Abstract
Objective: To identify the risk factors associated with postoperative hypoxemia in the postanesthesia care unit (PACU), providing evidence-based recommendations for its prevention. Methods: Observational studies examining the risk factors for postoperative hypoxemia in PACU patients were systematically searched in PubMed, Embase, Cochrane Library, and Web of Science databases from their inception to June 2024. Two independent reviewers screened the literature, extracted data, and assessed the quality of the studies. Meta-analysis was performed using RevMan 5.3 software, employing fixed or random effects models to calculate odds ratios (OR), relative risks (RR), and 95% confidence intervals (CI). Results: A total of 11 studies were included, comprising 8 cohort studies, 2 case-control studies, and 1 cross-sectional study, with a total of 24,147 subjects, of whom 5,587 experienced hypoxemia. The meta-analysis identified the following significant risk factors for postoperative hypoxemia in the PACU: Advanced age (OR=1.19, 95% CI: 1.11-1.29, P<0.001); Elevated body mass index (BMI) (OR=1.64, 95% CI: 1.36-1.97, P<0.001); Low preoperative oxygen saturation (OR=3.16, 95% CI: 2.56-3.91, P<0.001); Smoking status (OR=1.67, 95% CI: 1.15-2.43, P<0.05); Surgery duration >120 minutes (OR=1.43, 95% CI: 1.22-1.69, P<0.001); Opioid analgesic use (OR=1.51, 95% CI: 1.31-1.74, P<0.001). Conclusion: These findings highlight the need for targeted preventive strategies in patients at high risk for postoperative hypoxemia in the PACU.
Keywords: Postoperative, postanesthesia care unit, hypoxemia, risk factor, meta-analysis
Introduction
During surgical procedures, patients often require anesthetic intervention to mitigate the surgical stress response and ensure a smooth execution of the operation. Postoperatively, anesthetic agents are primarily metabolized and eliminated through the urinary and gastrointestinal tracts, minimizing potential cerebral toxicity. As a critical component of the perioperative anesthesia continuum, the postanesthesia care unit (PACU) plays a pivotal role in facilitating the transition of patients from anesthesia to full consciousness. However, various complications can arise during this period, with hypoxemia being one of the most prevalent.
Hypoxemia is defined as an insufficient oxygen level in the blood, characterized by arterial oxygen tension below the normal lower limit for the patient’s age group, accompanied by reduced oxygen saturation [1]. Studies have shown that 19.12% of patients experience hypoxemia during transport from the operating room to the PACU, and 69.8% develop hypoxemia within 30 minutes of PACU admission. Additionally, between 35% and 55% of adult patients experience at least one episode of hypoxemia during their stay in the PACU [2]. Prolonged or severe hypoxemia can lead to arrhythmias, abnormal blood pressure fluctuations, delayed postoperative wound healing, and even cardiac arrest or death [3,4]. Therefore, early identification of hypoxemia risk factors in PACU patients is crucial for developing targeted intervention strategies and ensuring a smooth recovery from anesthesia.
Previous studies have identified anesthetic, surgical, and patient-related factors associated with the occurrence of hypoxemia in the PACU [5]. However, inconsistencies in study populations, sample selection, research variables, and other methodological differences have resulted in conflicting findings, hindering the establishment of a unified understanding and standardized management protocols. Consequently, a systematic review and comprehensive meta-analysis of the existing literature are needed to investigate thoroughly the risk factors for postoperative hypoxemia in PACU patients. This review is to help clinicians to formulate individualized prevention strategies and optimize perioperative management to reduce postoperative hypoxemia in the PACU.
Materials and methods
Eligibility criteria
Inclusion criteria: (1) Observational study designs, including cohort studies, case-control studies, and cross-sectional studies; (2) Study participants were surgical patients transferred from the operating room to the PACU; (3) Hypoxemia was the exposure variable, with clearly defined diagnostic criteria; (4) The outcome measure was factors influencing postoperative hypoxemia in PACU patients.
Exclusion criteria: (1) Studies with incomplete or insufficient data; (2) Inability to access full-text articles or extract necessary data; (3) Duplicate publications; (4) Studies predominantly consisting of reviews, case reports, experience summaries, expert opinions, or animal studies.
Information sources
This review has been registered on PROSPERO (registration number: CRD42024587694). A comprehensive search was conducted for observational studies investigating the factors influencing postoperative hypoxemia in PACU patients using PubMed, Embase, Cochrane Library, and Web of Science databases from their inception to June 2024. Relevant references will also be collected for supplementary analysis.
Search strategy
The search terms included “postanesthesia care unit”, “PACU”, “hypoxia”, and “risk factors”. The strategy combined controlled vocabulary and free-text terms, tailored to each database. For instance, the PubMed search strategy was: (((postanestheaia care unit [Title/Abstract]) OR (PACU [Title/Abstract])) AND (((((((hypoxia [MeSH Terms])) OR (deficiencies, oxygen [Title/Abstract])) OR (oxygen deficiency [Title/Abstract])) OR (anoxemia [Title/Abstract])) OR (hypoxemia [Title/Abstract])) OR (anoxia [Title/Abstract]))) AND (((((((risk factors [MeSH Terms]) OR (factor, risk [Title/Abstract])) OR (populations at risk [Title/Abstract])) OR (risk sores [Title/Abstract])) OR (risk factor scores [Title/Abstract])) OR (correlates, health [Title/Abstract])) OR (factors, social risk [Title/Abstract])).
Selection process
Two authors independently screened the literature by initially reviewing article titles and abstracts to exclude studies that do not meet the inclusion criteria. They then performed a full-text review of the selected articles. Any discrepancies in the selection process were resolved through discussion or consultation with a third reviewer if necessary.
Data collection process
Two authors independently used a standardized data extraction form to collect relevant information, including the title, first author, publication year, country/region, study design, sample size, and identified risk factors. After data extraction, they cross-checked the collected data, and any discrepancies were resolved through consultation with a third reviewer if necessary.
Data items
The primary outcome variables were the independent variables for the binary logistic regression model for postoperative hypoxemia in PACU patients. From these, calculable odds ratios (OR) or relative risks (RR) and 95% confidence intervals (CI) were extracted.
Study risk of bias assessment
The two authors independently evaluated the quality of cohort and case-control studies using the Newcastle-Ottawa Scale (NOS). The NOS assesses studies based on three domains: selection (4 items), comparability (1 item), and exposure/outcome (3 items), for a total of 8 items. The scoring system uses a semi-quantitative star system, where each item, except for comparability (up to 2 stars), can receive 1 star. Each star corresponds to 1 point, with a maximum score of 9 points. A total score of ≥6 points indicates high-qualityarticle. The quality of cross-sectional studies was assessed using the Agency for Healthcare Research and Quality (AHRQ) tool, which consists of 11 items. A score of ≥8 out of 11 points was considered indicative of high-quality article.
Statistical analysis
Statistical analyses were performed using RevMan 5.3 software. Binary variables were expressed as log odds ratios (Log OR) with 95% CI. Heterogeneity among the included studies was assessed using the I2 statistic. If I2≤50% or P≥0.05, homogeneity was considered present, and a fixed-effects model was used for analysis. If I2 >50% or P<0.05, significant heterogeneity was indicated. Sensitivity analysis and subgroup analysis were performed to identify sources of heterogeneity. If heterogeneity could not be resolved, a random-effects model was applied. A significance level of P<0.05 was considered statistically significant. Publication bias was assessed using a funnel plot, with asymmetry suggesting potential bias.
Results
Study selection
The comprehensive search yielded 327 relevant articles. After removing 68 duplicates, 259 unique articles remained. A further 217 articles were excluded based on titles and abstracts. Following a full-text review, an additional 31 articles were excluded, leaving 11 studies that met the inclusion criteria. The selection process is illustrated in Figure 1.
Figure 1.
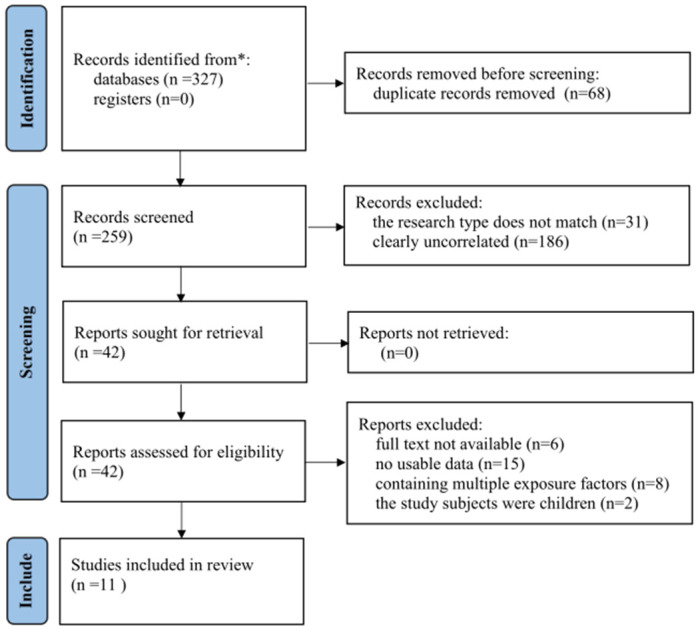
Process of literature screening.
Study characteristics
The 11 studies included were, published between 2011 and 2023. Ethiopia had the highest number of publications (three studies). The predominant study design was cohort analysis, with a combined sample size of 24,147 participants, including 5,587 cases of hypoxemia. Most studies investigated up to seven risk factors, while a few focused on a single factor. The fundamental characteristics of the included studies are presented in Table 1.
Table 1.
Fundamental characteristics of included literature
| Included study | Nation | Research type | Hypoxemia (n) | Total sample size (n) | Research factor |
|---|---|---|---|---|---|
| Liu SS 2011 [6] | America | Case-control study | 348 | 701 | ② |
| Aust H 2012 [7] | Germany | Cohort study | 163 | 959 | ① ② ⑧ |
| Labaste F 2016 [8] | France | Cohort study | 67 | 505 | ② |
| Kaushal A 2018 [9] | India | Cohort study | 61 | 452 | ① ② ③ |
| Quintero-Cifuentes IF 2018 [10] | Colombia | Cross-sectional study | 60 | 365 | ① |
| Taye MG 2021 [11] | Ethiopia | Cohort study | 194 | 424 | ② ③ ⑤ |
| Andualem AA 2022 [12] | Ethiopia | Cohort study | 225 | 298 | ① |
| Lin F 2022 [13] | China | Case-control study | 116 | 756 | ① ② |
| Bang YJ 2023 [14] | Korea | Cohort study | 842 | 12109 | ① ② ③ ④ ⑤ ⑥ ⑦ |
| Berhanu M 2023 [15] | Ethiopia | Cohort study | 149 | 352 | ① ⑥ ⑦ |
| Haller K 2023 [16] | Germany | Cohort study | 3362 | 6724 | ④ ⑧ |
①: Age; ②: Body Mass Index; ③: Smoking; ④: Gender; ⑤: Hypertension; ⑥: American Sociological Association grade ≥III; ⑦: Surgery duration over; ⑧: Administration of opioid analgesics.
Risk of bias in studies
Among the 11 included studies, 6 were prospective cohort studies and 4 were retrospective studies (including 2 case-control studies). All studies had Newcastle-Ottawa Scale (NOS) scores of ≥6, indicating appropriate selection of study subjects and well-defined groups. However, only half of the studies adjusted for the crude odds ratio in regression analysis, as detailed in Table 2. One study (Quintero-Cifuentes IF) received an Agency for Healthcare Research and Quality (AHRQ) score of 10, indicating high quality.
Table 2.
Bias risk assessment for cohort study/case-control study
| Included study | Selection | Comparability | Exposure | Score |
|---|---|---|---|---|
| Liu SS 2011 [6] | ☆☆☆ | ☆ | ☆☆ | 6 |
| Aust H 2012 [7] | ☆☆☆☆ | ☆ | ☆☆ | 7 |
| Labaste F 2016 [8] | ☆☆☆☆ | ☆ | ☆☆ | 7 |
| Kaushal A 2018 [9] | ☆☆☆☆ | ☆ | ☆☆ | 7 |
| Taye MG 2021 [11] | ☆☆☆☆ | ☆☆ | ☆☆ | 8 |
| Andualem AA 2022 [12] | ☆☆☆☆ | ☆☆ | ☆☆ | 8 |
| Lin F 2022 [13] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Bang YJ 2023 [14] | ☆☆☆ | ☆☆ | ☆☆ | 7 |
| Berhanu M 2023 [15] | ☆☆☆☆ | ☆☆ | ☆☆ | 8 |
| Haller K 2023 [16] | ☆☆☆ | ☆ | ☆☆ | 6 |
☆ symbolizes 1 point.
Analysis of factors related to postoperative hypoxemia in PACU patients
Age
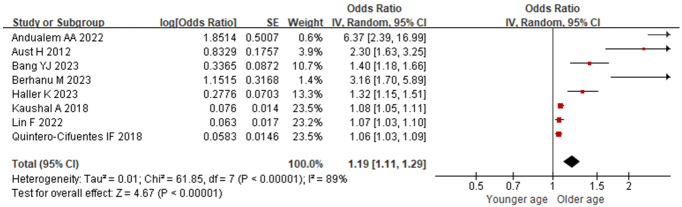
8 included studies examined age-related factors. Significant heterogeneity was observed (I2=89%, P<0.001), and sensitivity analysis revealed stable I2 values even when individual studies were excluded. No source of heterogeneity was identified, necessitating the use of a random-effects model for analysis. Advanced age was identified as a significant risk factor for postoperative hypoxemia in PACU patients [OR=1.19 (95% CI: 1.11-1.29), P<0.001], as depicted in Figure 2.
Figure 2.
Relationship between age and postoperative hypoxemia in PACU patients. PACU: postanesthesia care unit.
Body mass index (BMI)
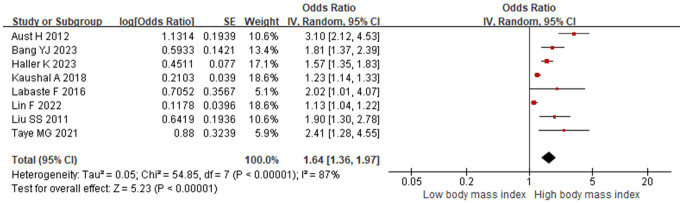
8 studies investigated BMI. Substantial heterogeneity was observed (I2 =87%, P<0.001), with stable I2 values across sensitivity analyses. The consistent P values indicated robust results. A random-effects model was used, revealing that elevated BMI was a risk factor for postoperative hypoxemia in PACU patients [OR=1.64 (95% CI: 1.36-1.97), P<0.001], as shown in Figure 3.
Figure 3.
Relationship between body mass index and postoperative hypoxemia in PACU patients. PACU: postanesthesia care unit.
Preoperative oxygen saturation
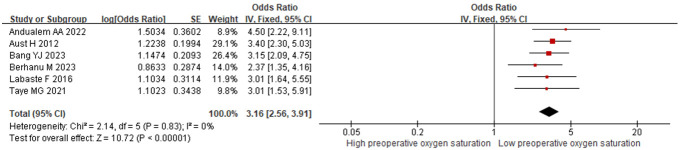
6 studies examined preoperative oxygen saturation. No significant heterogeneity was found (I2=0%, P=0.83), so a fixed-effects model was applied. The results showed that lower preoperative oxygen saturation was a risk factor for postoperative hypoxemia in PACU patients [OR=3.16 (95% CI: 2.56-3.91), P<0.001], as depicted in Figure 4.
Figure 4.
Relationship between preoperative oxygen saturation and postoperative hypoxemia in PACU patients. PACU: postanesthesia care unit.
Smoking status
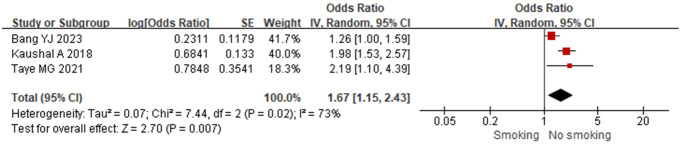
3 included studies analyzed smoking status as a risk factor. Substantial heterogeneity was present (I2=73%, P=0.02), with stable I2 values observed during sensitivity analyses. The consistent changes in P values indicated a degree of result stability, with no identifiable source of heterogeneity. A random-effects model was used, demonstrating that smoking status was a risk factor for postoperative hypoxemia in PACU patients [OR=1.67 (95% CI: 1.15-2.43), P=0.007], as shown in Figure 5.
Figure 5.
Relationship between smoking and postoperative hypoxemia in PACU patients. PACU: postanesthesia care unit.
Other factors
Two studies investigated additional factors, including gender, hypertension, American Society of Anesthesiologists grade, surgery duration, and opioid analgesic use. The meta-analysis identified surgery duration >120 minutes [OR=1.43 (95% CI: 1.22-1.69), P<0.001] and opioid analgesic use [OR=1.51 (95% CI: 1.31-1.74), P<0.001] as risk factors for postoperative hypoxemia, as shown in Table 3.
Table 3.
Meta-analysis of risk factors for postoperative hypoxemia in PACU patients
| Included study | Research factor | Heterogeneity test | Fixed effect model | Random effects model | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| I2 | P | OR | P | OR | P | ||
| Bang YJ 2023 [14], Haller K 2023 [16] | Male | 92% | <0.001 | - | - | 0.98 (0.65-1.47) | 0.92 |
| Taye MG 2021 [11], Bang YJ 2023 [14] | Hypertension | 89% | 0.003 | - | - | 1.98 (0.78-5.03) | 0.15 |
| Bang YJ 2023 [14], Berhanu M 2023 [15] | ASA grade ≥III | 93% | <0.001 | - | - | 4.32 (0.63-29.72) | 0.14 |
| Bang YJ 2023 [14], Berhanu M 2023 [15] | Surgery duration over 120 minutes | 46% | 0.18 | 1.43 (1.22-1.69) | <0.001 | - | - |
| Aust H 2012 [7], Haller K 2023 [16] | Opioid analgesic | 0% | 0.35 | 1.51 (1.31-1.74) | <0.001 | - | - |
ASA: American Society of Anesthesiologists; PACU: postanesthesia care unit.
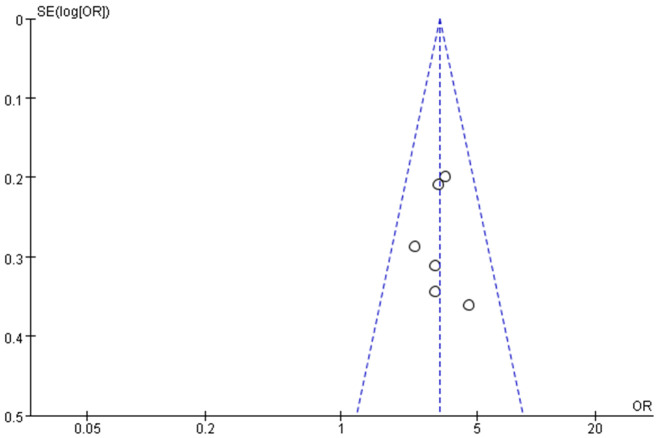
Publication bias
A funnel plot illustrating the correlation between preoperative oxygen saturation and postoperative hypoxemia in the PACU is presented in Figure 6. Although all studies fell within the 95% CI, notable asymmetry suggests possible publication bias.
Figure 6.
Funnel plot.
Discussion
Low oxygen saturation in the PACU is a critical respiratory adverse event. Its pathogenesis may be linked to inadequate ventilation, airway obstruction, ventilation/perfusion mismatch, increased alveolar-arterial oxygen gradient, impaired oxygen diffusion, elevated pulmonary shunt, and decreased cardiac output [17,18]. This meta-analysis aims to identify the risk factors for hypoxemia in the PACU and propose corresponding preventive measures. The findings are as follows.
This meta-analysis identified advanced age as an independent risk factor for postoperative hypoxemia in PACU patients. Previous studies [19] have shown that aging contributes to small vessel disease, resulting in decreased vascular reactivity and inadequate perfusion, ultimately leading to hypoxemia. Another study [20] suggested that with increasing age, imbalances in the renin-angiotensin system exacerbate inflammation and lung injury, reducing lung elasticity, increasing alveolar collapse, and ultimately causing hypoxemia. In summary, aging is associated with a decline in physiological function, reduced surgical tolerance, and increased risk of cerebral hypoxia, all of which heighten the risk of hypoxemia. Consequently, clinicians should consider extending recovery and extubation times for elderly patients while closely monitoring their respiratory function, assessing acid-base balance, ensuring regular clearance of tracheal secretions, and conducting real-time monitoring of exhaled carbon dioxide to facilitate early intervention and reduce the risk of respiratory depression.
An elevated BMI is also an independent risk factor for postoperative hypoxemia in PACU patients. A study [21] reported that the incidence of perioperative hypoxemia is 16% in patients with a normal BMI, compared to 28% and 35% in patients with a BMI >30 kg/m2 and >40 kg/m2, respectively. Obesity is characterized by chronic inflammation, leading to the release and dysregulation of fatty acids, which destabilizes pulmonary vasculature and alters the expression of endothelial adhesion molecules, thereby increasing the susceptibility to acute lung injury [22]. As such, patients with elevated BMI should receive individualized guidance on appropriate dietary practices and weight management strategies tailored to their specific needs. Preoperative assessments should include evaluations of oral tolerance and surgical risk, followed by enhanced postoperative monitoring of vital signs and early initiation of nasal cannula oxygen therapy.
A lower preoperative oxygen saturation level is an independent risk factor for postoperative hypoxemia in PACU patients. Decreased oxygen saturation may result from insufficient oxygen supply or impaired oxygen delivery due to factors such as inadequate ventilation, respiratory failure, circulatory disorders, anemia, or impaired gas exchange in the lungs. These conditions can adversely affect oxygen supply and transport. The patient’s preoperative health status is closely associated with the risk of postoperative hypoxemia. A low preoperative oxygen saturation level indicates that the patient may already have one or more of these issues before surgery, increasing the likelihood of deterioration postoperatively and leading to hypoxemia [23,24]. Therefore, for patients with reduced preoperative oxygen saturation, comprehensive assessment of the respiratory system should be conducted, and respiratory and circulatory measurements should be closely monitored. An individualized preoperative preparation plan, including oxygen therapy, respiratory management, and preoperative breathing exercises, should be developed to optimize oxygenation and reduce the risk of hypoxemia.
Smoking is another independent risk factor for postoperative hypoxemia in PACU patients. Long-term smoking results in the accumulation of tar, nicotine, and other substances in lung tissue, impairing mucus clearance and increasing pulmonary vascular resistance. Smoking also damages the bronchi and alveoli, causing necrosis and shedding of epithelial cells, which reduces lung clearance capacity and increases sputum production and viscosity, thereby elevating the risk of hypoxemia [25,26]. It is crucial to emphasize the harmful effects of smoking before surgery and provide smoking cessation counseling. Effective coughing techniques should be encouraged; if the patient’s coughing ability is inadequate, techniques such as suctioning and chest percussion can be used to facilitate secretion clearance. Additionally, appropriate pulmonary protective measures should be implemented.
Surgery duration exceeding 120 minutes and the administration of opioid analgesics have also been identified as independent risk factors for postoperative hypoxemia in PACU patients. Prolonged surgical procedures can increase mechanical ventilation time, possibly causing mucosal ulceration, organ necrosis, and a higher risk of pulmonary infection, ultimately leading to hypoxemia [27]. Opioids, although highly effective for pain management during the perioperative period, have adverse effects such as respiratory depression. A study [28] demonstrated that excessive intraoperative opioid use is associated with a higher incidence of postoperative hypoxemia. Therefore, a comprehensive preoperative assessment should be conducted for each patient, followed by the development of a surgical plan aimed at preventing hypoxemia associated with prolonged surgery. Close monitoring of sedation levels and ventilation is essential, and strategies such as preoperative preventive analgesia, intraoperative protective measures, optimized anesthesia, and early postoperative pain management should be implemented. Nonsteroidal anti-inflammatory drugs, local wound infiltration, and nerve blocks may be used to minimize the adverse effects of inadequate analgesia.
This meta-analysis was limited by the small number of included studies, which were predominantly cohort and case-control studies. Limitations in study design, research subjects, and data extraction may have introduced selection bias. Some risk factors were evaluated in only a few studies, potentially leading to biased results. The heterogeneity among some included studies and the variation in sample sizes could affect the accuracy of the findings. The inconsistencies in the identified risk factors prevented a comprehensive analysis. Therefore, high-quality, large-sample randomized controlled trials are needed to further validate these findings.
In summary, advanced age, elevated body mass index, low preoperative oxygen saturation, smoking status, surgery duration over 120 minutes, and opioid analgesic use are all associated with an increased risk of postoperative hypoxemia in the PACU. It is essential to conduct thorough risk assessments and implement preventive measures for any high-risk patients.
Disclosure of conflict of interest
None.
References
- 1.van Schaik EPC, Blankman P, Van Klei WA, Knape HJTA, Vaessen PHHB, Braithwaite SA, van Wolfswinkel L, Schellekens WM. Hypoxemia during procedural sedation in adult patients: a retrospective observational study. Can J Anaesth. 2021;68:1349–1357. doi: 10.1007/s12630-021-01992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui N, Arzola C, Teresi J, Fox G, Guerina L, Friedman Z. Predictors of desaturation in the postoperative anesthesia care unit: an observational study. J Clin Anesth. 2013;25:612–7. doi: 10.1016/j.jclinane.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Saab R, Wu BP, Rivas E, Chiu A, Lozovoskiy S, Ma C, Yang D, Turan A, Sessler DI. Failure to detect ward hypoxaemia and hypotension: contributions of insufficient assessment frequency and patient arousal during nursing assessments. Br J Anaesth. 2021;127:760–768. doi: 10.1016/j.bja.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Wong AI, Charpignon M, Kim H, Josef C, de Hond AAH, Fojas JJ, Tabaie A, Liu X, Mireles-Cabodevila E, Carvalho L, Kamaleswaran R, Madushani RWMA, Adhikari L, Holder AL, Steyerberg EW, Buchman TG, Lough ME, Celi LA. Analysis of discrepancies between pulse oximetry and arterial oxygen saturation measurements by race and ethnicity and association with organ dysfunction and mortality. JAMA Netw Open. 2021;4:e2131674. doi: 10.1001/jamanetworkopen.2021.31674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan XZ, Zhang X, Tong DK, Ji F, Xu KH, He RZ. Risk factors for and predictive nomogram of postoperative hypoxaemia in elderly patients with femoral neck fractures. J Int Med Res. 2020;48:300060520945132. doi: 10.1177/0300060520945132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SS, Chisholm MF, Ngeow J, John RS, Shaw P, Ma Y, Memtsoudis SG. Postoperative hypoxemia in orthopedic patients with obstructive sleep apnea. HSS J. 2011;7:2–8. doi: 10.1007/s11420-010-9165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aust H, Eberhart LH, Kranke P, Arndt C, Bleimüller C, Zoremba M, Rüsch D. Hypoxemia after general anesthesia. Anaesthesist. 2012;61:299–309. doi: 10.1007/s00101-012-2000-x. [DOI] [PubMed] [Google Scholar]
- 8.Labaste F, Silva S, Serin-Moulin L, Lefèvre E, Georges B, Conil JM, Minville V. Predictors of desaturation during patient transport to the postoperative anesthesia care unit: an observational study. J Clin Anesth. 2016;35:210–214. doi: 10.1016/j.jclinane.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal A, Goyal P, Dhiraaj S, Agarwal A, Singh PK. Identification of various perioperative risk factors responsible for development of postoperative hypoxaemia. Turk J Anaesthesiol Reanim. 2018;46:416–423. doi: 10.5152/TJAR.2018.82160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintero-Cifuentes IF, Pérez-López D, Victoria-Cuellar DF, Satizábal-Padridín N, Billefals-Vallejo ES, Castaño-Ramírez DA, Beltrán-Osorio LD. Incidence of early postanesthetic hypoxemia in the postanesthetic care unit and related factors. Colombian Journal of Anesthesiology. 2018;46:309–316. [Google Scholar]
- 11.Taye MG, Molla A, Teshome D, Hunie M, Kibret S, Fentie Y, Temesgen N, Engidaw MT, Fenta E. Predictors of hypoxemia after general anesthesia in the early postoperative period in a hospital in Ethiopia: an observational study. Multidiscip Respir Med. 2021;16:782. doi: 10.4081/mrm.2021.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andualem AA, Yesuf KA. Incidence and associated factors of postoperative hypoxemia among adult elective surgical patients at Dessie comprehensive specialized hospital: an observational study. Ann Med Surg (Lond) 2022;78:103747. doi: 10.1016/j.amsu.2022.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin F, Gong X, Lei G, Wang X, Chen C, Zhang L. Predictive model of hypoxemia after shoulder arthroscopy: a retrospective observational study. Medicine (Baltimore) 2022;101:e32275. doi: 10.1097/MD.0000000000032275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang YJ, Park IH, Jeong H. Frequency and risk factors for failed weaning from supplemental oxygen therapy after general anesthesia at a postanesthesia care unit: a retrospective cohort study. BMC Anesthesiol. 2023;23:231. doi: 10.1186/s12871-023-02192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berhanu M, Dadi N, Mengistu B, Muluken Z, Tolesa A, Tageza T, Kalbesa M, Tesfaye G, Zawdie B. Magnitude of early postoperative hypoxemia and its associated factors among adult patients who undergo emergency surgery under general anesthesia at Jimma Medical Center, Jimma, Southwest Ethiopia, 2021: a prospective observational study. Perioper Med (Lond) 2023;12:1. doi: 10.1186/s13741-022-00288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller K, Trauzeddel RF, Treskatsch S, Berger C. Risk factors for postoperative hypoxemia during transport to the postanesthesia care unit and influence of transport monitoring: a retrospective propensity score-matched databank analysis. Anaesthesiologie. 2023;72:488–497. doi: 10.1007/s00101-023-01296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry NR, Hanson AC, Schulte PJ, Warner NS, Manento MN, Weister TJ, Warner MA. Disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes. Crit Care Med. 2022;50:204–211. doi: 10.1097/CCM.0000000000005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, Scott JB, Jing G, Li J. Management of postoperative hypoxemia. Respir Care. 2021;66:1136–1149. doi: 10.4187/respcare.08929. [DOI] [PubMed] [Google Scholar]
- 19.Geraldes R, Esiri MM, DeLuca GC, Palace J. Age-related small vessel disease: a potential contributor to neurodegeneration in multiple sclerosis. Brain Pathol. 2017;27:707–722. doi: 10.1111/bpa.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schouten LR, Helmerhorst HJ, Wagenaar GT, Haltenhof T, Lutter R, Roelofs JJ, van Woensel JB, van Kaam AH, Bos AP, Schultz MJ, Walther T, Wösten-van Asperen RM. Age-dependent changes in the pulmonary renin-angiotensin system are associated with severity of lung injury in a model of acute lung injury in rats. Crit Care Med. 2016;44:e1226–e1235. doi: 10.1097/CCM.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 21.Kendale SM, Blitz JD. Increasing body mass index and the incidence of intraoperative hypoxemia. J Clin Anesth. 2016;33:97–104. doi: 10.1016/j.jclinane.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Cortese G, Brazzi L. Do we need a strategy to reduce postoperative hypoxemia in morbidity obese patients? Minerva Anestesiol. 2019;85:1044–1046. doi: 10.23736/S0375-9393.19.13881-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Ma J, Lin D, Dong X, Wu J, Bai Y, Zhang D, Gao J. The risk factors of postoperative hypoxemia in patients with Stanford type A acute aortic dissection. Medicine (Baltimore) 2023;102:e34704. doi: 10.1097/MD.0000000000034704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Z, Yin A, Lu L, Lu Y, Jiang B, Yin L. Risk factors for intraprocedural hypoxemia in patients with acute cerebral ischemia treated with vascular intervention and its impact on prognosis: a retrospective cohort study. Brain Circ. 2024;10:42–50. doi: 10.4103/bc.bc_50_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varga JT. Smoking and pulmonary complications: respiratory prehabilitation. J Thorac Dis. 2019;11(Suppl 5):S639–S644. doi: 10.21037/jtd.2018.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geube M, Mireles-Cabodevila E. Commentary: measure what matters in one lung ventilation. J Thorac Cardiovasc Surg. 2020;160:1123–1124. doi: 10.1016/j.jtcvs.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Bartels K, Kaizer A, Jameson L, Bullard K, Dingmann C, Fernandez-Bustamante A. Hypoxemia within the first 3 postoperative days is associated with increased 1-year postoperative mortality after adjusting for perioperative opioids and other confounders. Anesth Analg. 2020;131:555–563. doi: 10.1213/ANE.0000000000004553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Monguio R, Lun Z, Bongiovanni T, Chen CL, Seoane-Vazquez E. Postoperative respiratory events in surgical patients exposed to opioid analgesic shortages compared to fully matched patients non-exposed to shortages. Drug Saf. 2022;45:359–367. doi: 10.1007/s40264-022-01171-6. [DOI] [PubMed] [Google Scholar]