Abstract
Objective: To identify independent risk factors for protein-energy malnutrition (PEM) in children aged 8-10 years and to develop and validate a nomogram model for estimating PEM risk. Methods: In this retrospective study, a cohort of 1,412 children from The Fifth Affiliated Hospital of Guangzhou Medical University, spanning January 2022 to December 2023, was identified. Participants were randomly classified into a training set (n=988) and a validation set (n=424). Patients in the training set were divided into normal (n=667) and PEM (n=321) groups. Data collection involved demographic, sociological, physical, and biochemical assessments. Independent risk factors for PEM were identified using univariate and multivariate logistic regression. A nomogram risk model was constructed from significant predictors, and its performance was assessed using the receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA). An independent dataset further validated the nomogram model. Results: Among the 1,412 children, 497 (35.2%) had PEM, which included stunting (11.83%), underweight (11.61%), and wasting (11.76%). Multivariate analysis identified six independent risk factors for PEM: gestational age (OR (95% CI)=5.830 (3.604-9.431), P<0.001), household income (OR (95% CI)=0.383 (0.281-0.523), P<0.001), sleep duration (OR (95% CI)=1.800 (1.319-2.457), P<0.001), mood disorders (OR (95% CI)=6.924 (4.437-10.805), P<0.001), and physical activity time (OR (95% CI)=3.210 (2.342-4.400), P<0.001). The nomogram model demonstrated good predictive performance (AUC=0.803 (0.773-0.832)) and was validated well on an independent dataset (AUC=0.783 (0.739-0.828)). Conclusion: The study identified key independent risk factors for PEM in children and established a robust nomogram model for clinical risk assessment. The model’s high predictive accuracy and clinical applicability suggest it may be a valuable tool for the early identification and intervention strategies for PEM in clinical practice.
Keywords: Protein-energy malnutrition, predictive nomogram model, children
Introduction
Child growth and development are influenced by various factors, with nutrition playing a pivotal role. Protein-energy malnutrition (PEM) includes three aspects of malnutrition: stunted growth, low body weight, and wasting. Stunted growth reflects long-term chronic nutritional deficiencies, while low body weight and wasting reflect acute nutritional deficiencies in children [1]. Different types of malnutrition can co-exist in children. Specifically, malnutrition in children aged 8 to 10 years, during a critical period of physical development, can have irreversible immediate and long-term effects on health, including physical and cognitive developmental delays, and increased risk of infection and death [2-4]. PEM is considered by experts to be the most prevalent form of childhood malnutrition [5]. In 2016, the World Health Organization (WHO) reported that globally, 22.9% or 154.8 million children under 5 years of age had stunted growth [6,7]. Despite the development of social economy, the improvements in living environment, and the enhancement of health service levels, the nutritional status of children in China has been improving, but there are still obvious regional and urban-rural differences [5,8]. Therefore, early detection and targeted interventions are imperative to nurture children’s comprehensive development. Developing a nuanced understanding of the epidemiological patterns, deleterious effects, and risk factors associated with PEM is pivotal for preemptive action and effective mitigation.
PEM arises from multiple factors, including poverty, socioeconomic disparities, poor sanitation, and dietary inadequacies [6,7,9]. Furthermore, the unequal advancements in global economic conditions and living standards have led to nutritional imbalances, causing regions to face a dual burden of malnutrition and obesity, amplifying health challenges for children [5]. A balanced diet provides a range of essential nutrients in quantities sufficient to support and promote physical and mental health [10]. A cross-sectional cohort study in Switzerland showed that 10.9% and 20.2% of the 1,919 subjects analyzed had energy and protein intakes below the established dietary reference values, respectively [11]. It was also found that the lower the income level [12], the greater the likelihood of inadequate energy intake. In addition, the more children a family has, the higher the risk they may develop PEM [11]. Other findings suggest that variables such as age, gender, and specific dietary habits are influential in determining a person’s protein and energy consumption [13].
This study delves into the prevalence and determinants of PEM among 8- to 10-year-old school children in Guangzhou City from 2022 to 2024. Through systematic data gathering and rigorous analysis, this research aims to underpin PEM prevention and treatment strategies. Specifically, it underscores the importance of constructing an epidemiological model to identify and scrutinize key risk factors, assisting health professionals and policymakers in early risk identification and intervention. Additionally, the study introduces a nomogram prediction model based on essential variables to enhance the precision of PEM prevention and interventions. This model offers a quantifiable method to appraise the risk of PEM in children, facilitating the crafting of personalized interventions and helping policymakers optimize resource distribution and effective preventative measures.
Materials and methods
Study population
Children who underwent medical examination at The Fifth Affiliated Hospital of Guangzhou Medical University between January 2022 and December 2023 were included in this study. The inclusion criteria were: 1) children aged 8-10 years attending the outpatient and inpatient departments, and 2) parents or guardians who agreed to participate in the study. Exclusion criteria were: 1) children with chronic diseases affecting growth (e.g., chronic renal failure, congenital heart disease, malignant tumors, endocrine diseases, etc.), 2) children taking medication that affects their nutritional status for a long period, and 3) children diagnosed with malnutrition or having participated in nutrition-related intervention studies.
The children included in the study were randomly classified into a training set (n=988) and a validation set (n=424). Besides, patients in the training set were divided into normal (n=667) and PEM (n=321) groups. The training set was used to develop the nomogram model, while the validation set was used to assess its predictive performance and generalizability. The study was reviewed and approved by the medical ethics committee of the Fifth Affiliated Hospital of Guangzhou Medical University.
Data collection and definition
Data collected included demographic information (gender, age), birth history (including prematurity), sibling status (number of children), whether they were ‘left-behind children’ (parents working in the city and children left behind in the countryside), primary caregiver, dietary habits, milk intake, sleep duration, physical activity, and mental health status. In addition, the results of the children’s physical examinations and blood biochemical indicator tests were collected.
A penchant for sweets, beverages, irregular eating, picky eating, or overeating were considered bad eating habits [14]. The levels of calcium, iron and zinc trace elements were based on the results of the test report issued by the hospital laboratory on the same day of venous blood sampling [15]. Serum calcium <1.55 mmol/L was regarded as calcium deficiency, serum iron <7.36 mmol/L as iron deficiency, and serum zinc <67.72 mmol/L as zinc deficiency. Mental health status was assessed using the Screening Scale for Anxious Emotional Disorders in Children (SCARED) [16], with a total of 41 entries, covering generalized anxiety, separation anxiety, somatization/panic, social phobia, and school phobia. Each entry corresponded to a score of 0, 1, or 2 based on the child’s response. A total score of ≥25 was considered indicative of anxiety, which is classified as a mood disorder.
Criteria for PEM
We calculated length (height)/age, weight/age, weight/length (height) and body mass index/age in this study. PEM was diagnosed in children with one or more of: growth retardation, low body weight, and wasting [17]. The height/length for age Z score (HAZ), weight for age Z score (WAZ) and weight for height/length Z score (WHZ) were calculated. HAZ<-2 was considered growth retardation, WAZ<-2 was considered low weight, and WHZ<-2 was considered wasting [17].
Statistical analyses
Data analysis was performed using R-studio software (version 4.1.2). Categorical variables were expressed as frequencies and percentages, and the chi-square test was used for group comparisons. Continuous variables were expressed as mean and standard deviation (SD), with Student t-tests applied accordingly. Independent risk factors associated with the development of PEM in children were identified by univariate and multivariate logistic regression analyses. A predictive nomogram was then constructed based on the regression coefficients of the predictors in the multivariate logistic regression. The sum of the scores for each predictor enabled a total score calculation. The predictive performance of the model was assessed by the AUC of the ROC curve analyses, and calibration was evaluated by a calibration curve. Lastly, the clinical utility of the predictive nomogram was evaluated using decision curve analysis (DCA). Validation was performed, and the stability of the study findings was assessed using another independent dataset. A p-value of less than 0.05 was considered significant in a two-sided test.
Results
Comparison of characteristics of the study population
A total of 1,412 children aged 8-10 years who met the criteria were included in the study, of whom 497 (35.2%) were considered to have PEM. Among these participants, 167 children (11.83%) had stunting with an age-specific height Z score of <-2, 164 children (11.61%) were underweight with an age-specific weight Z score of <-2, and 166 children (11.76%) were wasting with a height-specific weight Z score of <-2. The participants were randomly divided into a training set (n=988) and a validation set (n=424). The results in Table 1 suggest that there was no significant difference between the two datasets in terms of demographic and sociological characteristics such as age and gender, indicating that the two sets of data were randomly distributed and comparable.
Table 1.
Comparisons of variables in training and validation sets
| Variable | Total (n=1,412) | Training set (n=988) | Validation set (n=424) | Χ2 | P |
|---|---|---|---|---|---|
| Group | 5.434 | 0.143 | |||
| Normal | 915 (64.8) | 638 (64.57) | 277 (65.33) | ||
| Stunting | 167 (11.83) | 113 (11.44) | 54 (12.74) | ||
| Underweight | 164 (11.61) | 109 (11.03) | 55 (12.97) | ||
| Wasting | 166 (11.76) | 128 (12.96) | 38 (8.96) | ||
| Age | 3.936 | 0.140 | |||
| 8 years | 454 (32.15) | 313 (31.68) | 141 (33.25) | ||
| 9 years | 469 (33.22) | 317 (32.09) | 152 (35.85) | ||
| 10 years | 489 (34.63) | 358 (36.23) | 131 (30.90) | ||
| Sex | 0.014 | 0.907 | |||
| Male | 726 (51.42) | 509 (51.52) | 217 (51.18) | ||
| Female | 686 (48.58) | 479 (48.48) | 207 (48.82) | ||
| Gestational Age | 0.136 | 0.712 | |||
| ≥37 weeks | 1226 (86.83) | 879 (88.97) | 347 (81.84) | ||
| <37 weeks | 186 (13.17) | 109 (11.03) | 77 (18.16) | ||
| Birth Weight | 0.294 | 0.588 | |||
| ≥2.5 kg | 1,096 (77.62) | 763 (77.23) | 333 (78.54) | ||
| <2.5 kg | 316 (22.38) | 225 (22.77) | 91 (21.46) | ||
| Residence | 0.146 | 0.702 | |||
| Rural | 667 (47.24) | 470 (47.57) | 197 (46.46) | ||
| Urban | 745 (52.76) | 518 (52.43) | 227 (53.54) | ||
| Left Behind Children | 2.364 | 0.124 | |||
| No | 983 (69.62) | 700 (70.85) | 283 (66.75) | ||
| Yes | 429 (30.38) | 288 (29.15) | 141 (33.25) | ||
| Household income | 0.092 | 0.761 | |||
| <100 thousand yuan | 558 (39.52) | 393 (39.78) | 165 (38.92) | ||
| ≥100 thousand yuan | 854 (60.48) | 595 (60.22) | 259 (61.08) | ||
| Number of children | 0.039 | 0.843 | |||
| >1 | 677 (47.95) | 472 (47.77) | 205 (48.35) | ||
| 1 | 735 (52.05) | 516 (52.23) | 219 (51.65) | ||
| Sleep Duration | 0.065 | 0.799 | |||
| ≥9 hours | 802 (56.80) | 559 (56.58) | 243 (57.31) | ||
| <9 hours | 610 (43.20) | 429 (43.42) | 181 (42.69) | ||
| Parents education level | 0.099 | 0.753 | |||
| Low level | 735 (52.05) | 517 (52.33) | 218 (51.42) | ||
| High level | 677 (47.95) | 471 (47.67) | 206 (48.58) | ||
| Bad eating habits | 0.193 | 0.66 | |||
| No | 897 (63.53) | 624 (63.16) | 273 (64.39) | ||
| Yes | 515 (36.47) | 364 (36.84) | 151 (35.61) | ||
| Calcium Deficiency | 0.045 | 0.831 | |||
| No | 1,190 (84.28) | 834 (84.41) | 356 (83.96) | ||
| Yes | 222 (15.72) | 154 (15.59) | 68 (16.04) | ||
| Iron Deficiency | 8.249 | 0.004 | |||
| No | 1,183 (83.78) | 846 (85.63) | 337 (79.48) | ||
| Yes | 229 (16.22) | 142 (14.37) | 87 (20.52) | ||
| Zinc Deficiency | 0.019 | 0.890 | |||
| No | 1,324 (93.77) | 927 (93.83) | 397 (93.63) | ||
| Yes | 88 (6.23) | 61 (6.17) | 27 (6.37) | ||
| Mood disorders | 0.002 | 0.966 | |||
| No | 1,223 (86.61) | 856 (86.64) | 367 (86.56) | ||
| Yes | 189 (13.39) | 132 (13.36) | 57 (13.44) | ||
| Milk Intake | 2.747 | 0.097 | |||
| <300 ml/d | 895 (63.39) | 640 (64.78) | 255 (60.14) | ||
| ≥300 ml/d | 517 (36.61) | 348 (35.22) | 169 (39.86) | ||
| Physical activity time | 1.167 | 0.28 | |||
| ≥2 hours/d | 717 (50.78) | 511 (51.72) | 206 (48.58) | ||
| <2 hours/d | 695 (49.22) | 477 (48.28) | 218 (51.42) |
Notes: Parental education levels: high level, have completed ≥ nine years of compulsory education; low level, have not completed nine years of compulsory education.
Comparison and multivariate logistic analysis between PEM and normal groups
A total of 17 potential predictors were included in the study, and intergroup comparisons were made between the normal and PEM groups (Table 2). Compared to children with normal growth and development, children in the PEM group showed significant differences in the variables of gestational age (χ2=74.229, P<0.001), household income (χ2=55.494, P<0.001), sleep duration (χ2=15.662, P<0.001), bad eating habits (χ2=4.736, P=0.030), calcium deficiency (χ2=3.879, P=0.048), mood disorders (χ2=96.023, P<0.001), and physical activity time (χ2=68.398, P<0.001).
Table 2.
Comparisons of variables between normal group and PEM group in training set
| Variable, n (%) | Total (n=988) | Normal (n=661) | PEM (n=327) | χ2 | P |
|---|---|---|---|---|---|
| Age | 0.477 | 0.788 | |||
| 8 years | 313 (31.68) | 214 (32.38) | 99 (30.28) | ||
| 9 years | 317 (32.09) | 211 (31.92) | 106 (32.42) | ||
| 10 years | 358 (36.23) | 236 (35.70) | 122 (37.31) | ||
| Sex | 0.118 | 0.732 | |||
| Male | 509 (51.52) | 338 (51.13) | 171 (52.29) | ||
| Female | 479 (48.48) | 323 (48.87) | 156 (47.71) | ||
| Gestational Age | 74.229 | <0.001 | |||
| ≥37 weeks | 879 (88.97) | 628 (95.01) | 251 (76.76) | ||
| <37 weeks | 109 (11.03) | 33 (4.99) | 76 (23.24) | ||
| Birth Weight | 1.109 | 0.292 | |||
| ≥2.5 kg | 763 (77.23) | 517 (78.21) | 246 (75.23) | ||
| <2.5 kg | 225 (22.77) | 144 (21.79) | 81 (24.77) | ||
| Residence | 0.362 | 0.547 | |||
| Rural | 470 (47.57) | 310 (46.90) | 160 (48.93) | ||
| Urban | 518 (52.43) | 351 (53.10) | 167 (51.07) | ||
| Left Behind Children | 0.300 | 0.584 | |||
| No | 700 (70.85) | 472 (71.41) | 228 (69.72) | ||
| Yes | 288 (29.15) | 189 (28.59) | 99 (30.28) | ||
| Household income | 55.494 | <0.001 | |||
| <100 thousand yuan | 393 (39.78) | 209 (31.62) | 184 (56.27) | ||
| ≥100 thousand yuan | 595 (60.22) | 452 (68.38) | 143 (43.73) | ||
| Number of children | 0.419 | 0.518 | |||
| >1 | 472 (47.77) | 311 (47.05) | 161 (49.24) | ||
| 1 | 516 (52.23) | 350 (52.95) | 166 (50.76) | ||
| Sleep Duration | 15.662 | <0.001 | |||
| ≥9 hours | 559 (56.58) | 403 (60.97) | 156 (47.71) | ||
| <9 hours | 429 (43.42) | 258 (39.03) | 171 (52.29) | ||
| Parents education level | 1.791 | 0.181 | |||
| Low level | 517 (52.33) | 336 (50.83) | 181 (55.35) | ||
| High level | 471 (47.67) | 325 (49.17) | 146 (44.65) | ||
| Bad eating habits | 4.736 | 0.030 | |||
| No | 624 (63.16) | 433 (65.51) | 191 (58.41) | ||
| Yes | 364 (36.84) | 228 (34.49) | 136 (41.59) | ||
| Calcium Deficiency | 3.879 | 0.048 | |||
| No | 834 (84.41) | 563 (85.17) | 271 (82.87) | ||
| Yes | 154 (15.59) | 98 (14.83) | 56 (17.13) | ||
| Iron Deficiency | 0.929 | 0.335 | |||
| No | 846 (85.63) | 571 (86.38) | 275 (84.10) | ||
| Yes | 142 (14.37) | 90 (13.62) | 52 (15.90) | ||
| Zinc Deficiency | 0.052 | 0.820 | |||
| No | 927 (93.83) | 621 (93.95) | 306 (93.58) | ||
| Yes | 61 (6.17) | 40 (6.05) | 21 (6.42) | ||
| Mood disorders | 96.023 | <0.001 | |||
| No | 856 (86.64) | 622 (94.10) | 234 (71.56) | ||
| Yes | 132 (13.36) | 39 (5.90) | 93 (28.44) | ||
| Milk Intake | 5.912 | 0.015 | |||
| <300 ml/d | 640 (64.78) | 411 (62.18) | 229 (70.03) | ||
| ≥300 ml/d | 348 (35.22) | 250 (37.82) | 98 (29.97) | ||
| Physical activity time | 68.398 | <0.001 | |||
| ≥2 hours/d | 511 (51.72) | 403 (60.97) | 108 (33.03) | ||
| <2 hours/d | 477 (48.28) | 258 (39.03) | 219 (66.97) |
Notes: Parental education levels: high level, have completed ≥ nine years of compulsory education; low level, have not completed nine years of compulsory education. PEM, protein-energy malnutrition.
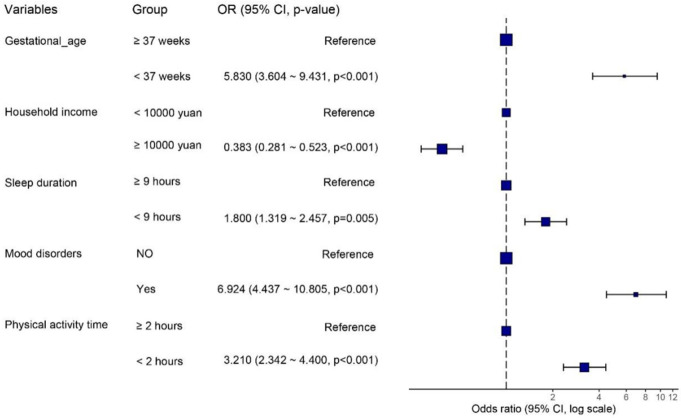
To further explore the risk factors for PEM in children, we performed univariate and multivariate logistic regression analyses of potential predictors (Supplementary Table 1). Gestational age (OR (95% CI)=5.830 (3.604-9.431), P<0.001), household income (OR (95% CI)=0.383 (0.281-0.523), P<0.001), sleep duration (OR (95% CI)=1.800 (1.319-2.457), P<0.001), mood disorders (OR (95% CI)=6.924 (4.437-10.805), P<0.001), and physical activity time (OR (95% CI)=3.210 (2.342-4.400), P<0.001) were significant and independent predictors of PEM. Mood disorders appeared to have the greatest effect on PEM (Figure 1).
Figure 1.
Forest plot of predictors of PEM in children. PEM, protein-energy malnutrition.
Construction and validation of the nomogram model
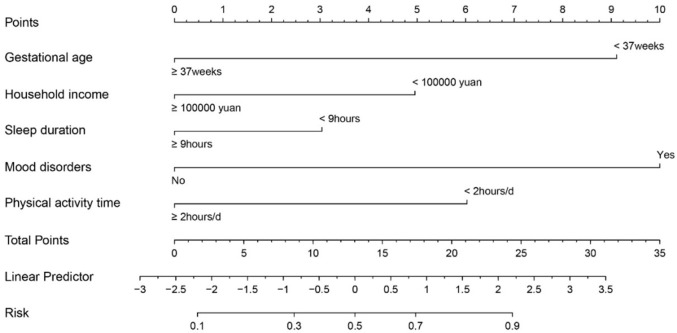
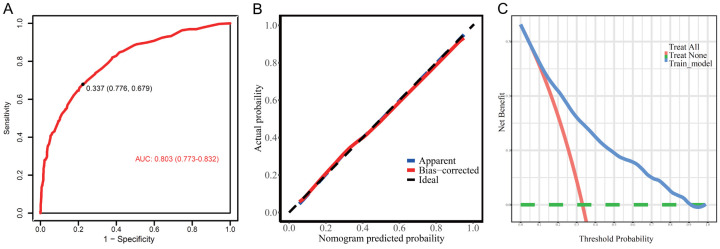
We screened independent predictors of children with PEM by multivariate logistic regression equations and constructed a risk score model based on these six predictors [Risk score =1.763 * Gestational age (<37 weeks =1, ≥37 weeks =0) + 0.959 * Household income (<100 thousand yuan per year =1, ≥100 thousand yuan per year =0) + 0.588 * Sleep Duration (<9 hours =1, ≥9 hours =0) + 1.935 * Mood disorders (Yes =1, No =0) + 1.166 * Physical activity time (<2 hours/d =1, ≥2 hours/d =0)]. A nomogram model was used to generate corresponding scores based on the weighting coefficients of the predictors. As shown in Figure 2, mood disorders had the highest score of 100, and the risk probability of PEM for each individual can be calculated by obtaining the total score from all variables. The nomogram model showed good performance in estimating the risk of PEM (AUC=0.803 (0.773-0.832)) (Figure 3A). Additionally, the calibration plot showed that the nomogram accurately predicted the risk of developing PEM in children (Figure 3B). The clinical decision curve suggested that the nomogram could benefit patients in the range of 0.1 to 0.9 probability thresholds for clinical use (Figure 3C).
Figure 2.
Nomogram predicting the occurrence of PEM.
Figure 3.
Evaluation of nomogram model in the training set. A. The ROC curve; B. The calibration curve; C. Clinical decision curve of the nomogram. PEM, protein-energy malnutrition.
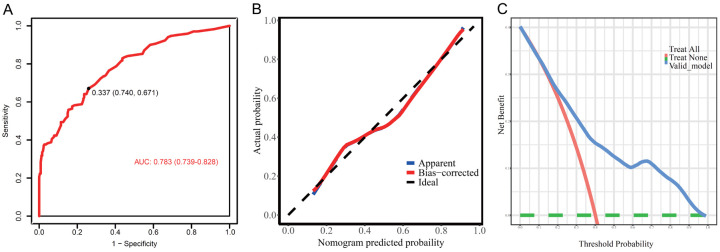
Furthermore, the nomogram model was validated using another independent dataset, and the results suggested that the model had good discrimination (AUC=0.783 (0.739-0.828)), calibration ability, and clinical applicability (Figure 4).
Figure 4.
Validation of nomogram model in the validation set. A. The ROC curve; B. The calibration curve; C. Clinical decision curve of the nomogram.
Discussion
The findings of this study contribute important insight into the risk factors and predictive modeling of PEM among children aged 8-10 years. With a significant proportion of the study population (35.2%) identified as experiencing PEM, the relevance of this research in pediatric health cannot be overstated. Our study demonstrates a strong association between PEM and several socio-demographic and health-related factors - specifically gestational age, family income, sleep duration, eating habits, calcium deficiency, mood disorders, and physical activity. Notably, mood disorders emerged as the most influential predictor of PEM, reflecting the complex interplay between mental health and nutritional status. This aligns with broader literature indicating that psychological well-being can significantly affect physical health, particularly during developmental years [18-21].
Previous studies have similarly identified risk factors for PEM in children. Preterm babies are a priority group for neonatal deaths and a high-risk group for long-term health problems, given the immaturity of their organs, which leads to poor immunity and susceptibility to diseases affecting growth and development [22-24]. Additionally, the influence of sleep on children’s growth and development lies in the fact that the pituitary gland secretes growth hormone during deep sleep. Sufficient sleep allows the body to fully enter the deep sleep state, thus promoting the release of growth hormone [25]. Sufficient sleep can also increase the metabolic rate of the body and promote the absorption and utilization of nutrients, contributing to growth and development [26,27]. Previous studies have suggested that even transient sleep problems may hinder children’s growth and development and may extend into adulthood, resulting in various complications [28]. Furthermore, exercise promotes the growth and healthy development of bones. Bones are stimulated by external forces during weight training, jumping, and impact sports, which stimulate the development of bone growth plates and increase the density and length of bones [29,30]. However, during the COVID-19 epidemic, the restriction of study to the home, and reduced travel activities reduced children’s physical activity, which was detrimental to their health.
In this study, we focused on life behaviors, growth and development indicators, and mental health aspects. Mental health problems can lead to cortisol secretion in children’s bodies, which affects the secretion of growth hormone [31,32]. Unhealthy psychological states can also lead to poor eating behaviors and sleep problems, which are detrimental to normal growth and development [31,32]. Mental health and growth and development affect each other, forming a vicious circle that is even more unfavorable to children’s physical and mental health [33,34]. This study further validates the significant influence of mental health on children’s growth and development. It suggests that proactive measures should be taken to improve this situation, such as interacting with children more often to make them feel the warmth of the family, giving them a sense of security and well-being. Additionally, reasonably and appropriately meeting the needs of children, accompanying them to face their emotional problems, and providing opportunities for them to engage in activities within their abilities are crucial.
The clinical significance of this study is multifaceted. First, it provides up-to-date epidemiological data on malnutrition among school-aged children in a specific age group (8-10 years) and geographic area (Guangzhou City), which will significantly deepen the understanding of malnutrition patterns in China. By identifying the major independent risk factors for PEM, this study provides valuable insight into which variables (e.g., gestational age, household income, sleep duration, dietary habits, emotional disorders, and physical activity) are most likely to contribute to malnutrition in this age group. Finally, our study used a predictive nomogram model that showed strong predictive power (AUC of 0.803). With this predictive model, healthcare providers can estimate a child’s risk of developing PEM based on individual characteristics, leading to earlier and more personalized interventions.
Despite its strengths, our study has some limitations. The cross-sectional design precludes causal interpretation, and self-reported data may introduce a reporting bias. Regional focus might limit the broader applicability of our findings, and the exclusion criteria for children with specific medical conditions might underestimate the true PEM risk landscape. Moreover, the SCARED tool, while validated for mood disorder assessment, may overlook mental health issues that affect nutrition. To address these limitations and build upon our understanding, future research should adopt a longitudinal approach to capture the evolution of PEM and its risk factors. Incorporating interventional strategies will further elucidate the efficacy of these preventive measures.
In conclusion, our study elucidates the independent predictors of PEM and emphasizes the consideration of both physical and mental health in prevention strategies. The validated predictive nomogram models provide healthcare providers with an important tool for the early identification of at-risk children, allowing timely interventions to avoid the detrimental effects of PEM.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Waghmare H, Chauhan S, Sharma SK. Prevalence and determinants of nutritional status among women and children in Pakistan. BMC Public Health. 2022;22:766. doi: 10.1186/s12889-022-13059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khani Jeihooni A, Mohammadkhah F, Razmjouie F, Harsini PA, Sedghi Jahromi F. Effect of educational intervention based on health belief model on mothers monitoring growth of 6-12 months child with growth disorders. BMC Pediatr. 2022;22:561. doi: 10.1186/s12887-022-03593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Yuan S, Fang H, Huang G, Huang Q, Wang H, Wang A. Prevalence and associated factors for stunting, underweight and wasting among children under 6 years of age in rural Hunan Province, China: a community-based cross-sectional study. BMC Public Health. 2022;22:483. doi: 10.1186/s12889-022-12875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi X, Zhu Y, Wang Y, He Q, Hee J, Chen W, Takesue R, Tang K. Socio-economic inequalities in children’s nutritional status in Democratic Republic of the Congo in 2017-2018: an analysis of data from a nationally representative survey. Public Health Nutr. 2022;25:257–268. doi: 10.1017/S1368980021004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jongpiputvanich S, Poomsuwan P, Phittayanon P. Prevalence and risk factors of protein energy malnutrition (PEM) in preschool children of Klong-Toey Slum, Bangkok, Thailand. J Med Assoc Thai. 1992;75:39–45. [PubMed] [Google Scholar]
- 6.Nahar B, Ahmed T, Brown KH, Hossain MI. Risk factors associated with severe underweight among young children reporting to a diarrhoea treatment facility in Bangladesh. J Health Popul Nutr. 2010;28:476–483. doi: 10.3329/jhpn.v28i5.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Reducing stunting in children: equity considerations for achieving the global nutrition targets 2025. Geneva: World Health Organization; 2018. [Google Scholar]
- 8.Zhang X, Zhang L, Pu Y, Sun M, Zhao Y, Zhang D, Wang X, Li Y, Guo D, He S. Global, regional, and national burden of protein-energy malnutrition: a systematic analysis for the global burden of disease study. Nutrients. 2022;14:2592. doi: 10.3390/nu14132592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahar B, Hossain M, Mahfuz M, Islam MM, Hossain MI, Murray-Kolb LE, Seidman JC, Ahmed T. Early childhood development and stunting: findings from the MAL-ED birth cohort study in Bangladesh. Matern Child Nutr. 2020;16:e12864. doi: 10.1111/mcn.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradhananga P, Shrestha A, Adhikari N, Shrestha N, Adhikari M, Ide N, Dhungel S, Bajracharya S, Aryal A. Double burden of malnutrition in Nepal: a trend analysis of protein-energy malnutrition and High Body Mass Index using the data from Global Burden of Disease 2010-2019. PLoS One. 2022;17:e0273485. doi: 10.1371/journal.pone.0273485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimmer R, Audétat A, Binggeli J, Schuetz P, Kaegi-Braun N. Association of sociodemographic, socioeconomic and lifestyle characteristics with low protein and energy intake in the healthy Swiss population. Nutrients. 2023;15:2200. doi: 10.3390/nu15092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fivian E, Harris-Fry H, Offner C, Zaman M, Shankar B, Allen E, Kadiyala S. The extent, range, and nature of quantitative nutrition research engaging with intersectional inequalities: a systematic scoping review. Adv Nutr. 2024;15:100237. doi: 10.1016/j.advnut.2024.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amoadu M, Abraham SA, Adams AK, Akoto-Buabeng W, Obeng P, Hagan JE Jr. Risk factors of malnutrition among in-school children and adolescents in developing countries: a scoping review. Children (Basel) 2024;11:476. doi: 10.3390/children11040476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolstenholme H, Kelly C, Heary C. ‘Fussy eating’ and feeding dynamics: school children’s perceptions, experiences, and strategies. Appetite. 2022;173:106000. doi: 10.1016/j.appet.2022.106000. [DOI] [PubMed] [Google Scholar]
- 15.Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Rhodes CJ, Valko M. Essential metals in health and disease. Chem Biol Interact. 2022;367:110173. doi: 10.1016/j.cbi.2022.110173. [DOI] [PubMed] [Google Scholar]
- 16.Rappaport BI, Pagliaccio D, Pine DS, Klein DN, Jarcho JM. Discriminant validity, diagnostic utility, and parent-child agreement on the Screen for Child Anxiety Related Emotional Disorders (SCARED) in treatment- and non-treatment-seeking youth. J Anxiety Disord. 2017;51:22–31. doi: 10.1016/j.janxdis.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tontisirin K, Valyasevi A. Protein energy malnutrition related to diarrhea in Thai children. J Nutr Sci Vitaminol (Tokyo) 1981;27:513–520. doi: 10.3177/jnsv.27.513. [DOI] [PubMed] [Google Scholar]
- 19.Bosch-Bayard J, Razzaq FA, Lopez-Naranjo C, Wang Y, Li M, Galan-Garcia L, Calzada-Reyes A, Virues-Alba T, Rabinowitz AG, Suarez-Murias C, Guo Y, Sanchez-Castillo M, Roger K, Gallagher A, Prichep L, Anderson SG, Michel CM, Evans AC, Bringas-Vega ML, Galler JR, Valdes-Sosa PA. Early protein energy malnutrition impacts life-long developmental trajectories of the sources of EEG rhythmic activity. Neuroimage. 2022;254:119144. doi: 10.1016/j.neuroimage.2022.119144. [DOI] [PubMed] [Google Scholar]
- 20.Mata LJ. Child malnutrition and deprivation--observations in Guatemala and Costa Rica. Food Nutr (Roma) 1980;6:7–14. [PubMed] [Google Scholar]
- 21.Galler JR, Bryce CP, Zichlin ML, Waber DP, Exner N, Fitzmaurice GM, Costa PT. Malnutrition in the first year of life and personality at age 40. J Child Psychol Psychiatry. 2013;54:911–919. doi: 10.1111/jcpp.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbacheva IV, Kuznetsova OU, Gilmiyarova FN, Pechkurov DV, Vinogradova LN. Characteristics of the metabolic status of children of the first year of life with protein-energy deficiency depending on the gestational age at birth. Klin Lab Diagn. 2020;65:405–410. doi: 10.18821/0869-2084-2020-65-7-405-410. [DOI] [PubMed] [Google Scholar]
- 23.Grantham-McGregor SM, Walker SP, Chang S. Nutritional deficiencies and later behavioural development. Proc Nutr Soc. 2000;59:47–54. doi: 10.1017/s0029665100000069. [DOI] [PubMed] [Google Scholar]
- 24.Roy P, Goel MK, Rasania SK. Designing new growth charts for low-birth weight babies: need of the hour in India. Indian J Public Health. 2014;58:110–112. doi: 10.4103/0019-557X.132286. [DOI] [PubMed] [Google Scholar]
- 25.Zaffanello M, Pietrobelli A, Cavarzere P, Guzzo A, Antoniazzi F. Complex relationship between growth hormone and sleep in children: insights, discrepancies, and implications. Front Endocrinol (Lausanne) 2024;14:1332114. doi: 10.3389/fendo.2023.1332114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medise BE, Julia M, Devaera Y, Sitaresmi MN, Asmarinah, Widjaja NA, Kalalo RT, Soesanti F, Friska D, Sirait WR, Azzopardi P, Sawyer S. Understanding the pubertal, psychosocial, and cognitive developmental trajectories of stunted and non-stunted adolescents: protocol of a multi-site Indonesian cohort study. Front Pediatr. 2024;12:1296128. doi: 10.3389/fped.2024.1296128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abuhammad S, Bani Younis A, Ahmed AH. Impact of a structured sleep education program on mothers’ knowledge and attitudes toward infant sleeping. Heliyon. 2024;10:e29885. doi: 10.1016/j.heliyon.2024.e29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensson L, Unossons M, Ek AC. Measurement of perceived health problems as a means of detecting elderly people at risk of malnutrition. J Nutr Health Aging. 2003;7:257–262. [PubMed] [Google Scholar]
- 29.Radó SI, Molnár M, Széll R, Szőllősi GJ, Törő V, Shehab B, Manios Y, Anastasiou C, Iotova V, Tsochev K, Chakarova N, Giménez-Legarre N, Miguel Berges ML, Schwarz PEH, Rurik I, Sárváry A. Association between screen time and sociodemographic factors, physical activity, and BMI among children in six European countries (Feel4Diabetes): a cross-sectional study. Children (Basel) 2024;11:458. doi: 10.3390/children11040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiraki M, Nishiguchi S, Saito M, Fukuzawa Y, Mizuta T, Kaibori M, Hanai T, Nishimura K, Shimizu M, Tsurumi H, Moriwaki H. Nutritional status and quality of life in current patients with liver cirrhosis as assessed in 2007-2011. Hepatol Res. 2013;43:106–112. doi: 10.1111/hepr.12004. [DOI] [PubMed] [Google Scholar]
- 31.De Bolle M, De Clercq B, Decuyper M, De Fruyt F. Affective determinants of anxiety and depression development in children and adolescents: an individual growth curve analysis. Child Psychiatry Hum Dev. 2011;42:694–711. doi: 10.1007/s10578-011-0241-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q. Fluctuations in maternal depressive symptoms, anxiety, and anger and children’s depression risks in middle childhood. Res Child Adolesc Psychopathol. 2024;52:1247–1260. doi: 10.1007/s10802-024-01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verrest L, Wilthagen EA, Beijnen JH, Huitema ADR, Dorlo TPC. Influence of malnutrition on the pharmacokinetics of drugs used in the treatment of poverty-related diseases: a systematic review. Clin Pharmacokinet. 2021;60:1149–1169. doi: 10.1007/s40262-021-01031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanmodi KK, Amzat J, Aminu K. Theories, determinants, and intervention models and approaches on inequalities of undernutrition amongst under fives: a literature review. Health Sci Rep. 2024;7:e2078. doi: 10.1002/hsr2.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.