Abstract
Background: Metastatic spread to the pancreas from renal cell carcinoma is a relatively rare event. Even rarer is the metachronous occurrence of clear cell renal cell carcinoma (ccRCC) and pancreatic ductal adenocarcinoma (PDAC). Our case report contributes to the existing literature by documenting the unusual occurrence of metachronous ccRCC and PDAC in a 69-year-old patient, and we review the literature.
Keywords: Metachronous primary carcinoma, clear cell renal cell carcinoma, pancreatic ductal adenocarcinoma, case report
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of RCC, accounting for approximately 70% of cases. Although ccRCC can often be detected early and effectively managed with surgical or ablative treatment, a proportion of patients will present with or develop metastases. The incidence of metastasis varies but is estimated to occur in up to one-third of ccRCC cases [1].
Pancreatic ductal adenocarcinoma (PDAC), is the most common form of pancreatic neoplasm, representing approximately 85% of all pancreatic tumors. Pancreatic cancer metastases are generally rare, with primary sources typically consisting of lung cancer and gastrointestinal cancer. Metastasis of RCC to the pancreas is even rarer, accounting for approximately 5% of all pancreatic metastases [2].
Our case report contributes to the existing literature by documenting the unusual occurrence of metachronous ccRCC and PDAC in a 69-year-old patient. We also review the literature data.
Case presentation
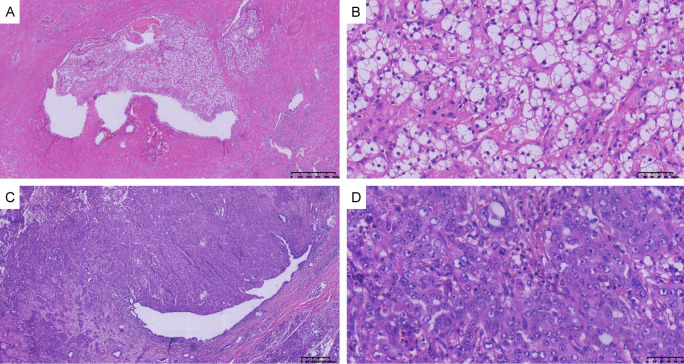
A 66-year-old male Chinese citizen was admitted to our hospital on December 24, 2019 due to the discovery of a left kidney mass during a physical examination. The patient had a medical history of liver cirrhosis and hypersplenism, as well as a previous splenectomy. Notably, there was no family history of cancer. Abdominal computed tomography (CT) revealed a left renal cystic lesion with septa, categorized as Bosniak grade III (Figure 1A). To obtain more information, a renal magnetic resonance imaging (MRI) examination was recommended and conducted 4 days later, which confirmed the presence of multilocular cystic lesions in the left kidney, also classified as Bosniak grade III (Figure 1B). Following the exclusion of surgical contraindications and under general anesthesia, a laparoscopic left partial nephrectomy was performed on December 30, 2019, resulting in a successful surgery. A postoperative pathologic examination identified ccRCC with cystic transformation, measuring 2.5×2.3×2.3 cm (Figure 2). The tumor stage was determined to be pT1N0M0, indicating localized disease without lymph node involvement or distant metastases. Immunohistochemical results showed positive expression of vimentin, CD10, epithelial membrane antigen (EMA), and P504s, 10% positive expression of Ki-67, and negative expression of CK7. Following discharge, the patient underwent regular outpatient follow-up visits for 2 years.
Figure 1.
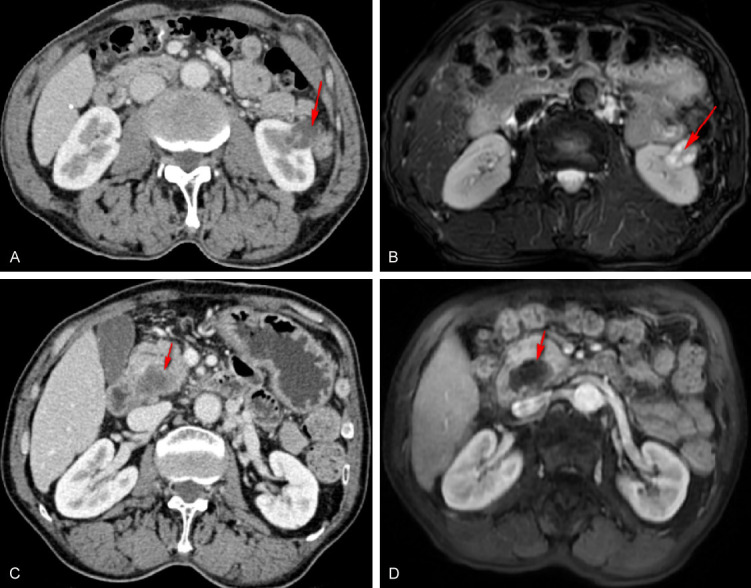
Left kidney tumor and pancreatic head tumor on abdominal CT and MRI. A: Left kidney tumor on abdominal CT; B: Left kidney tumor on abdominal MRI; C: Pancreatic head tumor on abdominal CT. D: Pancreatic head tumor on abdominal MRI. The red arrow indicates the location of the tumor.
Figure 2.
Histopathologic findings of postoperative pathology. A: Clear cell renal cell carcinoma (×40). B: Clear cell renal cell carcinoma (×400). C: Adenocarcinoma of the pancreatic head (×40). D: Adenocarcinoma of the pancreatic head (×400).
Thirty-three months later, on September 23, 2023, the patient was readmitted due to upper abdominal pain that had persisted for 1 week. Routine laboratory tests showed an elevation in the CA199 level (Table 1), which can be a marker of pancreatic and other gastrointestinal cancers. A CT scan of the abdomen revealed the presence of a cystic and solid mass in the head of the pancreas (Figure 1C). To further evaluate the pancreatic mass, a pancreatic MRI was performed (Figure 1D). The results showed a cystic and solid mass in the head of the pancreas along with dilation of the main pancreatic duct, indicating the possibility of pancreatic cancer. Considering the findings, the contraindication for surgery was removed, and on October 3, 2023, a radical pancreaticoduodenectomy was successfully performed while the patient was under general anesthesia.
Table 1.
Laboratory data of this patient during two hospitalizations
| Variable | Reference Range | 1st (kidney) | 2nd (pancreas) |
|---|---|---|---|
| White-cell count | (3.5-9.5)×109/L | 5.81 | 7.70 |
| Red-cell count | (4.3-5.8)×1012/L | 4.78 | 4.68 |
| Platelet count | (125-350)×109/L | 210 | 227 |
| Albumin | (40-55) g/L | 34.4 | 43.9 |
| Hemoglobin | (130-175) g/L | 146 | 140 |
| Carcinoembryonic antigen | (0-5) ng/ml | 5.69 | 4.46 |
| Alpha-fetoprotein | (0-20) ng/ml | 2.56 | 2.72 |
| Carbohydrate antigen 199 | (0-37) U/ml | 2.00 | 147.18 |
| Total bilirubin | (0-23) μmol/L | 16.8 | 13.6 |
| Direct bilirubin | (0-8.6) μmol/L | 6.3 | 5.9 |
| Alanine aminotransferase | (9-50) U/L | 14 | 15 |
Serum carbohydrate antigen 199 levels in pancreas cancer are significantly higher than normal; no obvious abnormalities were observed on other indicators.
The postoperative pathological findings revealed poorly differentiated adenocarcinoma of the pancreas head with infiltration into the duodenal mucosa (3.0×3.0 cm), and positive neural invasion (Figure 2). The pathological tumor stage was pT2N0M0. The postoperative pathological examination indicated the presence of poorly differentiated adenocarcinoma in the head of the pancreas, involving infiltration into the duodenal mucosa. The size of the tumor was measured at 3.0×3.0 cm. Additionally, the report mentioned positive neural invasion, suggesting the spread of cancer cells into the nerves. The pathological staging of the tumor was pT2N0M0, indicating that it was localized within the pancreas and had not spread to nearby lymph nodes or distant sites. Immunohistochemical results showed positive expression of CK7, P53, MUC1, CD10, CK19 and CA199, 50% positive expression of Ki-67, and negative expression of DPC4, MUC5, MUC2, and CDX-2. Three weeks later, the patient was discharged and scheduled for chemotherapy.
Discussion
Multiple primary cancers (MPCs) are defined as the occurrence of two or more tumors with different histologic features and originating from different body parts in a single individual [3,4]. MPCs can be classified into two main types: synchronous and metachronous. Synchronous MPCs refer to the development of multiple tumors either simultaneously or within 6 months of each other, whereas metachronous MPCs develop more than 6 months after the detection of the first primary cancer. The occurrence of MPCs is a complex phenomenon that may result from various factors, including genetic predisposition, environmental exposure, lifestyle behavior, and treatment-related factors [5].
Secondary tumors in the pancreas are often clinically inapparent. In a study of surgical specimens, the majority of resected pancreatic tumors were primary pancreatic tumors, such as pancreatic ductal adenocarcinoma. Only a small percentage, 3.9% of pancreatic surgical specimens showed tumors originating from outside the pancreas, indicating secondary involvement. The study also revealed that among the secondary tumors involving the pancreas, lymphomas accounted for 29% of cases, while carcinomas of the stomach and kidney accounted for 18% and 16% respectively [2]. This finding suggests that these sites of origin are relatively common sources of metastases to the pancreas. Furthermore, a systematic literature review identified RCC metastases as the most frequent type among patients who underwent pancreatectomy for metastatic tumors. RCC accounted for 63% of cases in this particular study [6]. Hence, it is important to recognize and distinguish between primary pancreatic tumors and secondary tumors in clinical practice. Careful evaluation and investigation are necessary to determine the origin of tumors involving the pancreas to guide appropriate treatment strategies.
However, synchronous or metachronous tumors involving both the kidney and pancreas are rare, and there have been limited reports of such cases. The first reported case of an association between renal and pancreatic cancer dates back to 1969, and since then, only a few case reports have been published through large-scale data research [7]. In a combined analysis of patients with pancreatic tumors and RCC, 12 patients were identified to have both renal cell cancer and primary pancreatic cancer [8]. Another analysis focusing solely on renal cell cancer patients found that out of 4,176 individuals, 219 developed a second primary malignancy [9]. However, the association between pancreatic cancer and renal cell cancer was determined not to be significant, as there were only 6 cases of pancreatic cancer identified. Furthermore, among individuals who underwent surgery for renal cancer, 209 developed a second primary malignant tumor. Although there was a significantly increased incidence of subsequent bladder and prostate cancer in patients with papillary RCC, no significant association was observed with pancreatic cancer.
The occurrence of simultaneous or subsequent tumors involving both ccRCC and PDAC is infrequent. A total of 9 patients, including the case we described, are identified in Table 2. The patients ranged in age from 67 to 78 years (median 71.5 years) and had a male predominance with six men and three women. In these patients, four metachronous and five synchronous double tumor patients were discovered. In 2003, Alexakis et al. [10] reported two cases involving ccRCC and PDAC. One patient demonstrated synchronous tumors, while the other patient presented with metachronous tumors. The patients underwent resection for both cancer types with a favorable outcome. Olgyai et al. [11] reported a case in 2004 involving synchronous primary PDAC and ccRCC. The patient underwent surgeries to remove both tumors and received subsequent oncologic therapy. A combined analysis conducted by Müller in 2012 identified three patients with ccRCC and synchronous or metachronous PDAC [8]. The time period of this analysis spanned from 2001 to 2008. All patients underwent nephrectomy and pancreaticoduodenectomy. In 2017, Tarik Mahfoud reported a case of synchronous primary cancers involving ccRCC and PDAC [12]. Unfortunately, the patient’s prognosis was poor, and they died 13 months after diagnosis due to disease progression. The treatment approach included chemotherapy for PDAC and tyrosine kinase inhibitors for ccRCC. In 2021, a patient who had a undergone left nephrectomy for ccRCC 20 years prior was diagnosed with PDAC [13]. The individual received neoadjuvant therapy for borderline-resectable PDAC, followed by total pancreatectomy. A postoperative pathologic examination revealed both PDAC and two metachronous metastases of ccRCC in the pancreatic body.
Table 2.
Overview of literature on concurrent pancreatic ductal adenocarcinoma (PDAC) and renal cell carcinoma (RCC)
| Age | Ref | Age | Sex | Localization of ccRCC | Procedure for ccRCC | Localization of PDAC | Procedure for PDAC | Timing (months) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 67 | [10] | 67 [10] | M | Left kidney | Nephrectomy | Pancreas head | Whipple | Synchronous | 15, dead |
| 76 | [10] | 76 [10] | M | Right kidney | Nephrectomy | Pancreas head | Whipple | Metachronous (9) | 6, alive |
| 70 | [8] | 70 [8] | M | Unknown | Partial neph. | Proximal pancreas | Whipple | Synchronous | 35, alive |
| 72 | [8] | 72 [8] | W | Unknown | Nephrectomy | Proximal pancreas | Whipple | Metachronous (94) | 14, dead |
| 70 | [8] | 70 [8] | M | Unknown | Partial neph. | Proximal pancreas | Whipple | Synchronous | 5.25, dead |
| 70 | [12] | 70 [12] | W | Right kidney | Chemotherapy | Pancreas body | Chemotherapy | Synchronous | 13, dead |
| 78 | [13] | 78 [13] | M | Left kidney | Nephrectomy | Pancreas head | Whipple | Metachronous (480) | Perioperative, death |
| 72 | [11] | 72 [11] | W | Unknown | Nephrectomy | Distal pancreas | Distal pancreatectomy | Synchronous | 6, alive |
| 69 | 69 | M | Left kidney | Partial neph. | Pancreas head | Whipple | Metachronous (33) | Present case |
In our report, the patient underwent a right nephrectomy for ccRCC, and 33 months later, was diagnosed with a second primary PDAC and underwent pancreaticoduodenectomy. The exact mechanism underlying the association between MPCs, such as ccRCC and PDAC, is not yet fully understood. However, research has suggested that genetic factors may play a role. For example, microsatellite instability is more common in MPCs than in sporadic cancers [14]. In some cases, multiple primary tumors may arise due to an inherited genetic predisposition. Von Hippel-Lindau (VHL) disease is an example of a familial multiple-cancer syndrome characterized by a large number of cystic and solid neoplasms. This condition is caused by mutations in the VHL gene and is associated with an increased risk of developing ccRCC [15]. It is difficult to draw general conclusions based on a single case report, as the relationship between ccRCC and PDAC has been documented only rarely in the literature. Nevertheless, given the complexities involved in managing MPCs, including the possibility of genetic factors, it is essential for healthcare professionals to evaluate each case on an individual basis and develop a personalized treatment plan.
Conclusion
Our case of metachronous primary ccRCC and PDAC is extremely rare. Awareness and interdisciplinary cooperation in the management of MPCs, such as ccRCC and PDAC, are important. Surgery is often the primary treatment method for small tumors associated with ccRCC and PDAC. However, the optimal management approach for MPCs may vary depending on factors such as tumor stage, resectability, and individual patient characteristics. Additional epidemiologic details and molecular investigation are required to understand the association between MPCs.
Disclosure of conflict of interest
None.
References
- 1.Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17:245–261. doi: 10.1038/s41581-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444:527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 3.Rovatti M, Gerosa E, Turi V, D’Abrosca F, De Cesare F. Multiple primary malignant neoplasms. Minerva Chir. 1995;50:949–958. [PubMed] [Google Scholar]
- 4.Kojima S, Sakamoto T, Nagai Y, Honda M, Ogawa F. Metachronous rectal metastasis from primary transverse colon cancer: a case report. Surg Case Rep. 2018;4:90. doi: 10.1186/s40792-018-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 6.Adler H, Redmond CE, Heneghan HM, Swan N, Maguire D, Traynor O, Hoti E, Geoghegan JG, Conlon KC. Pancreatectomy for metastatic disease: a systematic review. Eur J Surg Oncol. 2014;40:379–386. doi: 10.1016/j.ejso.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki E, Kushida S, Okinaka T, Sasaki K, Abe S. Case report of double cancer of the pancreas and the kidney with polyposis of the large intestine. Gan No Rinsho. 1969;15:203–206. [PubMed] [Google Scholar]
- 8.Müller SA, Pahernik S, Hinz U, Martin DJ, Wente MN, Hackert T, Leowardi C, Haferkamp A, Büchler MW, Schmied BM. Renal tumors and second primary pancreatic tumors: a relationship with clinical impact? Patient Saf Surg. 2012;6:18. doi: 10.1186/1754-9493-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor AF, McLaughlin JK, Curtis RE, Flannery JT, Fraumeni JF Jr. Risk of second malignancy after cancers of the renal parenchyma, renal pelvis, and ureter. Cancer. 1986;58:1158–1161. doi: 10.1002/1097-0142(19860901)58:5<1158::aid-cncr2820580530>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Alexakis N, Bosonnet L, Connor S, Ellis I, Sutton R, Campbell F, Hughes M, Garvey C, Neoptolemos JP. Double resection for patients with pancreatic cancer and a second primary renal cell cancer. Dig Surg. 2003;20:428–432. doi: 10.1159/000072711. [DOI] [PubMed] [Google Scholar]
- 11.Olgyai G, Haulik L, Oláh A. Double resection for synchronous pancreatic and renal cell cancer--case report. Magy Seb. 2004;57:287–289. [PubMed] [Google Scholar]
- 12.Mahfoud T, Tanz R, Khmamouche MR, Allaoui M, Belbaraka R, Khouchani M, Ichou M. Synchronous primary renal cell carcinoma and pancreatic ductal adenocarcinoma: case report and literature review. Case Rep Oncol. 2017;10:1050–1056. doi: 10.1159/000484552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeberle L, Busch M, Kirchner J, Fluegen G, Antoch G, Knoefel WT, Esposito I. Pancreatic ductal adenocarcinoma concomitant with pancreatic metastases of clear-cell renal cell carcinoma: a case report. J Med Case Rep. 2021;15:314. doi: 10.1186/s13256-021-02768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohtani H, Yashiro M, Onoda N, Nishioka N, Kato Y, Yamamoto S, Fukushima S, Hirakawa-Ys Chung K. Synchronous multiple primary gastrointestinal cancer exhibits frequent microsatellite instability. Int J Cancer. 2000;86:678–683. doi: 10.1002/(sici)1097-0215(20000601)86:5<678::aid-ijc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O’Toole D, Bernades P, Ruszniewski P, Richard S. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]