Abstract
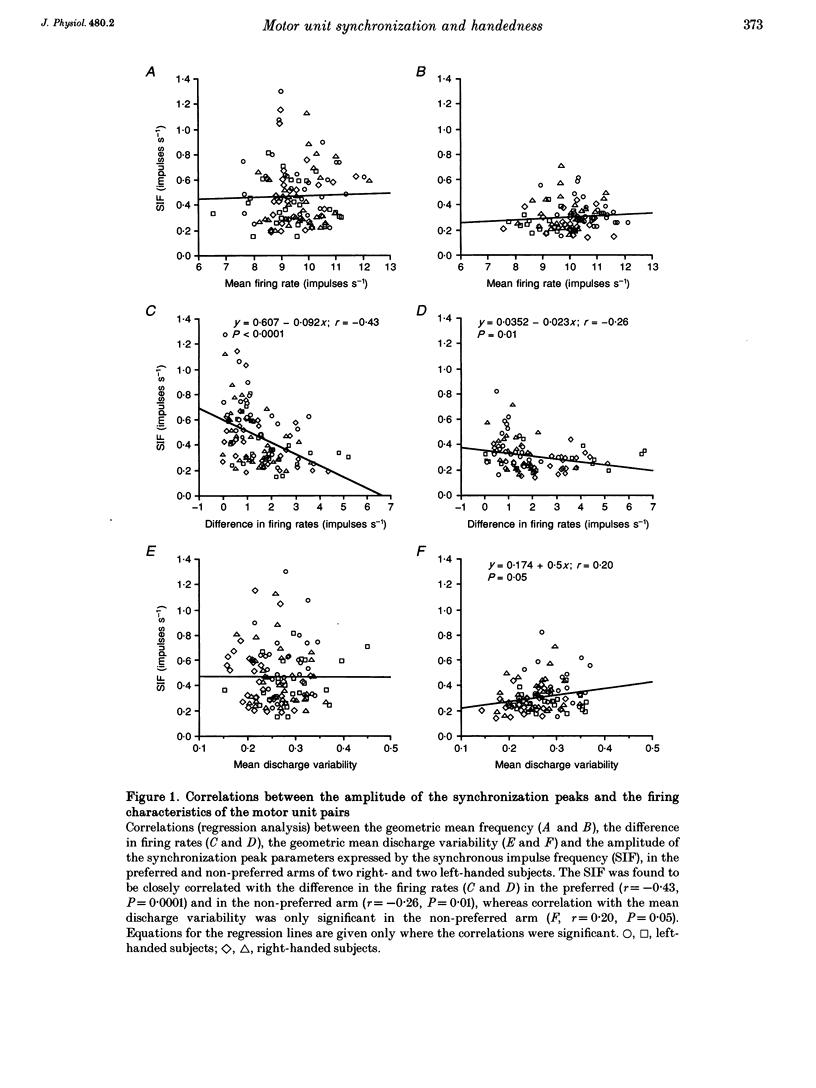
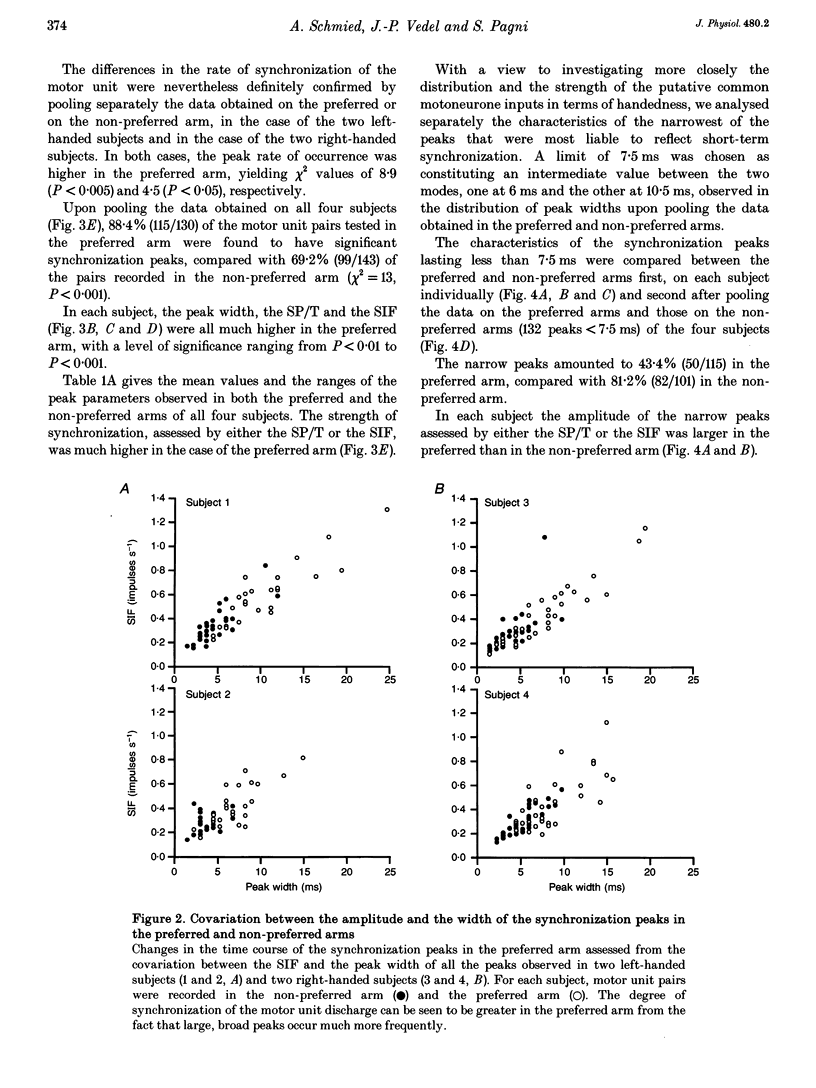
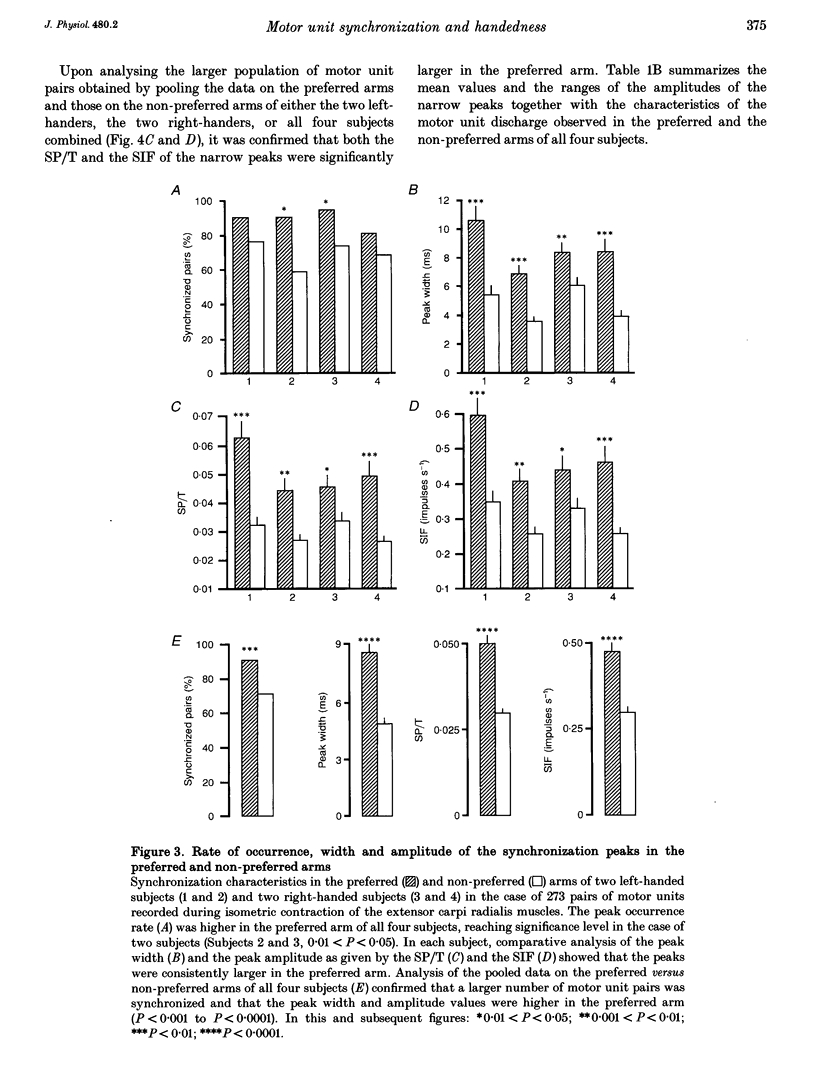
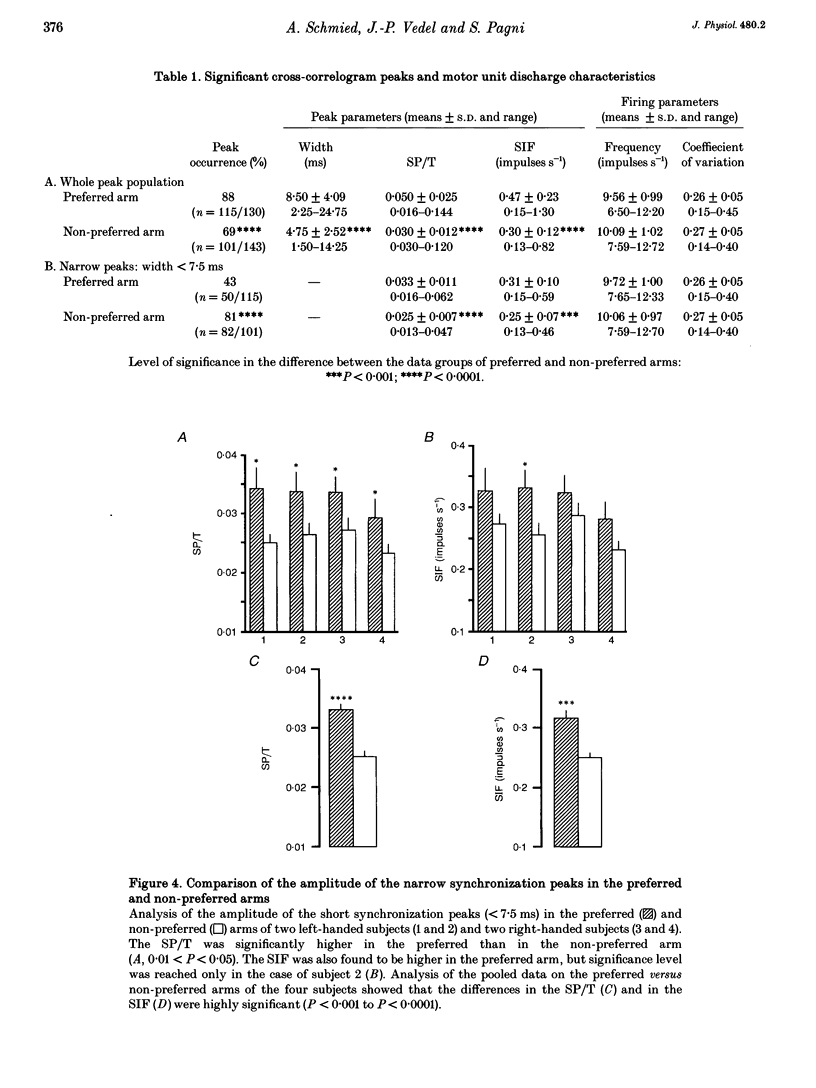
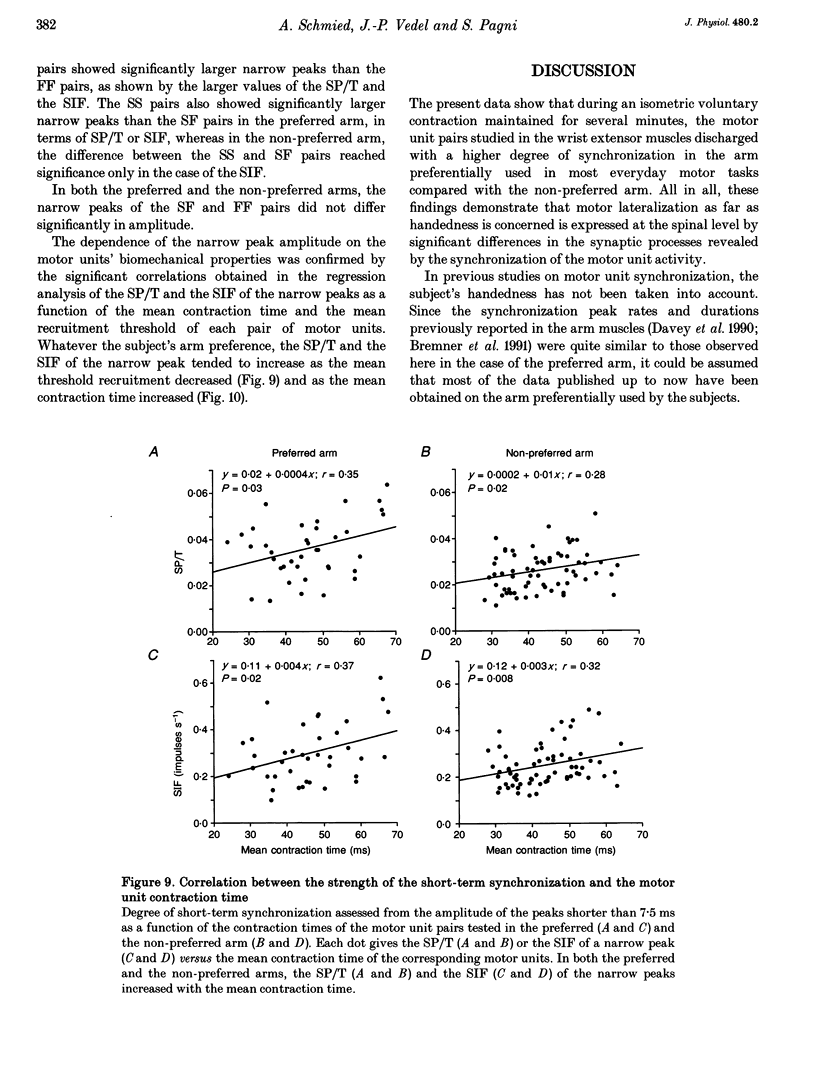
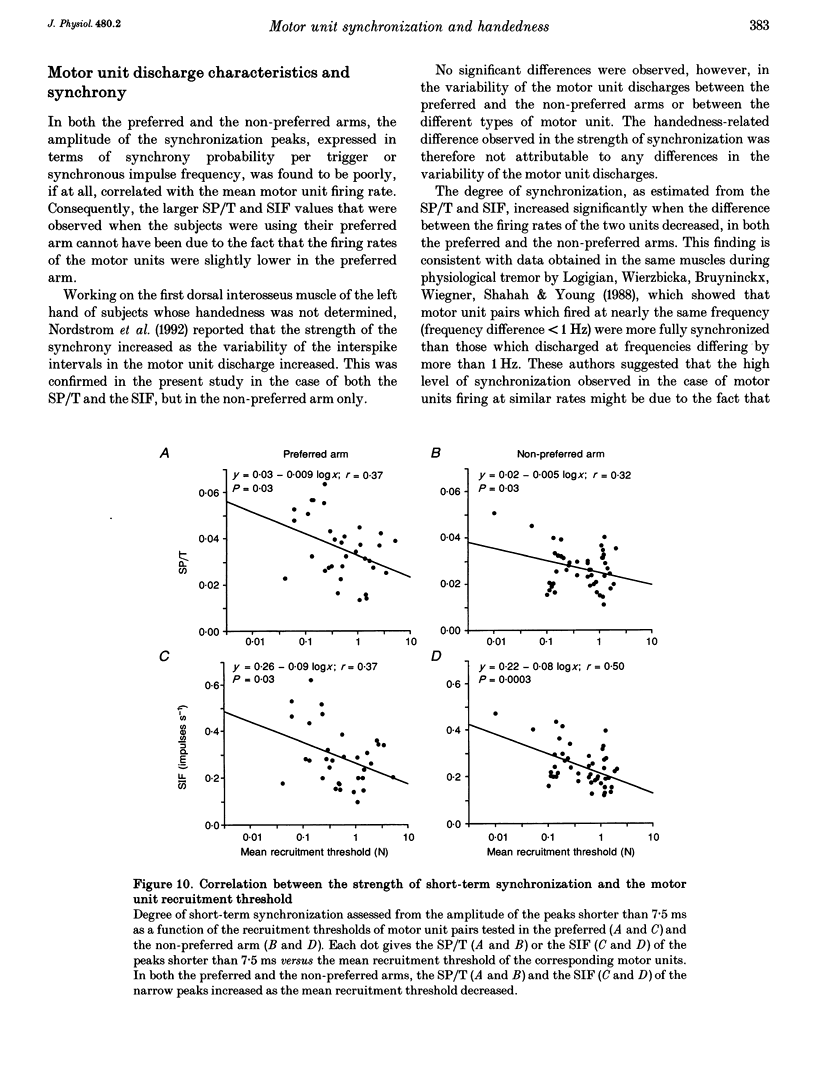
1. Motoneurone synchronization as a means of investigating synaptic connectivity was studied in the extensor carpi radialis muscles of the preferred and non-preferred arms of healthy right- and left-handed human subjects. The activities of pairs of motor units recorded during voluntary isometric contractions were analysed by cross-correlation to detect any synchronous motor unit firing in the form of central peaks in the cross-correlation histograms. 2. The synchronization peaks were compared first in the case of 273 motor unit pairs tested in the preferred and non-preferred arms of two left-handed subjects and two right-handed subjects. The percentage of synchronized motor unit pairs was found to be significantly higher in the preferred arm with synchronization peaks significantly larger and broader than in the non-preferred arm. The narrow peaks (< 7.5 ms) likely to reflect the activity of common inputs to motoneurones were also found to be significantly larger in the preferred arm of all four subjects. 3. The handedness-related differences in synchronization were definitely confirmed in a total of 275 pairs of motor units tested in the left extensor carpi radialis muscles of fourteen right-handed subjects using their non-preferred arm and six left-handed subjects using their preferred arm. In order to determine whether the differences in synchronization were dependent on the motor unit type, each motor unit was characterized on the basis of its recruitment threshold and on the basis of the contraction time of its twitch extracted from the overall muscle force using the spike-triggered averaging method. Two populations of motor units were distinguished, namely the 'slow' motor units (recruitment thresholds < 0.4 N, contraction times > 40 ms) and the 'fast' motor units (recruitment thresholds > 0.6 N, contraction times < or = 40 ms). 4. In the non-preferred arm, the synchronization peaks were always fairly narrow, whatever the motor unit's biomechanical properties; whereas in the preferred arm, broad peaks were found to be particularly common among the pairs including one or two fast motor units, which also showed the largest rate of synchronization occurrence. 5. The narrow peaks (< 7.5 ms) were found to be consistently larger in the preferred than the non-preferred arm whatever the categories of motor unit pairs. In both arms, however, the amplitude of the narrow peaks tended to increase as the recruitment threshold of the motor unit decreased and as their contraction time increased.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
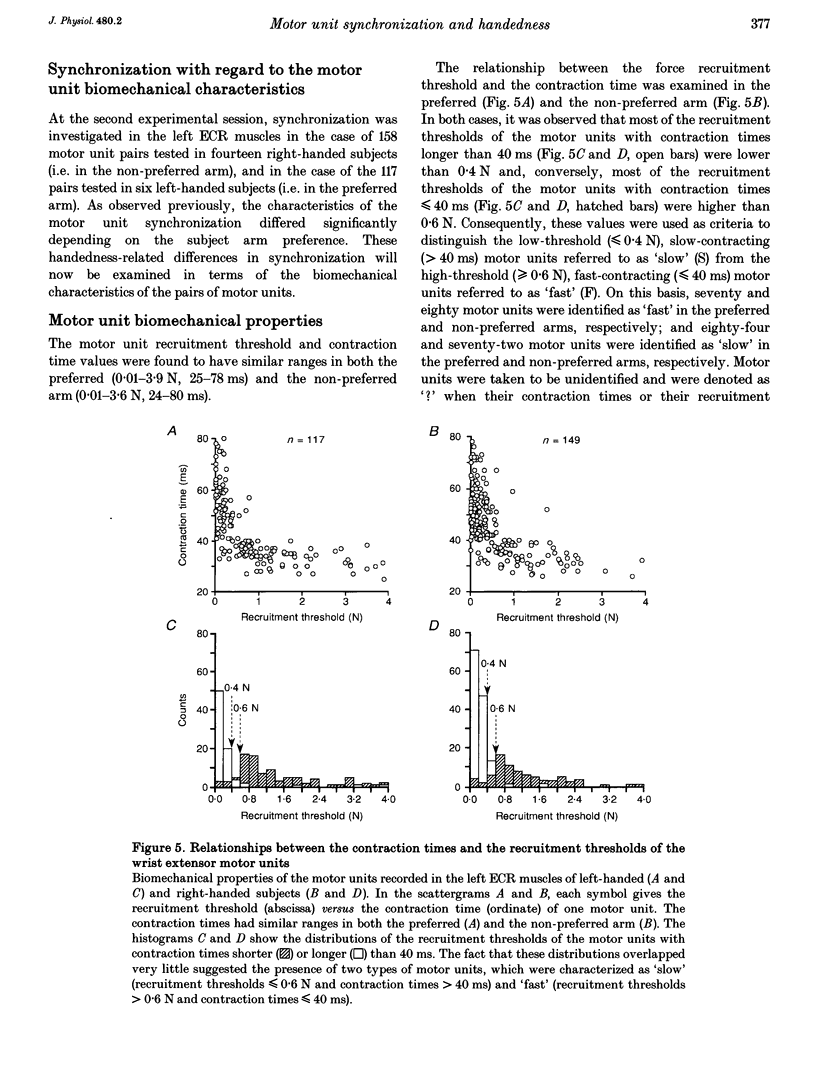
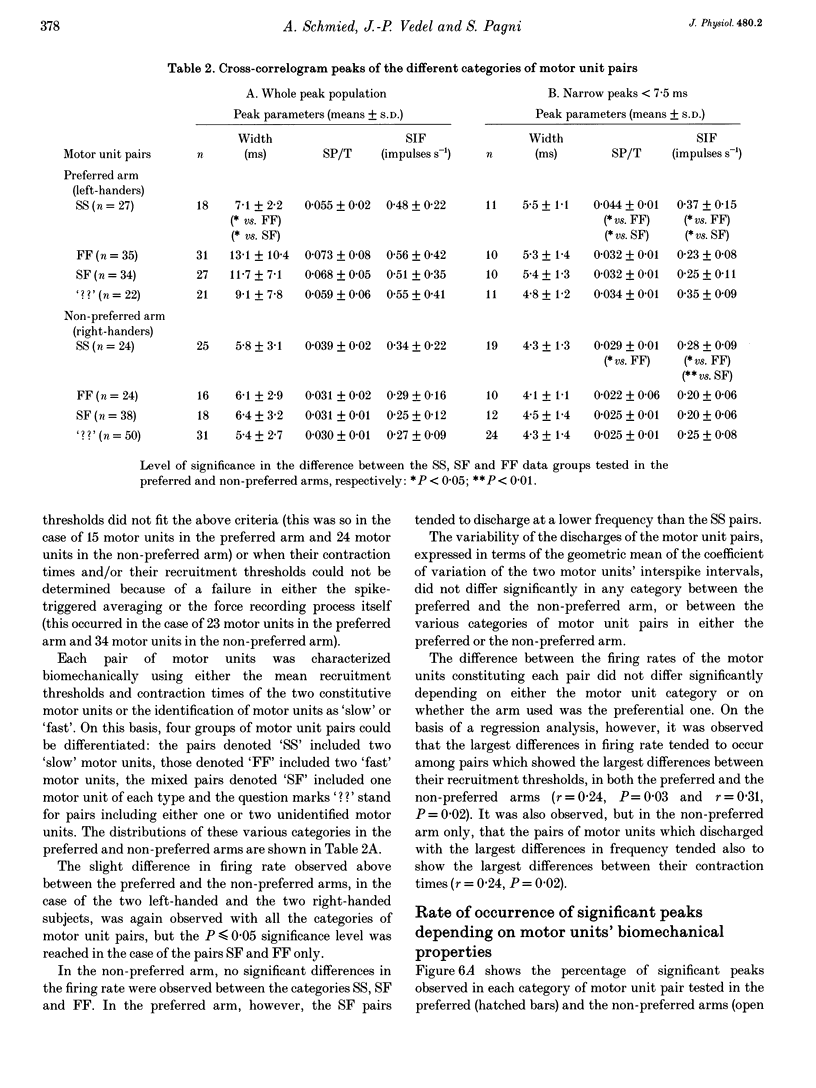
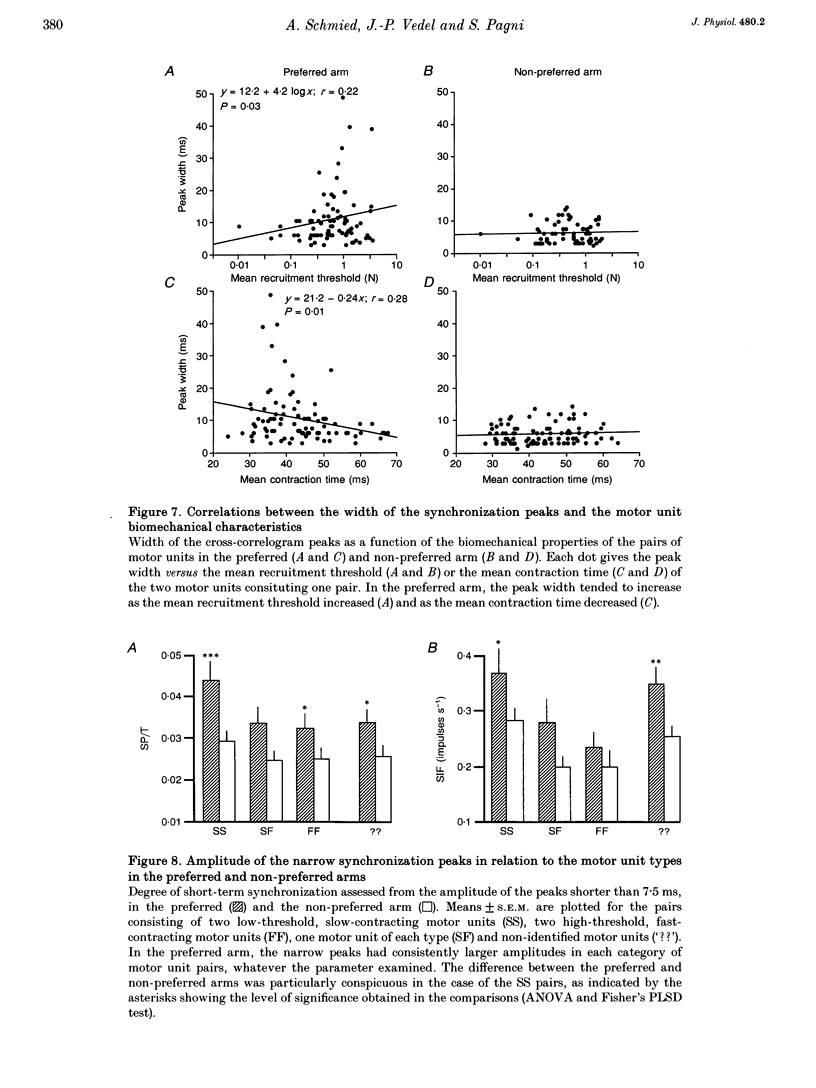
PDF



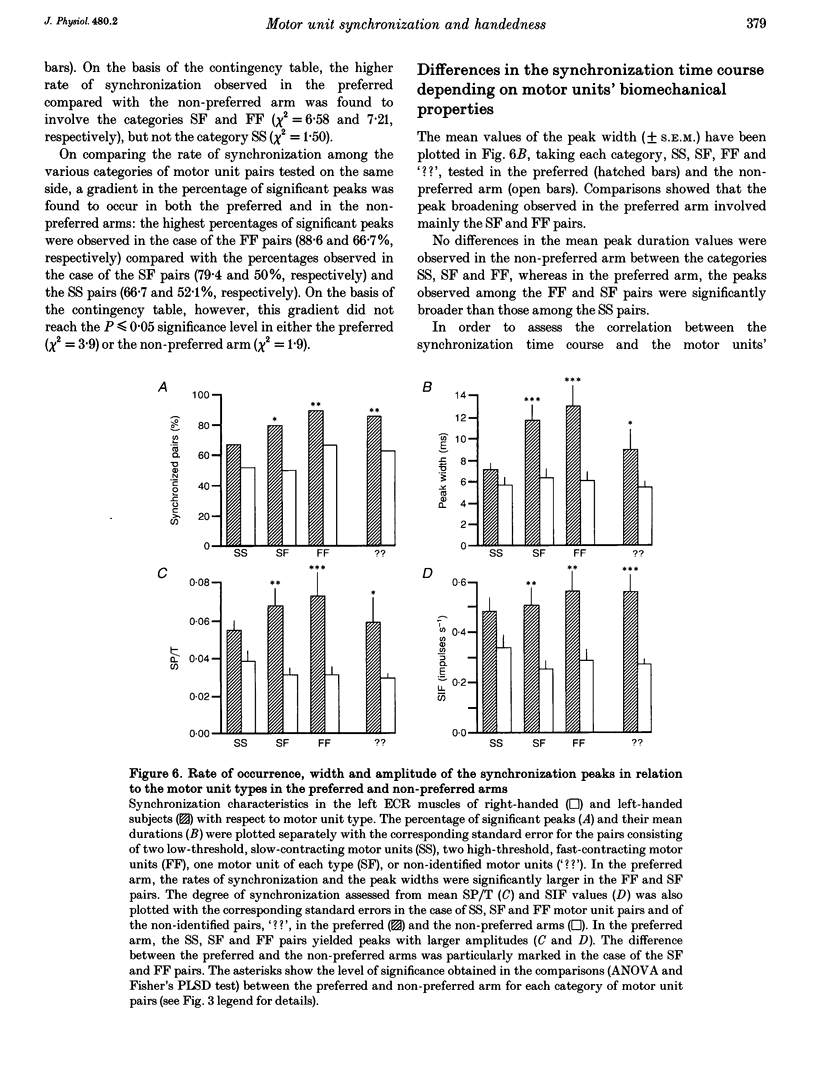





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L., Datta A. K., Guz A. Synchronization of motor unit firing during different respiratory and postural tasks in human sternocleidomastoid muscle. J Physiol. 1989 Jun;413:213–231. doi: 10.1113/jphysiol.1989.sp017650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHTHAL F., MADSEN A. Synchronous activity in normal and atrophic muscle. Electroencephalogr Clin Neurophysiol. 1950 Nov;2(4):425–444. doi: 10.1016/0013-4694(50)90079-4. [DOI] [PubMed] [Google Scholar]
- Bremner F. D., Baker J. R., Stephens J. A. Variation in the degree of synchronization exhibited by motor units lying in different finger muscles in man. J Physiol. 1991 Jan;432:381–399. doi: 10.1113/jphysiol.1991.sp018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rymer W. Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol. 1976 May;39(3):447–458. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope T. C., Fetz E. E., Matsumura M. Cross-correlation assessment of synaptic strength of single Ia fibre connections with triceps surae motoneurones in cats. J Physiol. 1987 Sep;390:161–188. doi: 10.1113/jphysiol.1987.sp016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Stephens J. A. Synchronization of motor unit activity during voluntary contraction in man. J Physiol. 1990 Mar;422:397–419. doi: 10.1113/jphysiol.1990.sp017991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H., Friedland C. L., Short D. J. Motor unit discharge characteristics and short term synchrony in paraplegic humans. J Neurol Neurosurg Psychiatry. 1990 Sep;53(9):764–769. doi: 10.1136/jnnp.53.9.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H., Stein R. B. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods. 1986 Aug;17(2-3):153–166. doi: 10.1016/0165-0270(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Dengler R., Wolf W., Birk P., Struppler A. Synchronous discharges in pairs of steadily firing motor units tend to form clusters. Neurosci Lett. 1984 Jun 15;47(2):167–172. doi: 10.1016/0304-3940(84)90424-5. [DOI] [PubMed] [Google Scholar]
- Dick T. E. Artifactual averaged 'twitch tension' waveforms resulting from synchronized activity: recording from feline diaphragmatic motor units. Neurosci Lett. 1990 Jun 22;114(1):57–62. doi: 10.1016/0304-3940(90)90428-c. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode D. J., Glenn S., Manning A. A., Middleton J. F. Lateral asymmetry of the Hoffmann reflex: relation to cortical laterality. J Neurol Neurosurg Psychiatry. 1980 Sep;43(9):831–835. doi: 10.1136/jnnp.43.9.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logigian E. L., Wierzbicka M. M., Bruyninckx F., Wiegner A. W., Shahahi B. T., Young R. R. Motor unit synchronization in physiologic, enhanced physiologic, and voluntary tremor in man. Ann Neurol. 1988 Mar;23(3):242–250. doi: 10.1002/ana.410230306. [DOI] [PubMed] [Google Scholar]
- Mantel G. W., Lemon R. N. Cross-correlation reveals facilitation of single motor units in thenar muscles by single corticospinal neurones in the conscious monkey. Neurosci Lett. 1987 Jun 1;77(1):113–118. doi: 10.1016/0304-3940(87)90617-3. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975 Mar;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973 Jan;228(2):285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom M. A., Fuglevand A. J., Enoka R. M. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol. 1992;453:547–574. doi: 10.1113/jphysiol.1992.sp019244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom M. A., Miles T. S., Türker K. S. Synchronization of motor units in human masseter during a prolonged isometric contraction. J Physiol. 1990 Jul;426:409–421. doi: 10.1113/jphysiol.1990.sp018146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom M. A., Miles T. S., Veale J. L. Effect of motor unit firing pattern on twitches obtained by spike-triggered averaging. Muscle Nerve. 1989 Jul;12(7):556–567. doi: 10.1002/mus.880120706. [DOI] [PubMed] [Google Scholar]
- Nudo R. J., Jenkins W. M., Merzenich M. M., Prejean T., Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992 Aug;12(8):2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Powers R. K., Rymer W. Z. Effects of acute dorsal spinal hemisection on motoneuron discharge in the medial gastrocnemius of the decerebrate cat. J Neurophysiol. 1988 May;59(5):1540–1556. doi: 10.1152/jn.1988.59.5.1540. [DOI] [PubMed] [Google Scholar]
- Romaiguère P., Vedel J. P., Pagni S., Zenatti A. Physiological properties of the motor units of the wrist extensor muscles in man. Exp Brain Res. 1989;78(1):51–61. doi: 10.1007/BF00230686. [DOI] [PubMed] [Google Scholar]
- Schmied A., Ivarsson C., Fetz E. E. Short-term synchronization of motor units in human extensor digitorum communis muscle: relation to contractile properties and voluntary control. Exp Brain Res. 1993;97(1):159–172. doi: 10.1007/BF00228826. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Mannard A., Yemm R. New methods for analysing motor function in man and animals. Brain Res. 1972 May 12;40(1):187–192. doi: 10.1016/0006-8993(72)90126-6. [DOI] [PubMed] [Google Scholar]
- Stein R. D., Weaver L. C. Multi- and single-fibre mesenteric and renal sympathetic responses to chemical stimulation of intestinal receptors in cats. J Physiol. 1988 Feb;396:155–172. doi: 10.1113/jphysiol.1988.sp016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan U. Spinal motor lateralization assessed by recovery curve of H reflex from wrist flexors in right-, and left-handed normal subjects. Int J Neurosci. 1989 Oct;48(3-4):309–312. doi: 10.3109/00207458909002175. [DOI] [PubMed] [Google Scholar]
- Thomas C. K., Bigland-Ritchie B., Westling G., Johansson R. S. A comparison of human thenar motor-unit properties studied by intraneural motor-axon stimulation and spike-triggered averaging. J Neurophysiol. 1990 Oct;64(4):1347–1351. doi: 10.1152/jn.1990.64.4.1347. [DOI] [PubMed] [Google Scholar]
- Wiegner A. W., Wierzbicka M. M. A method for assessing significance of peaks in cross-correlation histograms. J Neurosci Methods. 1987 Dec;22(2):125–131. doi: 10.1016/0165-0270(87)90006-9. [DOI] [PubMed] [Google Scholar]



