Abstract
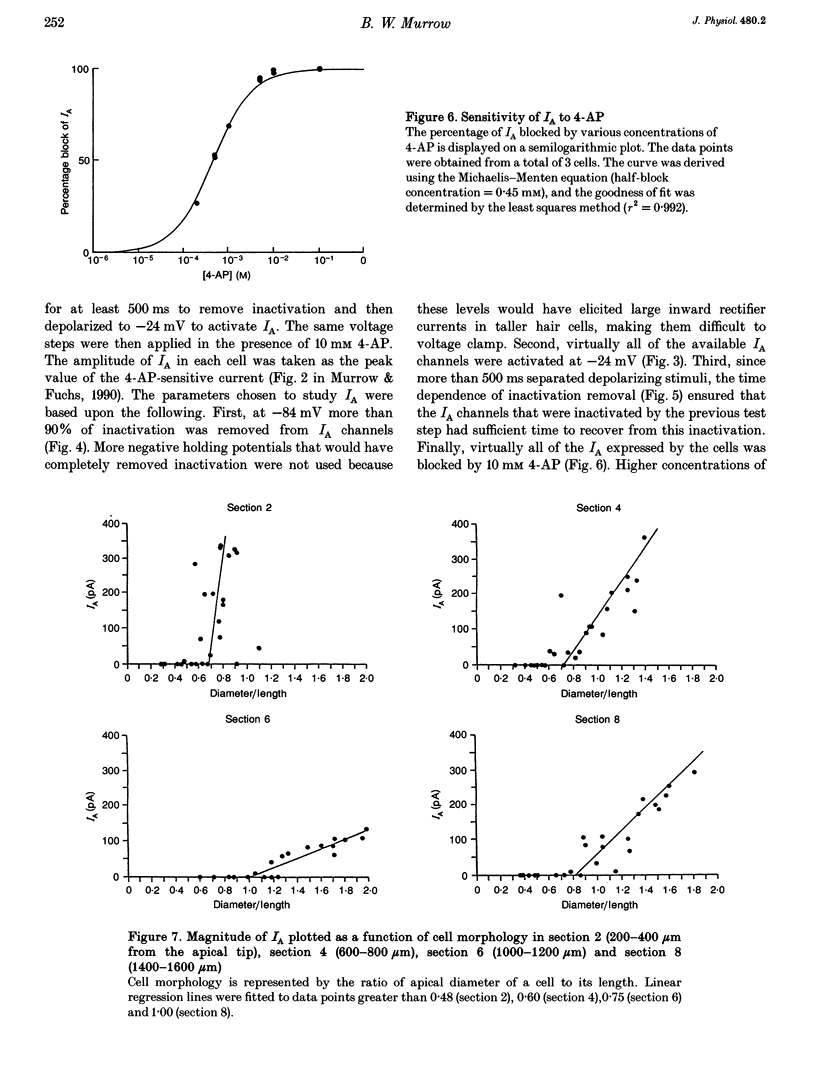
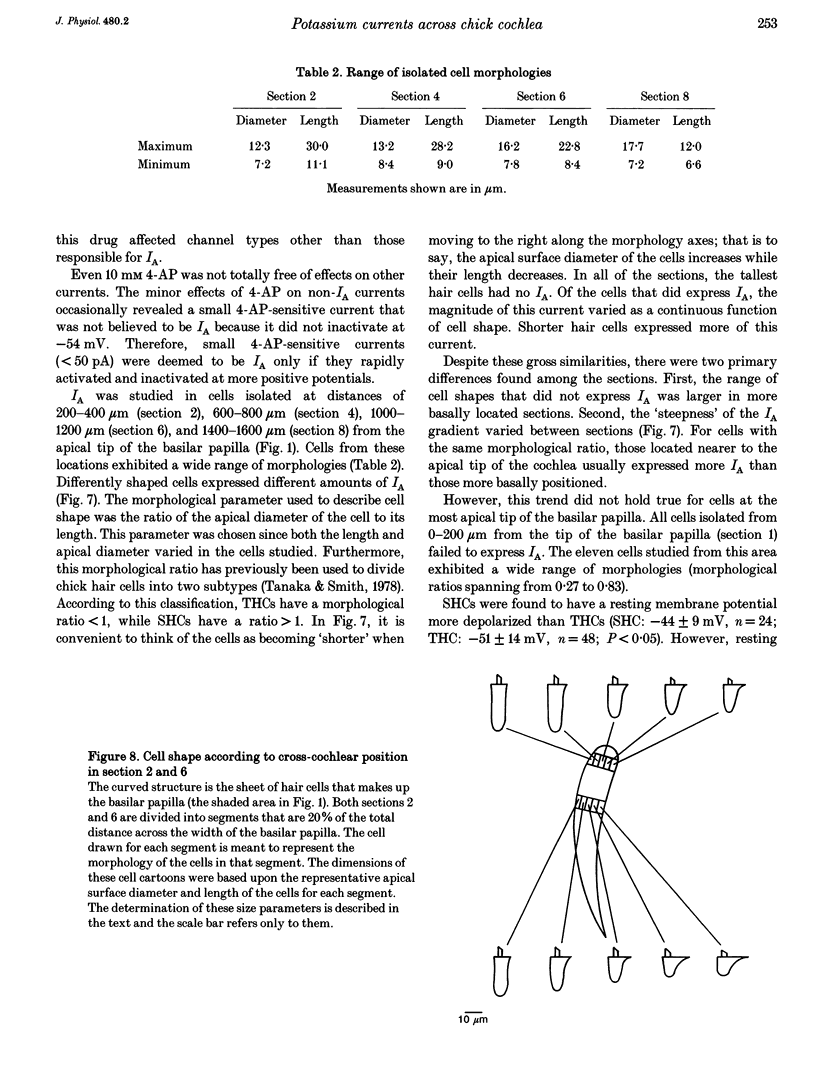
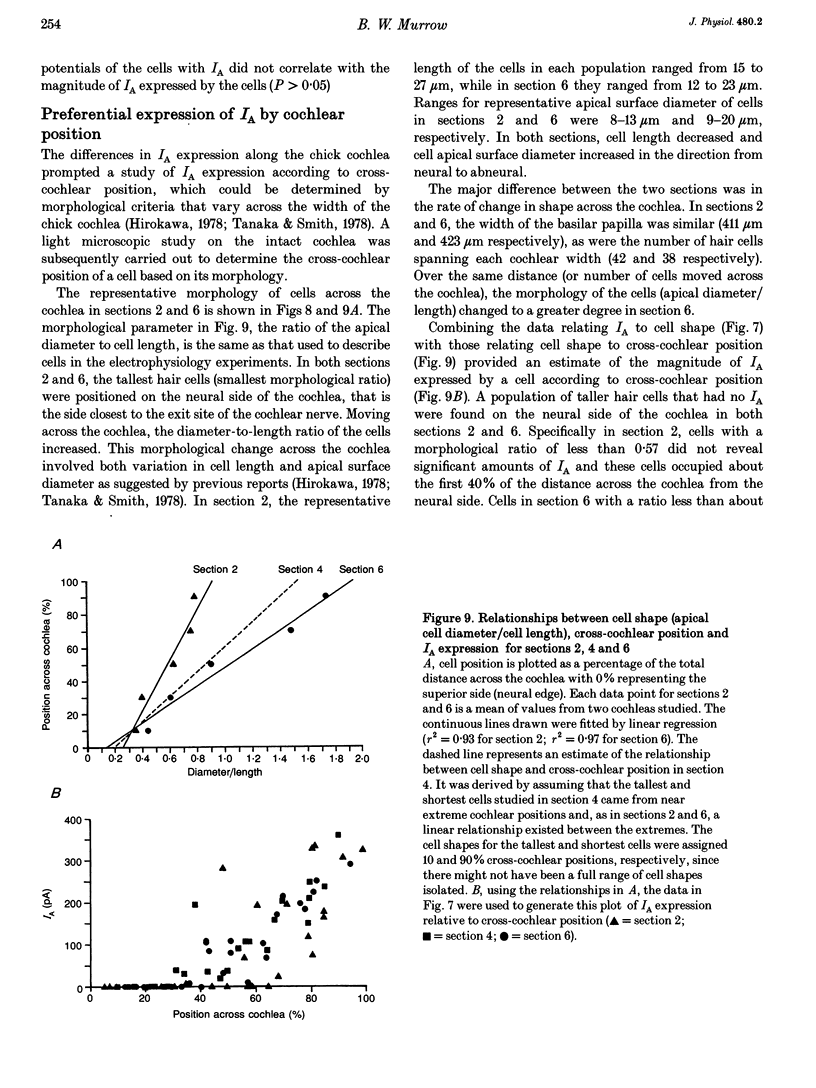
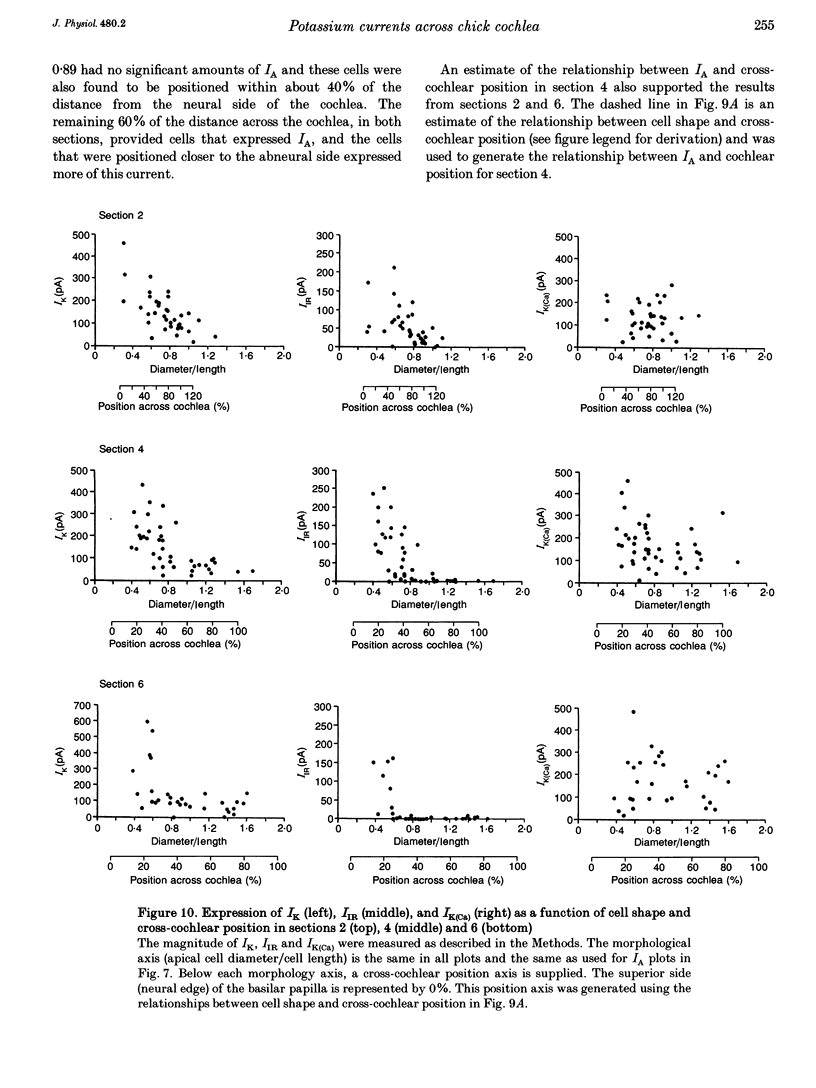
1. Potassium currents in chick cochlear hair cells were studied using whole-cell voltage clamp techniques. Cells were isolated from 200 microns-long segments of the apical half of the cochlea. In each segment, expression of potassium currents by cells positioned across the width ('inner-outer' hair cell axis) of the cochlea was examined. 2. A rapidly inactivating potassium current (IA) was found in some hair cells. At a membrane potential of -24 mV, IA activated to peak values within 7 +/- 1 ms and inactivated within 73 +/- 16 ms. The activation 'threshold' was around -50 mV and hyperpolarization more negative than -56 +/- 5 mV was required before significant removal of inactivation occurred (V 1/2 (half-inactivation potential) = -74 +/- 5 mV). The resting potential of cells with IA was -46 mV +/- 11 mV. This current was blocked by 4-aminopyridine with a Kd of 0.45 mM. 3. Cells that were isolated from the most apical tip of the cochlea expressed no IA. In areas more basal than 200 microns from the apex, the magnitude of IA correlated with cell morphology. In each area, the tallest hair cells (cells with the smallest ratio of apical surface diameter to length) had none of this current. Of the cells with IA, the shorter cells (larger ratio of apical surface diameter to length) had more of this current. 4. The magnitude of IA in a cell was dependent upon cross-cochlear position, and the relationship between IA and cell morphology was most probably a reflection of a differential distribution of cell shape across the cochlea. The tallest hair cells, occupying roughly the first 40% of the distance from the neural side of the basilar papilla, had no IA. Of the remaining cells, those nearer to the abneural edge expressed more IA, such that iso-magnitude lines ran approximately parallel to the long axis of the cochlea. 5. A delayed rectifier current (IK) and an inward rectifier current (IIR) were also differentially distributed among hair cells across the cochlea; however, their distribution differed from that of IA. IK and IIR were preferentially expressed by the taller hair cells, which were positioned nearer to the neural side of the cochlea. Ca(2+)-activated potassium current (IK(Ca)) did not vary systematically between cells of different shape or cross-cochlear position, and IK(Ca) could often be found in cells with IA.
Full text
PDF
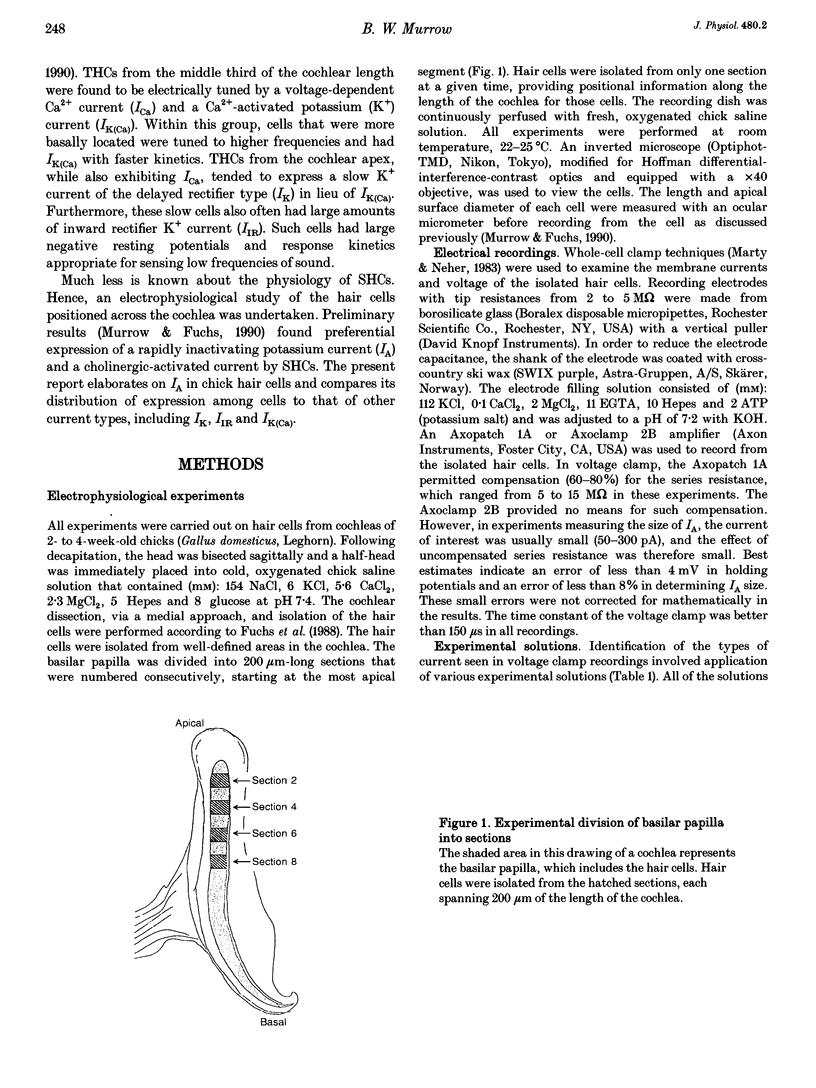

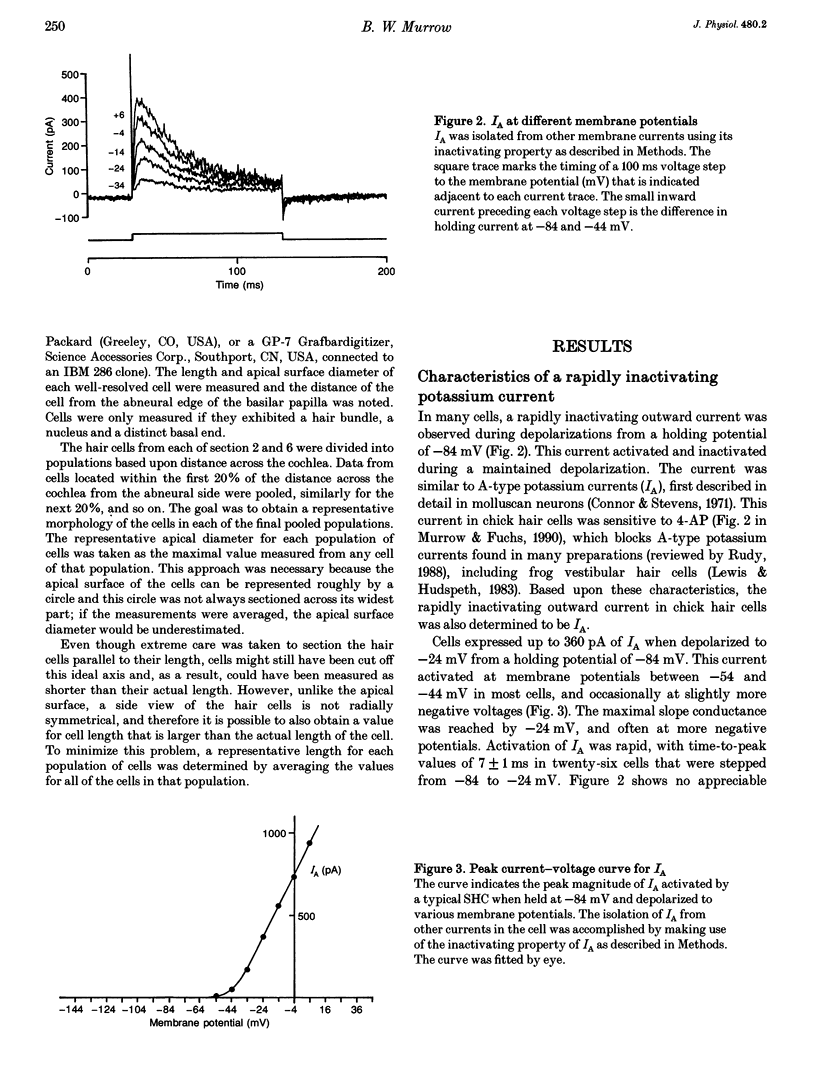
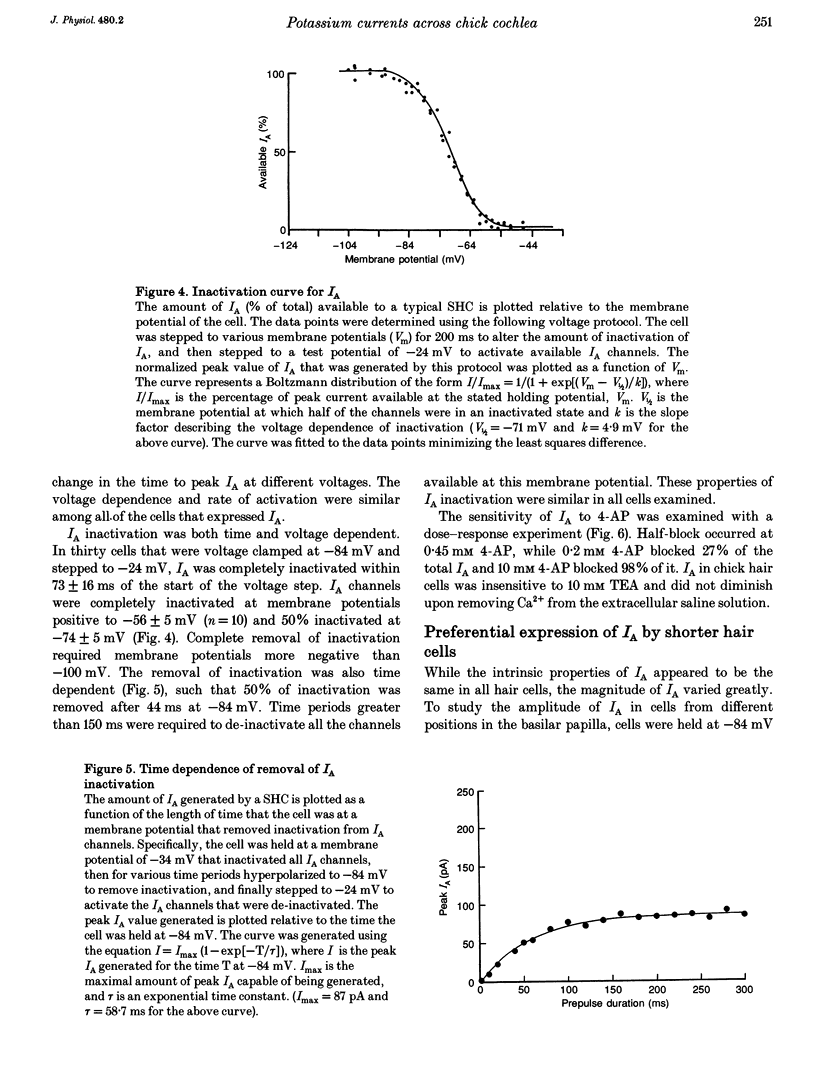




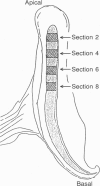
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firbas W., Müller G. The efferent innervation of the avian cochlea. Hear Res. 1983 Apr;10(1):109–116. doi: 10.1016/0378-5955(83)90021-7. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G., Murrow B. W. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:529–551. doi: 10.1113/jphysiol.1990.sp018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Murrow B. W. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc Biol Sci. 1992 Apr 22;248(1321):35–40. doi: 10.1098/rspb.1992.0039. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Murrow B. W. Cholinergic inhibition of short (outer) hair cells of the chick's cochlea. J Neurosci. 1992 Mar;12(3):800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Nagai T., Evans M. G. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988 Jul;8(7):2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. The ultrastructure of the basilar papilla of the chick. J Comp Neurol. 1978 Sep 15;181(2):361–374. doi: 10.1002/cne.901810208. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Ashmore J. F. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol. 1992 Mar;448:73–98. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley G. D., Norris C. H., Guth P. S. Electrophysiological properties and morphology of hair cells isolated from the semicircular canal of the frog. Hear Res. 1989 Apr;38(3):259–276. doi: 10.1016/0378-5955(89)90070-1. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984 Aug;52(2):340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Kros C. J., Crawford A. C. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990 Feb;421:263–291. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Correia M. J. Studies of solitary semicircular canal hair cells in the adult pigeon. II. Voltage-dependent ionic conductances. J Neurophysiol. 1989 Oct;62(4):935–945. doi: 10.1152/jn.1989.62.4.935. [DOI] [PubMed] [Google Scholar]
- Lavigne-Rebillard M., Cousillas H., Pujol R. The very distal part of the basilar papilla in the chicken: a morphological approach. J Comp Neurol. 1985 Aug 15;238(3):340–347. doi: 10.1002/cne.902380308. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Murrow B. W., Fuchs P. A. Preferential expression of transient potassium current (IA) by 'short' hair cells of the chick's cochlea. Proc Biol Sci. 1990 Dec 22;242(1305):189–195. doi: 10.1098/rspb.1990.0123. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Serrano E. E., Getting P. A. Diversity of the transient outward potassium current in somata of identified molluscan neurons. J Neurosci. 1989 Nov;9(11):4021–4032. doi: 10.1523/JNEUROSCI.09-11-04021.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc C. K., Zagotta W. N., Aldrich R. W. Single-channel and genetic analyses reveal two distinct A-type potassium channels in Drosophila. Science. 1987 May 29;236(4805):1094–1098. doi: 10.1126/science.2437657. [DOI] [PubMed] [Google Scholar]
- Strong J. A. Modulation of potassium current kinetics in bag cell neurons of Aplysia by an activator of adenylate cyclase. J Neurosci. 1984 Nov;4(11):2772–2783. doi: 10.1523/JNEUROSCI.04-11-02772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I., Furukawa T. Morphological and functional aspects of two different types of hair cells in the goldfish sacculus. J Neurophysiol. 1989 Dec;62(6):1330–1343. doi: 10.1152/jn.1989.62.6.1330. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Smith C. A. Structure of the chicken's inner ear: SEM and TEM study. Am J Anat. 1978 Oct;153(2):251–271. doi: 10.1002/aja.1001530206. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]