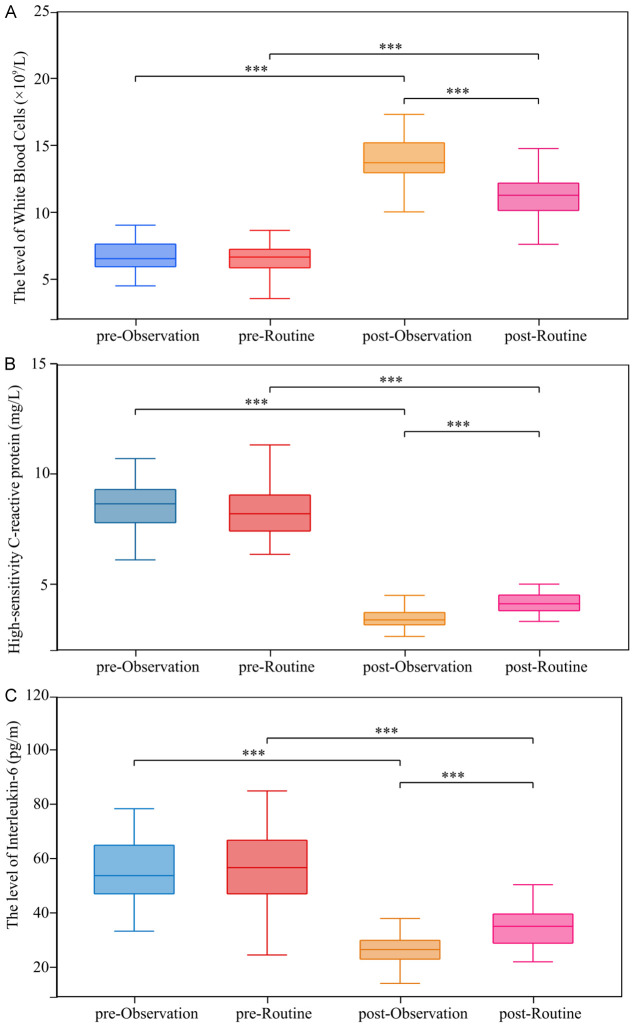
As shown in Figure 6, before intervention, WBC, Hs-CRP and IL-6 levels were similar between the two groups (all P > 0.05). After intervention, both groups exhibited improvements in their inflammatory status, with the observation group showing a significantly higher degree of remission (P < 0.05).
Figure 6.
Changes in inflammatory status. The level of (A) White blood cells, (B) High-sensitivity C-reactive protein, (C) Interleukin-6. ***P < 0.001.
Comparison of other complications between the two groups
As shown in Table 2, the incidence of complications, other than DVT, in the observation group (8.00%) was significantly lower than that in the routine group (21.33%) (P < 0.05). It can be concluded that implementing or adjusting nursing or prevention programs based on Caprini evaluation results can reduce the risk of multiple complications in patients with NS to a certain extent.
Table 2.
Occurrence of other complications
| Group | Infection | Dyslipidemia | Abnormal blood pressure | Electrolyte disturbance | Total incidence |
|---|---|---|---|---|---|
| Observation group | 2 (2.67) | 3 (4.00) | 2 (2.67) | 1 (1.33) | 6 (8.00) |
| Routine group | 4 (5.33) | 7 (9.33) | 5 (6.67) | 3 (4.00) | 16 (21.33) |
| χ2 | 5.327 | ||||
| P | 0.021 |
Patient satisfaction with care and treatment
As shown in Table 3, the proportion of patients who expressed being very satisfied or basically satisfied with the nursing and treatment provided were significantly higher than those in the routine group (Z=-1.965, P=0.049). This suggests that graded intervention based on Caprini evaluation results can improve patients’ satisfaction with the nursing and treatment provided by medical staff.
Table 3.
Patient satisfaction with care and treatment
| Group | Very satisfied | Basically satisfied | Less satisfied | Dissatisfied |
|---|---|---|---|---|
| Observation group | 31 (41.33) | 24 (32.00) | 16 (21.33) | 4 (5.33) |
| Routine group | 22 (29.33) | 21 (28.00) | 27 (36.00) | 5 (6.67) |
| Z | -1.965 | |||
| P | 0.049 | |||
Discussion
Current studies suggest that deep venous thrombosis (DVT), a common complication in patients with nephrotic syndrome (NS), is related to three factors: blood stasis, vascular injury and hypercoagulability [16,17]. In NS, patients often experience hypercoagulability, blood stasis and vascular injury. Firstly proteinuria in NS results in the loss of proteins involved in coagulation, anticoagulation and fibrinolysis, leading to a prolonged hypercoagulable state [18]. Secondly, increased platelet aggregation contributes to slower blood flow and an increased risk of thrombosis [19]. Additionally, disorders in inflammatory factors and hyperlipidemia can damage the vascular endothelial cells, further promoting thrombus formation. Finally, severe hypoalbuminemia, common in NS patients, leads to increased fluid leakage, interstitial edema, blocked venous return, and blood stasis. Furthermore, the need for a kidney biopsy to determine the underlying pathological type of NS can necessitate discontinuation of anticoagulant medications such as low molecular weight heparin for several days and strict bed rest post-surgery to prevent bleeding complications and exacerbate blood stasis.
Once DVT occurs in patients with NS, it not only delays the complete remission of NS but also lead to the progression of the condition into chronic kidney disease. Literature reports that DVT can develop into a pulmonary embolism, which can cause sudden death [20-22]. Early detection and prevention of DVT are crucial for the prognosis of NS patients. Current, research suggests that the thrombus assessment scales are beneficial for early screening and diagnosis of high-risk patients, providing a basis for the development of targeted intervention measures. Commonly used scales for assessing DVT risk include Padua score, Modified Geneva score, Wells score, and Caprini score [23-25]. The Padua score is a commonly used VTE risk assessment tool in internal medicine. However, the Padua score, Geneva score, and Wells score do not fully account for high-risk factors specific to NS patients, such as leg swelling, hyperlipidemia, hypoproteinemia, and chest pain. In contrast, the Caprini score is designed to assess the risk of thrombosis in hospitalized patients and serves as a VTE risk assessment tool for non-orthopedic surgical patients. Some studies have shown that the Caprini scale can better predict the risk of DVT formation in hospitalized patients in various clinical settings, including neurology, neurosurgery, internal medicine, and gynecological malignancies, and is commonly used for risk prediction in orthopedics, internal medicine, and critically ill patients [26-29]. The Caprini score includes more than 30 high-risk factors, including leg swelling, hormone therapy, and coagulation indicators, which are particularly relevant to the high-risk factors for thrombosis in nephrotic syndrome. Therefore, the Caprini score may have a significant advantage in the evaluation of DVT in NS patients.
This study retrospectively analyzed the clinical data of 150 patients with NS, with 75 patients assessed with Caprini scores at the initial admission, and the other 75 NS patients not receiving it before treatment. For patients without Caprini scoring, evaluations were based on collected clinical data. The initial Caprini scores and risk grading were similar between the two groups. However, the incidence of DVT in NS patients was significantly higher in the routine group than that in the observation group. Post-treatment analysis revealed that patients in the routine group had poorer renal function and more severe inflammatory reactions. This may be attributed to the graded care provided to patients in the observation group under the guidance based on Caprini risk assessments. The Caprini risk assessment divides patients into four levels: low risk, medium risk, high risk, and extremely high risk. Targeted measures based on the risk levels of DVT can not only reduce the incidence of DVT but also reduce the waste of medical resources. This study also found that early evaluation of DVT risk in NS patients using the Caprini score and graded intervention measures can reduce the incidence of complications such as infection, dyslipidemia, abnormal blood pressure, and electrolyte disorders in NS patients. Zhang et al. [30] applied the Caprini score to 34,893 orthopedic trauma patients, showing an area under the curve for DVT exceeding 0.7. Wang et al. [8] also found that the Caprini score effectively stratifies DVT risk in patients with high-energy thoracolumbar fractures. Moreover, the Caprini score has proven valuable in guiding interventions for deep vein thrombosis after laparoscopic radical colorectal cancer surgery [31]. This study’s results align with these findings, highlighting the Caprini scoring model’s effectiveness across diverse patient populations and its applicability to NS patients. Additionally, early DVT risk assessment in NS patients using the Caprini score and implementing graded intervention measures can also improve patient satisfaction with care and treatment. The findings of this study underscore the importance of early risk assessment in improving patient care experience and satisfaction, which may be related to the perceived level of attention, involvement in treatment decisions, and expectations for outcomes. These findings provide new perspectives for further optimizing clinical nursing processes, increasing patient engagement, and improving patient care outcomes.
Using the Caprini score as a guiding basis for predictive care in NS patients is effective in reducing the risk of DVT. At the same time, graded care based on the Caprini score can also improve the renal function of NS patients, reduce the incidence of complications, and enhance patients’ satisfaction with nursing and treatment. Therefore, the Caprini model demonstrates significant value in preventing DVT formation in NS patients. Still, this study is a retrospective analysis, and the findings need to be further verified by prospective studies.
Disclosure of conflict of interest
None.
References
- 1.Rodriguez-Ballestas E, Reid-Adam J. Nephrotic syndrome. Pediatr Rev. 2022;43:87–99. doi: 10.1542/pir.2020-001230. [DOI] [PubMed] [Google Scholar]
- 2.Gao XY, Liu YM, Zheng DN, Li YW, Li H, Xiong XL, Chen HY, Wang H, Yu XY, Qu K, Jin J, Lin B, He Q. Comparison of the prophylactic antithrombotic effect of indobufen and warfarin in patients with nephrotic syndrome: a randomized controlled trial. Ren Fail. 2023;45:2163505. doi: 10.1080/0886022X.2022.2163505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtta T, Jalanko H. Congenital nephrotic syndrome: is early aggressive treatment needed? Yes. Pediatr Nephrol. 2020;35:1985–1990. doi: 10.1007/s00467-020-04578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Tan TC, Chertow GM, Ordonez JD, Fan D, Law D, Yankulin L, Wojcicki JM, Zheng S, Chen KK, Khoshniat-Rad F, Yang J, Parikh RV. Primary Nephrotic syndrome and risks of ESKD, cardiovascular events, and death: the kaiser permanente nephrotic syndrome study. J Am Soc Nephrol. 2021;32:2303–2314. doi: 10.1681/ASN.2020111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright F, Gilchrist M. Nephrotic syndrome: delays in diagnosis and a cause of pulmonary embolism not to miss. Br J Hosp Med (Lond) 2023;84:1–4. doi: 10.12968/hmed.2023.0011. [DOI] [PubMed] [Google Scholar]
- 6.Kelddal S, Nykjaer KM, Gregersen JW, Birn H. Prophylactic anticoagulation in nephrotic syndrome prevents thromboembolic complications. BMC Nephrol. 2019;20:139. doi: 10.1186/s12882-019-1336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey D, Romero K, Patel R, Ouellette T, Anasseri S, Eftekhari P. Bilateral renal vein thrombosis in membranous nephropathy: hypoalbuminemia predictive of venous thromboembolism in nephrotic syndrome. Cureus. 2022;14:e30032. doi: 10.7759/cureus.30032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Lv B, Li W, Wang S, Ding W. Diagnostic performance of the Caprini risk assessment model combined with D-Dimer for preoperative deep vein thrombosis in patients with thoracolumbar fractures caused by high-energy injuries. World Neurosurg. 2022;157:e410–e416. doi: 10.1016/j.wneu.2021.10.106. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Wang Y, Li D, Jia Y, Li F, Chen T, Liu Y, Zeng Z, Wan Z, Zeng R, Wu H. The Caprini risk score for early prediction of mortality in patients with acute coronary syndrome. J Cardiovasc Nurs. 2023;38:472–480. doi: 10.1097/JCN.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akella S, Russo D, Bradley CS, Kowalski JT. Caprini model integration into an electronic medical record to improve perioperative venous thromboembolism prophylaxis. Obstet Gynecol. 2023;142:1135–1137. doi: 10.1097/AOG.0000000000005390. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Zheng R, Fu Y, Xiang Q, Yue Z, Li J, Yu C, Jiang Y. Assessing the thrombosis risk of peripherally inserted central catheters in cancer patients using Caprini risk assessment model: a prospective cohort study. Support Care Cancer. 2021;29:5047–5055. doi: 10.1007/s00520-021-06073-4. [DOI] [PubMed] [Google Scholar]
- 12.Politano SA, Colbert GB, Hamiduzzaman N. Nephrotic syndrome. Prim Care. 2020;47:597–613. doi: 10.1016/j.pop.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hazeltine MD, Scott EM, Dorfman JD. An abbreviated Caprini model for VTE risk assessment in trauma. J Thromb Thrombolysis. 2022;53:878–886. doi: 10.1007/s11239-021-02611-3. [DOI] [PubMed] [Google Scholar]
- 14.Lukaszuk RF, Nycz KP, Plens K, Undas A. Caprini VTE computerized risk assessment improves the use of thromboprophylaxis in hospitalized patients with pulmonary disorders. Adv Clin Exp Med. 2022;31:261–266. doi: 10.17219/acem/115080. [DOI] [PubMed] [Google Scholar]
- 15.Wilson S, Chen X, Cronin M, Dengler N, Enker P, Krauss ES, Laberko L, Lobastov K, Obi AT, Powell CA, Schastlivtsev I, Segal A, Simonson B, Siracuse J, Wakefield TW, McAneny D, Caprini JA. Thrombosis prophylaxis in surgical patients using the Caprini risk score. Curr Probl Surg. 2022;59:101221. doi: 10.1016/j.cpsurg.2022.101221. [DOI] [PubMed] [Google Scholar]
- 16.Duffett L. Deep venous thrombosis. Ann Intern Med. 2022;175:ITC129–ITC144. doi: 10.7326/AITC202209200. [DOI] [PubMed] [Google Scholar]
- 17.Uematsu H, Shinoda K, Saito A, Sakai K. Deep venous thrombosis in a kidney transplant recipient with COVID-19: a case report. CEN Case Rep. 2023;12:98–103. doi: 10.1007/s13730-022-00724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94:861–869. doi: 10.1016/j.kint.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Guo N, Chen X, Xing C. Low triiodothyronine syndrome is associated with platelet function in patients with nephrotic syndrome. Rev Assoc Med Bras (1992) 2019;65:988–992. doi: 10.1590/1806-9282.65.7.988. [DOI] [PubMed] [Google Scholar]
- 20.Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, Gervaise A, Poissy J, Susen S, Hekimian G, Artifoni M, Periard D, Contou D, Delaloye J, Sanchez B, Fang C, Garzillo G, Robbie H, Yoon SH. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298:E70–E80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Wang T, Yuan Y, Su H, Chen L, Huang H, Lu Z, Gu J. Silent pulmonary embolism in deep vein thrombosis: relationship and risk factors. Clin Appl Thromb Hemost. 2022;28:10760296221131034. doi: 10.1177/10760296221131034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhakta S, Erben Y, Sanghavi D, Fortich S, Li Y, Hasan MM, Dong Y, Brigham TJ, Edwards MA, Meschia JF, Franco PM. A systematic review and meta-analysis of racial disparities in deep vein thrombosis and pulmonary embolism events in patients hospitalized with coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2022;10:939–944. e933. doi: 10.1016/j.jvsv.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Hu Y, Li X, Wang L, Wang M, Xiao J, Yi Q. Assessment of the risk of venous thromboembolism in medical inpatients using the padua prediction score and Caprini risk assessment model. J Atheroscler Thromb. 2018;25:1091–1104. doi: 10.5551/jat.43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arpaia GG, Caleffi A, Marano G, Laregina M, Erba G, Orlandini F, Cimminiello C, Boracchi P. Padua prediction score and IMPROVE score do predict in-hospital mortality in internal medicine patients. Intern Emerg Med. 2020;15:997–1003. doi: 10.1007/s11739-019-02264-4. [DOI] [PubMed] [Google Scholar]
- 25.Girardi AM, Bettiol RS, Garcia TS, Ribeiro GLH, Rodrigues EM, Gazzana MB, Rech TH. Wells and geneva scores are not reliable predictors of pulmonary embolism in critically ill patients: a retrospective study. J Intensive Care Med. 2020;35:1112–1117. doi: 10.1177/0885066618816280. [DOI] [PubMed] [Google Scholar]
- 26.Hanh BM, Cuong LQ, Son NT, Duc DT, Hung TT, Hung DD, Giang TB, Hiep NH, Xuyen HTH, Nga NT, Chu DT. Determination of risk factors for venous thromboembolism by an adapted caprini scoring system in surgical patients. J Pers Med. 2019;9:36. doi: 10.3390/jpm9030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson BP, Sioshansi PC, Conway RM, Minutello K, Bojrab DI, Hong RS, Sargent EW, Schutt CA, Bojrab DI 2nd, Zappia JJ, Babu SC. Rate of development of venous thromboembolism in lateral skull base surgery. Laryngoscope. 2022;132:662–667. doi: 10.1002/lary.29889. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Quintanilla FJ, Azpiri-Lopez JR, Romero-Ibarguengoitia ME, Ponce-Sierra TH, Martinez-Gallegos EP. Improving thromboprophylaxis in the medical inpatients: the role of the resident in an academic hospital. Phlebology. 2023;38:91–95. doi: 10.1177/02683555221147472. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Liu Y, Chen S, Jin Y, Jiang H. The combination of Caprini risk assessment scale and thrombotic biomarkers to evaluate the risk of venous thromboembolism in critically ill patients. Medicine (Baltimore) 2018;97:e13232. doi: 10.1097/MD.0000000000013232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Hao A, Lu Y, Huang W. Deep vein thrombosis and validation of the Caprini risk assessment model in Chinese orthopaedic trauma patients: a multi-center retrospective cohort study enrolling 34,893 patients. Eur J Trauma Emerg Surg. 2023;49:1863–1871. doi: 10.1007/s00068-023-02265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Sun R, Hu X, Chen Z, Lai C. Caprini risk assessment model combined with D-dimer to predict the occurrence of deep vein thrombosis and guide intervention after laparoscopic radical resection of colorectal cancer. World J Surg Oncol. 2023;21:299. doi: 10.1186/s12957-023-03183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]