Abstract
Objective: To assess the efficacy of sacubitril-valsartan in the treatment of acute myocardial infarction (AMI) using meta-analysis methods. Methods: Relevant papers on sacubitril/valsartan for treating AMI were searched on PubMed, Embase, Medical Literature Analysis, and Retrieval System On-Line (MEDLINE), Science Direct, The Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Scientific Journal Database, and Chinese Biomedical Literature Database (CBM). The time range was from their inception to February 1, 2023. Results: A total of 10 articles involving 13,135 patients were included for this meta-analysis according to the inclusion and exclusion criteria. Among these patients, 6,581 were treated with sacubitril/valsartan, as the experimental group, and the other 6,554 patients were classified into the control group. After treatment, the risk of hospitalization for heart failure (HF) in the experimental group was lower than that of the control group (OR=0.77, 95% CI: 0.67-0.88, P=0.0002); the average left ventricular end diastolic diameter (LVEDD) (MD=-5.56, 95% CI: -7.92-3.20, P<0.0001) was significantly higher and 6-minute-walk distance (6MWD) (MD=95.86, 95% CI: 30.57-161.16, P=0.004) was significantly longer in the treatment group than in the control group. Besides, the left ventricular ejection fraction (LVEF) (MD=2.99, 95% CI: 0.47-5.51, P=0.02) was significantly lower than that of the control group. Conclusion: Sacubitril/Valsartan improves cardiac function in patients with AMI, reduces the risk of postoperative myocardial reinfarction, and reduces the risk of hospitalization for HF.
Keywords: Sacubitril/valsartan, acute myocardial infarction, effectiveness analysis, meta-analysis
Introduction
Myocardial infarction (MI) is a severe cardiovascular disease that can lead to heart failure (HF) [1]. Acute MI (AMI) is a prevalent form of heart attack, and early treatment is essential to improve patient outcome. Thrombolytic therapy and early revascularization techniques have successfully reduced the mortality rate of AMI in clinical practice [2]. Despite significant advancements in reperfusion therapy, managing the disease course following AMI remains a formidable challenge. Consequently, early detection and effective control of myocardial infarction (MI) size are imperative to mitigate adverse outcomes and enhance the prognosis of AMI patients [3,4].
Sarcupyrine/valsartan is a compound drug that is a co-crystallization of e Neprilysin Inhibitor (NEPI) Sarcupyrine and Angiotensin Receptor Blocker (ARB) valsartan in equal ratio. This combination effectively regulates both the Neprilysin (NEP) and renin-angiotensin systems, achieving the effect of regulating water/sodium balance, expanding capillaries, and thus inhibiting atrial reconstruction [5]. Studies noted that combining sacubitril with valsartan not only counteracts the potential toxic effects of NEPI but also exerts the positive effects of natriuretic peptides, thus bringing more medical benefit to HF patients. Such combined application can not only inhibit the negative effects of Angiotensin (Ang) but also effectively improve the symptoms of patients with cardiac exhaustion, thus obtaining better clinical outcomes [6-8]. A literature review reveals a paucity of studies investigating the prevention and treatment of HF following AMI using angiotensin receptor neprilysin inhibitors (ARNIs), with a notable absence of meta-analyses on sacubitril/valsartan [9]. Therefore, the purpose of this work was to further explore the early clinical efficacy of ARNI, Sarcupyrine/valsartan, in HF after AMI to better understand the application field of ARNI and their early clinical efficacy. In addition, the advantages of ARNI and their mechanism of action are summarized so that HF patients can gain more prevention and treatment benefits after AMI.
Data and methods
Methods for screening the literature
PubMed, Embase, Medical Literature Analysis and Retrieval System On-Line (MEDLINE), Web of Science, Google Scholar, Science Direct, The Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Science and Technology Journal Database, and Chinese Biomedical Literature Database (CBM) were searched to screen the literature focusing on sacubitril/valsartan treatment of HF after AMI from the establishment of the database to February 1, 2023. Chinese database searching terms included AMI, HF, enkephalin inhibitors, sacubitril/valsartan, nohindal, and HF. The English database searching terms included heart failure, HF, sacubitril/valsartan, AMI, enkephalase inhibitors, sacubitril/valsartan, and nohintal. The search strategies were refined after multiple iterations, and professional journals were searched manually to avoid omissions. We focused on studies involving human subjects only. The literature search was conducted using a combination of subject words and free words to ensure comprehensive coverage. To ensure thoroughness, the search process included tracking citations of each relevant document. The literature quality was assessed using RevMan5.3 software provided by the Cochrane Collaboration Network.
Inclusion and exclusion criteria for literature screening
The study employed the PICOS framework (Patient, Intervention, Comparison, Outcome, and Study design) to select randomized controlled trials (RCTs).
The inclusion criteria: 1. RCTs. 2. All patients diagnosed with AMI. 3. Patients received treatment with sacubitril/valsartan. 4. Studies with available relevant clinical outcome measures.
Exclusion criteria: 1. Studies with a sample size of less than 10 cases; a small sample size may lead to bias and inadequate power. 2. Literature types such as duplicate reports, conference proceedings, and abstracts. 3. Non-RCTs. 4. In vitro experiments, animal experiments, experiments involving healthy populations, or pre-experiments.
Two experienced researchers (Jianfei Ye and Weifen Zheng) independently conducted the literature selection process. Initially, articles were selected based on their titles and content of the references. Subsequently, potentially relevant studies underwent a comprehensive review to assess their eligibility according to predetermined inclusion and exclusion criteria. Data extraction encompassed details such as the first author, publication year, subject characteristics, control agents, duration of follow-up, and clinical outcome measures related to the study (including changes in mortality, risk of hospitalization, and cardiac function indicators). In cases of disagreement, a third reviewer was consulted to adjudicate the inclusion of relevant literature.
Methods for data extraction
The data were input into a unified Microsoft Excel (Microsoft, USA) platform for data extraction by the above two researchers independently, followed by a crosscheck. Disagreements were resolved through discussion. The data included basic information (title, first author, year, country, publication journal, literature source); basic characteristics of the subjects: sex ratio, age, sample size in different groups; key elements of bias risk assessment: random method, application of blinded method, allocation hiding; and concerned outcome indicators and outcome measurement data, such as odds ratio (OR), complete response (CR), partial response (PR), safety results, and adverse events (AEs).
Methods for evaluating the literature quality
RevMan5.3 was utilized to evaluate the included literature with reference to the RCT bias risk assessment method under the Cochrane Manual of Systematic Review 5.3 [10]. The specific assessment content is shown in Table 1. Based on the potential for bias, assessments were categorized into low, moderate, high, or unknown risk. In instances of discordance, the two researchers engaged in joint discussion, or a third researcher was consulted for intervention.
Table 1.
Criteria for literature quality assessment
| No. | Aspects | Requirements |
|---|---|---|
| 1 | Random allocation method | Whether the method for generating randomly assigned sequences was described in detail |
| 2 | Allocation hiding | Whether the method of hiding the random assignment sequence was described in detail to determine whether the allocation of interventions was predictable before the outcome of the experiment |
| 3 | Blind method | Whether subjects, researchers, and outcome assessors were blinded to the assigned interventions |
| 4 | Incomplete data | Whether each of the primary outcome data was completely described and whether incomplete outcome data was properly processed |
| 5 | Selective reporting | Whether all outcomes of predetermined primary outcome measures were fully reported |
| 6 | Other sources | Whether there were other factors that could cause a high risk of bias in the experiment |
Statistical analysis
The statistical analysis was conducted using RevMan5.3, with baseline patient characteristics and clinical outcomes reported in the form of mean ± standard deviation or counts (percentages).
R language was employed to select effect sizes that accurately reflected the entire dataset based on the characteristics of the data types. The inverse variance method and Mantel-Haenszel method were utilized for dichotomous and continuous outcomes, respectively. Heterogeneity was assessed using the chi-square test for statistical significance and the I2 statistic to evaluate the degree of heterogeneity among the results included in the meta-analysis. The primary measure of heterogeneity used for assessment was the I2 value, ranging from 0% to 100%. P-value <0.05 or I2>50% indicated heterogeneity exists among the studies included, thus a random effect model was applied; otherwise a fixed effect model was used.
Results
The screened literature and a brief introduction
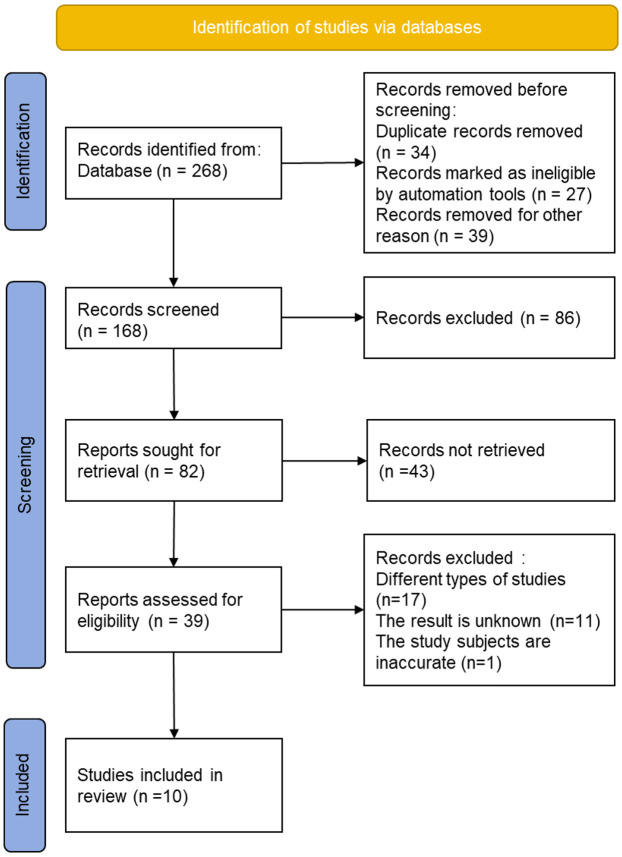
A total of 268 studies were obtained by searching the databases. The initial screening process excluded 34 duplicates, 27 unqualified studies, and 39 for other reasons. By reading abstracts and titles, an additional 86 studies were excluded. After excluding 43 research reports and review articles, 39 studies remained for further review. After thoroughly reading the full texts, 17 studies with incorrect research types, 11 studies with incomplete or unavailable treatment outcomes, and 1 paper focusing on animals were excluded. 10 studies were included in the final analysis. The detailed screening process is depicted in Figure 1.
Figure 1.
The process for screening eligible studies.
By reviewing the literature, basic information from 10 included studies [11-20] was extracted, as listed in Table 2. These studies collectively included 13,135 patients: 12,504 with AMI, 200 with STEMI, and 881 with TIMI. Among these patients, 6,581 were treated with sacubitril/valsartan, as the experimental group, and the other 6,554 patients were classified as the control group. Seven RCTs reported mortality, and six reported HF-induced hospitalization risk after treatment.
Table 2.
Data extraction for included literature
| Author | Year | Type of MI | Sample size | Male/female | Age (Year old) | Intervention measures | Length of treatment (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| Control | Experimental | Control | Experimental | Control | Experimental | Control | Experimental | ||||
| Chen [11] | 2021 | AMI | 39 | 42 | 24/15 | 27/15 | 51.3 ± 6.21 | 51.28 ± 6.27 | Bisoprolol 5 mg, qd | Sacubitril/valsartan 50-200 mg, bid | 4 weeks |
| Docherty [12] | 2021 | AMI | 46 | 47 | 43/3 | 42/5 | 59.7 ± 10.1 | 61.8 ± 10.6 | Valsartan 160 mg, bid | Sacubitril/valsartan 97/103 mg, bid | 13 months |
| Dong [13] | 2022 | AMI | 66 | 65 | 53/13 | 51/14 | 60.4 ± 10.0 | 60.2 ± 9.8 | Enalapril 10 mg, bid | Sacubitril/valsartan 97/103 mg, bid | 6 months |
| Fan [14] | 2023 | AMI | 39 | 39 | 31/8 | 28/11 | 68.0 ± 11.5 | 71.3 ± 10.5 | Irbesartan 150 mg, qd | Sacubitril/valsartan 200 mg, bid | 3 months |
| Halle [15] | 2021 | AMI | 98 | 103 | 77/21 | 86/17 | 67.6 ± 10.0 | 66.1 ± 10.8 | Enalapril 10 mg, bid | Sacubitril/valsartan 97/103 mg, bid | 8 months |
| Jering [16] | 2021 | AMI | 2,831 | 2,830 | 64 | 64 | 55.7 ± 9.7 | 55.7 ± 9.8 | Ramipril 5 mg, bid | Sacubitril/valsartan 100 mg, bid | 23 months |
| Rezq [17] | 2021 | STEMI | 100 | 100 | 88/12 | 86/14 | 57.0 ± 11.6 | 52.0 ± 9.2 | Ramipril 5 mg, bid | Sacubitril/valsartan 100 mg, bid | 6 months |
| Velazquez [18] | 2018 | TIMI | 441 | 440 | 63 | 61 | 55.7 ± 9.7 | 55.7 ± 9.8 | Enalapril 10 mg, bid | Sacubitril/valsartan 97/103 mg, bid | 8 weeks |
| Pfeffer MA [19] | 2021 | AMI | 2,831 | 2,830 | 2,131/700 | 2,167/663 | 63.5 ± 11.4 | 64.0 ± 11.6 | Ramipril (5 mg per day) | Sacubitril/valsartan 97/103 mg, bid | 4 months |
| Yang [20] | 2023 | AMI | 63 | 85 | 57/6 | 75/10 | 59.92 ± 12.02 | 59.07 ± 11.53 | Valsartan 80 mg once daily | Sacubitril/valsartan 100 mg twice daily | 6 months |
Bias risk
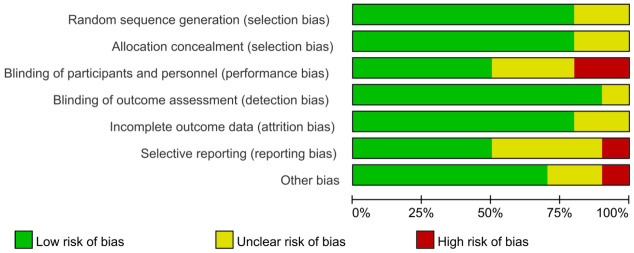
Figure 2 illustrates the bias risk of the 10 studies, which suggested that 8 studies were rated as grade A (75.00%), and 2 were rated as grade B (12.5%). Figure 3 summarizes the bias risk.
Figure 2.
Bias risk of the included literature.
Figure 3.
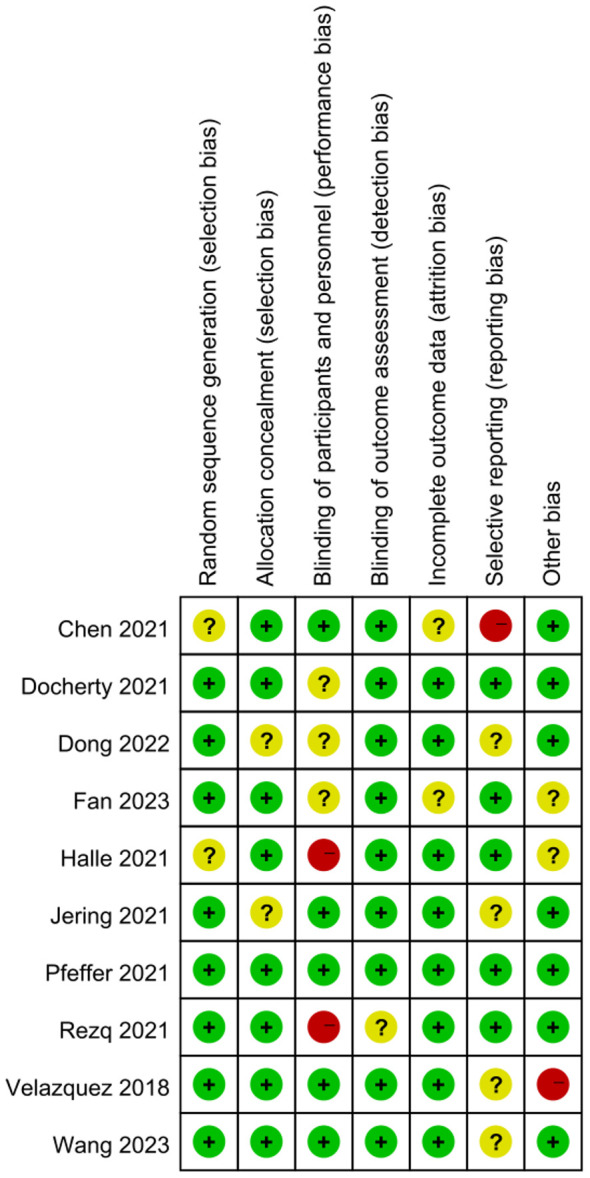
Summary of bias risk. Note: +, -, and ? refer to low, high, and unclear risk, respectively.
Mortality of patients
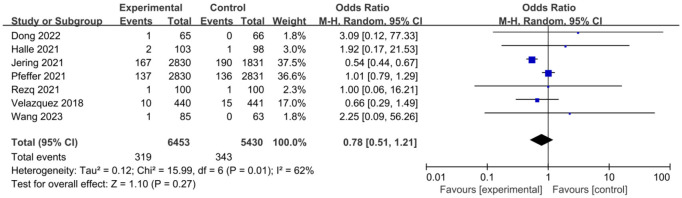
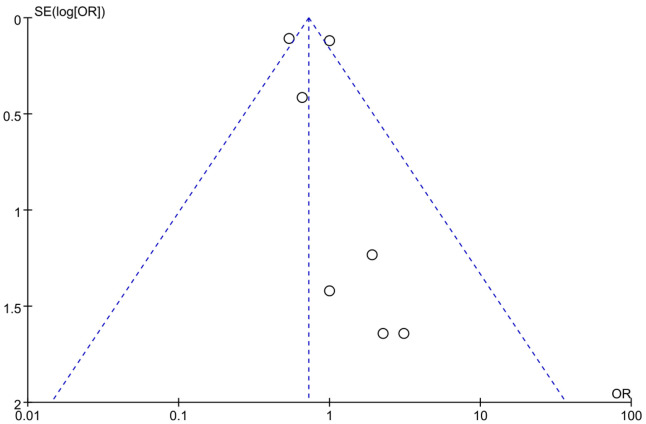
Using OR as an indicator of clinical outcome, the OR for posttreatment mortality was 0.87 across 7 studies. There was heterogeneity in mortality rates among different studies (I2=62%, P=0.01) (Figure 4), thus a random effects model was used, and the results showed that there was no significant difference in posttreatment mortality rate between the control group and the experimental group (P=0.27). The OR was 0.78, with a 95% CI of 0.51 to 1.21. The lowest and highest OR values were 0.54 and 3.09, respectively, with 95% CIs of (0.44, 0.67) and (0.12, 77.33). The funnel plot of posttreatment mortality (Figure 5) revealed low risk of publication bias across the 7 studies. According to these results, it was concluded that sacubitril/valsartan can lower the posttreatment mortality of patients with AMI.
Figure 4.
Forest plot of posttreatment mortality of patients.
Figure 5.
Funnel plot of studies reporting post-treatment mortality.
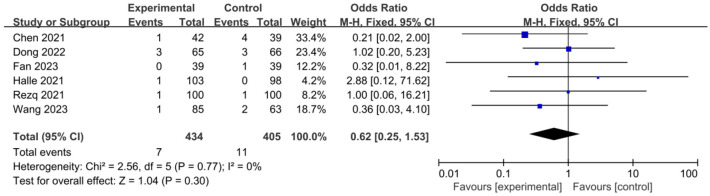
Myocardial re-infarction
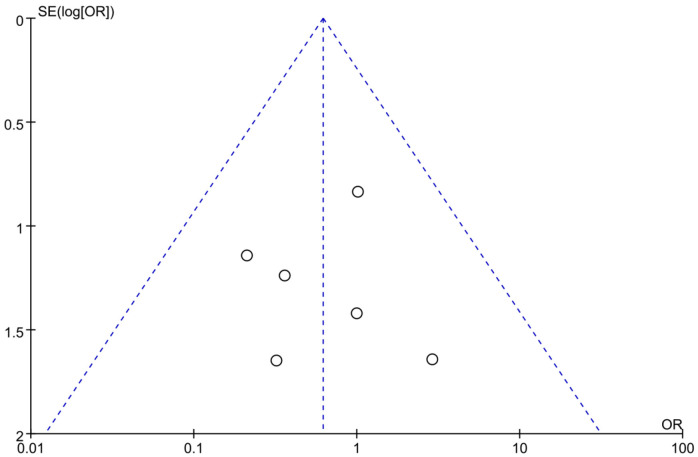
OR was used as an indicator of clinical outcome in this work. As demonstrated in Figure 6, there was no heterogeneity (I2=0%, P=0.77) among studies reporting the myocardial re-infarction count, thus a fixed effects model was used for analysis. The results showed that there was no significant difference (P=0.30) in the myocardial reinfarction count between the control group and the experimental group after treatment. The OR was 0.62, with a 95% CI of 0.25 to 1.53. The lowest and highest OR values were 0.21 and 2.88, respectively, with corresponding 95% CIs of (0.02, 2.00) and (0.12, 71.62). The funnel plot of myocardial re-infarction (Figure 7) displays a slight risk of publication bias. Based on these findings, sacubitril/valsartan treatment has the ability to decrease the incidence of myocardial re-infarction following treatment.
Figure 6.
Forest plot of myocardial re-infarction counts after treatment.
Figure 7.
Funnel plot of studies reporting myocardial reinfarction counts.
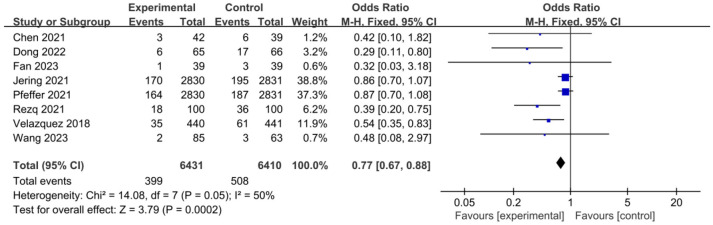
Risk of HF hospitalization
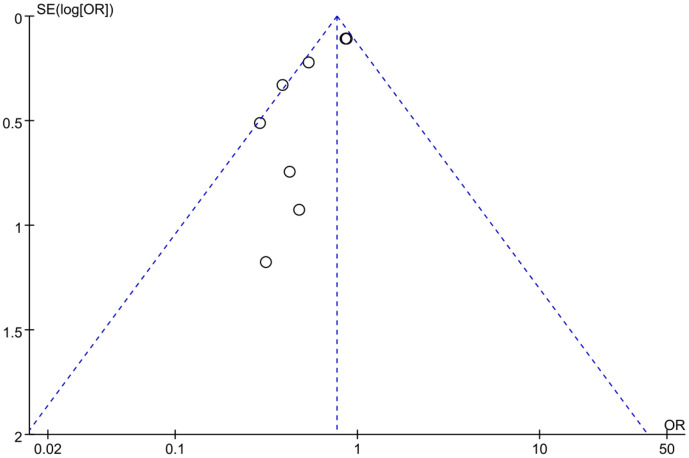
In Figure 8, the risk of HF hospitalization in 8 studies showed an I2 value of 50% and a P value of 0.05. There was no significant heterogeneity in the incidence of hospitalization for HF among different studies. Thus, a fixed effects model was used for analysis, and the results showed that the risk of hospitalization for HF in the experimental group was significantly lower than that of the control group after treatment (P=0.0002). The overall OR was 0.77, with a 95% CI of 0.67 to 0.88. The highest and lowest OR values were 0.86 and 0.29, with corresponding 95% CIs of (0.70, 1.07) and (0.11, 0.80), respectively. The funnel plot (Figure 9) for the HF hospitalization shows low risk for publication bias among the included literature. According to the above results, sacubitril/valsartan can lower the risk of HF hospitalization when applied to treat AMI.
Figure 8.
Forest plot of risk of heart failure (HF) hospitalization.
Figure 9.
Funnel plot of studies reporting heart failure (HF) hospitalization.
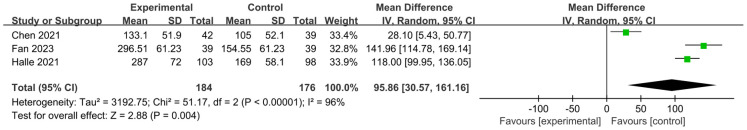
Patients’ six-minute walking distance (6MWD)
The mean difference (MD) was used to evaluate the 6MWD of patients in this work. As shown in Figure 10, the 6MWD of patients across the 3 studies exhibited an I2 value of 96% and a P value less than 0.00001, indicating significant heterogeneity among different studies. Hence, a random effects model was used for analysis. The results showed that the average 6MWD of patients in the experimental group after treatment was significantly longer than that of the control group (P=0.004, MD=95.86, 95% CI: 30.57-161.16). The minimal and maximal MD values were 28.10 and 141.96, respectively, with corresponding 95% CIs of (5.43, 50.77) and (114.78, 169.14), respectively.
Figure 10.
Forest plot of 6-minute walking distance (6MWD).
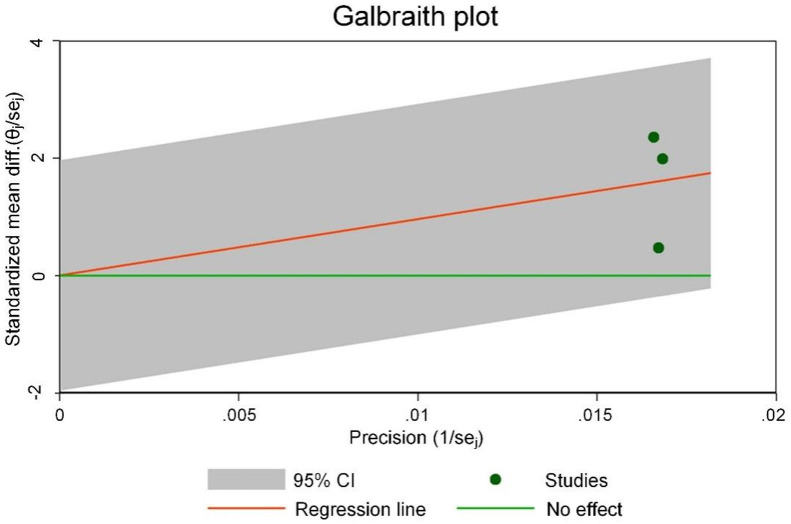
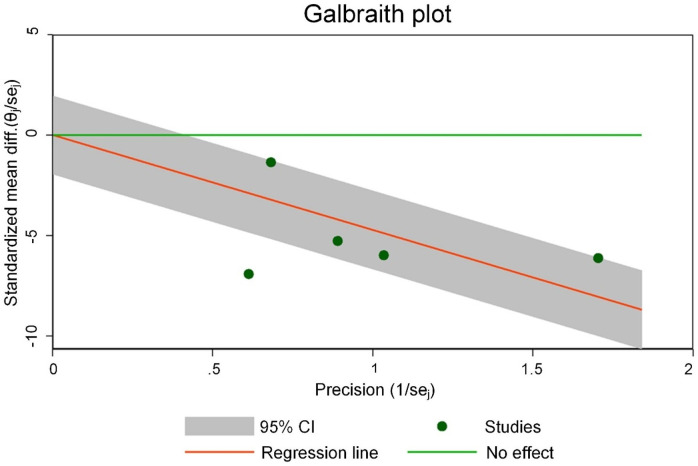
Figure 11 is the Galbraith chart for the heterogeneity test of 6MWD of each study. The results show that none of the three included studies showed significant deviation from other points (or were distributed at the edge of the graph), indicating small heterogeneity among the studies. Figure 12 shows the funnel plot of publication bias analysis of 6MWD related studies. The three included studies were all distributed outside the funnel plot, indicating that there was certain publication bias among the studies.
Figure 11.
Galbraith heterogeneity test results for the 6-minute walking distance (6MWD).
Figure 12.
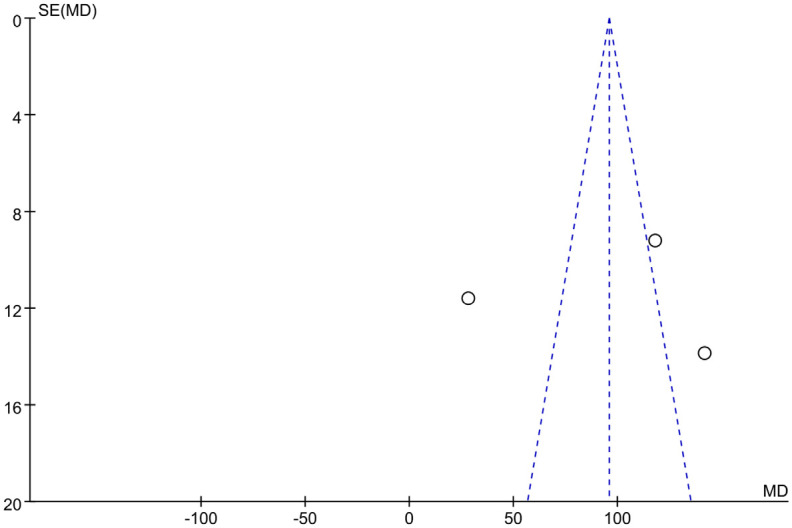
Funnel plot of studies reporting 6-minute walking distance (6MWD).
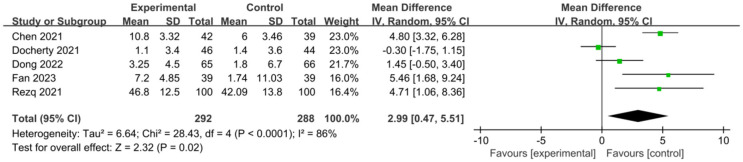
Patients’ left ventricular ejection fraction (LVEF)
In Figure 13, the LVEF of patients across 5 studies exhibited an I2 of 86% and a P value less than 0.0001, indicating significant heterogeneity among different studies. Hence, a random effects model was used for analysis, and the results showed that the average LVEF of patients in the experimental group was significantly higher than that of the control group after treatment (P=0.02, MD=2.99, 95% CI: 0.47-5.51). The lowest and highest MD values were -0.30 and 5.46, respectively, with 95% CIs of (-1.75, 1.15) and (1.68, 9.24), respectively.
Figure 13.
Forest plot of left ventricular ejection fraction (LVEF).
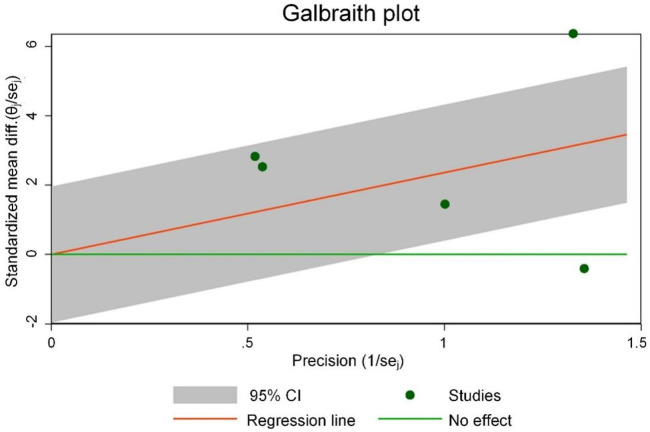
Figure 14 shows the Galbraith chart for the heterogeneity test of LVEF in each study. The results showed that among the five studies included, two studies significantly deviated from other points (or were distributed at the edge of the chart), indicating significant heterogeneity. Figure 15 shows the funnel plot of publication bias analysis of LVEF-related studies. Among the five included studies, two studies were distributed outside the funnel plot, indicating that there was certain publication bias among the studies. Based on these findings, it is speculated that sacubitril/valsartan may enhance LVEF in the treatment of acute myocardial infarction. However, further research is needed for verification.
Figure 14.
Galbraith heterogeneity for left ventricular ejection fraction (LVEF).
Figure 15.
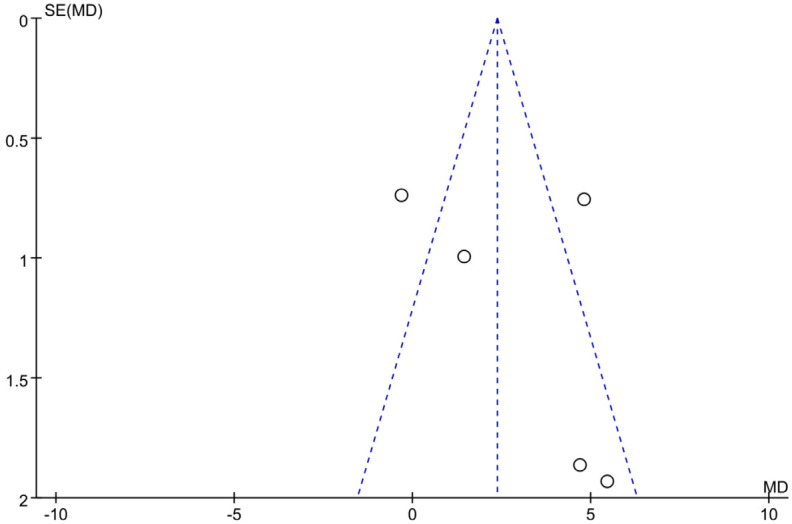
Funnel plot of studies reporting left ventricular ejection fraction (LVEF).
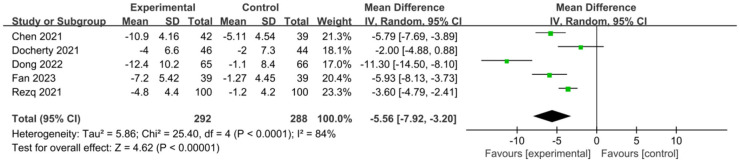
Patients’ left ventricular end-diastolic diameter (LVEDD)
In Figure 16, the LVEDD across 5 studies showed an I2 of 84% and a P value less than 0.0001, indicating significant heterogeneity among different studies. Therefore, a random effects model was used for analysis, and the results showed that the average LVEDD of patients in the experimental group was significantly lower than that of the control group after treatment (P<0.00001, MD=-5.56, 95% CI: -7.92-3.20). The lowest and largest MD values were -11.30 and -2.00, respectively, with corresponding 95% CIs of (-14.50, -8.10) and (-4.88, 0.88), respectively.
Figure 16.
Forest plot of left ventricular end-diastolic diameter (LVEDD).
Figure 17 is the Galbraith chart for the heterogeneity test of LVEDD of each study. The results show that among the five studies included, one study significantly deviates from other points (or is distributed at the edge of the figure). These results indicate that there is some heterogeneity among LVEDD studies. Figure 18 shows the funnel plot of publication bias analysis of LVEDD related studies. Among the five included studies, one study was distributed outside the funnel plot, indicating that there was certain publication bias among the studies. Based on these findings, sacubitril/valsartan is speculated to improve LVEDD in patients with acute myocardial infarction, but further studies are needed to confirm this.
Figure 17.
Galbraith heterogeneity test results in left ventricular end-diastolic diameter (LVEDD).
Figure 18.
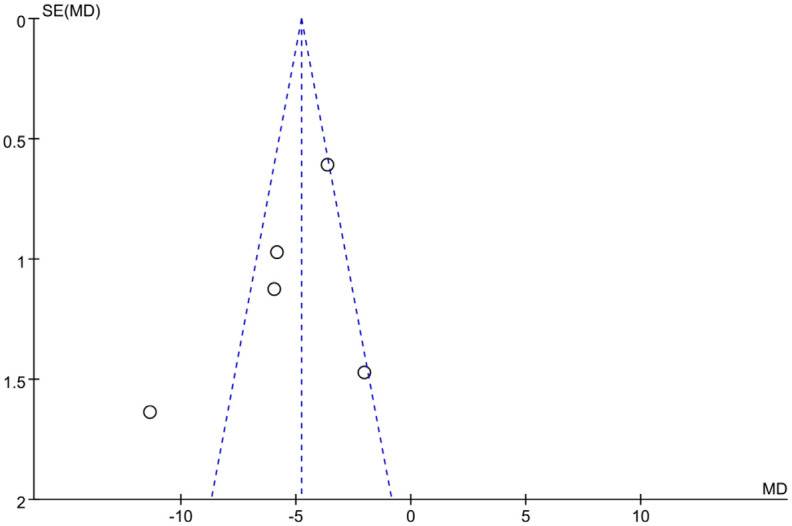
Funnel plot of studies reporting left ventricular end-diastolic diameter (LVEDD).
Discussion
Sacubitril/valsartan is becoming increasingly widespread for AMI in clinical practice. Studies have shown that compared to traditional treatments, sacubitril/valsartan significantly improves myocardial function and therapeutic outcome, thus providing greater clinical treatment value [21]. Furthermore, research indicates that sacubitril/valsartan not only improves cardiac function but also reduces cardiovascular mortality [22]. Based on this, the present study included 10 studies on the efficacy and safety of sacubitril/valsartan in the treatment of AMI.
The preliminary results of the meta-analysis indicated that the OR for myocardial reinfarction from six studies was 0.62, OR for HF hospitalization from eight studies was 0.77, and OR for post-treatment mortality from seven studies was 0.78. These findings suggest that the use of sacubitril/valsartan significantly reduced the risk of post-AMI myocardial reinfarction, HF hospitalization, and post-treatment mortality. These results are consistent with the findings of Desai et al. [23] and Fröhlic et al. [24], who had demonstrated that sacubitril/valsartan effectively improves the prognosis of HF patients. Additionally, Kido et al. [25] showed that higher doses of sacubitril/valsartan effectively reduced HF hospitalization rates (16.10% vs. 29.14%, 19.51%) and all-cause mortality (9.27% vs. 29.63%, 17.58%). Acanfora et al. [26] and Correale et al. [27] also confirmed that sacubitril/valsartan significantly reduced mortality in HF patients, substantially improving prognosis and providing clinicians with more effective clinical treatment guidance. The global PARADISE-MI study also indicated the significant efficacy of ARNI in post-MI HF patients, and further evidence was expected to support sacubitril/valsartan as the preferred treatment option for these patients [28,29].
The meta-analysis further found that the MD of 6MWD in three studies was 95.86, and the MD of LVEDD was -5.56, suggesting that sacubitril/valsartan significantly improved patients’ cardiac function, increased 6MWD, and reduced LVEDD. These results are consistent with the findings of McMurray et al. [30], whose study demonstrated that sacubitril/valsartan improved LVEF and cardiac remodeling (reduced LVEDD). Researchers have also confirmed that sacubitril/valsartan treatment reduced LVEF and left atrial volume index (LAVIs) in patients with chronic HF [31,32]. Additionally, it has been confirmed that the reduction of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTNT) are closely associated with the improvement of LVEF and LAVIs. Rossignol et al. [33] discovered that sacubitril/valsartan had a significant impact on preventing and treating refractory HF, proposing that it can effectively reduce concentrations of NT-proBNP, aldosterone (ALD), and intercellular adhesion molecule 1 (ICAM-1), prevent changes in cardiac function, prolong the 6MWD, and have fewer side effects. Another study indicated that regardless of whether patients underwent vascular reconstruction surgery, sacubitril/valsartan significantly improved their 6MWD [34]. Although some studies suggested limited improvement in LVEF after AMI with sacubitril/valsartan [35], others, including those with larger sample sizes, demonstrated significant improvements in LVEF in HF patients after AMI [36,37]. This suggests that future research is needed to validate the efficacy and safety of sacubitril/valsartan in different patient populations to better guide clinical practice.
Sacubitril is a neprilysin inhibitor that inhibits the activity of NEP, leading to an increase in levels of brain natriuretic peptide (BNP). BNP is an endogenous diuretic, natriuretic, vasodilator, and antifibrotic substance. By increasing BNP levels, sacubitril promotes diuresis, reduces salt and water retention, vasodilates, and inhibits fibrosis. Valsartan is an angiotensin II receptor antagonist that blocks the binding of angiotensin II to its receptors, thereby inhibiting the renin-angiotensin system (RAS). Angiotensin II is a potent vasoconstrictor that also promotes salt and water retention and cardiac remodelling. By blocking angiotensin II receptors, valsartan reduces vasoconstriction, decreases salt and water retention, and reduces the workload on the heart. Combining the effects of these two drugs, sacubitril/valsartan acts synergistically in HF patients through two pathways. Sacubitril/valsartan, as a combination therapy, exerts synergistic effects on the treatment of HF through multiple mechanisms, making it one of the important therapeutic options in cardiovascular disease management. However, this meta-analysis did not specifically analyze the mechanism of action of sacubitril/valsartan, hence further exploration is needed.
Conclusion
Sacubitril/valsartan has demonstrated significant efficacy in patients after acute myocardial infarction (AMI). First, sacubitril/valsartan can effectively improve cardiac function and reduce the incidence of myocardial reinfarction after treatment. Second, it can reduce the risk of hospitalization for HR in patients, thereby improving quality of life. In addition, sacubitril/valsartan treatment can reduce mortality and enhance survival after treatment. Functionally, sacubitril/valsartan treatment significantly increased the six-minute walk distance and improved LVEF and LVEDD in patients with AMI, further confirming its role in cardiac function recovery and improvement in quality of life. However, more studies and long-term follow-up data are still needed to validate this conclusion and provide more evidence for clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Guan T, Zhu X, Tian L, Shen T. Evaluation of the effect of nano-sodium alginate-bioglass on cardiac function of myocardial infarction based on cardiac ultrasound. Cell Mol Biol (Noisy-le-grand) 2022;68:67–76. doi: 10.14715/cmb/2022.68.3.9. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim NE, Piña IL, Camacho A, Bapat D, Felker GM, Maisel AS, Butler J, Prescott MF, Abbas CA, Solomon SD, Januzzi JL Jr Prospective Study of Biomarkers, Symptom Improvement and Ventricular Remodeling During Entresto Therapy for Heart Failure (PROVE-HF) Study Investigators. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur J Heart Fail. 2020;22:2018–2025. doi: 10.1002/ejhf.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, Shi V, Zhao Z, Cowie MR PARALLAX Investigators and Committee members. Effect of sacubitril/valsartan vs. standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021;326:1919–1929. doi: 10.1001/jama.2021.18463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salinas-Casado J, Méndez-Rubio S, Esteban-Fuertes M, Gómez-Rodríguez A, Vírseda-Chamorro M, Luján-Galán M, Rituman G. Efficacy and safety of D-mannose (2 g), 24 h prolonged release, associated with Proanthocyanidin (PAC), versus isolate PAC, in the management of a series of women with recurrent urinary infections. Arch Esp Urol. 2018;71:169–177. [PubMed] [Google Scholar]
- 5.Senni M, Wachter R, Witte KK, Straburzynska-Migaj E, Belohlavek J, Fonseca C, Mueller C, Lonn E, Chakrabarti A, Bao W, Noe A, Schwende H, Butylin D, Pascual-Figal D TRANSITION Investigators. Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2020;22:303–312. doi: 10.1002/ejhf.1670. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, Herrmann-Lingen C, Garfias Macedo T, Koziolek M, Noutsias M, Schulze PC, Wachter R, Hasenfuß G, Laufs U. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. 2021;23:92–113. doi: 10.1002/ejhf.1838. [DOI] [PubMed] [Google Scholar]
- 7.Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, Chaaban S, Bøhmer E, Pouleur AC, Mueller C, Tribouilloy C, Lonn E, A L Buraiki J, Gniot J, Mozheiko M, Lelonek M, Noè A, Schwende H, Bao W, Butylin D, Pascual-Figal D TRANSITION Investigators. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. doi: 10.1002/ejhf.1498. [DOI] [PubMed] [Google Scholar]
- 8.Wada J, Nakatsuka A. Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama. 2016;70:151–8. doi: 10.18926/AMO/54413. [DOI] [PubMed] [Google Scholar]
- 9.Bao J, Kan R, Chen J, Xuan H, Wang C, Li D, Xu T. Combination pharmacotherapies for cardiac reverse remodeling in heart failure patients with reduced ejection fraction: a systematic review and network meta-analysis of randomized clinical trials. Pharmacol Res. 2021;169:105573. doi: 10.1016/j.phrs.2021.105573. [DOI] [PubMed] [Google Scholar]
- 10.Barcot O, Boric M, Poklepovic Pericic T, Cavar M, Dosenovic S, Vuka I, Puljak L. Risk of bias judgments for random sequence generation in Cochrane systematic reviews were frequently not in line with Cochrane handbook. BMC Med Res Methodol. 2019;19:170. doi: 10.1186/s12874-019-0804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Wu X, Li Y, Peng Y. Study on the application effect of bisoprolol combined with sacubitril valsartan sodium tablets in the cardiac rehabilitation of patients with acute myocardial infarction combined with left heart failure after percutaneous coronary intervention (PCI) Ann Palliat Med. 2021;10:5455–5461. doi: 10.21037/apm-21-877. [DOI] [PubMed] [Google Scholar]
- 12.Docherty KF, Campbell RT, Brooksbank KJM, Dreisbach JG, Forsyth P, Godeseth RL, Hopkins T, Jackson AM, Lee MMY, McConnachie A, Roditi G, Squire IB, Stanley B, Welsh P, Jhund PS, Petrie MC, McMurray JJV. Effect of neprilysin inhibition on left ventricular remodeling in patients with asymptomatic left ventricular systolic dysfunction late after myocardial infarction. Circulation. 2021;144:199–209. doi: 10.1161/CIRCULATIONAHA.121.054892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Xu Y, Ding C, Yu Z, Yu Z, Xia X, Chen Y, Jiang X. Comparing the efficacy of angiotensin receptor-neprilysin inhibitor and enalapril in acute anterior STEMI patients after primary percutaneous coronary intervention: a prospective randomized trial. Cardiovasc Diagn Ther. 2022;12:42–54. doi: 10.21037/cdt-21-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan H, Wang Y, Wang X, Dong X, Shao X, Yang F. Effect of emergency percutaneous coronary intervention combined with sacubitril and valsartan on the cardiac prognosis in patients with acute myocardial infarction. Int J Gen Med. 2023;16:499–505. doi: 10.2147/IJGM.S389216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halle M, Schöbel C, Winzer EB, Bernhardt P, Mueller S, Sieder C, Lecker LSM. A randomized clinical trial on the short-term effects of 12-week sacubitril/valsartan vs. enalapril on peak oxygen consumption in patients with heart failure with reduced ejection fraction: results from the ACTIVITY-HF study. Eur J Heart Fail. 2021;23:2073–2082. doi: 10.1002/ejhf.2355. [DOI] [PubMed] [Google Scholar]
- 16.Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, van der Meer P, Wernsing M, Carter K, Guo W, Zhou Y, Lefkowitz M, Gong J, Wang Y, Merkely B, Macin SM, Shah U, Nicolau JC, Braunwald E. Prospective ARNI vs. ACE inhibitor trial to Determine Superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021;23:1040–1048. doi: 10.1002/ejhf.2191. [DOI] [PubMed] [Google Scholar]
- 17.Rezq A, Saad M, El Nozahi M. Comparison of the efficacy and safety of sacubitril/valsartan versus ramipril in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2021;143:7–13. doi: 10.1016/j.amjcard.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E PIONEER-HF Investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Claggett B, Lewis EF, Granger CB, Køber L, Maggioni AP, Mann DL, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, Berwanger O, Cikes M, De Pasquale CG, East C, Fernandez A, Jering K, Landmesser U, Mehran R, Merkely B, Vaghaiwalla Mody F, Petrie MC, Petrov I, Schou M, Senni M, Sim D, van der Meer P, Lefkowitz M, Zhou Y, Gong J, Braunwald E PARADISE-MI Investigators and Committees. Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. N Engl J Med. 2021;385:1845–1855. doi: 10.1056/NEJMoa2104508. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Li X, Wang L, Wu X, Wang C, Li T, Wang H. Effects of sacubitril/valsartan on cardiac reverse remodeling and cardiac resynchronization in patients with acute myocardial infarction. Front Cardiovasc Med. 2023;9:1059420. doi: 10.3389/fcvm.2022.1059420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapelios CJ, Lainscak M, Savarese G, Laroche C, Seferovic P, Ruschitzka F, Coats A, Anker SD, Crespo-Leiro MG, Filippatos G, Piepoli MF, Rosano G, Zanolla L, Aguiar C, Murin J, Leszek P, McDonagh T, Maggioni AP, Lund LH Heart Failure Long-Term Registry Investigators. Sacubitril/valsartan eligibility and outcomes in the ESC-EORP-HFA heart failure long-term registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM-HF trial, ESC guidelines, and real world. Eur J Heart Fail. 2019;21:1383–1397. doi: 10.1002/ejhf.1532. [DOI] [PubMed] [Google Scholar]
- 22.Qiao GH, Zhu P, Yue L, Yue S. MiR-125b improves acute myocardial infarction in rats by regulating P38/Sirtl/P53 signaling pathway. J Biol Regul Homeost Agents. 2020;34:1297–1306. doi: 10.23812/20-177-A. [DOI] [PubMed] [Google Scholar]
- 23.Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K, Abbas CA, Rocha R, Mitchell GF EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs. enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–1084. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fröhlich H, Frey N, Estler B, Mäck M, Schlegel P, Beckendorf J, Frankenstein L, Täger T. Haemodynamic effects of sacubitril/valsartan initiation in outpatients with chronic heart failure. Am J Cardiovasc Drugs. 2022;22:695–704. doi: 10.1007/s40256-022-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kido K, Bianco C, Caccamo M, Fang W, Sokos G. Evaluating sacubitril/valsartan dose dependence on clinical outcomes in patients with heart failure with reduced ejection fraction. Ann Pharmacother. 2021;55:1069–1075. doi: 10.1177/1060028020983522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acanfora D, Scicchitano P, Acanfora C, Maestri R, Goglia F, Incalzi RA, Bortone AS, Ciccone MM, Uguccioni M, Casucci G. Early initiation of sacubitril/valsartan in patients with chronic heart failure after acute decompensation: a case series analysis. Clin Drug Investig. 2020;40:493–501. doi: 10.1007/s40261-020-00908-4. [DOI] [PubMed] [Google Scholar]
- 27.Correale M, Mallardi A, Mazzeo P, Tricarico L, Diella C, Romano V, Ferraretti A, Leopizzi A, Merolla G, Di Biase M, Brunetti ND. Sacubitril/valsartan improves right ventricular function in a real-life population of patients with chronic heart failure: the Daunia heart failure registry. Int J Cardiol Heart Vasc. 2020;27:100486. doi: 10.1016/j.ijcha.2020.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan Y, Chen JQ, Li XY, Jiang SN. ClyA enhances LPS-induced IL-1β secretion in human macrophages through TLR4 and NLRP3 signaling. J Biol Regul Homeost Agents. 2021;35:495–504. doi: 10.23812/20-500-A. [DOI] [PubMed] [Google Scholar]
- 29.El-Battrawy I, Akin I. Impact of sacubitril/valsartan on cardiac arrest event rate. Letter regarding the article ‘Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics’. Eur J Heart Fail. 2022;24:1324. doi: 10.1002/ejhf.2444. [DOI] [PubMed] [Google Scholar]
- 30.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diao K, Wang D, Chen Z, Wu X, Ma M, Zhu Y, Zhang L, Wang H, Wang M, He S, Li C, Deng Q, Yan T, Wu T, Tang L, Huang B, Sun J, He Y. Rationale and design of a multi-center, prospective randomized controlled trial on the effects of sacubitril-valsartan versus enalapril on left ventricular remodeling in ST-elevation myocardial infarction: the PERI-STEMI study. Clin Cardiol. 2021;44:1709–1717. doi: 10.1002/clc.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dos Santos MR, Alves MNN, Jordão CP, Pinto CEN, Correa KTS, de Souza FR, da Fonseca GWP, Tomaz Filho J, Costa M, Pereira RMR, Negrão CE, Barretto ACP. Sacubitril/valsartan versus enalapril on exercise capacity in patients with heart failure with reduced ejection fraction: a randomized, double-blind, active-controlled study. Am Heart J. 2021;239:1–10. doi: 10.1016/j.ahj.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol P, Lainscak M, Crespo-Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH Heart Failure Long-Term Registry Investigators Group. Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP heart failure long-term registry. Eur J Heart Fail. 2020;22:1378–1389. doi: 10.1002/ejhf.1793. [DOI] [PubMed] [Google Scholar]
- 34.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, Rodriguez-Cordero A, Zafar MU, Fergus I, Atallah-Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ EMPA-TROPISM (ATRU-4) Investigators. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Shuang L, Jidong W, Hongjuan P, Zhenwei Y. Effects of apelin on proliferation and apoptosis in rat ovarian granulosa cells. Clin Exp Obstet Gynecol. 2016;43:409–13. [PubMed] [Google Scholar]
- 36.Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Investigators. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang XM, Li JJ, Yin W, Fu HL, Yu F, Gu LQ, Zhang Y, Du M, Ye Z, Xu L. Effect of sacubitril valsartan on heart failure with mid-range or preserved ejection fraction in patients on maintenance hemodialysis: real-world experience in a single-center, prospective study. BMC Cardiovasc Disord. 2024;24:79. doi: 10.1186/s12872-024-03744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]