Abstract
BACKGROUND
Spastic equinus (plantar flexed) foot is a common postural pattern in patients who suffer from post-stroke spasticity. To date, some clinicians use the Silfverskiöld Test in their practice to differentiate between gastrocnemius and soleus muscle overactivity in patients with spastic equinus (plantar flexed) foot. This use of the Silfverskiöld Test goes beyond its original aim, which was to distinguish isolated gastrocnemius contracture in patients with equinus deformity.
AIM
The aim of this study was to investigate the Silfverskiöld Test validity for evaluating spastic equinus (plantar flexed) foot (i.e., differentiation between gastrocnemius and soleus muscle overactivity) by checking its outcome against those of selective diagnostic nerve block of tibial motor nerve branches to the soleus, gastrocnemius and tibialis posterior muscles.
DESIGN
The design of the study was retrospective observational.
SETTING
The study was set in a university hospital.
POPULATION
Sixty-seven adult stroke patients with spastic equinus (plantar flexed) foot.
METHODS
Each patient underwent selective diagnostic nerve block of tibial motor nerve branches to the soleus, gastrocnemius and tibialis posterior muscles. All patients were evaluated before diagnostic nerve block by means of the Silfverskiöld Test which was considered positive when ankle joint passive dorsiflexion was greater with the knee flexed than extended. Furthermore, they were assessed before and after nerve block by means of the modified Ashworth Scale and the Tardieu Scale.
RESULTS
Our sample included 41 males and 26 females (mean age 57.6 years) suffering from spastic equinus (plantar flexed) foot due to chronic stroke (mean time from onset 2.4 years). Forty-eight patients out of 67 presented with positive Silfverskiöld Test. The χ2 test showed no association between the Silfverskiöld Test and spastic overactivity of the gastrocnemius (P=0.253), soleus (P=0.605) and tibialis posterior (P=0.462) muscles as evaluated by serial selective diagnostic block of the tibial nerve motor branches.
CONCLUSIONS
Our findings do not support the Silfverskiöld Test as a valid tool for evaluating spastic equinus (plantar flexed) foot to differentiate between gastrocnemius, soleus and tibialis posterior spastic muscle overactivity in adult patients with stroke.
CLINICAL REHABILITATION IMPACT
The choice for an appropriate management of spastic equinus (plantar flexed) foot in adults with stroke should not be mainly defined on the base of Silfverskiöld Test.
Key words: Equinus deformity, Muscle spasticity, Nerve block, Rehabilitation
Stroke is a leading cause of disability worldwide.1 Spasticity is a main sequela of stroke, which manifests “as velocity- and muscle length-dependent increase in resistance to externally imposed muscle stretch”. From a pathophysiological point of view “it results from hyperexcitable descending excitatory brainstem pathways and from the resultant exaggerated stretch reflex responses”. From a clinical point of view “other related motor impairments, including abnormal synergies, inappropriate muscle activation, and anomalous muscle coactivation, coexist with spasticity and share similar pathophysiological origins.”2 The prevalence of post-stroke spasticity (PSS) is 4-27% within the first 6 weeks, 19% at 3 months, 21.7-42.6% between 4 and 6 months, and 17-38% at 12 months from onset.3 Spastic equinus (plantar flexed) foot is a common postural pattern in patients who suffer from PSS.4 It may be due to several conditions, including calf muscles spastic overactivity, calf muscle-tendon shortening (i.e. equinus contracture deformity), ankle dorsiflexor muscles weakness or imbalance.5 In line with his clinical experience, Nils Silfverskiöld described two types of equinus contracture deformity. The first one consisted of equinus that is essentially inflexible with the knee fully extended, but capable of being corrected passively when the knee is flexed. As to this clinical presentation, the gastrocnemius muscle was supposed to be the primary deforming factor. The second type of deformity defined by Nils Silfverskiöld consisted of persistent equinus, whether the knee is extended or flexed. This condition was assumed to involve the calf and ankle as a whole by including calf muscles contracture, ankle joint posterior capsule retraction or bony deformities, such as anterior bony block or talus deformation.6, 7 Accordingly, in 1924, Nils Silfverskiöld proposed a clinical test based on knee flexion in order to distinguish between isolated gastrocnemius contracture vs. combined shortening of the gastro-soleus complex, ankle joint capsule retraction and bony deformity.7 So, if the equinus is mainly due to an isolated gastrocnemius contracture, it can be overcome by flexing the knee and consequently, relaxing the gastrocnemius (positive test).6, 7 If this is not the case (negative test), the entire “calf and ankle mechanism” is involved.7 Unfortunately, over-time the use of Silfverskiöld Test has gone beyond its original aim.7 So, to date some clinicians use this test in their real-life practice not to distinguish the clinical presentations of equinus contracture deformity but to differentiate between gastrocnemius and soleus muscle overactivity in patients with spastic equinus (plantar flexed) foot instead.8 According to this way of thinking, if no change occurs to the ankle joint position in response to knee flexion is supposed to indicate soleus muscle overactivity. On the other hand, a marked decrease of the ankle dorsiflexion angle while you straighten the leg is assumed to imply gastrocnemius muscle overactivity.8 To the best of our knowledge, the Silfverskiöld Test has not been yet validated for the evaluation of calf muscles spastic overactivity (i.e. to distinguish between gastrocnemius muscle spastic overactivity vs. soleus muscle spastic overactivity). This is relevant from a clinical point of view, because a misinterpretation of the Silfverskiöld Test may lead to incorrect therapeutical decisions as to the management of PSS, such as an inaccurate selection of muscles to inject with botulinum toxin type A (BoNT-A).9 So, in order to improve PSS management by avoiding the misuse of clinical tests we conducted this study, which aims to investigate the validity of Silfverskiöld for evaluating calf muscles spastic overactivity by checking its outcome against those of selective diagnostic nerve block (DNB) of tibial motor nerve branches to the calf muscles in stroke patients suffering from spastic equinus (plantar flexed) foot.
Materials and methods
This single center retrospective (chart review), observational study analyzed data from 67 stroke patients with PSS suffering from spastic equinus (plantar flexed) foot, who were evaluated with serial selective DNB of the tibial nerve motor branches to the soleus, gastrocnemius and tibialis posterior muscles at our Clinical Unit, from January 2020 to December 2022. Inclusion criteria were as follows: age greater than 18 years; spastic equinus (plantar flexed) foot consequent to first-ever unilateral ischemic or hemorrhagic stroke (documented by a computerized tomography scan or magnetic resonance imaging; subarachnoid hemorrhage excluded); calf muscles spasticity graded at least 1 on the Modified Ashworth Scale (MAS);10 at least 3 months since stroke onset. Exclusion criteria were as follows: participation in other trials; previous treatment of spastic equinovarus foot with neurolytic or surgical procedures; other neurological or orthopedic conditions involving the affected lower limb. All participants were outpatients. All patients provided written informed consent, which included consent for data extraction from chart review as needed. The study was carried out according to the Declaration of Helsinki and approved by the local ethics committee.
Clinical evaluation procedures
As a part of our clinical routine, all patients were evaluated by means of the Silfverskiöld Test before serial selective DNB assessment. It was performed with the patient in supine position using the “two-hand technique” (one hand neutralizes and locks the subtalar joint, while the other stabilized the talonavicular joint and forefoot to isolate the ankle joint motion).11 The maximal degree of ankle passive dorsiflexion was measured at slow speed with the knee extended and flexed at 90° using a handheld goniometer. The test was considered positive when ankle joint dorsiflexion was greater with the knee flexed than extended.8 Considering the minimal detectable change defined for this methodology at 7.7°12 and the current literature about its clinical use,11 the sensitivity of the measurement was set at 10°. The dorsiflexion angle was defined as positive and the plantar flexion angle as negative, taking 0° as the neutral position of the joint.13 Furthermore, all patients, were evaluated before and after DNB assessment considering the MAS10 and the Tardieu Scale (TS).14 Patients remained in the supine position with their knees extended during these evaluations. The MAS was used to evaluate spastic calf muscles tone. Is a 6-point scale grading the resistance of a relaxed limb to rapid passive stretch (0 = no increase in muscle tone; 1 = slight increase in muscle tone at the end of the range of motion; 1+ = slight increase in muscle tone through less than half of the range of motion; 2 = more marked increase in muscle tone through most of the range of motion; 3 = considerable increase in muscle tone; 4 = joint is rigid).10 The TS was used to evaluate spastic calf muscle tone according to the spasticity grade, which measures the gain in muscle reaction to fast stretch in dorsiflexion from (0 – no resistance throughout passive movement; 1 – slight resistance throughout passive movement; 2 – clear catch at a precise angle, interruption of the passive movement, followed by release; 3 – unsustained clonus occurring at a precise angle; 4 – sustained clonus occurring at a precise angle), and the spasticity angle, which measures the difference (R2-R1) between the ankle dorsiflexion passive range of motion (R2) and ankle dorsiflexion angle at which the reaction to fastest stretch (i.e., angle of catch) occurs (R1).14
Diagnostic nerve block procedures
All patients included in this retrospective observational study underwent a serial selective DNB evaluation, which started by blocking the tibial nerve motor branch for the soleus muscle, followed by selective DNB of the tibial nerve branches to the gastrocnemius (medialis and lateralis) and tibialis posterior muscles. This is based on the current expert opinion about the management of spastic equinus (plantar flexed) foot with BoNT-A, which reached 100% consensus for injection into the soleus and gastrocnemius muscles as well as 50% consensus for injection into the tibialis posterior muscle.9 Patients laid in the prone position during the whole procedure. A 22-gauge, 80 mm, ultrasound faceted tip echogenic needle for nerve block (SonoPlex STIM, Pajunk, Geisingen, Germany) was guided to the motor branches of tibial nerve by means of ultrasound (MyLab 70 XVision, Esaote, Genoa, Italy) and electrical nerve stimulation (Plexygon,Vygon, Padua, Italy). Anatomic landmarks were used for linear transducer (scanning frequency of 15 MHz) and needle tip positioning.15 Once the tibial nerve motor branches were identified by ultrasound, following elicitation of appropriate muscular response to a 1 Hz, 100 μs, 0.5 mA electrical stimulus, 1-2 mL of lidocaine 2% was injected (aspiration was done before injection to ensure the absence of vessels at the needle tip).
Statistical analysis
Statistical analysis was carried out by means of the Statistical Package for Social Science for Macintosh, version 26.0 (SPSS Inc, Armonk, NY, USA). Descriptive statistics were used for demographic and clinical features of our sample. From a statistical analysis perspective, the Silfverskiöld Test was considered as positive or negative according to the description above. Also, the outcome of DNB was considered in a dichotomic way as relief (i.e., reduction of at least one point of the MAS Score and/or reduction of at least one point of the TS grade and/or reduction of at least 18 degrees of the TS angle) or scant (i.e., reduction of the TS angle less than 18 degrees) / no relief (i.e., no change of the MAS Score, TS grade and TS angle) of spastic muscle overactivity.16, 17 The paired sample t-test was performed to compare the outcome of DNB evaluation with baseline condition. To assess the association between these two categorical variables we used the χ2 test. The alpha level for significance was set at P<0.05.
Results
Demographic and clinical features of patients are reported in Table I. At the end of DNB evaluation, significant improvements were observed as to the MAS (P=0.001), the TS grade (P<0.001) and the TS angle (P<0.001). See Table I for further details about the baseline and post-DNB conditions. As to the χ2 test, no association was found between the Silfverskiöld Test and the spastic overactivity of gastrocnemius (P=0.253), soleus (P=0.605) and tibialis posterior (P=0.462) muscles as evaluated by serial DNB of the tibial nerve motor branches. Changes in the condition of patients after selective DNB are also shown in Figure 1.
Table I. —Demographic and clinical features of patients.
| Variables | Values |
|---|---|
| Age (years), mean (SD) | 57.6 (12.3) |
| Gender (N.), male/female | 41/26 |
| Time since stroke onset (years), mean (SD) | 2.6 (1.4) |
| Silfverskiöld Test (N.), positive/negative | 48/19 |
| Calf muscles spastic overactivity at baseline | |
| MAS (0-5), median (IQR) | 2.0 (2.0; 3.0) |
| TS grade (0-4), median (IQR) | 2.0 (2.0; 4.0) |
| TS angle (°), mean (SD) | 13.4 (8.1) |
| Calf muscles spastic overactivity after DNB evaluation | |
| MAS (0-5), median (IQR) | 1.0 (1.0; 3.0) |
| TS grade (0-4), median (IQR) | 1.0 (1.0; 2.0) |
| TS angle (°), mean (SD) | 2.8 (3.1) |
SD: standard deviation; N.: number; PROM: passive range of motion; MAS: Modified Ashworth Scale; TS: Tardieu Scale; IQR: interquartile range; DNB: diagnostic nerve block.
Figure 1.
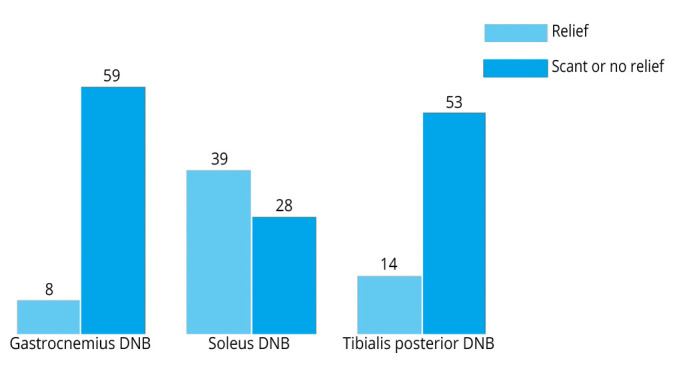
—Changes in the condition of patients after selective DNB. DNB: diagnostic nerve block.
Discussion
This observational study was based on a chart review and aimed to retrospectively investigate the Silfverskiöld Test validity for differentiating gastrocnemius from soleus spastic muscle overactivity. The results showed no association between the outcomes of Silfverskiöld Test and those of tibial motor nerve branches selective DNB to the gastrocnemius, soleus and tibialis posterior muscles in stroke patients suffering from spastic equinus (plantar flexed) foot. Nils Silfverskiöld defined two clinical presentations of the equinus contracture deformity: 1) equinus foot that is essentially inflexible with the knee fully extended but passively correctable when the knee is flexed; and 2) equinus foot that persists whether the knee is extended or flexed.6 He suggested to treat the first clinical presentation (i.e., equinus foot passively correctable when the knee is flexed) by transferring the gastrocnemius heads (muscle origin) from the femoral condyles to the tibia (below the knee joint level) (the so-called “Silfverskiöld’s operation”). On the other hand, the second clinical presentation (i.e., persistent equinus foot) should receive Achilles tendon lengthening.7 Consistently, Nils Silfverskiöld developed a clinical test that aims to evaluate equinus contracture deformity and differentiate its clinical presentations (i.e., to distinguish between isolated gastrocnemius contracture vs. combined shortening of the gastro-soleus complex, ankle joint capsule retraction and bony deformity) on a surgical approach perspective.6, 7 The triceps surae is a structure made of the soleus (i.e., one-joint muscle crossing the ankle joint) and the (medial and lateral) gastrocnemius muscles (i.e., two-joint muscle crossing both the knee and ankle joints).18 As a whole, its contraction induces foot plantar flexion and stabilization of the ankle joint. In particular, the soleus muscle mainly assists ankle plantar flexion (i.e., pushing down the foot) when the knee is bent. Instead, considering it is bi-articular, the gastrocnemius muscle plantar flexes the ankle and assists in flexing leg at the knee joint. So, in order to critically discuss its use in PSS patients without equinus contracture deformity (i.e., to distinguish between gastrocnemius and soleus spastic muscle overactivity), from a functional anatomy perspective the Silfverskiöld Test may not reflect an altered interaction between the triceps muscles affected by spastic overactivity considering that a simultaneous activation of both soleus and gastrocnemius muscles were found in patients with spastic equinus (plantar flexed) foot evaluated by means of electromyography.19 Furthermore, from an architectural point of view, the triceps surae muscle complex assessed using ultrasonography was found altered in hemiplegic patients with spastic equinus (plantar flexed) foot.20-22 In particular, during the extended knee condition of Silfverskiöld Test, the fascicle length was found longer in the lateral gastrocnemius, unmodified in the medial gastrocnemius and shorter in the soleus muscles when compared to the healthy (not affected) leg. Moreover, the pennation angle was found smaller in the (medial and lateral) gastrocnemius and unmodified in the soleus.20 On the other hand, during the flexed knee condition of the Silfverskiöld Test, the fascicle length was found increased and the pennation angle decreased in the soleus muscle. Opposite findings were observed in the (medial and lateral) gastrocnemius muscle.20 These architectural modifications are not considered by the Silfverskiöld Test, which is mainly based on a mechanical concept, and represent a further reason for explaining the concerns about its validity for differentiating gastrocnemius and soleus muscle overactivity in stroke patients suffering from spastic equinus (plantar flexed) foot. As to our results, another interesting finding regards the large proportion of patients who showed an improvement of the soleus muscle spastic overactivity after selective DNB, which might appear surprising if compared to the outcome of gastrocnemius and tibialis posterior motor nerve branches DNB. However, this observation is in line with our clinical experience and with the current literature that describes spastic overactivity of the soleus muscle as the main responsible for spastic equinus (plantar flexed) foot in 75% of cases, whereas the gastrocnemius muscle would be predominantly involved in only 12.5% of cases.23
Limitations of the study
This study has a number of limitations. First, the design of the study was retrospective. In our view, the current findings should be considered preparatory for larger, future, prospective studies on the same field (i.e., investigate the validity of the main clinical tests commonly used for PSS evaluation). Furthermore, due to the retrospective design, the patients were not standardized, and we did not provide information about the spastic muscle echo intensity because the database was incomplete with respect to this outcome.21, 22, 24 However, despite this information might be relevant for having wider evidence about modifications of spastic muscles and their clinical manifestations, the results of this study would not be affected by the absence of ultrasound data considering the relation between spastic muscle ultrasound parameters and their response to DNB.24 Second, this study did not include data about treatments, such as BoNT-A. In particular, we avoided the injection of “wrong muscles” because of the DNB evaluation, which is routinely performed in our clinical practice. So, we cannot draw any conclusion about the clinical outcome of patients treated with BoNT-A only based on the Silfverskiöld Test compared to those evaluated by means of the DNB. Third, we did not report information about the ankle dorsiflexion active range of motion because, according to the retrospective design of this study, we did not find enough data in our records on this outcome. Furthermore, we did not perform neurophysiological evaluations such as (surface) electromyography at rest or during walking.
Conclusions
Our findings do not support the Silfverskiöld Test as a valid tool for evaluating spastic equinus (plantar flexed) foot in order to differentiate between gastrocnemius, soleus and tibialis posterior spastic muscle overactivity in adult patients with stroke. On this line, a use of the Silfverskiöld Test that goes beyond its original aims (i.e., to distinguish between isolated gastrocnemius contracture vs. combined shortening of the gastro-soleus complex, ankle joint capsule retraction and bony deformity) should be discouraged to avoid the misinterpretation of clinical condition in PSS patients. So, the choice of an appropriate management of spastic equinus (plantar flexed) foot in adults with stroke should not be defined mainly on the base of the Silfverskiöld Test. Future larger prospective studies on this issue are needed, considering (and overcoming) the limitations reported above.
Footnotes
Conflicts of interest: Alessandro Picelli, Andrea Santamato, Alessio Baricich, and Nicola Smania received research funds, honoraria, donations and financial supports, and have consultant relationship topically but not directly related to the manuscript.
References
- 1.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795–820. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34487721&dopt=Abstract 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Francisco GE, Rymer WZ. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil Neural Repair 2021;35:601–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33978513&dopt=Abstract 10.1177/15459683211011214 [DOI] [PubMed] [Google Scholar]
- 3.Picelli A, Santamato A, Cosma M, Baricich A, Chisari C, Millevolte M, et al. Early botulinum toxin type A injection for post-stroke spasticity: a longitudinal cohort study. Toxins (Basel) 2021;13:374. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34073918&dopt=Abstract 10.3390/toxins13060374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esquenazi A, Bavikatte G, Bandari DS, Jost WH, Munin MC, Tang SF, et al. Long-term observational results from the ASPIRE study: OnabotulinumtoxinA treatment for adult lower limb spasticity. PM R 2021;13:1079–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33151636&dopt=Abstract 10.1002/pmrj.12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deltombe T, Wautier D, De Cloedt P, Fostier M, Gustin T. Assessment and treatment of spastic equinovarus foot after stroke: guidance from the Mont-Godinne interdisciplinary group. J Rehabil Med 2017;49:461–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28451697&dopt=Abstract 10.2340/16501977-2226 [DOI] [PubMed] [Google Scholar]
- 6.Silfverskiöld N. Reduction of the uncrossed two-joints muscles of the leg to one-joint muscles in spastic conditions. Acta Chir Scand 1924;56:315–30. [Google Scholar]
- 7.Singh D. Nils Silfverskiöld (1888-1957) and gastrocnemius contracture. Foot Ankle Surg 2013;19:135–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23548458&dopt=Abstract 10.1016/j.fas.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 8.Kovalenko A, Misikov V, Sinelnikov K, Shamigulov V, Iskra DE, Khatkova S, et al. Spasticity: diagnosis and treatment. London: IntechOpen; 2020. [Google Scholar]
- 9.Esquenazi A, Alfaro A, Ayyoub Z, Charles D, Dashtipour K, Graham GD, et al. OnabotulinumtoxinA for lower limb spasticity: guidance from a Delphi panel approach. PM R 2017;9:960–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28286053&dopt=Abstract 10.1016/j.pmrj.2017.02.014 [DOI] [PubMed] [Google Scholar]
- 10.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987;67:206–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3809245&dopt=Abstract 10.1093/ptj/67.2.206 [DOI] [PubMed] [Google Scholar]
- 11.Goss DA, Jr, Long J, Carr A, Rockwell K, Cheney NA, Law TD, Sr. Clinical implications of a one-hand versus two-hand technique in the Silfverskiöld Test for gastrocnemius equinus. Cureus 2020;12:e6555. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32042528&dopt=Abstract 10.7759/cureus.6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konor MM, Morton S, Eckerson JM, Grindstaff TL. Reliability of three measures of ankle dorsiflexion range of motion. Int J Sports Phys Ther 2012;7:279–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22666642&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini M, Iocco M, Molteni F, Santamato A, Smania N, Italian Spasticity Study Group . Management of stroke patients submitted to botulinum toxin type A therapy: a Delphi survey of an Italian expert panel of specialist injectors. Eur J Phys Rehabil Med 2014;50:525–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24963604&dopt=Abstract [PubMed] [Google Scholar]
- 14.Banky M, Clark RA, Pua YH, Mentiplay BF, Olver JH, Williams G. Inter- and intra-rater variability of testing velocity when assessing lower limb spasticity. J Rehabil Med 2019;51:54–60. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30483723&dopt=Abstract 10.2340/16501977-2496 [DOI] [PubMed] [Google Scholar]
- 15.Picelli A, Chemello E, Verzini E, Ferrari F, Brugnera A, Gandolfi M, et al. Anatomical landmarks for tibial nerve motor branches in the management of spastic equinovarus foot after stroke: an ultrasonographic study. J Rehabil Med 2019;51:380–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30843081&dopt=Abstract https://doi.org/ 10.2340/16501977-2543 [DOI] [PubMed] [Google Scholar]
- 16.Paulis WD, Horemans HL, Brouwer BS, Stam HJ. Excellent test-retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture 2011;33:185–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21131203&dopt=Abstract 10.1016/j.gaitpost.2010.10.094 [DOI] [PubMed] [Google Scholar]
- 17.Naghdi S, Ansari NN, Ghorbani-Rad S, Senobari M, Sahraian MA. Intra-rater reliability of the Modified Tardieu Scale in patients with multiple sclerosis. Neurol Sci 2017;38:93–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27620726&dopt=Abstract 10.1007/s10072-016-2714-7 [DOI] [PubMed] [Google Scholar]
- 18.Herman R. Function of the gastrocnemius and soleus muscles. A preliminary study in the normal human subject. Phys Ther 1967;47:105–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6045280&dopt=Abstract 10.1093/ptj/47.2.105 [DOI] [PubMed] [Google Scholar]
- 19.Perry J, Hoffer MM, Giovan P, Antonelli D, Greenberg R. Gait analysis of the triceps surae in cerebral palsy. A preoperative and postoperative clinical and electromyographic study. J Bone Joint Surg Am 1974;56:511–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4822513&dopt=Abstract 10.2106/00004623-197456030-00008 [DOI] [PubMed] [Google Scholar]
- 20.Park KB, Joo SY, Park H, Rhee I, Shin JK, Abdel-Baki SW, et al. Architecture of the triceps surae muscles complex in patients with spastic hemiplegia: implication for the limited utility of the Silfverskiöld Test. J Clin Med 2019;8:2096. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31805732&dopt=Abstract 10.3390/jcm8122096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picelli A, Bonetti P, Fontana C, Barausse M, Dambruoso F, Gajofatto F, et al. Is spastic muscle echo intensity related to the response to botulinum toxin type A in patients with stroke? A cohort study. Arch Phys Med Rehabil 2012;93:1253–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22502807&dopt=Abstract 10.1016/j.apmr.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 22.Picelli A, Tamburin S, Cavazza S, Scampoli C, Manca M, Cosma M, et al. Relationship between ultrasonographic, electromyographic, and clinical parameters in adult stroke patients with spastic equinus: an observational study. Arch Phys Med Rehabil 2014;95:1564–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24792138&dopt=Abstract 10.1016/j.apmr.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Decq P, Cuny E, Filipetti P, Kéravel Y. Role of soleus muscle in spastic equinus foot. Lancet 1998;352:118. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9672287&dopt=Abstract 10.1016/S0140-6736(98)85025-3 [DOI] [PubMed] [Google Scholar]
- 24.Filippetti M, Di Censo R, Varalta V, Baricich A, Santamato A, Smania N, et al. Is the outcome of diagnostic nerve block related to spastic muscle echo intensity? A retrospective observational study on patients with spastic equinovarus foot. J Rehabil Med 2022;54:jrm00275. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35266004&dopt=Abstract 10.2340/jrm.v54.85 [DOI] [PMC free article] [PubMed]


