Abstract
Vascular thrombosis and hypercoagulability are frequently observed in individuals with nephrotic syndrome (NS), particularly those diagnosed with membranous nephropathy (MN). It is extensively recognized that venous thrombosis occurs far more frequently than arterial thrombosis. However, we report a patient who experienced two significant arterial and venous thrombotic events within the past two years, and he was a 35-year-old man with nephrotic syndrome secondary to membranous nephropathy. In contrast to most previous similar studies that had shown only a single demonstration of venous embolism or arterial embolism, this case highlighted the occurrence of both venous and arterial embolic events. This suggests and prompts considerations for future research avenues and anticoagulant treatment strategies.
Keywords: Membranous nephropathy, nephrotic syndrome, thrombosis, anticoagulant treatment
Introduction
Nephrotic syndrome (NS) is a common manifestation of various glomerular diseases, characterized by heavy proteinuria, hypoalbuminemia, hyperlipidemia, and edema. Among the different causes of NS, membranous nephropathy (MN) is one of the most prevalent in adults [1]. Patients with NS, especially those with MN, have an elevated risk of thromboembolic events, including venous thromboembolism (VTE) and arterial thromboembolism (ATE), which significantly contribute to the morbidity and mortality of the condition [2-8]. Studies indicate that 10-30% of NS patients may develop venous thromboembolic complications, with a higher incidence seen in MN [9-11]. The most common sites of thrombosis include the deep veins of the lower extremities, pulmonary arteries, and renal veins. While arterial thromboembolic events are less frequent, they can still lead to significant morbidity [1,12]. The underlying mechanisms involve a complex interplay of hypercoagulability, loss of anticoagulant factors in the urine, endothelial dysfunction, and platelet activation, all contributing to a prothrombotic state [13].
Managing thromboembolic risk in NS, particularly in MN patients, typically involves anticoagulant therapy. Prophylactic anticoagulation is generally recommended for patients with severe hypoalbuminemia (<2.5 g/dL), which is associated with an increased risk of thrombotic complications [1,12]. Warfarin is widely used; however, direct oral anticoagulants (DOACs) have gained attention due to their ease of use and potentially improved safety profile [1,14]. Nevertheless, there is no consensus on the optimal initiation criteria, duration, or selection of anticoagulants for these patients. Balancing the reduction of thrombotic risk with minimizing bleeding complications remains a key challenge for clinical practice [15].
The potential role of antiplatelet therapy in managing thromboembolic risk in NS has also gained interest. Platelet hyperactivity, commonly seen in NS, may predispose patients to arterial thromboembolic events, leading to the investigation of antiplatelet agents such as aspirin for ATE prevention [1,12]. However, current evidence is limited, and large-scale randomized controlled trials are lacking. Some observational studies suggest that aspirin may reduce arterial events, particularly in patients with severe hypoalbuminemia or other high-risk features, but concerns regarding bleeding risk, especially in those already on anticoagulation therapy, remain unresolved [15].
Despite advancements in understanding thromboembolic risks in NS and MN, significant gaps remain regarding optimal prevention strategies. Emerging data on combining anticoagulation and antiplatelet therapy suggest the possibility for more personalized thromboprophylaxis approaches in this patient population. Further research is needed to clarify the role of antiplatelet agents, define the best anticoagulant regimens, and assess the effect of these therapies on long-term outcome, including renal and cardiovascular complications [1,8,14].
Unlike most previous case reports [2,3,7,8], this is a rare case report in which the patient, despite receiving a standard anticoagulation regimen, experienced both severe venous and arterial thromboembolic events within a short period. This unique case challenges the current understanding of anticoagulation strategies and provides new insight into the complexity of thromboembolic management in nephrotic syndrome. It also underscores the need for further research to refine anticoagulation protocols and explore novel approaches in the prevention and treatment of thromboembolic complications in this patient population.
Case presentation
Medical history
A 35-year-old male patient was hospitalized on March 6, 2024, due to recurrent bilateral lower limb edema persisting for over two years, with recent exacerbation over the past week. Previously, in September 2022, he had undergone surgery at the First Affiliated Hospital of Kunming Medical University. The procedure involved extracting an inferior vena cava thrombus, placing/removing a filter to prevent further exacerbation of pulmonary embolism, extracting an iliac vein thrombus, and performing lower extremity deep vein cannulation thrombolysis. At that time, the diagnosis was bilateral lower extremity deep vein thrombosis, iliac vein thrombosis, inferior vena cava thrombosis, and pulmonary artery embolism. After a thorough history-taking, his blood biochemistry revealed an albumin level of 31 g/L, and his urinalysis showed a urinary protein 2+ in September 2022. The lower extremity edema improved post-surgery, but some swelling persisted. He only took rivaroxaban for 3 months and took diuretics intermittently post-surgery, and he did not seek further consultation about edema and urinary protein.
Three months before admission, he was also diagnosed with acute myocardial infarction in February 2024 and underwent coronary intervention with stent placement. After surgery, he was prescribed atorvastatin calcium tablets 20 mg once daily, aspirin enteric-coated tablets 100 mg once daily, and ticagrelor tablets 90 mg once daily. During this period, his urine routine showed urinary protein 4+, and his renal function exhibited a blood creatinine level of 120 μmol/L (Normal reference value is 40 to 110) and albumin of 19 g/L. It was only at this time that he was advised to consult a nephrologist for further evaluation. Other than that, he had no specific medical history of diabetes or hypertension.
Diagnosis and treatment process
On admission, his vital signs were normal, and physical examination revealed no yellowing of the skin or sclera, no palmar erythema or spider naevi, no jugular vein distension, no dry or wet rales in both lungs, and no heart enlargement. His liver and spleen were not palpable, and no shifting dullness was observed. His lower extremities were moderately swollen. Additionally, laboratory tests showed a hemoglobin concentration of 102 g/L, urinary protein 3+, a 24-hour urinary protein quantification of 13.225 g/L, a urine albumin-to-creatinine ratio of 3532.19 mg/g, a calcium level of 1.94 mmol/L, a blood creatinine level of 98 μmol/L, a urea nitrogen level of 4.48 mmol/L, a total protein level of 35.1 g/L, and an albumin level of 17.5 g/L. Furthermore, his anti-phospholipase A2 receptor antibody (PLA2R) was 164.52 U/ml (reference range <20). Autoantibody spectrum, rheumatic panel, thyroid function, coagulation function and factors, and tumor markers were all within normal range. Besides, his electrocardiogram showed sinus rhythm, abnormal Q waves, and ST-segment elevation. His chest computed tomography (CT) on March 6, 2024, presented slight exudative changes in the right lower lobe and a small nodule in the left upper lobe. Additionally, changes were observed in the coronary stent intervention as shown in Figure 1A, and a high-density shadow was detected in the inferior vena cava as shown in Figure 1B. His urinary system color Doppler ultrasound indicated enlarged kidneys and a thickened renal cortex.
Figure 1.
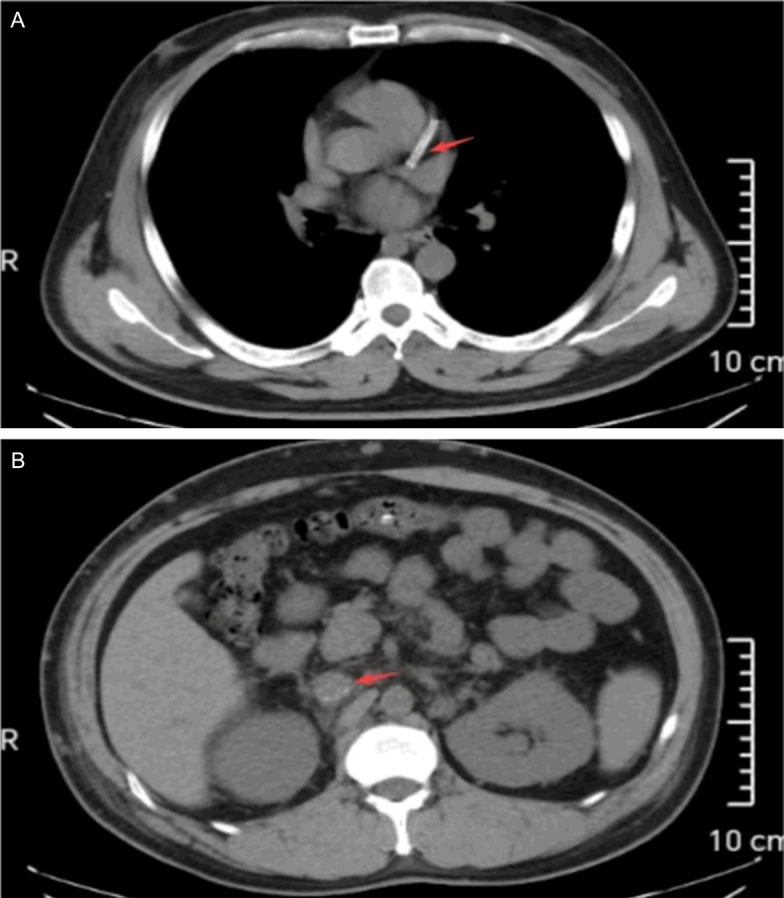
Partial chest CT image: The red arrow in (A) indicates a coronary stent, while the red arrow in (B) highlights thrombosis in the inferior vena cava.
The diagnosis included membranous nephropathy, nephrotic syndrome, personal history of inferior vena cava thrombosis and pulmonary artery embolism, and post-coronary artery stent implantation status. Given the elevated bleeding risk linked to dual antiplatelet therapy, a renal puncture biopsy was omitted. Following a comprehensive discussion of treatment options for membranous nephropathy, the patient opted for rituximab in conjunction with tacrolimus immunosuppressive therapy. On March 9, 2024, the patient was treated with rituximab injection (1 g). The patient was readmitted on April 11, 2024, for a rituximab injection and disease evaluation. Given the patient’s personal history of inferior vena cava thrombosis and pulmonary artery embolism, coupled with indications of inferior vena cava thrombosis in the last chest CT on March 6, 2024, a vascular surgery consultation was arranged for further treatment guidance. A whole abdominal computed tomography scan on April 11, 2024, was recommended by a vascular surgeon, which revealed high-density shadows in the inferior vena cava and left renal vein as shown in Figure 2. The vascular surgeon, contacted by phone to review the scan, recommended lifelong anticoagulation therapy. Given the patient’s recent coronary stenting, we consulted with a cardiovascular specialist to develop a more tailored anticoagulation regimen. On April 19, 2024, the patient received a rituximab injection (1 g) and was prescribed rivaroxaban (15 mg once daily) and clopidogrel (75 mg once daily) for anticoagulation and antiplatelet therapy, based on the multidisciplinary consultations.
Figure 2.
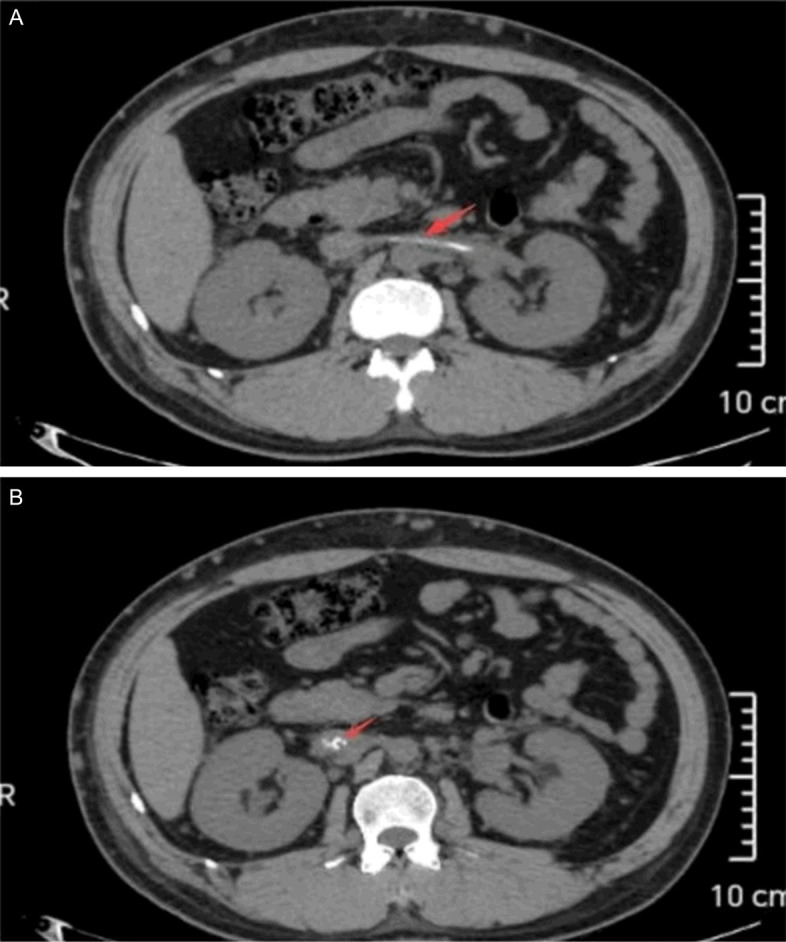
Whole abdomen CT partial image: The red arrow in (A) indicates renal vein thrombosis, while the red arrow in (B) shows thrombosis in the inferior vena cava.
Subsequent treatment plan and follow-up
According to KDIGO guidelines, immunosuppressive treatment for membranous nephropathy should be tailored to the patient’s risk profile, primarily defined by proteinuria levels and renal function. High-risk patients (proteinuria >8 g/day or declining renal function) require more aggressive immunosuppression. First-line therapy in this group includes rituximab or a cyclophosphamide-corticosteroid regimen [1]. In this case, the patient’s 24-hour urinary protein quantification was 13.225 g/L, exceeding the 8 g/day threshold and categorizing him as high-risk. Thus, aggressive immunosuppressive therapy was indicated. After a comprehensive discussion of the potential benefits and risks of various treatment options, the patient chose a combination therapy with rituximab and tacrolimus.
On April 19, 2024, the patient’s treatment regimen was initiated, consisting of rivaroxaban (15 mg once daily), clopidogrel hydrogensulfate (75 mg once daily), fenofibrate (0.2 g once daily), sacubitril/valsartan (100 mg twice daily), spironolactone (40 mg once daily), furosemide (20 mg once daily), and tacrolimus (1.5 mg twice daily). During a follow-up visit on June 30, 2024, significant improvement in edema was observed, with urine protein levels at 2+ and albumin at 31 g/L. A subsequent telephone follow-up on September 8, 2024, confirmed that the edema in both lower extremities had completely resolved, allowing for discontinuation of diuretics. Laboratory tests at this point showed a urine protein level of 1+ and an albumin level of 35 g/L. These findings indicated that the patient’s membranous nephropathy was effectively managed through the combination of rituximab (1 g, administered in two doses on March 9 and April 19) and tacrolimus (1.5 mg twice daily), leading to complete remission using the current therapeutic approach.
Discussion
In nephrotic syndrome, the heightened risk of thromboembolic events arises from multiple interconnected mechanisms. The urinary loss of key antithrombotic proteins, such as antithrombin III, protein C, and protein S, leads to a prothrombotic state. Concurrently, increased synthesis of procoagulant factors-including factors V, VIII, fibrinogen, and plasminogen activator inhibitor 1-occurs, largely as a compensatory response to hypoalbuminemia. This imbalance between coagulation and fibrinolysis facilitates thrombus formation and persistence. Additionally, platelet hyperactivity and hyperfibrinogenemia further exacerbate the hypercoagulable state, significantly increasing the risk of thrombosis in nephrotic syndrome [13]. In this case, the patient was a young male who had been in a state of hypoproteinemia. He presented in a hypercoagulable state, and no relevant cardiovascular risk factors were noticed before the onset of the disease. However, he had suffered from two serious arterial and venous thrombotic events in the past two years. Based on his medical history and examination results, the two severe embolic events were closely associated with nephrotic syndrome secondary to membranous nephropathy.
As shown by the results in Table 1, venous embolic events dominate embolic events in patients with nephrotic syndrome. These studies have predominantly focused on isolated venous thromboembolism or arterial thromboembolism in patients with nephrotic syndrome. However, the case we report is rare, since severe venous and arterial thrombotic events occurred together in the same patient. This dual manifestation of thrombotic complications highlights the complex interplay between the pathophysiology of MN and its associated prothrombotic state, which is less commonly observed and reported in the literature.
Table 1.
Reported thromboembolic events and management in nephrotic syndrome and membranous nephropathy
| Author | Year | Study type | Embolic events | Anticoagulation strategy | Antiplatelet strategy |
|---|---|---|---|---|---|
| Shi-Jun Li [10] | 2012 | Prospective Study | VTE in 36% of MN patients, mainly RVT and PE. | LMWH for 1 month, then warfarin for ≥6 months. | Platelet activation noted; not the primary focus. |
| Sophia Lionaki [11] | 2012 | Retrospective Cohort | VTE in 7% of MN patients, mostly within 2 years of diagnosis. | Anticoagulation post-VTE; not used prophylactically. | No significant protective effect noted. |
| Joseph Loscalzo [13] | 2013 | Clinical Review | Thrombotic events in ~25% of NS patients. | Highlights hypercoagulable state; emphasizes monitoring and anticoagulation for high-risk patients. | Platelet hyperactivity discussed; statins may reduce aggregation. |
| Kavita Krishna [2] | 2015 | Case Report | AMI in a 28-year-old with NS. | Urgent PCI and unfractionated heparin. | Tirofiban during PCI; aspirin post-procedure. |
| Julia M. Hofstra [12] | 2016 | Commentary | High risk of venous and arterial thrombosis in MN. | Recommends prophylactic warfarin for albumin <2.5 g/dL. | Aspirin may reduce arterial events; bleeding risk needs assessment. |
| Herve’ Lobbes [17] | 2021 | Editorial | VTE as a critical complication in NS. | LMWH or heparin followed by VKAs for 3-6 months. | Lacks evidence for antiplatelet use; more trials needed. |
| KDIGO 2021 Guidelines [1] | 2021 | Clinical Guideline | Increased VTE/ATE risk in NS patients. | Prophylactic anticoagulation for high-risk patients; options include LMWH, warfarin, DOACs. | Antiplatelet therapy for specific high-risk scenarios. |
| Federica De Pascali [15] | 2024 | Systematic Review and Meta-Analysis | VTE incidence: PE at 1.8%, DVT at 0.9% with prophylaxis. | Prophylactic anticoagulation reduces VTE but has bleeding risk. | Limited evidence for aspirin in VTE prevention in primary NS. |
Note: NS: Nephrotic Syndrome; MN: Membranous Nephropathy; VTE: Venous Thromboembolism; RVT: Renal Vein Thrombosis; PE: Pulmonary Embolism; ATE: Arterial Thromboembolism; LMWH: Low-Molecular-Weight Heparin; DOACs: Direct Oral Anticoagulants; PCI: Percutaneous Coronary Intervention; AMI: Acute Myocardial Infarction; VKA: Vitamin K Antagonist; KDIGO: Kidney Disease Improving Global Outcomes.
Thrombotic and embolic events are more common in patients with membranous nephropathy when albumin levels are 25 g/L or lower. Therefore, anticoagulant therapy is often recommended for these patients once albumin levels fall to 25 g/L or below [1,16,17]. Venous thromboembolism is the primary concern, while arterial embolism is less common. As a result, clinical practice primarily emphasizes anticoagulant therapy for the prevention and management of venous thromboembolism. In this case, following the initial embolic event (a serious deep vein thrombosis event), the patient received rivaroxaban for three months in accordance with guideline recommendations. However, one year later, he experienced a severe acute myocardial infarction. Following the second embolic event, the patient was treated with aspirin enteric-coated tablets and Tegretol antiplatelet therapy. However, the patient continued to suffer from renal vein and inferior vena cava thrombosis two months later. Finally, the patient was prescribed rivaroxaban (15 mg once daily) and clopidogrel (75 mg once daily) for combined anticoagulation and antiplatelet therapy, following a multidisciplinary consultation. Compared to most anticoagulation strategies studied (Table 1), this combination regimen deviated from traditional protocols and merits attention. The patient’s severe venous thrombosis, coupled with arterial involvement, necessitated a more individualized approach, carefully balancing the risk of bleeding against the need to prevent further thromboembolic events. The decision-making process involved a thorough evaluation of both antiplatelet and anticoagulant therapies, guided by the patient’s specific clinical presentation and treatment response. This case suggests that in certain situations, antiplatelet therapy may be as crucial as anticoagulation, despite the typical clinical focus on anticoagulant therapy alone. Therefore, individual anticoagulation and antiplatelet therapeutic strategies (for whom, when to start, how to do it) should still be necessarily considered for patients with nephrotic syndrome, so as to reduce the incidence of serious embolic events and to avoid serious bleeding events [18].
Conclusion
This case suggests that in certain circumstances, antiplatelet therapy may be just as important as anticoagulation, despite the usual clinical emphasis on anticoagulants alone. Therefore, individualized strategies for both anticoagulation and antiplatelet therapy (including patient selection, timing, and method) should be carefully considered for patients with nephrotic syndrome. Further research is needed to explore the combined use of anticoagulants and antiplatelet therapies in complex cases involving both arterial and venous thrombosis in NS patients.
The insights gained from this case are as follows: 1) Earlier medical attention and prompt diagnosis, coupled with early prophylactic anticoagulation, may prevent severe thrombotic and embolic events. However, early prophylactic antiplatelet also holds considerable importance for patients with membranous nephropathy sometimes, while there are many uncertainties regarding its exact implementation [14,16,19]. As a result, future research in this field should aim to maintain consistent treatment plans to facilitate steady data collection and better guide early anticoagulation and antiplatelet treatment. 2) Previously, venous embolism was considered the main type of thrombotic event in nephrotic syndrome patients, with arterial embolism events being overlooked [20]. However, some recent studies have reported arterial embolism events [2,3]. Therefore, the necessity to simultaneously intensify antiplatelet therapy in some patients with membranous nephropathy during early anticoagulation remains to be clarified. To date, there has been no large-scale, multi-center, open study on the epidemiologic characteristics of thrombotic events in nephrotic syndrome patients. Future research may enhance clinical medication strategies in this scenario.
Acknowledgements
The authors extend their gratitude for the patient’s consent, the approval of the hospital ethics committee, and the support of the information department. They also acknowledge the anonymous reviewers for their contributions in editing and reviewing the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Krishna K, Hiremath S, Lakade S, Davakhar S. Acute myocardial infarction in nephrotic syndrome. J Assoc Physicians India. 2015;63:67–68. [PubMed] [Google Scholar]
- 3.Xie L, Tang Y, Liu J, He SQ, Li JH, Zhu Y, Liu ZB, Cheng Z, Gong JB. Acute myocardial infarction in patients of nephrotic syndrome: a case series. J Geriatr Cardiol. 2017;14:481–484. doi: 10.11909/j.issn.1671-5411.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn SG, Tahk SJ, Tahk SJ, Whang JC, Yoo SY, Jang HJ, Xun LZ, Choi SY, Hwang KS, Yoon MH, Shin JH, Kim HS, Choi BI, Kim DH. Intracoronary thrombosis treated with stent and abciximab in patient with membranous glomerulonephritis. Korean Circ J. 2000;30:1307–1311. [Google Scholar]
- 5.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, Navis G, van der Meer J. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117:224–30. doi: 10.1161/CIRCULATIONAHA.107.716951. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Tan TC, Chertow GM, Ordonez JD, Fan D, Law D, Yankulin L, Wojcicki JM, Zheng S, Chen KK, Khoshniat-Rad F, Yang J, Parikh RV. Primary nephrotic syndrome and risks of ESKD, cardiovascular events, and death: the kaiser permanente nephrotic syndrome study. J Am Soc Nephrol. 2021;32:2303–2314. doi: 10.1681/ASN.2020111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han X, Zhao P, Wang Z, Ji X, Zhao M. Acute lower extremity arterial thrombosis associated with nephrotic syndrome in adults: case series and literature review. BMC Nephrol. 2023;24:318. doi: 10.1186/s12882-023-03374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed HN, Bashir AM, Mohamed YG. Multiple venous and pulmonary artery thrombosis as a presenting complaint of nephrotic syndrome-case report and challenges in management. Vasc Health Risk Manag. 2022;18:589–593. doi: 10.2147/VHRM.S371373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513–520. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li SJ, Guo JZ, Zuo K, Zhang J, Wu Y, Zhou CS, Lu GM, Liu ZH. Thromboembolic complications in membranous nephropathy patients with nephrotic syndrome-a prospective study. Thromb Res. 2012;130:501–505. doi: 10.1016/j.thromres.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Lionaki S, Derebail VK, Hogan SL, Barbour S, Lee T, Hladunewich M, Greenwald A, Hu Y, Jennette CE, Jennette JC, Falk RJ, Cattran DC, Nachman PH, Reich HN. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofstra JM, Wetzels JFM. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Kidney Int. 2016;89:981–983. doi: 10.1016/j.kint.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Loscalzo J. Venous thrombosis in the nephrotic syndrome. N Engl J Med. 2013;368:956–958. doi: 10.1056/NEJMcibr1209459. [DOI] [PubMed] [Google Scholar]
- 14.Tian Y, Sun B, Sun G. Research progress of nephrotic syndrome accompanied by thromboembolism. Int Urol Nephrol. 2023;55:1735–1745. doi: 10.1007/s11255-023-03474-8. [DOI] [PubMed] [Google Scholar]
- 15.De Pascali F, Brunini F, Rombolà G, Squizzato A. Efficacy and safety of prophylactic anticoagulation in patients with primary nephrotic syndrome: a systematic review and meta-analysis. Intern Med J. 2024;54:214–223. doi: 10.1111/imj.16227. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, McDonald G, Jolly T, Batten A, Chacko B. A systematic review of prophylactic anticoagulation in nephrotic syndrome. Kidney Int Rep. 2019;5:435–447. doi: 10.1016/j.ekir.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobbes H, Mainbourg S, Lega JC. Prevention of venous thromboembolism in nephrotic syndrome: the quest towards precision medicine. Nephrol Dial Transplant. 2020 doi: 10.1093/ndt/gfaa337. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Lee T, Biddle AK, Lionaki S, Derebail VK, Barbour SJ, Tannous S, Hladunewich MA, Hu Y, Poulton CJ, Mahoney SL, Charles Jennette J, Hogan SL, Falk RJ, Cattran DC, Reich HN, Nachman PH. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420. doi: 10.1038/ki.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutkowska E, Fułek M, Fułek K, Fortuna P, Madziarska K. Nephrotic syndrome - different risk of venous thromboembolism with different approaches to justify prophylactic anticoagulation. Angiology. 2023;74:519–525. doi: 10.1177/00033197221126248. [DOI] [PubMed] [Google Scholar]
- 20.Wolf O, Didier R, Chagué F, Bichat F, Rochette L, Zeller M, Fauchier L, Bonnotte B, Cottin Y. Nephrotic syndrome and acute coronary syndrome in children, teenagers and young adults: systematic literature review. Arch Cardiovasc Dis. 2023;116:282–290. doi: 10.1016/j.acvd.2023.03.002. [DOI] [PubMed] [Google Scholar]


