Abstract
Mercury content is a critical indicator for quality control in cosmetics. Historically, mercury poisoning was primarily linked to occupational exposures. However, with the widespread household exposure to mercury, especially in cosmetics, non-occupational mercury poisoning has become increasingly prevalent. Mercury poisoning can damage major organs such as the heart, liver, and kidneys. Therefore, this article intends to summarize current knowledge on the harms and underlying mechanisms associated with mercury poisoning from cosmetics, in order to provide clinical insight to enable tertiary prevention.
Keywords: Cosmetics, mercury poisoning, organ damage, review
Introduction
Mercury, a heavy metal with known antibacterial and whitening properties, has been historically used in various industries and cosmetics. Despite its beneficial effects, it is often illegally added to whitening products [1-5]. However, mercury poses significant health risks, including the potential for skin absorption and long-term exposure leading to diverse health issues. Symptoms of mercury poisoning can range from mild allergic reactions to severe neurological damage [6,7]. Initial signs may include skin rashes, peeling, and itching, but with prolonged exposure, more severe symptoms can develop, such as cognitive decline, mood fluctuations, muscle weakness, and even renal impairment. Research indicates that approximately 70% of mercury poisoning cases are attributable to cosmetics [8,9]. These symptoms are often subtle in the early stages and can progressively worsen over time, adversely affecting quality of life. Accordingly, this paper provides a comprehensive review of the multisystem damage and mechanisms associated with mercury poisoning from cosmetics.
Cosmetics and mercury poisoning
Mercury, with its notable physical and chemical properties, is used across various industries, including cosmetics. Its inherent toxicity, however, becomes a significant concern when it comes into contact with the human body. In whitening products, mercury predominantly manifests as mercury salts, which act by inhibiting tyrosinase activity in the skin. This inhibition reduces melanin production, thereby achieving a whitening effect [10,11]. This direct mechanism of action on melanin production in the skin allows mercury-containing whitening products to deliver visible whitening effects in a short period, making them popular among individuals seeking immediate results. Recent studies have shown that some commercially available whitening products contain metallic mercury (Figure 1). According to national cosmetic hygiene standards (GB7916-87), the maximum permissible content of mercury in cosmetics should not exceed 1 mg/kg. Products exceeding this threshold are considered to contain excessive mercury levels, which may pose health risks [12]. In recent years, there has been an increasing number of clinical cases of mercury poisoning due to cosmetics use. Research has indicated that the primary organ systems affected by mercury poisoning include the oral mucosa, nervous system, digestive system, and kidneys, reflecting the broad spectrum of potential harm associated with mercury in cosmetics [13] (Table 1).
Figure 1.
Types of cosmetics associated with mercury poisoning.
Table 1.
Manifestations of organ damage from cosmetic-related mercury poisoning
| Tissue and organ | Clinical presentation |
|---|---|
| Oral cavity | Oral mucosal inflammation, excessive salivation, dysphagia, gum pain, and ulcers of the lips and tongue. |
| Digestive system | Hepatocyte necrosis, cholestatic liver disease, organic or functional gastrointestinal diseases. |
| Nervous system | Tremors, emotional instability, insomnia, memory loss, muscle twitching, headaches and abnormal sensory perceptions. Long-term mercury exposure may lead to gait instability, tremulous speech, blurred vision, and hearing loss. |
| Kidney | Membranous kidney disease, with mercury forming metallothionein complexes that damage renal tubules. |
| Others | Abnormal thyroid function, hypercoagulability, aplastic anemia, accelerated aging, and infertility. |
Diagnostic criteria for cosmetics-related mercury poisoning
Currently, the diagnostic criteria for mercury poisoning related to cosmetics use are primarily adapted from those for occupational mercury poisoning [13]. The criteria include: 1. A history of cosmetic use for more than 6 months, with chronic mercury poisoning meeting the diagnostic standards outlined in the Diagnostic Criteria of Occupational Mercury Poisoning (GBZ89-2007); 2. Urinary mercury levels exceeding 0.50 µmol/L; 3. Absence of recent use of mercury-containing medications.
Mercury absorption and metabolism
Mercury metabolism generally follows a similar pattern across its various forms. Upon absorption into the body, mercury undergoes a redox cycle. Specifically, mercury vapor is converted into divalent mercury ions within red blood cells through the hydrogen peroxidase pathway. The primary routes of mercury excretion are through urine and feces, though the predominant pathway can vary based on exposure duration and environmental factors. In the initial week following exposure, mercury is predominantly excreted through feces. However, in individuals with long-term occupational exposure, urinary excretion becomes the main route of elimination [14,15]. Additionally, mercury can also be excreted through gaseous exchange, body fluids, and breast milk.
Organ damage from cosmetic-related mercury poisoning
Research has demonstrated that mercury poisoning can lead to multi-organ damage. Mercury vapor, inhaled through the respiratory tract, is absorbed predominantly by the alveoli. Due to its ability to easily permeate lipid-rich cell membranes of the alveolar walls, mercury combines with blood lipids and is distributed by means of the bloodstream [16,17]. Once in the body, mercury binds rapidly with thiol groups in red blood cells and is oxidized into divalent mercury ions by hydrogen peroxidase. Mercury induces systemic toxicity through various toxic mechanisms, including direct injury or damage to biomembranes, oxidative stress, intracellular calcium overload, immune system impairment, and apoptosis. The nature and extent of organ damage can vary, depending on the specific organ and the mechanisms involved. This review presents a comprehensive analysis of recent research on the organ-specific damage and mechanisms associated with cosmetic-related mercury poisoning.
Cardiovascular complications associated with cosmetic-related mercury poisoning
Current research indicates that mercury poisoning can affect the myocardium, though it typically does not cause myocardial cell necrosis in the early stages. Initial manifestations include an extension of the QRS interval on an electrocardiogram (ECG). Despite treatment to expel mercury, the QRS interval often remains prolonged compared to normal values, suggesting that mercury’s toxic effects on myocardial sodium channels may not be fully reversible even after mercury levels are normalized [18-20]. Additionally, cosmetic-related mercury poisoning has been linked to the development of hypertension in affected individuals [21-24]. Detailed information is provided in Table 2.
Table 2.
Mechanism of cardiovascular complications associated with cosmetic-related mercury poisoning
| Clinical presentation | Potential mechanism |
|---|---|
| Hypertension | Mercury ions induce calcium overload in vascular smooth muscle cells, activate the excitation-contraction coupling mechanism, and stimulate phospholipase A2. This process results in vasoconstriction mediated by vasoactive substances such as thromboxane, leading to hypertension. |
| Arrhythmia (QRS wave prolongation) | Mercury inhibits myocardial sodium pumps by binding to the P-ring domain I Cys373 of sodium channels, oxidizing cysteine residues, and forming disulfide bonds between sulfhydryl groups. This oxidation creates disulfide compounds, which impair sodium channel permeability. Consequently, the depolarization rate of myocardial cells in phase 0 slows, and the conduction rate of action potentials decreases, leading to prolonged QRS complexes on ECG. |
Respiratory system damage from cosmetic-related mercury poisoning
Elemental mercury vapor, due to its high volatility, can directly cause severe respiratory conditions such as chemical-induced bronchitis, interstitial pneumonia and pulmonary edema. Patients primarily present with chest pain, difficulty breathing, and cough, which may be accompanied by hemoptysis. Auxiliary tests often indicate a reduced lung capacity and impaired lung function. In severe cases, these conditions can escalate to respiratory failure, posing life-threatening risks [25,26]. Possible mechanisms underlying these effects are shown in Table 3.
Table 3.
Mechanisms of respiratory system damage from cosmetic-related mercury poisoning
| Clinical presentation | Potential mechanism |
|---|---|
| Chemical-induced bronchitis | Mercury vapor induces corrosive inflammation of the airways, leading to the shedding and necrosis of tracheal epithelial cells at various levels. |
| Pulmonary edema | Damage to epithelial cells results in their detachment and subsequent obstruction of the airway lumen. This is accompanied by tissue edema, telangiectasia, and inflammatory cell infiltration. |
| Interstitial pneumonia | Infiltration of inflammatory cells and extensive proliferation of fibroblasts in the interstitial lung tissue are observed [27,28]. |
Digestive system complications associated with cosmetic-related mercury poisoning
Current evidence suggests that mercury poisoning primarily affects the digestive system through direct injury to the oral cavity, liver damage, and gastrointestinal dysfunction. Liver injury is often evidenced by elevated liver enzymes, while gastrointestinal symptoms may include abdominal pain, distension, nausea, vomiting, diarrhea, and constipation. Some patients may experience chronic liver damage due to impaired gallbladder function, potentially progressing to cirrhosis. The mechanisms underlying these digestive system complications are summarized in Table 4.
Table 4.
Mechanisms of digestive system complications associated with cosmetic-related mercury poisoning
| Clinical presentation | Potential mechanism |
|---|---|
| Damage to the oral mucosa and tongue | It is evidenced by ulcers and inflammation of oral mucosa, tongue and lips, accompanied by excessive salivation, dysphagia and gum pain [29]. |
| Liver damage | Mercury disrupts hepatic redox functions by binding to reduced glutathione. This interaction, combined with intracellular calcium overload that activates phospholipase, leads to cell membrane peroxidation and free radical production. These effects further deplete reduced glutathione and ultimately result in liver damage [30]. |
| Inhibition of digestive enzyme function | Mercury-induced structural changes in enzymes, due to interactions with amino, carboxyl, and hydroxyl groups, impair enzyme function [31]. |
| Gastrointestinal tract injury | Injury to the gastric mucosa and accelerated smooth muscle peristalsis cause both organic and functional changes in the stomach. |
| Gallbladder disorder | Mercury poisoning disrupts the energy balance of capillary bile duct membranes and impairs bile transport capacity, leading to congestion and subsequent liver changes [32]. |
Renal injury associated with cosmetic-related mercury poisoning
In the early stages of renal injury, due to low concentrations of mercury in cosmetics, damage is typically limited to the proximal convoluted tubules, with clinical manifestations including mild proteinuria. As mercury accumulation increases, injury extends to the distal convoluted tubules and collecting ducts, impairing urine concentration and resulting in increased nocturia. With significant mercury accumulation and progression to moderate to severe poisoning, patients may develop glomerular damage, marked proteinuria, nephrotic syndrome, and eventually renal insufficiency. The mechanisms contributing to renal injury are detailed in Table 5.
Table 5.
Mechanisms of renal injury associated with cosmetic-related mercury poisoning
| Clinical presentation | Potential mechanism |
|---|---|
| Direct injury | Mercury binds to proteins in the glomerular basement membrane, causing damage that leads to proteinuria, hematuria, and potentially chronic kidney injury [33,34]. |
| Secondary membranous nephropathy | High concentrations of mercury cause direct chemical damage to renal tubular epithelial cells in the distal convoluted tubules and collecting ducts. Microscopic changes include vacuolation, degeneration, and necrosis. Prolonged exposure may also induce immune complex nephritis, with membranous nephropathy being a common outcome [35]. |
| Renal tubulointerstitial damage | It is characterized by swelling of tubular epithelial cells, flattening of the brush border, necrosis, and detachment of tubules. Distal convoluted tubules may show a reduced number of clear tubules [36]. |
| Interstitial nephritis | Expansion of the glomerular capillary plexus, local thickening of the glomerular basement membrane, and abnormal proliferation of mesangial cells and matrix are observed. Microscopic findings include narrowing of small balloon cavities, protein exudation, fibrous tissue hyperplasia, and hyaline deposition around individual glomeruli. Interstitial damage features epithelial cell swelling, shedding of the brush border, focal necrosis, and transparent casts in the glomeruli [37,38]. |
Neurological system
Central nervous system
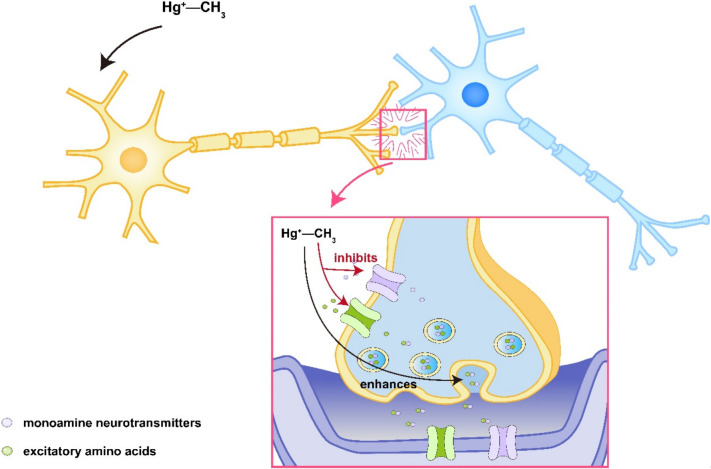
Exposure to mercury vapor primarily manifests neurologically as tremors. Other neurological symptoms may include mood swings, sleep disturbances, memory loss, cognitive decline, involuntary muscle twitching, headaches, and sensory abnormalities [39]. Prolonged exposure can lead to more severe complications, such as unsteady gait, tremulous speech, blurred vision, and hearing impairments, potentially leading to complete loss of these functions. The central nervous system damage predominantly affects the cerebral cortex and cerebellum. Some researchers believe that widespread cellular damage in areas such as the somatosensory and visual cortices might be the cause of impairments in sensory, auditory, visual, and olfactory functions. Methylmercury exposure has been shown to reduce adrenaline levels throughout the nervous system, with notable effects in the cerebral cortex and pontine medulla [40]. Additionally, there is a decrease in dopamine levels in the substantia nigra, hypothalamus, striatum, hippocampus, and olfactory bulb, while serotonin levels increase. In vitro studies have shown that methylmercury can stimulate the release of monoamine neurotransmitters and excitatory amino acids in synaptosomes, with release levels correlating with the concentration of methylmercury and duration of exposure. Methylmercury also inhibits the synaptic uptake of these neurotransmitters and excitatory amino acids, leading to increased levels in the synaptic cleft [41]. This persistent receptor stimulation triggers a cascade of responses that ultimately results in neuronal damage, as shown in Figure 2.
Figure 2.
Schematic diagram of central nervous system injury associated with mercury poisoning.
Peripheral nervous system
The impact of mercury on the peripheral nervous system varies depending on the type and dose of mercury exposure. Low doses typically cause damage to peripheral nerve fibers, leading to axonal degeneration [42]. In contrast, high doses can damage dorsal root ganglia and anterior horn motor neurons, leading to the activation of glial cells and severe, persistent peripheral neuropathic pain. Possible mechanisms underlying these effects are detailed in Table 6.
Table 6.
Mechanisms of peripheral nerve tissue damage associated with cosmetic-related mercury poisoning
| Nerve injury | Potential mechanism |
|---|---|
| Pain | Activation of glial cells leads to the release of proinflammatory cytokines and other active substances. These factors interact with neurons, facilitating the transmission and progression of neuropathic pain [43,44]. |
| Aging | Methylmercury impairs neurons by blocking e2-tubulin, disrupting the internal structure and biochemical balance of neurons. This interference affects cell growth, differentiation, and survival through the PKC cascade, ultimately leading to abnormal energy metabolism, impaired mitochondrial function, and disrupted signal transmission. Over time, these effects contribute to accelerated neuronal aging [45,46]. |
Immune system
Mercury exposure adversely affects immune system function, particularly through its impact on T cells. Mercury inhibits T cell proliferation and affects the expression of interleukin 1 (IL-1) synthesis and secretion, as well as IL-3 receptors and transferrin receptors on T cells, thus impairing T cell function. Both in vitro and in vivo studies have demonstrated that mercury can promote the proliferation of Th3 cells and increase IL-4 production, while simultaneously suppressing Th1 cell proliferation [47]. This Th1/Th3 imbalance is a key factor in the development of many autoimmune diseases. Mercury can disrupt the normal expression of Major Histocompatibility Complex II (MHC II) on target cells, affecting T cell production and inducing the production of heat shock proteins, which further contribute to autoimmune disease progression. Additionally, mercury vapor can bind directly to MHC molecules on antigen-presenting cells or to T cells, thereby inducing and exacerbating various autoimmune diseases.
Other systems
Mercury can also directly impair the pituitary, thyroid, and adrenal glands. One study has confirmed that workers exposed to mercury have higher mercury levels in the thyroid and pituitary glands compared to the kidneys, brain, and other tissues. This accumulation leads to reduced oxytocin production and is associated with mood disorders, such as depression and suicidal tendencies. In the thyroid gland, mercury poisoning primarily results in suppressed thyroid function, as evidenced by reduced thyroid hormone levels in peripheral blood [48]. The hematologic system may also be affected, exhibiting a secondary hypercoagulable state due to disruption of the thrombomodulin/protein C system and an imbalance between tissue plasminogen activator and its inhibitor. Mercury exposure can increase oxidative free radicals and lipid damage, which impacts the coagulation/anticoagulation system and causes aplastic anemia. The reproductive system is similarly affected, with potential outcomes including infertility, miscarriages, preterm labor, and low birth weight. This is due to that mercury exposure can inhibit the secretion of progesterone by ovarian granulosa cells and adversely affect sperm production, motility, and morphology, though it does not appear to affect testosterone production [49].
Conclusion
The use of mercury in cosmetics is becoming increasingly common, and long-term exposure to this heavy metal can cause damage to various organs, including the cardiovascular, digestive, nervous, and immune systems, primarily due to the mercury’s corrosive nature and its involvement in redox reactions. However, due to the lack of precise diagnostic markers, early detection of mercury poisoning is challenging, leading to chronic damage from prolonged exposure. To mitigate these risks, it is crucial to strengthen the monitoring of mercury content in cosmetics, thereby improving the safety of cosmetic products and reducing the potential for mercury-related health problems.
Disclosure of conflict of interest
None.
References
- 1.Pramanik S, Kumar M, Qureshi A. Mercury in skin-care products in India and consumer exposure risks. Regul Toxicol Pharmacol. 2021;121:104870. doi: 10.1016/j.yrtph.2021.104870. [DOI] [PubMed] [Google Scholar]
- 2.Bamidele OD, Kayode BA, Eniayewu OI, Adegbola AJ, Olatoye RS, Njinga NS, Abdullahi ST, Bakare-Odunola MT. Quality assessment of hydroquinone, mercury, and arsenic in skin-lightening cosmetics marketed in Ilorin, Nigeria. Sci Rep. 2023;13:20992. doi: 10.1038/s41598-023-47160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Podgórska A, Puścion-Jakubik A, Grodzka A, Naliwajko SK, Markiewicz-Żukowska R, Socha K. Natural and conventional cosmetics-mercury exposure assessment. Molecules. 2021;26:4088. doi: 10.3390/molecules26134088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo M, Wang J, Du R, Liu Y, Chi J, He X, Huang K, Luo Y, Xu W. A test strip platform based on a whole-cell microbial biosensor for simultaneous on-site detection of total inorganic mercury pollutants in cosmetics without the need for predigestion. Biosens Bioelectron. 2020;150:111899. doi: 10.1016/j.bios.2019.111899. [DOI] [PubMed] [Google Scholar]
- 5.Wan Mohamed Radzi CWJ, Nordin FNM. Status of cosmetic safety in malaysia market: mercury contamination in selected skin whitening products. J Cosmet Dermatol. 2022;21:6875–6882. doi: 10.1111/jocd.15429. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Viso P, Domene A, Sánchez A, Vélez D, Monedero V, Devesa V, Zúñiga M. Challenges and strategies for preventing intestinal damage associated to mercury dietary exposure. Toxicology. 2023;494:153580. doi: 10.1016/j.tox.2023.153580. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw JL, Hall AJ. Mercury in cetaceans: exposure, bioaccumulation and toxicity. Sci Total Environ. 2019;694:133683. doi: 10.1016/j.scitotenv.2019.133683. [DOI] [PubMed] [Google Scholar]
- 8.Rojas-Franco P, Franco-Colín M, Torres-Manzo AP, Blas-Valdivia V, Thompson-Bonilla MDR, Kandir S, Cano-Europa E. Endoplasmic reticulum stress participates in the pathophysiology of mercury-caused acute kidney injury. Ren Fail. 2019;41:1001–1010. doi: 10.1080/0886022X.2019.1686019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran QK, Najafali D, Tiffany L, Tanveer S, Andersen B, Dawson M, Hausladen R, Jackson M, Matta A, Mitchell J, Yum C, Kuhn D. Effect of blood pressure variability on outcomes in emergency patients with intracranial hemorrhage. West J Emerg Med. 2021;22:177–185. doi: 10.5811/westjem.2020.9.48072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barit JJG, Chamberlin CVS, Young PS, Obbus SFV, Abalos-Babaran S, Lucero-Orillaza HE, Encarnacion LA, Ramirez-Quizon MN. Excessive mercury levels in an unregistered cosmetic whitening product causing Allergic contact dermatitis. Dermatitis. 2020;31:e18–e19. doi: 10.1097/DER.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 11.Ashraf T, Taneez M, Kalsoom S, Irfan T, Shafique MA. Experimental calculations of metals content in skin-whitening creams and theoretical investigation for their biological effect against tyrosinase enzyme. Biol Trace Elem Res. 2021;199:3562–3569. doi: 10.1007/s12011-020-02441-z. [DOI] [PubMed] [Google Scholar]
- 12.Ye BJ, Kim BG, Jeon MJ, Kim SY, Kim HC, Jang TW, Chae HJ, Choi WJ, Ha MN, Hong YS. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med. 2016;28:5. doi: 10.1186/s40557-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan TYK, Chan APL, Tang HL. Nephrotic syndrome caused by exposures to skin-lightening cosmetic products containing inorganic mercury. Clin Toxicol (Phila) 2020;58:9–15. doi: 10.1080/15563650.2019.1639724. [DOI] [PubMed] [Google Scholar]
- 14.Vogel N, Murawski A, Schmied-Tobies MIH, Rucic E, Doyle U, Kämpfe A, Höra C, Hildebrand J, Schäfer M, Drexler H, Göen T, Kolossa-Gehring M. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany - human biomonitoring results of the german environmental survey 2014-2017 (GerES V) Int J Hyg Environ Health. 2021;237:113822. doi: 10.1016/j.ijheh.2021.113822. [DOI] [PubMed] [Google Scholar]
- 15.Yin L, Lin S, Summers AO, Roper V, Campen MJ, Yu X. Children with amalgam dental restorations have significantly elevated blood and urine mercury levels. Toxicol Sci. 2021;184:104–126. doi: 10.1093/toxsci/kfab108. [DOI] [PubMed] [Google Scholar]
- 16.Carrasco P, Estarlich M, Iñiguez C, Ferrero A, Murcia M, Esplugues A, Vioque J, Marina LS, Zabaleta C, Iriarte G, Fernández-Somoano A, Tardon A, Vrijheid M, Sunyer J, Ballester F, Llop S. Pre and postnatal exposure to mercury and respiratory health in preschool children from the Spanish INMA birth cohort study. Sci Total Environ. 2021;782:146654. doi: 10.1016/j.scitotenv.2021.146654. [DOI] [PubMed] [Google Scholar]
- 17.Wankhede AG. Attempted homicide by instilling elemental mercury in the respiratory tract: a two-year follow-up. Med Sci Law. 2022;62:154–157. doi: 10.1177/00258024211067847. [DOI] [PubMed] [Google Scholar]
- 18.Martins AC, Santos AAD, Lopes ACBA, Skalny AV, Aschner M, Tinkov AA, Paoliello MMB. Endothelial dysfunction induced by cadmium and mercury and its relationship to hypertension. Curr Hypertens Rev. 2021;17:14–26. doi: 10.2174/1573402117666210121102405. [DOI] [PubMed] [Google Scholar]
- 19.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, Rudan I. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154–1163. doi: 10.1001/jamapediatrics.2019.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan J, Pan Y, Tang Z, Song Y. Mercury poisoning presenting with hypertension: report of 2 cases. Am J Med. 2019;132:1475–1477. doi: 10.1016/j.amjmed.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Hu XF, Singh K, Chan HM. Mercury exposure, blood pressure, and hypertension: a systematic review and dose-response meta-analysis. Environ Health Perspect. 2018;126:076002. doi: 10.1289/EHP2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regencia ZJG, Dalmacion GV, Baja ES. Effect of heavy metals on ventricular repolarization and depolarization in the metropolitan manila development authority (MMDA) traffic enforcers’ health study. Arch Environ Occup Health. 2022;77:87–95. doi: 10.1080/19338244.2020.1853017. [DOI] [PubMed] [Google Scholar]
- 23.Bello KAS, Wilke MCB, Simões RP, Landim-Vieira M, Langa P, Stefanon I, Vassallo DV, Fernandes AA. Chronic exposure to mercury increases arrhythmia and mortality post-acute myocardial infarction in rats. Front Physiol. 2023;14:1260509. doi: 10.3389/fphys.2023.1260509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos Ruybal MCP, Gallego M, Sottani TBB, Medei EH, Casis O, Nascimento JHM. Methylmercury poisoning induces cardiac electrical remodeling and increases arrhythmia susceptibility and mortality. Int J Mol Sci. 2020;21:3490. doi: 10.3390/ijms21103490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smiechowicz J, Skoczynska A, Nieckula-Szwarc A, Kulpa K, Kübler A. Occupational mercury vapour poisoning with a respiratory failure, pneumomediastinum and severe quadriparesis. SAGE Open Med Case Rep. 2017;5:2050313X17695472. doi: 10.1177/2050313X17695472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pile JE, Truong J. 48-year-old with altered mental status and respiratory failure: a case report. Clin Pract Cases Emerg Med. 2021;5:502–506. doi: 10.5811/cpcem.2021.3.51331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ismail OI, El-Meligy MMS. Could vitamin C protect against mercuric chloride induced lung toxicity in the offspring rat: a histological and immunohistochemical study. Ultrastruct Pathol. 2021;45:197–211. doi: 10.1080/01913123.2021.1954118. [DOI] [PubMed] [Google Scholar]
- 28.Koopsamy Naidoo SV, Bester MJ, Arbi S, Venter C, Dhanraj P, Oberholzer HM. Oral exposure to cadmium and mercury alone and in combination causes damage to the lung tissue of sprague-dawley rats. Environ Toxicol Pharmacol. 2019;69:86–94. doi: 10.1016/j.etap.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Ma H, Yao W, Peng B, Liu X, Chen J, Lin Y, Di T, Li P, He X. Mercury-containing preparations attenuate neutrophil extracellular trap formation in mice and humans through inhibiting the ERK1/2 pathway. J Ethnopharmacol. 2024;321:117421. doi: 10.1016/j.jep.2023.117421. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi-Bardbori A, Rannug A. Arsenic, cadmium, mercury and nickel stimulate cell growth via NADPH oxidase activation. Chem Biol Interact. 2014;224:183–188. doi: 10.1016/j.cbi.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Chung JW, Acharya D, Singh JK, Sakong J. Association of blood mercury level with liver enzymes in Korean adults: an analysis of 2015-2017 Korean national environmental health survey. Int J Environ Res Public Health. 2023;20:3290. doi: 10.3390/ijerph20043290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang C, Ma X, Zhao J, Li S, Liu C, Tang Y, Zhou J, Chen J, Li X, Li W. Oleanolic acid inhibits mercury chloride induced-liver ferroptosis by regulating ROS/iron overload. Ecotoxicol Environ Saf. 2023;258:114973. doi: 10.1016/j.ecoenv.2023.114973. [DOI] [PubMed] [Google Scholar]
- 33.Said S, Hernandez GT. Environmental exposures, socioeconomics, disparities, and the kidneys. Adv Chronic Kidney Dis. 2015;22:39–45. doi: 10.1053/j.ackd.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Mishra M, Nichols L, Dave AA, Pittman EH, Cheek JP, Caroland AJV, Lotwala P, Drummond J, Bridges CC. Molecular mechanisms of cellular injury and role of toxic heavy metals in chronic kidney disease. Int J Mol Sci. 2022;23:11105. doi: 10.3390/ijms231911105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caza TN, Al-Rabadi LF. What can mercury teach us about membranous nephropathy and minimal change disease? Kidney Int Rep. 2022;7:1157–1160. doi: 10.1016/j.ekir.2022.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yawei C, Jing S, Wenju S, Yupeng L, Ping Z, Liping H. Mercury as a cause of membranous nephropathy and Guillain-Barre syndrome: case report and literature review. J Int Med Res. 2021;49:300060521999756. doi: 10.1177/0300060521999756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doshi M, Annigeri RA, Kowdle PC, Subba Rao B, Varman M. Membranous nephropathy due to chronic mercury poisoning from traditional Indian medicines: report of five cases. Clin Kidney J. 2018;12:239–244. doi: 10.1093/ckj/sfy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Z, Wu N, Du X, Li H, Mei X, Song Y. Toxic nephropathy secondary to chronic mercury poisoning: clinical characteristics and outcomes. Kidney Int Rep. 2022;7:1189–1197. doi: 10.1016/j.ekir.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philibert A, Fillion M, Da Silva J, Lena TS, Mergler D. Past mercury exposure and current symptoms of nervous system dysfunction in adults of a first nation community (Canada) Environ Health. 2022;21:34. doi: 10.1186/s12940-022-00838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury exposure and heart diseases. Int J Environ Res Public Health. 2017;14:74. doi: 10.3390/ijerph14070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma Ghimire P, Tripathee L, Zhang Q, Guo J, Ram K, Huang J, Sharma CM, Kang S. Microbial mercury methylation in the cryosphere: progress and prospects. Sci Total Environ. 2019;697:134150. doi: 10.1016/j.scitotenv.2019.134150. [DOI] [PubMed] [Google Scholar]
- 42.Ekinci M, Ceylan E, Keleş S, Cağatay HH, Apil A, Tanyıldız B, Uludag G. Toxic effects of chronic mercury exposure on the retinal nerve fiber layer and macular and choroidal thickness in industrial mercury battery workers. Med Sci Monit. 2014;20:1284–1290. doi: 10.12659/MSM.890756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun B, Fan S, Yao K, Li Y, Huang X. Changes in intraepidermal nerve fiber and Langerhans cell densities in the plantar skin of rats after mercuric chloride exposure. J Peripher Nerv Syst. 2018;23:17–22. doi: 10.1111/jns.12246. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Xu G, Weinreb RN, Yu M, Leung CK. Optic nerve head deformation in glaucoma: a prospective analysis of optic nerve head surface and lamina cribrosa surface displacement. Ophthalmology. 2015;122:1317–1329. doi: 10.1016/j.ophtha.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 45.Pamphlett R. The prevalence of inorganic mercury in human cells increases during aging but decreases in the very old. Sci Rep. 2021;11:16714. doi: 10.1038/s41598-021-96359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He Y, Yang X, Li Z, Wang T, Ma C, Wen X, Chen W, Zhang C. Aging rice straw reduces the bioavailability of mercury and methylmercury in paddy soil. Chemosphere. 2023;339:139711. doi: 10.1016/j.chemosphere.2023.139711. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Zhao Y, Li Q, Shao Y, Yu X, Cong W, Jia X, Qu W, Cheng L, Xue P, Zhou Z, He M, Zhang Y. Developmental exposure to mercury chloride impairs social behavior in male offspring dependent on genetic background and maternal autoimmune environment. Toxicol Appl Pharmacol. 2019;370:1–13. doi: 10.1016/j.taap.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Pamphlett R, Doble PA, Bishop DP. Mercury in the human thyroid gland: potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PLoS One. 2021;16:e0246748. doi: 10.1371/journal.pone.0246748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu F, Chen C, Zhang Y, Chen S, Huang X, Li J, Wang Y, Liu X, Deng G, Gao J. Elevated blood mercury level has a non-linear association with infertility in U.S. women: Data from the NHANES 2013-2016. Reprod Toxicol. 2020;91:53–58. doi: 10.1016/j.reprotox.2019.11.005. [DOI] [PubMed] [Google Scholar]