Abstract
Objective: To evaluate the therapeutic effect of sacubitril/valsartan compared to enalapril in managing heart failure (HF) after percutaneous coronary intervention (PCI). Methods: From January 2018 to December 2021, 63 hospitalized patients diagnosed with HF following acute myocardial infarction (AMI) were enrolled in this prospective clinical trial. The observation group was comprised of 31 patients treated with sacubitril/valsartan (LCZ696) sodium tablets, while the control group, including 32 patients, received enalapril maleate tablets. All patients received standard HF therapy, including water-soluble aspirin, hydroclopidogrel sulfate, once-daily bivalirudin calcium, twice-daily metoprolol tartrate (dose titrated based on heart rate), once-daily spironolactone (dose adjusted for electrolytes), and once-daily dehydroimidazole (dose adjusted for electrolytes). HF symptom control, N-terminal B-type natriuretic peptide precursor (NT-proBNP) levels, cardiac anatomical parameters, heart rate, blood pressure, and 6-minute walking distance over a 90-day follow-up were assessed. The study is registered under ClinicalTrials.gov [ChiCTR2100042944]. Results: On the 30th day post-discharge, the observation group exhibited a marked decrease in NT-proBNP levels and an improvement in left ventricular end-diastolic diameter, in contrast to the control group (both P<0.05). By the 90th day, the observation group showed significant improvements in left ventricular ejection fraction and left ventricular end-systolic diameter index, along with reduced blood pressure and serum creatinine levels (all P<0.05). Furthermore, the observation group displayed a more favorable New York Heart Association class distribution and enhanced performance in the 6-minute walk test (both P<0.05). No significant difference in the incidence of major adverse cardiovascular events was observed between the two groups during the 90-day follow-up period (P>0.05). Conclusion: Our findings indicate that sacubitril/valsartan (LCZ696) Sodium Tablets effectively enhance ventricular remodeling and cardiac function in patients with HF post-AMI, following a short-term treatment regimen. This therapeutic approach holds promise for improving clinical outcomes in this patient population.
Keywords: Sacubitril/valsartan, enalapril, heart failure, acute myocardial infarction, ventricular remodeling
Introduction
Heart failure (HF) is a condition characterized by impaired systolic and/or diastolic function of the heart muscle, resulting in inefficient venous return from the body. HF leads to inadequate perfusion of tissues and organs for normal metabolism, causing various organ dysfunctions and abnormal hemodynamics [1]. Clinically, it manifests as symptoms stemming from systemic or pulmonary circulatory congestion. Over time, HF patients experience declining activity tolerance and quality of life. In China, the number of HF patients has reached 13.7 million, including 6.5-8.75 million over 60 years old, representing a 44% increase over the past 15 years [1,2]. Additionally, the hospitalized mortality rate for HF patients in China stands at 4.1% [3,4]. Despite significant advances in medicine, the treatment of HF has stagnated over the past decade, posing a significant public health concern due to the aging population [5]. HF patients exhibit diverse clinical symptoms and have a higher 5-year mortality rate than several cancers. Despite improvements in living standards, diagnostics, and therapeutic techniques, HF remains a leading cause of morbidity and mortality in the elderly. Therefore, the management of HF continues to be a critical challenge in clinical research.
Over the past two decades, significant advancements have been achieved in the diagnosis and management of HF [5]. Nevertheless, the long-term prognosis remains unsatisfactory, with 5-year survival rates trailing behind those of malignant tumors. The traditional “golden triangle” of HF treatment, despite its utilization of higher doses of Renin-angiotensin-aldosterone system (RAAS) inhibitors, has demonstrated limited effectiveness in reducing readmission and mortality rates. To address this pressing issue, a novel class of drugs, angiotensin receptor endopeptidase inhibitors (ARNIs), has emerged.
ARNIs, exemplified by sacubitril/valsartan (LCZ696), exhibit a unique mechanism of action by inhibiting the RAAS system while activating the natriuretic peptide system [6,7]. This innovative approach modulates neuroendocrine function, resulting in various physiological benefits such as vasodilation, diuresis, and inhibition of sympathetic nerve activity.
The current study aims to provide evidence-based medical guidance on the use of LCZ696 in the treatment of HF patients following acute myocardial infarction (AMI). To achieve this, we compare the representative Angiotensin-converting enzyme inhibitor (ACEI) drug, enalapril maleate tablets, with the representative ARNI drug, sacubitril/valsartan (LCZ696) Sodium Tablets. The findings of this study will contribute to a deeper understanding of the efficacy and adverse reactions associated with these two drug classes in this specific patient population.
Materials and methods
Research subjects
A total of 63 inpatients diagnosed with HF following AMI at Yueyang People’s Hospital of Hunan Province from January 2018 to December 2021 were included. Participants included 45 males and 18 females, with an age range of 40-83 years (mean age: 63.63±10.31 years). The study was approved by the Medical Research Ethics Committee of Yueyang People’s Hospital of Hunan Province, in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from the patients or their legal representatives. The study is registered under ClinicalTrials.gov (ChiCTR2100042944).
Inclusion criteria
(1) Age ≥18 years, with no gender restrictions [8,9]. (2) High-risk for non-ST-segment elevation myocardial infarction (NSTEMI) as assessed by NSTEMI risk stratification [10]. (3) Fulfillment of diagnostic criteria for ST-segment elevation myocardial infarction (STEMI) based on the “Fourth Universal Definition of Myocardial Infarction, 2018”: Type I AMI defined by markers of myocardial injury (e.g., troponin) exceeding the 99th percentile upper reference limit at least once, accompanied by at least one of the following ischemic manifestations: Typical ischemic symptoms; New ischemic changes on ECG (e.g., left bundle branch block, ST-T changes) [10]; New pathological Q waves on ECG; Imaging evidence of new regional wall motion abnormalities or loss of viable myocardium; Coronary thrombosis on angiography, imaging, or autopsy. (4) Successful emergency percutaneous coronary intervention (PCI) with direct culprit vessel opening and postoperative heart rate >80 beats/min. (5) Development of HF post-PCI, classified as Killip I-III, and fulfilling NT-proBNP diagnostic criteria for HF [9,11]. (6) Fulfillment of diagnostic criteria for HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), or HF with preserved ejection fraction (HFpEF) post-PCI [12]. (7) Timely medication adherence during the observation period, regular outpatient follow-up, and good drug compliance. (8) Informed and willing participants, having signed the informed consent form.
Exclusion criteria
(1) Patients with known intolerance or allergy to ARNI/ACEI drugs. (2) Patients with unstable hemodynamic indices, such as Killip class IV or blood pressure <90/60 mmHg. (3) Hospital potassium concentration (K*) levels >5.5 mmol/L. (4) Concurrent use of drugs that interfere with LCZ696 metabolism, such as aliskiren. (5) Patients with poor compliance, difficulty in follow-up, or communication difficulties. (6) Pregnant or lactating women. (7) Patients with mental disorders or malignancies. (8) Coexisting coagulation dysfunction, liver and kidney insufficiency, or organic lesions (e.g., creatine kinase-MB >265 umol/L, Child-Pugh class C) [13]. (9) Patients with organic heart diseases, including heart valve disease, hypertensive heart disease, myocarditis, pericarditis, and congenital heart disease.
Main equipment, instruments and main therapeutic drugs
For blood pressure measurements, we utilized electronic sphygmomanometers (Dalian Omron Co., Ltd.) and desktop sphygmomanometers (Yuyue Medical Equipment Co., Ltd.). Cardiac ultrasonography was completed using color ultrasonic diagnostic equipment (Zhixinheng Technology Co., Ltd.). A digital 12-channel electrocardiography machine (Li Bang Precision Instrument Co., Ltd.) was employed for 12-lead electrocardiogram recordings. Receptor-ligand interactions were analyzed using the Receptor-Ligand Interaction Assay (ReLIA) Multifunctional Immunoassay system (Ruilai Bioengineering (Shenzhen) Co., Ltd.).
Coronary angiograms were obtained using the Philips Digital Subtraction Angiography (DSA) system from Royal Philips of the Netherlands. Biochemical data analysis was conducted with the Hitachi 7600 Automated Biochemistry Analyzer (Dahua Group Co., Ltd.).
In terms of therapeutic drugs, Sacubitril/Valsartan Sodium Salt Tablets (LCZ696) (Novartis Pharmaceutical Co., Ltd.), Enalapril Maleate Tablets (Shanghai Modern Pharmaceutical Co., Ltd.), Valsartan Capsules (Tianda Pharmaceuticals Limited), furosemide tablets (Tianjin Lisheng Pharmaceutical Co., Ltd.), spironolactone tablets (Jiangsu Changjiang Pharmaceutical Co., Ltd.) and baispiride enteric-coated tablets (Bayer healthcare Co., Ltd.) were administered for HF treatment.
For AMI treatment, clopidogrel bisulfate tablets (Sanofi Pharmaceutical Co., Ltd.), pitavastatin calcium tablets (Jiangsu Wanbang Biochemical Pharmaceutical Co., Ltd.), metoprolol tartrate tablets (Suzhou Yushi Pharmaceutical Co., Ltd.) and spironolactone tablets were administered.
Research grouping
Eligible patients were randomly assigned into two groups using a random number table, based on their admission order. The observation group received oral sacubitril/valsartan (LCZ696) Sodium Tablets, while the control group received oral enalapril maleate tablets. Other oral treatment drugs remained largely unchanged between the two groups. Detailed research protocols spanning from admission to endpoint are outlined below.
Inspection on admission
On the first day of admission, following emergency PCI, comprehensive patient data were recorded. This encompassed age, gender, body weight, comorbidities (including diabetes, hypertension, stroke, hyperlipidemia), smoking history, Killip classification, NSTEMI/STEMI status, involvement of other diseased blood vessels, post-PCI thrombolysis in myocardial infarction (TIMI) blood flow classification, and current oral medications (such as dual antiplatelet therapy (DAPT), statins, receptor antagonists, mineralocorticoid receptor antagonists (MRA), and diuretics). Additionally, NT-proBNP, liver function, routine biochemical, and blood lipid tests were conducted. Blood pressure and heart rate were measured, and a comprehensive transthoracic echocardiography was performed to assess left ventricular ejection fraction (LVEF), left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic diameter (LVEDD) [14-16]. Once the patient’s condition stabilized, the 6-minute walk test (6MWT) was administered to measure walking distance. The standard oral treatment regimen for post-PCI patients, as recommended by guidelines, included aspirin enteric-coated tablets (100 mg once daily), clopidogrel hydrogen sulfate tablets (75 mg once daily), pitavastatin calcium tablets (2 mg once daily), metoprolol tartrate tablets (initially 12.5 mg twice daily, with dose titrated based on heart rate), spironolactone (20 mg once daily, with dose adjusted according to electrolytes), and semide (20 mg once daily, with dose adjusted based on electrolytes). Sacubitril/Valsartan (LCZ696) Sodium Tablets Administration: If currently taking ACEIs, discontinue for 36 hours before initiating sacubitril/valsartan (LCZ696) Sodium Tablets. For patients not previously taking ACEIs, initiate sacubitril/valsartan on the first day of admission (following emergency PCI). Begin with an initial oral dose of 25 mg twice daily, doubling the dose every 14 days until reaching the target dose of 200 mg twice daily. If intolerance (e.g., asymptomatic hypotension) occurs, maintain the current maximum tolerated dose.
Enalapril Maleate Tablets Administration: On the first day of admission (post-emergency PCI), administer enalapril maleate tablets orally in addition to conventional treatment. Start with an initial dose of 2.5 mg twice daily, doubling the dose every 14 days until reaching the target dose of 10 mg twice daily. Maintain the current maximum tolerated dose if intolerance occurs [17].
Follow-up examinations
On the 30th and 90th days post-discharge, liver and kidney function, electrolytes, NT-proBNP levels were assessed in outpatient clinics. Echocardiography was performed, and blood pressure, heart rate, 6MWT, and cardiac function (New York Heart Association (NYHA) class) were measured. During this period, all patients were monitored via telephone for any adverse reactions, including symptomatic hypotension, angioedema, dry cough, impaired liver and kidney function, and major adverse cardiovascular events. These were recorded and tallied. The drug regimen was adjusted accordingly based on patients’ test results and drug tolerance.
Observation indicators and follow-up after discharge
Collect general information
General data was collected for all subjects, including gender, weight, age, comorbidities (hypertension, stroke, hyperlipidemia, diabetes), smoking history, blood lipid status, Killip classification, diseased blood vessels, NSTEMI/STEMI count, post-PCI TIMI blood flow classification, and other oral medications (DAPT, statins, receptor antagonists, MRA, diuretics).
NT-pro BNP levels
Collect fasting venous blood in the morning on the 1st day of admission, 30th day of discharge, and 90th day of discharge. Measure NT-proBNP levels using a ReLIA multifunctional immunoassay instrument and record the values.
Transthoracic doppler echocardiography
In this study, transthoracic Doppler echocardiography was employed to assess LVEF, LVESD, and LVEDD by echocardiography. LVEF measurements were derived using the Simpson method. Each parameter was measured twice by the same sonographer in our hospital’s B-ultrasound department, and the average value was recorded.
Heart rate
After a 10-minute resting period in a seated position, heart rate was measured twice, and the average value was calculated.
Blood pressure
Prior to measurement, patients were instructed to sit and rest for 10 minutes. Blood pressure was measured twice in both left and right arms, and the average value was taken.
6MWT
The 6-minute walking distance was recorded for all patients in a calm state when their condition was stable, upon admission, on the 30th day, and on the 90th day post-discharge.
Adverse reactions
Fasting venous blood samples were collected from all patients on the 1st day, 30th day, and 90th day post-discharge for biochemical analysis. The Hitachi 7600 automatic biochemical analyzer was used to determine uric acid (UA), blood urea nitrogen (BUN), alanine aminotransferase (ALT), serum creatinine (SCr), serum potassium ion concentration (K*), and aspartate transaminase (AST) levels [18,19]. Adverse reactions included: K*>5.5 mmol/L, creatine kinase-MB (CK-MB) >265 μmol/L or an increase of >10% compared to pre-treatment values, angioedema, dry cough, ALT and AST levels increased more than 3 times compared to pre-treatment values, and blood pressure <90/60 mmHg (accompanied by symptoms such as dizziness or headache).
Major adverse cardiovascular events
MACE comprises readmission for HF, unstable angina, recurrent myocardial infarction, repeat revascularization, cardiac death, and malignant arrhythmia.
Evaluation of therapeutic efficacy
Post-discharge follow-up assessments were conducted on the 30th and 90th days using the NYHA class. Therapeutic efficacy was defined according to the Chinese Medical Association Cardiovascular Diseases Branch (CSC) criteria: markedly effective (NYHA improvement of ≥II grades), effective (NYHA improvement of I grade but not to ≤II), and ineffective (NYHA improvement of <I grade or worsening of condition) [18]. The total effective rate was calculated as (markedly effective + effective) cases/total cases × 100% [20].
Statistical methods
Data analysis was conducted using SPSS software (version 25.0). Continuous variables were expressed as mean ± standard deviation and compared between groups using independent samples t-tests, assuming normal distribution confirmed by the Shapiro-Wilk test. Categorical variables were presented as frequencies and percentages, and group comparisons were performed using chi-square tests or Fisher’s exact tests where appropriate. The primary outcomes, including NT-proBNP levels and cardiac anatomical parameters, were analyzed using mixed-effects models to account for repeated measures over the 90-day follow-up. Adjustments for multiple comparisons were made using the Bonferroni method. The effect size was calculated for significant findings using Cohen’s d. A two-tailed P-value of <0.05 was considered statistically significant.
Results
During the 90-day follow-up, all 31 patients in the observation group completed the trial. Three patients experienced asymptomatic hypotension, but after adjusting the dosages of sacubitril/valsartan and trandolapril, their blood pressure remained within the normal range. Among the 32 patients in the control group. Two patients withdrw due to a significant increase in muscle and liver enzyme values. Additionally, two patients developed dry cough and were subsequently switched from enalapril maleate to sacubitril/valsartan for continued treatment. Therefore, 30 people in the control group finally completed the trial.
Comparison of general data and blood vessels
Baseline comparisons between the two groups included age, sex, weight, comorbidities (hypertension, stroke, hyperlipidemia, diabetes), smoking history, blood lipids, Killip classification, type of myocardial infarction (NSTEMI/STEMI), affected vessels, and surgical procedures. Post-TIMI blood flow assessments, use of other oral medications (DAPT, statins, β-blockers, MRA, diuretics), and other clinical data were also compared. See Tables 1, 2 and Figure 1.
Table 1.
Comparison of general data
| Observation index | Observation (n=31) | Control (n=30) | P |
|---|---|---|---|
| Male | 24 (77.7) | 21 (70) | 0.513 |
| Age (years) | 61.7±11.2 | 68.6±9.4 | 0.103 |
| Body weight (kg) | 68.8±6.6 | 70.5±6.4 | 0.295 |
| Smoking history | 23 (74.4) | 24 (80.0) | 0.592 |
| Diabetes | 8 (25.6) | 7 (23.5) | 0.825 |
| Hypertension | 13 (41.7) | 15 (50.0) | 0.525 |
| Cerebral apoplexy | 7 (22.4) | 9 (30.0) | 0.513 |
| Hyperlipidemia | 11 (35.3) | 16 (53.5) | 0.163 |
| Killip III number | 13 (41.7) | 15 (50.0) | 0.525 |
| NSTEMI number | 8 (25.6) | 11 (36.6) | 0.363 |
| Postoperative TIMI blood flow grade 3 | 31 (100) | 30 (100) | 1.000 |
| DAPT | 31 (100) | 30 (100) | 1.000 |
| Pitavastatin Calcium Tablets | 31 (100) | 30 (100) | 1.000 |
| Metoprolol tartrate tablets | 31 (100) | 30 (100) | 1.000 |
| Spironolactone tablets | 31 (100) | 30 (100) | 1.000 |
| Furosemide tablets | 31 (100) | 30 (100) | 1.000 |
| TG (mmol/L) | 1.8±1.3 | 1.7±1.8 | 0.726 |
| CHOL (mmol/L) | 5.2±1.6 | 4.6±1.2 | 0.097 |
| HDL-C (mmol/L) | 1.5±0.5 | 1.4±0.5 | 0.064 |
| LDL-C (mmol/L) | 3.3±1.3 | 2.6±0.6 | 0.082 |
| FPG (mmol/L) | 5.5±1.5 | 5.5±1.3 | 0.155 |
Note: NSTEMI: Non-ST-segment Elevation Myocardial Infarction, DAPT: Dual Antiplatelet Therapy, TIMI: Thrombolysis in Myocardial Infarction, TG: Triglycerides, CHOL: Cholesterol, HDL-C: High-Density Lipoprotein Cholesterol, LDL-C: Low-Density Lipoprotein Cholesterol, FPG: Fasting Plasma Glucose.
Table 2.
Comparison of diseased blood vessels
| Observation index | Observation (n=31) | Control (n=30) | P |
|---|---|---|---|
| LAD | 20 | 22 | 0.746 |
| LCX | 15 | 12 | 0.635 |
| RCA | 10 | 7 | 0.482 |
| LM | 4 | 2 | 0.548 |
Note: LAD: Left Anterior Descending Artery, LCX: Left Circumflex Artery, RCA: Right Coronary Artery, LM: Left Main Coronary Artery or Left Main Stem.
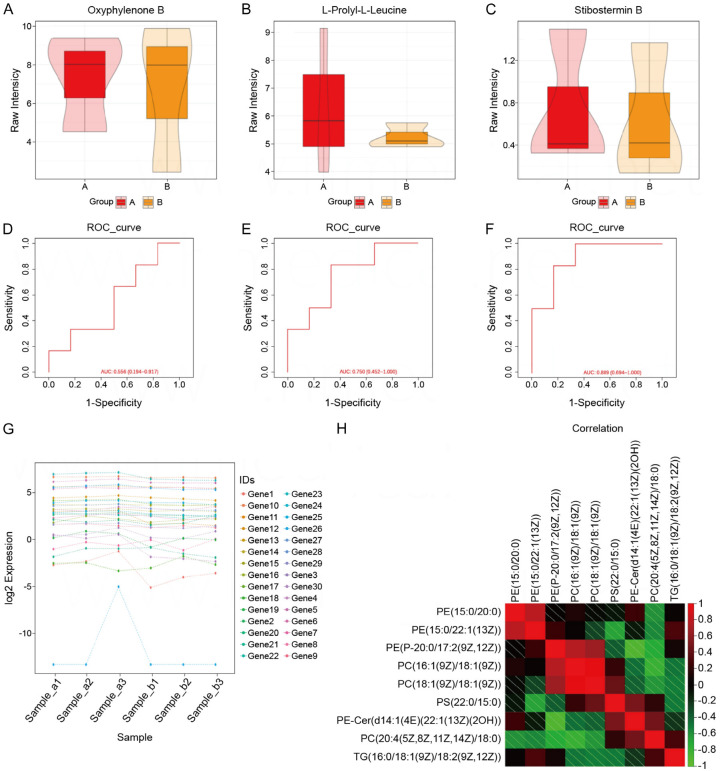
Figure 1.
Comparison of general data. A. Comparison of box plots of Oxyphylenone B in observation and control groups. B. Box plot comparison of L-Prolyl-L-Leucine in observation and control groups. C. Box plot comparison of Stibostemin B in observation and control groups. D. ROC curves based on Oxyphylenone B prediction models. E. ROC curve based on L-Prolyl-L-Leucine predictive modeling. F. ROC curve based on Stibostemin B predictive modeling. G. Log2 expression of 30 genes in six samples. H. Correlation analysis between 9 parameters.
Comparison of NT-proBNP levels
Analysis revealed no significant difference in NT-proBNP levels between the observation and control groups upon admission (P>0.05). The study demonstrated improved therapeutic efficacy in the observation group compared to the control group across different post-treatment periods (P<0.05). See Table 3.
Table 3.
Comparison of NT-proBNP levels
| Group (number of cases) | Day 1 of admission | Day 30 of discharge | 90th day of discharge |
|---|---|---|---|
| Observation (n=31) | 5379.7±2026.9 | 2204.6±1549.7 | 605.4±581.6 |
| Control (n=30) | 5652.7±2004.8 | 3862.7±1580.5 | 1950.5±1051.3 |
| P | 0.651 | <0.01 | <0.01 |
Note: NT-proBNP: N-terminal B-type natriuretic peptide precursor.
Comparison of cardiac anatomical parameters
After thorough analysis, it was determined that on the first day of hospitalization, there were no significant differences in LVEF, LVESD and LVEDD between the observation and control groups (P=0.976, P=0.539, P=0.368). By the 30th day post-discharge, compared to the first day of hospitalization, LVEF had increased, while LVESD and LVEDD had decreased. While LVEF and LVESD remained similar between groups (both P>0.05), a significant difference in LVEDD was observed (P<0.05). By the 90th day post-discharge, both LVEF, LVESD, and LVEDD had further improved compared to the first day of hospitalization and the 30th day post-discharge (P<0.001) [21]. The study results indicate that cardiac anatomical parameters in the observation group improved over time, with more significant improvements on the 90th day post-discharge compared to the 30th day. See Table 4 and Figure 2.
Table 4.
Comparison of cardiac parameters
| Group (number of cases) | Period | LVEF (%) | LVESD (mm) | LVEDD (mm) | P value (LVEF) | P value (LVESD) | P value (LVEDD) |
|---|---|---|---|---|---|---|---|
| Observation (n=31) | Day 1 of admission | 39.6±6.6 | 49.2±6.6 | 58.8±5.6 | - | - | - |
| Day 30 of discharge | 42.3±5.4 | 43.3±5.4 | 52.6±4.2 | - | - | - | |
| 90th day of discharge | 47.5±6.6 | 36.5±6.4 | 46.5±6.4 | - | - | - | |
| Control (n=30) | Day 1 of admission | 39.6±7.2 | 47.1±5.7 | 60.2±5.6 | 0.99 | 0.84 | 0.06 |
| Day 30 of discharge | 41.3±7.5 | 45.4±5.5 | 58.6±5.5 | 0.52 | 0.03 | 0.001 | |
| 90th day of discharge | 43.5±6.6 | 43.7±5.5 | 54.4±5.5 | 0.47 | 0.02 | 0.04 |
Note: LVEF: Left Ventricular Ejection Fraction, LVESD: Left Ventricular End-Systolic Diameter, LVEDD: Left Ventricular End-Diastolic Diameter.
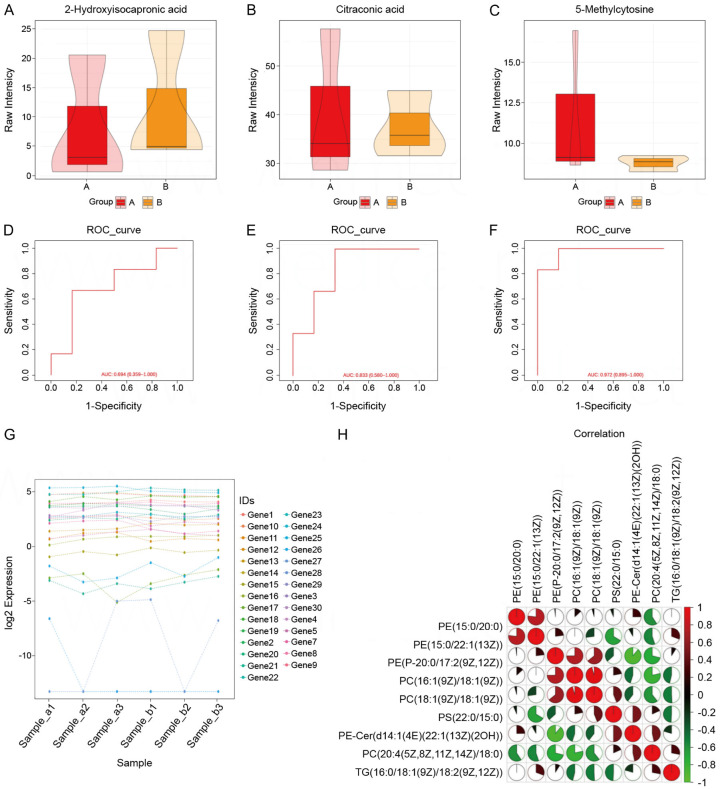
Figure 2.
Comparison of cardiac parameters. A. Comparison of box plots of 2-hydroxyisocaproic acid between observation and control groups. B. Box plot comparison of Citraconic acid in observation and control groups. C. Box plot comparison of 5-Methylcytosine in observation and control groups. D. ROC curve based on the prediction model of 2-hydroxyisocaproic acid. E. ROC curve based on the prediction model of Citraconic acid. F. ROC curve based on the prediction model of 5-Methylcytosine. G. Log2 expression of 30 genes in six samples. H. Correlation analysis between nine parameters.
Comparison of heart rate and blood pressure changes
Similarly, on the first day of hospitalization, there were no significant differences in heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) between the two groups (all P>0.05). However, from the follow-up to the 30th and 90th days post-discharge, HR was lower than on the first day of hospitalization. On the 30th day post-discharge, SBP and DBP were lower than on the first day of admission (P<0.05). By the 90th day post-discharge, SBP and DBP in the observation group were further reduced compared to the 30th day post-discharge (P<0.05). These results suggest that with an increased oral dosage of the drug, blood pressure in the observation group decreased more significantly than in the control group. See Table 5 and Figure 3.
Table 5.
Comparison of heart rate and blood pressure of patients
| Group (number of cases) | Time | HR (beats/min) | SBP (mmHg) | DBP (mmHg) | P value (HR) | P value (SBP) | P value (DBP) |
|---|---|---|---|---|---|---|---|
| Observation (n=31) | Day 1 of admission | 88.2±10.2 | 126.4±13.5 | 80.4±8.6 | - | - | - |
| Day 30 of discharge | 75.5±5.8 | 109.9±8.4 | 70.4±8.5 | - | - | - | |
| 90th day of discharge | 67.5±5.2 | 98.6±5.3 | 65.4±4.3 | - | - | - | |
| Control (n=30) | Day 1 of admission | 87.3±8.6 | 123.3±10.6 | 77.4±10.6 | 0.85 | 0.58 | 0.76 |
| Day 30 of discharge | 74.5±9.3 | 109.5±7.3 | 69.6±6.6 | 0.01 | 0.02 | 0.12 | |
| 90th day of discharge | 67.5±4.6 | 105.7±8.3 | 68.7±6.3 | <0.01 | <0.01 | 0.04 |
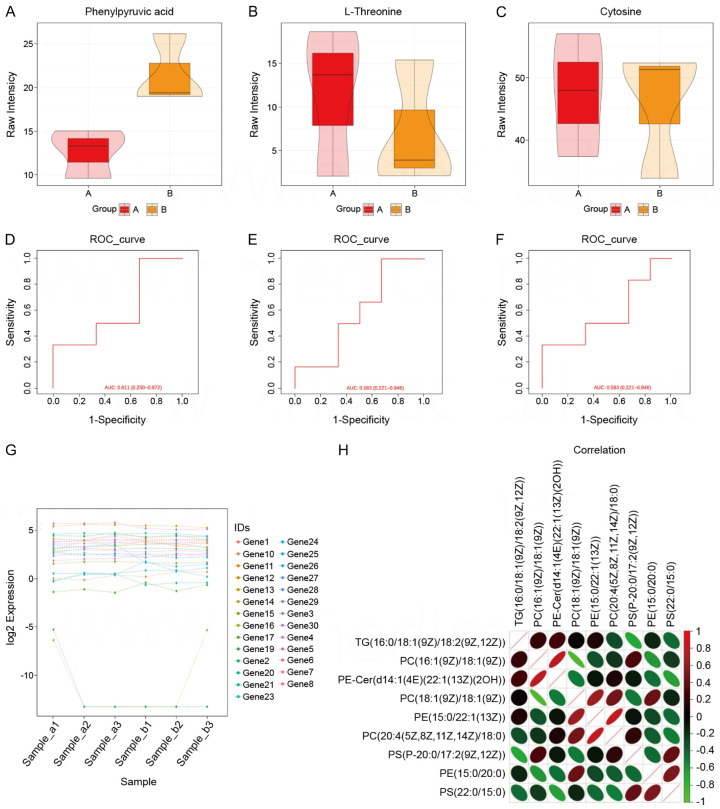
Figure 3.
Comparison of heart rate and blood pressure of patients. A. Comparison of box plots of Phenylpyruvic acid in the observation and control groups. B. Box plot comparison of L-Threonine in observation and control groups. C. Box plot comparison of Cytosine in observation and control groups. D. ROC curves based on Phenylpyruvic acid prediction models. E. ROC curve based on L-Threonine prediction model. F. ROC curve based on Cytosine prediction model. G. Log2 expression of 30 genes in six samples. H. Correlation analysis between nine parameters.
Comparison of 6MWT outcomes
Upon analysis, it was ascertained that upon initial admission, there was no significant difference in 6MWD between the observation and control groups (P>0.05). However, by the 30th day post-discharge, the 6MWD had significantly increased compared to the initial admission day (P<0.05). Furthermore, on the 90th day post-discharge, the 6MWD continued to improve, surpassing both the in-hospital value and the 30th-day post-discharge measurement. The results indicate that treatment led to an enhancement in walking distance during the 6MWT, and this improvement was more pronounced at the 90th day post-discharge compared to the 30th day. See Table 6.
Table 6.
Comparison of 6MWT of patients
| Group (number of cases) | Admission (m) | Discharge day 30 (m) | 90 days after discharge (m) |
|---|---|---|---|
| Observation (n=31) | 333.5±32.3 | 387.2±49.3 | 471.02±32.3 |
| Control (n=30) | 324.5±44.5 | 370.2±37.7 | 417.4±23.1 |
| P | 0.512 | 0.345 | 0.03 |
Note: 6MWT: 6-minute walk test.
Comparison of medication dosage and compliance
During the 90-day follow-up period post-discharge, the final oral dosages in the observation group were as follows: 20 patients received the target dose of 200 mg, twice daily, while 8 patients only tolerated a lower dose of 100 mg, twice daily. In the control group, 25 patients received the target dose, comprising 23 patients on enalapril maleate tablets (20 mg, twice daily) and 2 patients on valsartan capsules (80 mg, once daily). Additionally, 3 patients required a reduced dose of enalapril maleate (10 mg, twice daily).
Comparison of renal and liver function monitoring
Analysis revealed no difference in UA, BUN, SCr, K*, AST levels between the groups on the first day of hospitalization. Through the 90-day follow-up, UA, BUN, and K* levels remained unchanged compared to admission (all P>0.05). However, SCr, ALT, and AST levels were higher in the observation group compared to admission. Conversely, in the control group, ALT and AST levels decreased significantly (both P<0.001). On the 90th day post-discharge, no significant changes were observed in other indices (P>0.05), except for SCr, which was statistically different between the observation and control groups (P<0.05). See Table 7 and Figure 4.
Table 7.
Comparison of liver and kidney functions and electrolytes
| Group (number of cases) | Time | UA (mmol/L) | BUN (mmol/L) | SCr (mmol/L) | K* (mmol/L) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|---|---|---|
| Observation (n=31) | Admission day 1 | 378.4±93.5 | 6.7±2.3 | 89.7±28.3 | 4.2±0.5 | 284.4±225.6 | 69.6±44.7 |
| 90 days after discharge | 358.3±81.6 | 5.4±1.3 | 67.4±18.5 | 4.3±0.6 | 21.3±7.8 | 20.5±8.8 | |
| Control (n=30) | Admission day 1 | 335.6±86.8 | 5.7±2.3 | 80.25±25.5 | 4.3±0.5 | 226.3±13.05 | 61.4±27.5 |
| 90 days after discharge | 337.2±52.3 | 5.6±1.7 | 81.6±15.4 | 4.3±0.6 | 23.5±7.8 | 21.5±8.8 | |
| P | Admission day 1 | 0.76 | 0.34 | 0.08 | 0.99 | 0.65 | 0.47 |
| 90 days after discharge | <0.01 | 0.36 | <0.01 | 0.75 | <0.01 | 0.45 |
Note: UA: uric acid, BUN: blood urea nitrogen, ALT: alanine aminotransferase, SCr: serum creatinine, K*: serum potassium ion concentration, AST: aspartate transaminase.
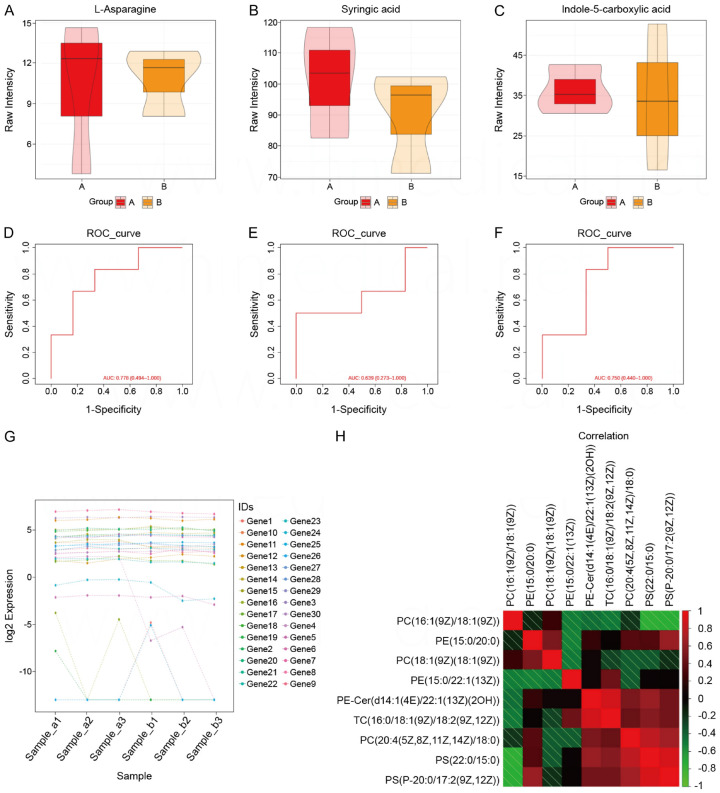
Figure 4.
Comparison of liver and kidney functions and electrolytes. A. Comparison of box plots of L-Asparagine in observation and control groups. B. Comparison of box plots for Syringic acid in observed and control groups. C. Box plot comparison of Indole-5-carboxylic acid for observation and control groups. D. ROC curve based on L-Asparagine prediction model. E. ROC curve based on Syringic acid prediction model. F. ROC curve based on Indole-5-carboxylic acid prediction model. G. Log2 expression of 30 genes in six samples. H. Correlation analysis between nine parameters.
Comparison of NYHA class and clinical efficacy
Based on the analysis, on the first day of hospitalization, the total proportion of NYHA class I-II patients was similar between the groups (16% vs. 10%), with no significant difference (P=0.482). By the 30th day post-discharge, this proportion increased to 42% in the observed group and 30% in the control group. By the 90th day, the proportion further increased to 90% in the observed group and 77% in the control group, indicating an improvement in cardiac function over time. See Table 8 and Figure 5.
Table 8.
Cardiac function classification (NYHA classification)
| Group | Class I, cases (%) | Grade II, cases (%) | Grade III, cases (%) | |
|---|---|---|---|---|
| Observation (n=31) | Day 1 of admission | 0 (0) | 5 (16) | 26 (84) |
| Day 30 of discharge | 4 (13) | 9 (29) | 12 (39) | |
| 90th day of discharge | 13 (42) | 15 (48) | 3 (10) | |
| Control (n=30) | Day 1 of admission | 0 (0) | 3 (10) | 27 (90) |
| Day 30 of discharge | 1 (3) | 8 (27) | 16 (53) | |
| 90th day of discharge | 6 (20) | 17 (57) | 7 (23) | |
Note: NYHA: New York Heart Association.
Figure 5.
Cardiac function classification (NYHA classification). A. Comparison of box plots of Dimethylmalonic acid in observation and control groups. B. Comparison of box plots forL-seryl-L-lsoleucine in observed and control groups. C. Box plot comparison of 3-Hydroxy-1-14-hydroxy-3,5-dimethoxyphenylipropan-1-one for observation and control groups. D. ROC curve based on Dimethylmalonic acid prediction model. E. ROC curve based on L-seryl-L-lsoleucine prediction model. F. ROC curve based on 3-Hydroxy-1-14-hydroxy-3,5-dimethoxyphenylipropan-1-one prediction model. G. Log2 expression of 30 genes in six samples. H. Correlation analysis between nine parameters. NYHA: New York Heart Association.
Over the 90-day follow-up period, the patients’ clinical efficacy was assessed using the NYHA classification. In the observation group, 9 patients (29%) demonstrated marked effectiveness, 18 (58%) showed effectiveness, and 4 (13%) were ineffective. In the control group, 5 patients (17%) were marked as effective, 16 (53%) were effective, and 9 (30%) were ineffective. The total effective rate was 87% in the observed group and 70% in the control group, indicating no significant difference in clinical efficacy between the groups. See Table 9.
Table 9.
Comparison of clinical efficacy
| Group | Effective | Remarkable effect | Invalid | Total effective rate |
|---|---|---|---|---|
| Observation (n=31) | 18 (58) | 9 (29) | 4 (13) | 27 (87) |
| Control (n=30) | 16 (53) | 5 (17) | 9 (30) | 21 (70) |
Discussion
In recent years, the incidence and mortality of AMI have surged significantly, which may be attributed to the rising consumption of fatty foods and irregular lifestyles. Epidemiological studies in China reveal that AMI patients tend to be younger and older, with a higher prevalence in rural areas [22]. The pathological mechanism of AMI involves the rupture of unstable lipid plaques, exposing endothelial collagen and triggering the formation of platelet thrombi or white blood cell clots. Concurrently, the coagulation cascade is rapidly activated, accelerating fibrin production and massive fibrin deposition, ultimately capturing red blood cells to form red thrombi. This process leads to acute stenosis or occlusion of the coronary artery lumen. Consequently, antiplatelet and antithrombotic therapy plays a pivotal role in the prevention and management of AMI.
Previous studies have demonstrated that cardiomyocyte necrosis commences from the endocardium within 18 minutes after coronary artery occlusion, with irreversible injury occurring between 20 and 40 minutes [23,24]. Over two-thirds of cardiomyocytes can sustain obstruction for more than three hours, leading to transverse wall myocardial necrosis within six hours, resulting in infarcted myocardial tissue. Each 30-minute extension in total myocardial ischemia time increases the patient’s mortality risk by 7.5%, indicating a negative correlation with prognosis [23,24]. Crucially, managing total myocardial ischemia time within 120 minutes is vital for improving survival rates. Prompt vessel reopening and restoration of coronary reperfusion via stent implantation within this timeframe can minimize necrosis and thus mortality.
While timely ischemia-reperfusion reduces myocardial necrosis, it can also inflict severe damage upon reperfusion, known as myocardial ischemia-reperfusion injury (MIRI). This injury is mediated by various cellular signaling pathways and substances, including calcium overload, oxygen free radical production, and activation of multiple signaling cascades. Notably, the accumulation of excessive reactive oxygen species is a pivotal factor in MIRI [25]. MIRI can exacerbate disease progression, hasten cardiomyocyte demise, and foster HF. Although advancements in chest pain centers have significantly lowered AMI mortality, post-infarction complications, including HF, arrhythmia, cardiogenic shock, and papillary muscle/tendon rupture, still detract from patients’ quality of life. HF following AMI, particularly, remains a critical public health concern demanding clinicians’ attention [15,16]. Over half of HF cases stem from ischemic heart disease due to myocardial infarction. Despite notable improvements in myocardial reperfusion therapies in China, particularly thrombolysis and PCI, many patients miss the optimal reperfusion window or suffer reperfusion injury post-PCI, resulting in myocardial ischemia-hypoxia, aberrant ventricular wall motion, diastolic-systolic dysfunction, and ultimately ventricular wall remodeling and HF [26]. Clinical studies investigating LCZ696’s efficacy in treating HF post-AMI are scarce, with most research focused on animal models [17,18]. LCZ696 is proposed to attenuate cardiac hypertrophy markers by mitigating inflammatory cell responses and collagen synthesis, while fostering early degradation and weakening of the extracellular matrix, thereby remodeling damaged heart muscles post-AMI and minimizing acute/chronic myocardial injuries. Studies have demonstrated the effectiveness of LCZ696 in treating acute anterior myocardial infarction with HF, enhancing cardiac function [18,19]. However, contrasting reports suggest that short-term treatment with LCZ696 or ACEI drugs yield similar prognostic and therapeutic outcomes, though these conclusions remain contentious.
The present study’s findings indicate that LCZ696 reduces NT-proBNP levels, increases LVEF, and decreases LVEDD and LVESD in the short-term, suggesting its application improves cardiac function in patients with HF following AMI [22,23]. Collectively, these studies suggest that LCZ696 facilitates cardiac function improvement in patients with AMI complicated by HF. This study exhibits both congruencies and disparities with previous research, potentially attributed to factors such as the limited sample size, specific infarction location, varying definitions of MACE in HF post-AMI, therapeutic drug follow-up duration, and regional/genetic variations. Notably, the present study is a single-center investigation, and although the center serves as the largest medical facility in the region with extensive outreach and a high patient volume, it cannot comprehensively represent the entire Chinese population. Therefore, a large-scale, prospective, randomized, double-blind clinical trial is imperative to validate the efficacy of LCZ696 in treating HF post-AMI.
Several limitations should be considered when interpreting these results. The limitations include a relatively small sample size, which may affect the generalizability of the findings, and a short follow-up duration that may not capture long-term treatment effects. Additionally, the study was conducted at a single center, which could introduce center-specific biases.
Collectively, the findings indicate that LCZ696 effectively promotes ventricular remodeling and cardiac function recovery in patients with HF following AMI, suggesting its potential to improve clinical outcomes in this patient population.
Disclosure of conflict of interest
None.
References
- 1.She J, Lou B, Liu H, Zhou B, Jiang GT, Luo Y, Wu H, Wang C, Yuan Z. ARNI versus ACEI/ARB in reducing cardiovascular outcomes after myocardial infarction. ESC Heart Fail. 2021;8:4607–4616. doi: 10.1002/ehf2.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jering KS, Claggett B, Pfeffer MA, Granger C, Køber L, Lewis EF, Maggioni AP, Mann D, McMurray JJV, Rouleau JL, Solomon SD, Steg PG, van der Meer P, Wernsing M, Carter K, Guo W, Zhou Y, Lefkowitz M, Gong J, Wang Y, Merkely B, Macin SM, Shah U, Nicolau JC, Braunwald E. Prospective ARNI vs. ACE inhibitor trial to determine superiority in reducing heart failure events after myocardial infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail. 2021;23:1040–1048. doi: 10.1002/ejhf.2191. [DOI] [PubMed] [Google Scholar]
- 3.Abboud A, Januzzi JL. Reverse cardiac remodeling and ARNI therapy. Curr Heart Fail Rep. 2021;18:71–83. doi: 10.1007/s11897-021-00501-6. [DOI] [PubMed] [Google Scholar]
- 4.Kuchulakanti PK. ARNI in cardiovascular disease: current evidence and future perspectives. Future Cardiol. 2020;16:505–515. doi: 10.2217/fca-2019-0089. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Fan Y, Li J, Chen M, Chen A, Yang D, Guan X, Cao Y. Combination of LCZ696 and ACEI further improves heart failure and myocardial fibrosis after acute myocardial infarction in mice. Biomed Pharmacother. 2021;133:110824. doi: 10.1016/j.biopha.2020.110824. [DOI] [PubMed] [Google Scholar]
- 6.Rubattu S, Gallo G, Volpe M. Sacubitril/valsartan: potential impact of ARNi “beyond the wall” of ACE2 on treatment and prognosis of heart failure patients with coronavirus disease-19. Front Cardiovasc Med. 2020;7:616564. doi: 10.3389/fcvm.2020.616564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg B. Angiotensin receptor-neprilysin inhibition (ARNI) in heart failure. Int J Heart Fail. 2020;2:73–90. doi: 10.36628/ijhf.2020.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefer DJ, Sharp TE 3rd. Angiotensin receptor-neprilysin inhibitors emerge as potential treatment for acute myocardial infarction. J Am Coll Cardiol. 2018;72:2357–2359. doi: 10.1016/j.jacc.2018.08.2170. [DOI] [PubMed] [Google Scholar]
- 9.Volpe M, Rubattu S, Battistoni A. ARNi: a novel approach to counteract cardiovascular diseases. Int J Mol Sci. 2019;20:2092. doi: 10.3390/ijms20092092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Zaiti SS, Martin-Gill C, Zègre-Hemsey JK, Bouzid Z, Faramand Z, Alrawashdeh MO, Gregg RE, Helman S, Riek NT, Kraevsky-Phillips K, Clermont G, Akcakaya M, Sereika SM, Van Dam P, Smith SW, Birnbaum Y, Saba S, Sejdic E, Callaway CW. Machine learning for ECG diagnosis and risk stratification of occlusion myocardial infarction. Nat Med. 2023;29:1804–13. doi: 10.1038/s41591-023-02396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khariton Y, Fonarow GC, Arnold SV, Hellkamp A, Nassif ME, Sharma PP, Butler J, Thomas L, Duffy CI, DeVore AD, Albert NM, Patterson JH, Williams FB, McCague K, Spertus JA. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:933–941. doi: 10.1016/j.jchf.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, Felker GM, Piña IL, O’Connor CM, Lindenfeld J, Januzzi JL, Cohen LS, Ahmad T. Clinical implications of the New York Heart Association Classification. J Am Heart Assoc. 2019;8:e014240. doi: 10.1161/JAHA.119.014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajra A, Ujjawal A, Sud K, Chakraborty S, Bandyopadhyay D. Sacubitril/valsartan averts post-myocardial infarction ventricular remodeling and preserves heart function. Int J Cardiol Heart Vasc. 2019;22:218–219. doi: 10.1016/j.ijcha.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, Nestler JA, Devarakonda T, Ghosh S, Das A, Salloum FN. Sacubitril/Valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol. 2018;72:2342–2356. doi: 10.1016/j.jacc.2018.07.102. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zhong C, Si J, Chen S, Kang L, Xu B. The impact of Sacubitril/Valsartan on cardiac fibrosis early after myocardial infarction in hypertensive rats. J Hypertens. 2022;40:1822–1830. doi: 10.1097/HJH.0000000000003230. [DOI] [PubMed] [Google Scholar]
- 16.Kompa AR, Lu J, Weller TJ, Kelly DJ, Krum H, von Lueder TG, Wang BH. Angiotensin receptor neprilysin inhibition provides superior cardioprotection compared to angiotensin converting enzyme inhibition after experimental myocardial infarction. Int J Cardiol. 2018;258:192–198. doi: 10.1016/j.ijcard.2018.01.077. [DOI] [PubMed] [Google Scholar]
- 17.O’Meara E, McDonald M, Chan M, Ducharme A, Ezekowitz JA, Giannetti N, Grzeslo A, Heckman GA, Howlett JG, Koshman SL, Lepage S, Mielniczuk LM, Moe GW, Swiggum E, Toma M, Virani SA, Zieroth S, De S, Matteau S, Parent MC, Asgar AW, Cohen G, Fine N, Davis M, Verma S, Cherney D, Abrams H, Al-Hesayen A, Cohen-Solal A, D’Astous M, Delgado DH, Desplantie O, Estrella-Holder E, Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, LeBlanc MH, Lee D, Masoudi FA, McKelvie RS, Rajda M, Ross HJ, Sussex B. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36:159–169. doi: 10.1016/j.cjca.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Xiong B, Nie D, Qian J, Yao Y, Yang G, Rong S, Zhu Q, Du Y, Jiang Y, Huang J. The benefits of sacubitril-valsartan in patients with acute myocardial infarction: a systematic review and meta-analysis. ESC Heart Fail. 2021;8:4852–4862. doi: 10.1002/ehf2.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kario K. The sacubitril/valsartan, a first-in-class, angiotensin receptor neprilysin inhibitor (ARNI): potential uses in hypertension, heart failure, and beyond. Curr Cardiol Rep. 2018;20:5. doi: 10.1007/s11886-018-0944-4. [DOI] [PubMed] [Google Scholar]
- 20.Chacko KA. AHA Medical/Scientific Statement: 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation. 1995;92:2003–5. [PubMed] [Google Scholar]
- 21.Bao J, Kan R, Chen J, Xuan H, Wang C, Li D, Xu T. Combination pharmacotherapies for cardiac reverse remodeling in heart failure patients with reduced ejection fraction: a systematic review and network meta-analysis of randomized clinical trials. Pharmacol Res. 2021;169:105573. doi: 10.1016/j.phrs.2021.105573. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Liu Q, Jiang Q, Ren W, Lam KM, Zhang W. Underwater image restoration via adaptive dark pixel prior and color correction. Int J Comput Vis. 2023;18:53–63. [Google Scholar]
- 23.D’Amario D, Rodolico D, Rosano GMC, Dahlström U, Crea F, Lund LH, Savarese G. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: data from the Swedish Heart Failure Registry. Eur J Heart Fail. 2022;24:871–884. doi: 10.1002/ejhf.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastać D, Joksimović Z, Pavlović S, Bastać M, Raščanin A, Đorđioski I. Paradigm change in the treatment of chronic heart failure according to ESC Guide 2021: new innovative drugs in focus. Timočki Medicinski Glasnik. 2022;47:40–47. [Google Scholar]
- 25.Rezq A, Saad M, El Nozahi M. Comparison of the efficacy and safety of sacubitril/valsartan versus ramipril in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2021;143:7–13. doi: 10.1016/j.amjcard.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Berwanger O, Pfeffer M, Claggett B, Jering KS, Maggioni AP, Steg PG, Mehran R, Lewis EF, Zhou Y, van der Meer P, De Pasquale C, Merkely B, Filippatos G, McMurray JJV, Granger CB, Solomon SD, Braunwald E. Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: win-ratio analysis of the PARADISE-MI trial. Eur J Heart Fail. 2022;24:1918–1927. doi: 10.1002/ejhf.2663. [DOI] [PubMed] [Google Scholar]