Abstract
Objective: To investigate the protective effect of Peimine (PM) against lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice and the underlying mechanisms. Methods: KM mice were randomly divided into five groups: Control, LPS, Peimine low-dose (PM-L, 0.1 mg/kg), medium-dose (PM-M, 1 mg/kg), and high-dose (PM-H, 10 mg/kg) groups. Mice in the PM treatment groups received intraperitoneal injection of Peimine at different doses, while the mice in control and LPS groups received physiological saline. Afterwards, mice in the LPS and PM groups were subjected to intranasal instillation of LPS to establish the model of acute lung injury. The wet-to-dry (W/D) weight ratio of lung tissues was calculated, and H&E staining was performed to observe pathological changes in the lung tissues. Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), and MDA were measured using ELISA kits. Western blot was employed to assess the expression of NF-κB, IκBα, phospho-IκBα, Nrf2, HO-1, and SOD2 in lung tissues. RT-PCR quantified the mRNA levels of Nrf2 and its downstream genes HO-1 and NQO1. Additionally, RAW264.7 cells were treated with various drug concentrations for 24 hours followed by LPS exposure (100 ng/mL) for another 24 hours. Prior to treatment of RAW264.7 cells with PM and LPS, the ML385+PM group was pre-treated with ML385 (3 μM) for 4 hours. ELISA kits were used to measure TNF-α, IL-6, IL-1β, and MDA in cell supernatants, while ROS levels were determined using a ROS assay kit. Results: Compared with the model group, pretreatment with PM significantly reduced the lung tissue W/D weight ratio, ameliorated lung tissue pathological changes, and inhibited the secretion of TNF-α, IL-6, and IL-1β in bronchoalveolar lavage fluid. PM inhibited the LPS-induced elevation in lung tissue MDA levels, SOD2 consumption, and ROS levels. Furthermore, PM suppressed LPS-induced NF-κB activation and nuclear translocation, while significantly enhancing the protein expression of Nrf2 and HO-1 and increasing the mRNA levels of Nrf2 and its downstream genes, such as HO-1 and NQO1. In RAW264.7 cells, LPS induction led to elevated IL-1β, IL-6, TNF-α, MDA, and ROS levels, which were significantly suppressed by PM treatment. However, the antioxidative and anti-inflammatory effects of PM were effectively blocked by inhibiting the Nrf2 pathway. Conclusion: PM effectively ameliorates LPS-induced lung injury, primarily through inhibition of the NF-κB pathway, and activation of the Nrf2 pathway, alongside a reduction in the release of inflammatory factors.
Keywords: Acute lung injury, Peimine, NF-κB, Nrf2
Introduction
Acute lung injury (ALI), a common complication in patients with sepsis, is characterized by damaged alveolar epithelial and endothelial cells, infiltration of inflammatory cells, and the appearance of congestion and edema. ALI is a critical condition frequently encountered in clinical practice, noted for its sudden onset and high mortality rate [1,2]. Currently, western medical treatment primarily focuses on addressing the underlying disease and providing respiratory support. However, there is a notable lack of specific pharmaceuticals and therapeutic techniques that consistently yield optimal outcomes. In addition, commonly used treatments like antibiotics and glucocorticoids have significant adverse reactions and potential dependency [3], making them unsuitable for long-term use. Therefore, it is meaningful to delve into the mechanisms underlying ALI and identify novel therapeutic agents for its prevention and treatment.
Despite extensive research on ALI, the underlying molecular mechanisms remain poorly understood. Inflammation/anti-inflammation and oxidation/antioxidation imbalances play crucial roles in the pathogenesis of ALI [4,5]. NF-κB is a vital molecule in the inflammatory process, essential for the optimal transcription and production of several crucial pro-inflammatory cytokines, which are activated by lipopolysaccharide (LPS) via the NF-κB pathway in the early stages of ALI [6,7]. Simultaneously, activation of the Nrf2 pathway, which regulates antioxidant defenses, is regarded as a pivotal factor in mitigating the inflammatory damage induced by LPS. It has been reported that Nrf2 can inhibit NF-κB pathway, thus effectively reducing the adverse reactions of excessive NF-κB activation under LPS exposure [8].
Fritillaria, a perennial herb in the lily family, is traditionally used in Chinese medicine for treating lung inflammation. It is known for its properties in clearing heat, resolving phlegm, and detoxification [9,10]. Peimine (PM), a major alkaloid component found in Fritillaria, has demonstrated protective effects in various disease models, including cerebral ischemia-reperfusion injury, liver cancer, and arthritis [11-14]. While previous research has shown that PM exerts a protective effect on ALI [15-17], its potential to modulate the inflammatory response and ALI induced by LPS through the Nrf2 and NF-κB pathways remains to be fully explored. LPS, the main toxic component of most foodborne pathogens such as Escherichia coli, is widely used to induce ALI caused by bacterial infections [18]. Upon pathogen detection, the innate immune system in the lungs is activated. RAW264.7 cells, as the most abundant innate immune cells in the distal lung, initiate a robust immune response by recognizing pathogens through cell membrane receptors. This activation leads to the production of reactive oxygen species (ROS), polarization of RAW264.7 cells towards the M1 phenotype, and secretion of pro-inflammatory mediators, thereby playing a critical role in regulating the body’s immune system [19,20]. This study aims to establish a RAW264.7 cell injury model and a mouse model of LPS-induced ALI. The primary objective is to explore the effects and mechanisms of PM on LPS-induced ALI in mice, focusing on its regulatory impacts on the Nrf2 and NF-κB pathways.
Materials and methods
Animals
Fifty SPF-grade Kunming (KM) male mice, weighing between 18 and 22 grams, were procured from Sibeifu (Beijing) Biotechnology Co., Ltd., under the license number SCXK (Beijing) 2019-0010. The mice were acclimatized to the new environment for one week at a controlled temperature of 25±2°C. All experimental protocols were approved by the Animal Experiment Committee of Wenzhou University (WZU2022-113), adhering to ethical guidelines described in the committee’s guidelines for the care and use of laboratory animals.
Cell culture
The mouse macrophage cell line RAW264.7 (Catalog No. CL-0190) was obtained from Procell (Wuhan, China). The cell line was verified via STR identification. Cells were cultured in supplemented DMEM and maintained in a 37°C incubator with 5% CO2. Passaging was performed based on cell growth status.
Chemicals and reagents
Peimine (PM, No. 23496-41-5) and lipopolysaccharide (LPS, No. L381866) were purchased from Aladdin (Shanghai, China). DMEM medium (No. A4192002) and fetal bovine serum (No. 10099) were ordered from Gibco (Grand Island, NY, USA). The 1% penicillin-streptomycin mixture (No. Y0001615) and ML385 (Nrf2 inhibitor, No. 846557-71-9) were sourced from Sigma-Aldrich (St. Louis, MO, USA). Universal tissue fixative (No. HJ193604) was purchased from Servicebio (Wuhan, China). The BCA protein quantification assay kit (No. 042820200917) and the reactive oxygen species detection assay kit (No. S0033M) were ordered from Beyotime (Shanghai, China). The mouse ELISA kits for determining TNF-α (No. H052-1-2), IL-6 (No. H007-1-2), and IL-1β (No. H002-1-2) levels, the MDA detection assay kit (No. A003-1-2), and the BCA protein detection assay kit (No. A045-4-2) were sourced from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). All antibodies used in this experiment, including TNF-α (No. ab183218), IL-6 (No. ab303458), IL-1β (No. ab315084), HMGB1 (No. ab18256), SOD2 (No. ab68155), NF-κB p65 (No. ab207297), IκBα (No. ab32518), p-IκBα (No. ab133462), Nrf2 (No. ab62352), HO-1 (No. ab305290), Lamin B (No. ab232731), and GAPDH (No. ab8245), were purchased from Abcam (Cambridge, MA, USA). The ECL luminescence detection kit (No. 1606902) was ordered from Merck Millipore (Billerica, MA, USA). The Trizol reagent (No. 15596018) and SuperScript III reverse transcriptase (No. 18080844) were sourced from Invitrogen (Carlsbad, CA, USA). The fluorescence quantitative PCR kit (No. 218073) was purchased from QIAGEN (Duesseldorf, Germany).
Establishment of animal models and drug administration
The KM mice were randomly divided into five groups: a normal control group, an LPS group, and three PM groups - PM-L (0.1 mg/kg), PM-M (1 mg/kg), PM-H (10 mg/kg) [17]. Drugs were administered by intraperitoneal injection 24 hours ahead of LPS exposure at a volume of 10 mL/kg. The control and LPS groups received an equivalent volume of physiological saline. An additional dose of the respective treatments was administered one hour prior to the experimental induction. Isoflurane gas anesthesia was then performed using a small animal anesthesia machine one hour after the drug administration. To induce the acute lung injury (ALI) model, animals were administered with LPS solution (20 μg dissolved in 50 μL of PBS) [17,21], while the control group received nasal instillation with PBS. The successful establishment of the ALI model was verified by measuring the lung wet-to-dry (W/D) weight ratio and assessing the degree of lung injury in the model group through H&E staining. At 12 hours post-LPS instillation, the animals were euthanized via an intraperitoneal injection of 1.0% (w/v) sodium pentobarbital at a dose of 50 mg/kg. Following euthanasia, the trachea was exposed for intubation, and 1.5 mL of pre-cooled PBS was injected into the lungs in three doses. The bronchoalveolar lavage fluid (BALF) was collected and placed on ice, with a target recovery rate of 80%.
H&E staining
First, tissue sections were deparaffinized using xylene, then rehydrated through a series of gradient alcohols (100%, 95%, 90%, 80%, and 70%), and finally rinsed in water. The slides were then stained with hematoxylin for 10 minutes, followed by differentiation with 2% hydrochloric acid for 10 seconds. The sections were counterstained with eosin for 10 seconds, and subsequently dehydrated, transparent, and sealed. The stained sections were subsequently examined under a microscope.
The content of MDA in lung homogenate
The MDA levels in lung tissue were evaluated using an MDA detection assay kit, with spectrophotometric measurements taken at 532 nm.
CCK-8 assay
Log-phase Raw264.7 cells were seeded into a 96-well plate and allowed to adhere. The control and model groups were refreshed with serum-containing medium, while the PM group received medium supplemented with various concentrations of PM ranging from 0 to 25 μM [22]. After a 24-hour treatment period, with 6 replicate wells up for each condition, the plate was incubated in a cell culture incubator for 1 to 4 hours. Absorbance at 450 nm was then measured using a Varioskan LUX plate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cell viability was calculated using the following formula: Viability (%) = (ODtreatment - ODblank)/(ODcontrol - ODblank) × 100%.
Intracellular ROS measurement
Log-phase Raw264.7 cells were treated with different drugs according to their respective groups and incubated for 24 hours. After this period, LPS (100 ng/mL) was added and incubated for another 24 hours [23]. The ML385+PM group was pre-treated with ML385 (3 μM) for 4 hours prior to treatment of PM and LPS [24]. Subsequently, a minimum of 1 mL of DCFH-DA was added to each well. The cells were then observed under a fluorescence microscope, capturing images from three randomly selected fields to assess the intracellular ROS levels.
Measurement of cytokine levels
Log-phase Raw264.7 cells were seeded at a density of 6 × 10^4 cells/well. Following this, cells were treated with different drugs according to various group assignments and incubated for 24 hours. LPS (100 ng/mL) was subsequently added and incubated for another 24 hours. The ML385+PM group underwent pre-treated with ML385 (3 μM) for 4 hours before the addition with PM and LPS. The supernatant was collected from the culture medium for analysis. Following the manufacturer’s instructions, the levels of the cytokines TNF-α, IL-1β, and IL-6 in both BALF and the supernatant from the cells were measured by using ELISA kits.
Western blotting
Approximately 100 mg of lung tissue was resuspended in RIPA buffer and incubated for 30 minutes for lysis, followed by centrifugation of the lysate. Equal amounts of protein (30 μg per well) were loaded onto an SDS-PAGE gel and subjected to electrophoresis at 120 V. Proteins were then transferred onto a PVDF membrane at 220 mA for 1 hour, and the membrane was blocked with 5% skim milk. It was then incubated overnight at 4°C with primary antibodies at appropriate dilutions, washed twice with TBST, and incubated with the secondary antibodies. Protein bands were visualized using an ECL detection kit.
RNA extraction and RT-PCR
Total RNA was extracted from lung tissue using TRIZOL, followed by a DNaseI treatment. The RNA was converted into cDNA utilizing a reverse transcription kit. The primers used for PCR amplification are listed in Table 1 of this study.
Table 1.
Primers for RT-PCR
| Gene | Primer sequence (5’-3’) |
|---|---|
| Nrf2 | F: GGTATTTGACTTCAGTCAA |
| R: ATGTCCACCAGGGTCTCAAT | |
| HO-1 | F: CGTAAATGACTTCAGTCAA |
| R: CGCAGAGACTAGTACAGTT | |
| NQO1 | F: CATGGCGGTCAGAAAAGCAC |
| R: ATGGCATACAGGTCCGACAC | |
| Gapdh | F: TGTGTCCGTCGTGGATCTGA |
| R: TTGCTGTTGAAGTCGCAGGAG |
Data analysis
Statistical results are presented as mean ± standard deviation (x±s). Analysis of variance (ANOVA) was used to compare differences across multiple groups, while the t-test was applied for comparisons between two specific groups. A P-value of less than 0.05 was considered statistically significant.
Results
Pre-treatment with PM ameliorated LPS-induced ALI
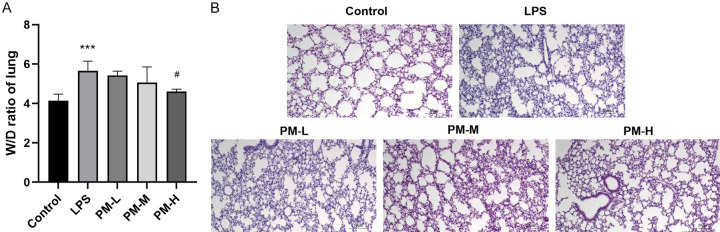
Lung edema is a hallmark of ALI, and the lung W/D ratio was employed to evaluate pulmonary edema. As illustrated in Figure 1A, the W/D ratio in the LPS group was significantly higher than in the control group (P < 0.01). However, treatment with high-dose PM (PM-H) (P < 0.01) markedly inhibited this increase, indicating that PM could effectively reduce the degree of LPS-induced ALI pulmonary edema.
Figure 1.
The effects of Peimine (PM) on lipopolysaccharide (LPS)-induced acute lung injury (ALI). A. The effect of PM on lung W/D ratio. B. The representative image of lung tissues (200×). The statistical results are presented as mean ± standard deviation (x±s, n=10 in each group). Compared with control group, ***P < 0.001; compared with LPS group, #P < 0.05.
H&E staining was conducted to investigate the protective effects of PM on lung tissue. As shown in Figure 1B, compared to the control group, LPS induced significant pathological changes, such as inflammatory cell infiltration, hemorrhage, alveolar wall thickening, and capillary congestion. In contrast, the lung structure alterations and damage were markedly improved in the PM-M and PM-H groups. The findings indicate that pretreatment with PM can substantially mitigate the pathological alterations associated with LPS-induced ALI.
Pre-treatment with PM inhibited the inflammatory response in LPS-induced ALI mice
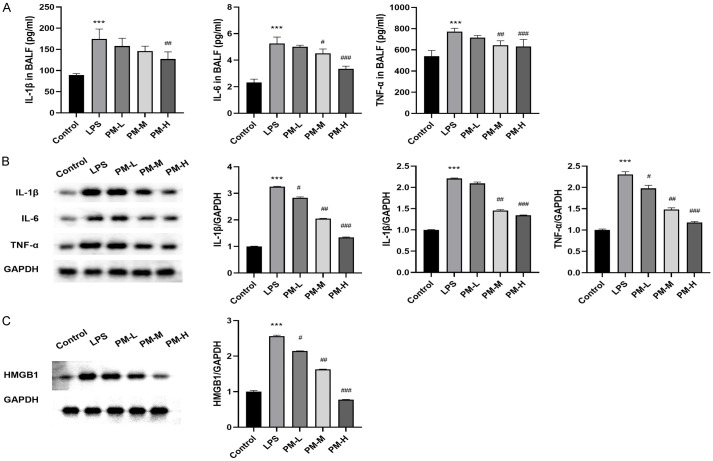
To evaluate the effect of PM on the cytokine production induced by LPS, an ELISA was conducted to quantify the concentrations of TNF-α, IL-6, and IL-1β in BALF. As depicted in Figure 2A, cytokine levels in the LPS group were significantly higher compared to the control group (all P < 0.01). These results were further validated by Western blot analysis, which revealed that LPS treatment significantly elevated the expression levels of inflammatory proteins in lung tissue. However, pretreatment with PM effectively attenuated these elevations (all P < 0.01) (Figure 2B). Additionally, the protein expression levels of HMGB1 were evaluated across all groups. LPS treatment increased HMGB1 expression in mice, whereas PM effectively inhibited the protein expression of HMGB1 (Figure 2C). Collectively, these findings demonstrate that PM can attenuate the inflammatory response in LPS-induced ALI mice.
Figure 2.
The effects of PM on LPS-induced ALI inflammation. A. The levels of IL-1β, IL-6, and TNF-α in BALF were determined by ELISA. B. The levels of inflammatory proteins in lung tissues was determined by Western blot analysis. C. The levels of HMGB1 protein in lung tissues was determined by Western blot analysis. The statistical results are presented as mean ± standard deviation (x±s, n=10 in each group). Compared with control group, ***P < 0.001; compared with LPS group, #P < 0.05, ##P < 0.01, ###P < 0.001. BALF: bronchoalveolar lavage fluid.
Pre-treatment with PM alleviated the oxidative stress response in LPS-induced ALI mice
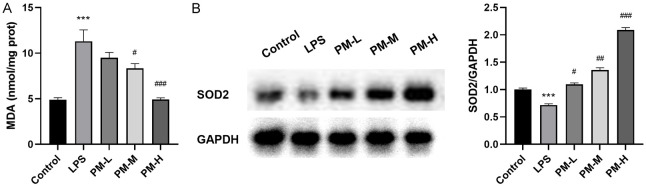
To further explore the potential impact of PM on oxidative stress in ALI lung tissue induced by LPS, MDA levels were evaluated in lung tissue. There was a significant increase in MDA levels in the LPS-stimulated group (P < 0.01) (Figure 3A). However, these increases were significantly reduced in mice pre-treated with PM (P < 0.01). WB revealed that SOD2 levels in the lung tissues of ALI mice induced by LPS was significantly decreased, whereas PM pre-treatment effectively countered this depletion of SOD2 (Figure 3B).
Figure 3.
The effects of PM on oxidative response induced by LPS in ALI. A. Effect of PM on MDA level in lung tissues. B. The SOD2 expression in lung tissues. The statistical results are presented as mean ± standard deviation (x±s, n=10 in each group). Compared with control group, ***P < 0.001; compared with LPS group, #P < 0.05, ##P < 0.01, ###P < 0.001. MDA: Malondialdehyde; SOD: Superoxide Dismutase.
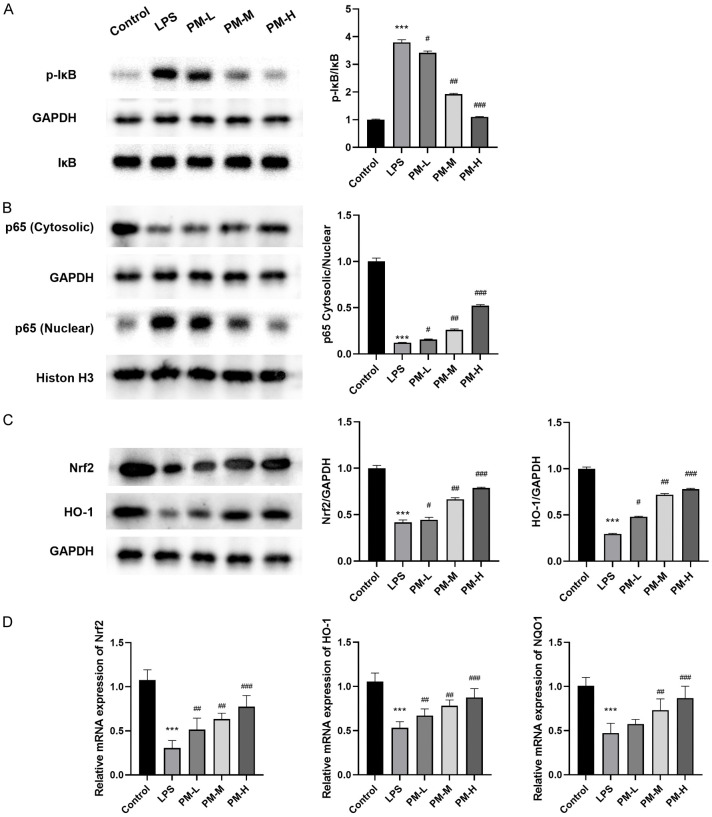
Pre-treatment with PM activated the Nrf2 pathway and inhibited NF-κB activation induced by LPS
The genes associated with the inflammatory response induced by LPS are critically dependent on the activation of NF-κB. To investigate the mechanisms by which PM modulates the production of pro-inflammatory mediators, we assessed the levels of NF-κB p65, IκBα, and p-IκB. Figure 4A illustrates that LPS administration significantly reduced IκBα levels and increased p-IκBα levels. However, pretreatment with PM notably mitigated these alterations. Furthermore, Figure 4B demonstrates that LPS stimulation enhanced the nuclear levels of NF-κB p65, whereas PM administration prevented the translocation of NF-κB p65 from the cytoplasm to the nucleus compared to the LPS group (Figure 4C, 4D). These findings indicate that PM efficiently suppresses the activation of IκBα and hinders the nuclear translocation of NF-κB p65 induced by LPS.
Figure 4.
The effects of PM on Nrf2 and NF-κB pathways in LPS-induced ALI mice. A. PM inhibited the activation of NF-κB. B. The inhibitory effect of PM on NF-κB nuclear translocation induced by LPS. C. Effects of PM treatment on the expression of Nrf2 and HO-1. D. Effects of PM treatment on the mRNA expression of Nrf2, NQO1, and HO-1. The statistical results are presented as mean ± standard deviation (x±s, n=10 in each group). Compared with control group, ***P < 0.001; compared with LPS group, #P < 0.05, ##P < 0.01, ###P < 0.001.
Pre-treatment of PM ameliorated LPS-induced inflammation and oxidative stress
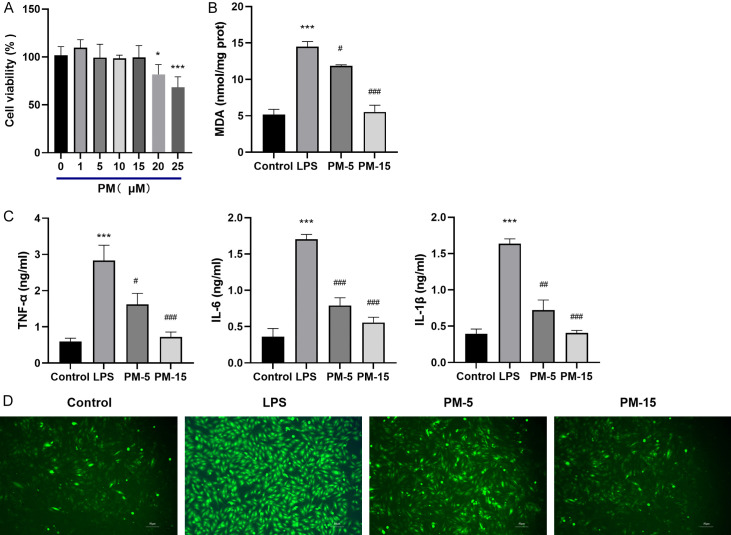
Compared to the control, PM doses exceeding 15 μM were found to moderately inhibit cell growth and affect cell viability. Consequently, doses of 15 μM or lower were used in subsequent experiments to avoid significant effects on cell viability, as demonstrated in Figure 5A.
Figure 5.
PM inhibited the inflammatory response and oxidative stress induced by LPS in Raw264.7 cells. A. The effect of PM on cell viability. B. The effect of PM on the levels of MDA in Raw264.7 cells induced by LPS. C. The effect of PM on the secretion of IL-1β, IL-6, and TNF-α from Raw264.7 cells induced by LPS. D. The representative image of Raw264.7 cells with LPS and PM treatment (200×). The statistical results are presented as mean ± standard deviation (x±s, n=6 in each group). Compared with control group, ***P < 0.001; compared with LPS group, #P < 0.05, ##P < 0.01, ###P < 0.001.
In RAW264.7 cells, LPS induction (100 ng/mL) significantly increased the levels of IL-1β, IL-6, TNF-α, MDA, and ROS. However, treatment with PM at 5 or 15 μM significantly reduced the production of these inflammatory mediators and ROS (Figure 5B-D), illustrating that PM exhibits anti-inflammatory and antioxidative effects on LPS-induced RAW264.7 cells.
PM inhibited LPS-induced inflammation and oxidative stress by activating the Nrf2 pathway
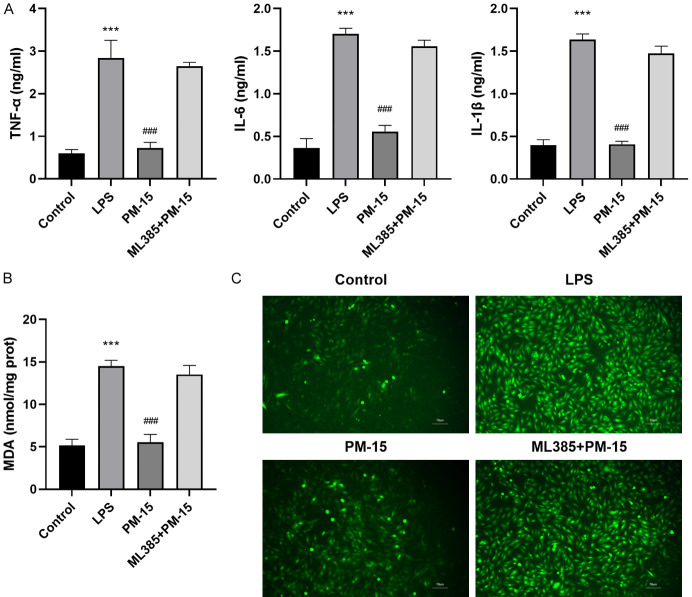
After treating RAW264.7 cells with the Nrf2 inhibitor ML385, the levels of IL-1β, IL-6, TNF-α, MDA, and ROS were increased (Figure 6A-C). This suggests that the inhibition of the Nrf2 pathway could serve to block the antioxidant and anti-inflammatory effects of PM.
Figure 6.
Blocking Nrf2 pathway reversed the effects of PM on LPS-induced inflammation and oxidative stress in Raw264.7 cells. A. Blocking Nrf2 pathway reversed the effects of PM on LPS-induced IL-1β, IL-6, and TNF-α secretion in Raw264.7 cells. B. Blocking Nrf2 pathway reversed the effects of PM on LPS-induced MDA levels in Raw264.7 cells. C. Blocking the Nrf2 pathway reversed the effects of PM on LPS-induced ROS levels in Raw264.7 cells, as evidenced by immune staining (200×). The statistical results are presented as mean ± standard deviation (x±s, n=6 in each group). Compared with control group, ***P < 0.001; compared the LPS group, ###P < 0.001.
Discussion
Acute lung injury (ALI), as well as its progression to acute respiratory distress syndrome (ARDS), has emerged as a significant life-threatening challenge in clinical settings. Traditional Chinese medicine, recognized for its numerous benefits in treating ALI, has emerged as a promising approach for intervention [25].
Inflammatory responses in ALI can lead to diffuse pulmonary edema affecting both the lung parenchyma and interstitium, accompanied by widening of the gaps between endothelial cells [26]. The W/D ratio of the lungs is a critical indicator of pulmonary edema and a common symptom of inflammation [27]. In this study, PM significantly reduced the LPS-induced increase in W/D ratio of the lungs in mice, indicating its effect on improving pulmonary edema caused by LPS. Concurrently, PM pretreatment also ameliorated the deteriorating histopathological changes in the lungs, indicating an improvement in lung injury.
LPS can initiate and amplify the body’s inflammatory response, leading to the accumulation of inflammatory cells such as neutrophils, a key factor in the onset of ALI [28,29]. Inflammatory cytokines like TNF-α, IL-6, and IL-1β play crucial roles in ALI pathogenesis by promoting inflammation [30]. TNF-α acts as a pivotal multifunctional cytokine involved in the pathogenesis of ALI, triggering innate immune responses by inducing the release of other cytokines, such as IL-6 from macrophages. IL-6 further intensifies the inflammatory cascade, resulting in significant pulmonary damage [31,32]. In addition, IL-1β serves as an important prognostic marker in ALI, contributing to alveolar epithelial repair [33]. The results of this study demonstrates a significant reduction in the levels of TNF-alpha, IL-6, and IL-1β in the presence of PM, indicating that the improvement observed in the LPS-induced ALI mouse model by PM is, at least in part, attributable to the negative regulation of pro-inflammatory cytokine secretion.
HMGB1 is another molecule closely associated with ALI. It not only promotes the activation of inflammatory cells, prompting macrophages and neutrophils to synthesize and release inflammatory factors, thus accelerating inflammation, but also participates in oxidative stress responses that further aggravate lung injury [34]. These findings reveal that PM significantly reduces the protein levels of HMGB1, suggesting that PM may negatively regulate pro-inflammatory cytokine secretion through modulation of HMGB1.
Oxidative stress and inflammation are often interdependent, with numerous studies suggesting that oxidative stress is directly triggered by inflammatory stimulation [35]. Oxidative stress typically involves an excess of ROS, leading to increased levels of MDA. In this study, pretreatment with PM significantly inhibited the LPS-induced increase in MDA levels. Superoxide dismutases (SODs) represent a primary defense against reactive oxygen species (ROS) by acting as scavenging enzymes [36]. SOD2, primarily distributed in the mitochondria, serves as a crucial protein for maintaining the stability of the mitochondrial environment by neutralizing oxygen radicals within the mitochondria. This protein also supports anti-apoptotic functions in response to oxidative stress, ionizing radiation, and inflammatory cytokines [37]. Similar research on the effects of curcumin on ventilator-induced lung injury (VILI) has revealed that curcumin pretreatment can mitigate VILI by increasing Nrf2 and SOD2 levels in lung tissue [38]. In this study, pretreatment with PM effectively reduced the depletion of SOD2 and significantly increased SOD2 levels in the lungs. This suggests that PM pretreatment effectively enhances the function of the antioxidant system, preventing oxidative tissue damage from free radicals and maintaining normal levels of SOD2 expression.
NF-κB plays a pivotal role in numerous cellular processes [39] and remains inactive in the cytoplasm under unstimulated conditions. However, upon LPS stimulation, the degradation and phosphorylation of IκBα are augmented, ultimately leading to the release and nuclear translocation of NF-κB [40]. Notably, NF-κB serves as a crucial transcription factor for the expression of various genes essential to the inflammatory response in the lung tissue. Consequently, targeted inhibition of the NF-κB pathway emerges as a promising therapeutic approach for the treatment of ALI. Additionally, the Nrf2 pathway represents a major endogenous antioxidant defense system [41] that can inhibit the activation of the NF-κB pathway by enhancing antioxidant defenses, thereby effectively counteracting the adverse effects of NF-κB overactivation after LPS exposure. Disruption of the NF-κB/Nrf2 axis by LPS contributes to the onset and development of ALI [42]. Our results demonstrate that pretreatment with PM inhibits NF-κB activation while simultaneously activating Nrf2, suggesting that PM exerts a protective effect against LPS-induced ALI through these mechanisms. Similar results have been reported previously. For instance, β-linalool, extracted from patchouli oil, has been demonstrated to mitigate LPS-induced ALI through the inhibition of NF-κB and the activation of the Nrf2 signaling pathway [43]. Furthermore, strictosamide has been shown to alleviate LPS-induced inflammation by targeting ERK2 and decreasing its phosphorylation, which in turn inhibits NF-κB signaling [44]. Macrophages, key coordinators in the pathogenesis of ALI, serve as the first line of defense against airborne particles and microbes. Their functions are traditionally classified into “M1 classically activated macrophages (CAM)” and “M2 alternatively activated macrophages (AAM)”. M1 macrophages are characterized by the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α, which promote autoimmune diseases [45]. Our study found that PM pretreatment suppressed the production of ROS, MDA, and inflammatory mediators in LPS-induced Raw264.7 cells, exerting significant antioxidative and anti-inflammatory effects. Importantly, PM effectively inhibited M1 polarization in RAW264.7 cells, reducing excessive secretion of IL-1β, IL-6, and TNF-α. These effects were effectively blocked by the Nrf2 inhibitor ML385, suggesting that PM attenuates LPS-induced ALI through Nrf2 signaling.
In conclusion, PM exerts a protective effect against LPS-induced ALI by inhibiting NF-κB p65 activation, activating the Nrf2 pathway, reducing the generation of inflammatory factors, and decreasing oxidative stress levels, ultimately improving lung repair after injury. These results support PM as a potential candidate for further development in the research of ALI treatment.
Acknowledgements
This study was supported in part by funding for “Fritillin A (PME) ameliorating LPS-induced acute lung injury by regulating the Nrf2 and NF-κB pathways” (grant number 2022Y1488). We acknowledge and appreciate our colleagues for their valuable suggestions and technical assistance in this study.
Disclosure of conflict of interest
None.
References
- 1.Xu H, Qi Q, Yan X. Myricetin ameliorates sepsis-associated acute lung injury in a murine sepsis model. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:165–175. doi: 10.1007/s00210-020-01880-8. [DOI] [PubMed] [Google Scholar]
- 2.Jin C, Chen J, Gu J, Zhang W. Gut-lymph-lung pathway mediates sepsis-induced acute lung injury. Chin Med J (Engl) 2020;133:2212–2218. doi: 10.1097/CM9.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R, Xie Y, Qiu J, Chen J. The effects of dexmedetomidine in a rat model of sepsis-induced lung injury are mediated through the adenosine monophosphate-activated protein kinase (AMPK)/silent information regulator 1 (SIRT1) pathway. Med Sci Monit. 2020;26:e919213. doi: 10.12659/MSM.919213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong W, Jia L, Cai Y, Chen Y, Gao M, Jin J, Zhu J. Evaluation of the anti-inflammatory effects of PI3Kδ/γ inhibitors for treating acute lung injury. Immunobiology. 2023;228:152753. doi: 10.1016/j.imbio.2023.152753. [DOI] [PubMed] [Google Scholar]
- 5.Tian Y, Zhu CL, Li P, Li HR, Liu Q, Deng XM, Wang JF. Nicotinamide mononucleotide attenuates LPS-induced acute lung injury with anti-inflammatory, anti-oxidative and anti-apoptotic effects. J Surg Res. 2023;283:9–18. doi: 10.1016/j.jss.2022.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Lou T, Jiang W, Xu D, Chen T, Fu Y. Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation. 2015;38:1213–1220. doi: 10.1007/s10753-014-0087-8. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Shan C, Wu Z, Yu H, Yang A, Tan B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm Res. 2020;69:365–373. doi: 10.1007/s00011-020-01331-3. [DOI] [PubMed] [Google Scholar]
- 8.Xu LT, Wang T, Fang KL, Zhao Y, Wang XN, Ren DM, Shen T. The ethanol extract of flower buds of Tussilago farfara L. attenuates cigarette smoke-induced lung inflammation through regulating NLRP3 inflammasome, Nrf2, and NF-κB. J Ethnopharmacol. 2022;283:114694. doi: 10.1016/j.jep.2021.114694. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZY, Yang J, Qi ZM. Advances in studies on Fritillaria tantrum. West China J Pharm Sci. 2017;45:9–13. [Google Scholar]
- 10.Wu F, Tian M, Sun Y, Wu C, Liu X. Efficacy, chemical composition, and pharmacological effects of herbal drugs derived from Fritillaria cirrhosa D. Don and Fritillaria thunbergii Miq. Front Pharmacol. 2022;13:985935. doi: 10.3389/fphar.2022.985935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Guo T, Du X, Yao JB, Shen LJ. Determination of content of methyl protodioscin and protodioscin in Zhebeimu formula granules by high-performance liquid chromatography. Medical Tribune. 2018;37:477–479. [Google Scholar]
- 12.Guo SL, Li JN, Xiao SX. Effects of methyl protodioscin on inflammatory response and autophagy in cerebral ischemia-reperfusion injury in mice. Chin J Gerontol. 2019;39:5347–5350. [Google Scholar]
- 13.Chen WS, Xiong W, Xiong S. Study on the effects of Peimine on the biological behavior of liver cancer cells. Heilongjiang Science. 2022;13:16–18. [Google Scholar]
- 14.Zhou J, Mao Y, Shi X, Zhang Y, Yu X, Liu X, Diao L, Yang X, Liu C, Liu D, Tan X, Liu M. Peimine suppresses collagen-induced arthritis, activated fibroblast-like synoviocytes and TNFα-induced MAPK pathways. Int Immunopharmacol. 2022;111:109181. doi: 10.1016/j.intimp.2022.109181. [DOI] [PubMed] [Google Scholar]
- 15.Gui GX, Guo X. The effect of methyl protodioscin on the time expression of COX-2, PGE2, and NO in mice with acute lung injury. Chinese Clinical Research in Traditional Chinese Medicine. 2016;8:11–13. [Google Scholar]
- 16.Gui GX. Experimental study on the protective effect of methyl protodioscin on acute lung injury in mice. Chinese Clinical Research in Traditional Chinese Medicine. 2016;8:4–6. [Google Scholar]
- 17.Gui GX. The effect of methyl protodioscin on the spatiotemporal expression of TNF-α, IL-4, IL-10, and PGE_2 in mice with acute lung injury. Chinese Clinical Research in Traditional Chinese Medicine. 2017;9:1–4. [Google Scholar]
- 18.He W, Kapate N, Shields CW 4th, Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2020;165-166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Joshi N, Walter JM, Misharin AV. Alveolar macrophages. Cell Immunol. 2018;330:86–90. doi: 10.1016/j.cellimm.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Bazzan E, Turato G, Tinè M, Radu CM, Balestro E, Rigobello C, Biondini D, Schiavon M, Lunardi F, Baraldo S, Rea F, Simioni P, Calabrese F, Saetta M, Cosio MG. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir Res. 2017;18:40. doi: 10.1186/s12931-017-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han XZ, Ma R, Chen Q, Jin X, Jin YZ, An RB, Piao XM, Lian ML, Quan LH, Jiang J. Anti-inflammatory action of Athyrium multidentatum extract suppresses the LPS-induced TLR4 signaling pathway. J Ethnopharmacol. 2018;217:220–227. doi: 10.1016/j.jep.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Yi PF, Wu YC, Dong HB, Guo Y, Wei Q, Zhang C, Song Z, Qin QQ, Lv S, Wu SC, Fu BD. Peimine impairs pro-inflammatory cytokine secretion through the inhibition of the activation of NF-κB and MAPK in LPS-induced RAW264.7 macrophages. Immunopharmacol Immunotoxicol. 2013;35:567–572. doi: 10.3109/08923973.2013.822508. [DOI] [PubMed] [Google Scholar]
- 23.Zhou MJ, Chen LY, Hu HH, Luo YY, Fang C, Zhang N. Protective effect of pulsatilla saponin B4 on lipopolysaccharide-induced acute lung injury. Traditional Chinese Drug Research and Clinical Pharmacology. 2019;30:664–670. [Google Scholar]
- 24.Yao P, Zhang Z, Cao J. Isorhapontigenin alleviates lipopolysaccharide-induced acute lung injury via modulating Nrf2 signaling. Respir Physiol Neurobiol. 2021;289:103667. doi: 10.1016/j.resp.2021.103667. [DOI] [PubMed] [Google Scholar]
- 25.Ye XY, Liu T. Research progress on experimental models of acute lung injury in vivo and in vitro. Occupational and Health. 2020;36:269–274. [Google Scholar]
- 26.Jin SZ, Hu B, Luan ZG. Research progress on the role of high mobility group protein 1 in sepsis-induced acute lung injury. Chin J Emerg Med. 2018;27:459–462. [Google Scholar]
- 27.Li T, Xiao G, Tan S, Shi X, Yin L, Tan C, Gu J, Liu Y, Deng H, Liu K, Liu M, Zhang H, Xiao X. HSF1 attenuates LPS-induced acute lung injury in mice by suppressing macrophage infiltration. Oxid Med Cell Longev. 2020;2020:1936580. doi: 10.1155/2020/1936580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen DW, Kang T, Xu XZ, Xia WJ, Ye X, Wu YB, Xu YR, Liu J, Ren H, Deng J, Chen YK, Ding HQ, Aslam M, Zelek WM, Morgan BP, Kapur R, Santoso S, Fu YS. Mechanism and intervention of murine transfusion-related acute lung injury caused by anti-CD36 antibodies. JCI Insight. 2023;8:e165142. doi: 10.1172/jci.insight.165142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeeuw van der Laan EAN, van der Velden S, Porcelijn L, Semple JW, van der Schoot CE, Kapur R. Update on the pathophysiology of transfusion-related acute lung injury. Curr Opin Hematol. 2020;27:386–391. doi: 10.1097/MOH.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 30.Doganyigit Z, Eroglu E, Akyuz E. Inflammatory mediators of cytokines and chemokines in sepsis: from bench to bedside. Hum Exp Toxicol. 2022;41:9603271221078871. doi: 10.1177/09603271221078871. [DOI] [PubMed] [Google Scholar]
- 31.Tian G, Li C, Zhai Y, Xu J, Feng L, Yao W, Bao B, Zhang L, Ding A. GC-MS based metabolomic profiling of lung tissue couple with network pharmacology revealed the possible protection mechanism of Pudilan Xiaoyan Oral Liquid in LPS-induced lung injury of mice. Biomed Pharmacother. 2020;124:109833. doi: 10.1016/j.biopha.2020.109833. [DOI] [PubMed] [Google Scholar]
- 32.Garbers C, Rose-John S. Dissecting interleukin-6 classic and trans-signaling in inflammation and cancer. Methods Mol Biol. 2023;2691:207–224. doi: 10.1007/978-1-0716-3331-1_16. [DOI] [PubMed] [Google Scholar]
- 33.Atmowihardjo LN, Heijnen NFL, Smit MR, Hagens LA, Filippini DFL, Zimatore C, Schultz MJ, Schnabel RM, Bergmans DCJJ, Aman J, Bos LDJ DARTS Consortium. Biomarkers of alveolar epithelial injury and endothelial dysfunction are associated with scores of pulmonary edema in invasively ventilated patients. Am J Physiol Lung Cell Mol Physiol. 2023;324:L38–L47. doi: 10.1152/ajplung.00185.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou HY, Tao J, Gai L, Yan BQ. Observation on the effect of low molecular weight heparin on oxygenation index and inflammatory reaction in ICU septic patients with ALI/ARDS. Chinese Pharmacist. 2019;22:106–108. [Google Scholar]
- 35.Wadley AJ, Veldhuijzen van Zanten JJ, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age (Dordr) 2013;35:705–718. doi: 10.1007/s11357-012-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang LC, Wang Y, Liu W, Zhang XM, Fan M, Zhao M. Protective effects of SOD2 overexpression in human umbilical cord mesenchymal stem cells on lung injury induced by acute paraquat poisoning in rats. Life Sci. 2018;214:11–21. doi: 10.1016/j.lfs.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Becuwe P, Ennen M, Klotz R, Barbieux C, Grandemange S. Manganese superoxide dismutase in breast cancer: from molecular mechanisms of gene regulation to biological and clinical significance. Free Radic Biol Med. 2014;77:139–151. doi: 10.1016/j.freeradbiomed.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Su K, Li XT, Tian M, Xue FS. Effects of pretreatment with borneol on mechanical ventilation-associated lung injury and expression of Nrf2 and SOD2 in lung tissue of mice. Journal of International Anesthesiology and Resuscitation. 2023;44:575–579. [Google Scholar]
- 39.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: evidence for LITAF-dependent LPS signaling pathways. Proc Natl Acad Sci U S A. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Huang B, Zhao F, Lian J, He L, Zhang Y, Ji L, Zhang J, Yan X, Zeng T, Ma C, Liang Y, Zhang C, Lin J. p38-mediated FOXN3 phosphorylation modulates lung inflammation and injury through the NF-κB signaling pathway. Nucleic Acids Res. 2023;51:2195–2214. doi: 10.1093/nar/gkad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 42.Choudhury S, Ghosh S, Gupta P, Mukherjee S, Chattopadhyay S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-κB and SAPK/JNK pathway. Free Radic Res. 2015;49:1371–1383. doi: 10.3109/10715762.2015.1075016. [DOI] [PubMed] [Google Scholar]
- 43.Chen XY, Dou YX, Luo DD, Zhang ZB, Li CL, Zeng HF, Su ZR, Xie JH, Lai XP, Li YC. β-Patchoulene from patchouli oil protects against LPS-induced acute lung injury via suppressing NF-κB and activating Nrf2 pathways. Int Immunopharmacol. 2017;50:270–278. doi: 10.1016/j.intimp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Geng Q, Liu B, Fan D, Cao Z, Li L, Lu P, Lin L, Yan L, Xiong Y, He X, Lu J, Chen P, Lu C. Strictosamide ameliorates LPS-induced acute lung injury by targeting ERK2 and mediating NF-κB signaling pathway. J Ethnopharmacol. 2024;322:117593. doi: 10.1016/j.jep.2023.117593. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Wang JC, Han L, Chen T. Palmatine attenuated lipopolysaccharide-induced acute lung injury by inhibiting M1 phenotype macrophage polarization via NAMPT/TLR2/CCR1 signaling. J Agric Food Chem. 2024;72:9087–9101. doi: 10.1021/acs.jafc.3c05597. [DOI] [PubMed] [Google Scholar]