Abstract
Background
Lung cancer ranks as the leading cause of cancer-related deaths worldwide. There is evidence that second-hand smoke (SHS) exposure is a risk factor for the development of lung cancer in never-smokers. This systematic review and meta-analysis aims to provide the most accurate quantification of the association between SHS exposure and lung cancer risk in never-smokers.
Materials and methods
Through the use of an innovative method to identify original publications, we conducted a systematic review of the literature, with corresponding meta-analysis, of all epidemiological studies evaluating the association between SHS exposure and lung cancer risk among never-smokers, published up to May 2023. Pooled relative risks were obtained using random-effects models. Dose–response relationships were derived using log-linear functions or cubic splines.
Results
Out of 126 identified eligible studies, 97 original articles were included in the meta-analysis. The pooled relative risk for lung cancer for overall exposure to SHS was 1.24 (95% CI 1.16–1.32, number of articles, n=82). Setting-specific relative risks were 1.20 (95% CI 1.12–1.28, n=67) for SHS exposure at home, 1.38 (95% CI 1.28–1.62, n=30) at a workplace, 1.37 (95% CI 1.22–1.53, n=28) at home or a workplace and 1.27 (95% CI 1.11–1.44, n=24) in nonspecified settings. The risk of lung cancer significantly increased with the duration, intensity and pack-years of SHS exposure.
Conclusions
This meta-analysis shows that exposure to SHS increases by more than 20% the risk of lung cancer among never-smokers, providing definitive evidence of the association between SHS exposure and lung cancer risk.
Shareable abstract
Lung cancer risk significantly increases by 24% in never-smokers exposed to second-hand smoke (SHS). In addition, the risk of lung cancer significantly increased with the duration, intensity and pack-years of SHS exposure. https://bit.ly/3MPKzbr
Introduction
According to the World Health Organization, lung cancer ranks as the first most commonly diagnosed malignancy worldwide, with an estimated 2.5 million new cases in 2022 [1]. Additionally, it holds the leading position for cancer-related deaths worldwide, accounting for approximately 18% of all cancer deaths. Although the unequivocal link between active smoking and lung cancer has been firmly established in the last century [2, 3], a non-negligible proportion of lung cancer cases occurs among never-smokers [4, 5].
Lung cancer in never-smokers is more frequently observed in women and is distinguished by a greater prevalence of the adenocarcinoma histological subtype when compared to lung cancer in smokers [6, 7]. Several risk factors for the development of lung cancer in never-smokers have been identified, including exposure to environmental pollution, radon, asbestos and having a family history of lung cancer [8–12]. In addition, second-hand smoke (SHS) exposure has been recognised as a significant risk factor for the development of lung cancer among never-smokers [13–15]. The biological basis for the association between SHS exposure and lung cancer risk in never-smokers lies in the presence of carcinogens and toxicants in sidestream smoke and exhaled mainstream smoke, which are the two components of SHS [13, 14].
Although the link between SHS exposure and lung cancer risk has been thoroughly investigated in the literature through observational studies, as well as over 50 systematic reviews, meta-analyses and pooled analyses, the most recent comprehensive systematic review on the topic was published more than 10 years ago [14]. Subsequent meta-analyses or systematic reviews on the topic have only included studies focusing on specific population subgroups or specific sources of SHS [15, 16].
The primary objective of this meta-analysis is to quantify the precise magnitude of the association between any type of SHS exposure and lung cancer risk among never-smokers, thus filling the existing gap from recent meta-analyses on the topic. Furthermore, this meta-analysis aims to unravel the association of interest using dose–response analyses and stratified analyses, with specific stratifications including setting of SHS exposure, sex, study design and cancer subtype.
Methods
The current review is one in a sequence of systematic reviews and meta-analyses exploring the association between cigarette smoking or SHS exposure and cancer risk [17–22]. This approach, which has already been described in detail in previous articles, takes advantage of an innovative methodology for identifying original articles, incorporating both umbrella and traditional review techniques [18, 23]. This efficient approach, which is highly effective when the studied issue has been highly investigated through systematic reviews, as in this case, ensures a thorough assessment of the existing literature on SHS exposure and lung cancer risk. The research protocol was formally registered with the International Prospective Registry of Systematic Reviews (PROSPERO), with the registration number CRD42017063991.
Search strategy
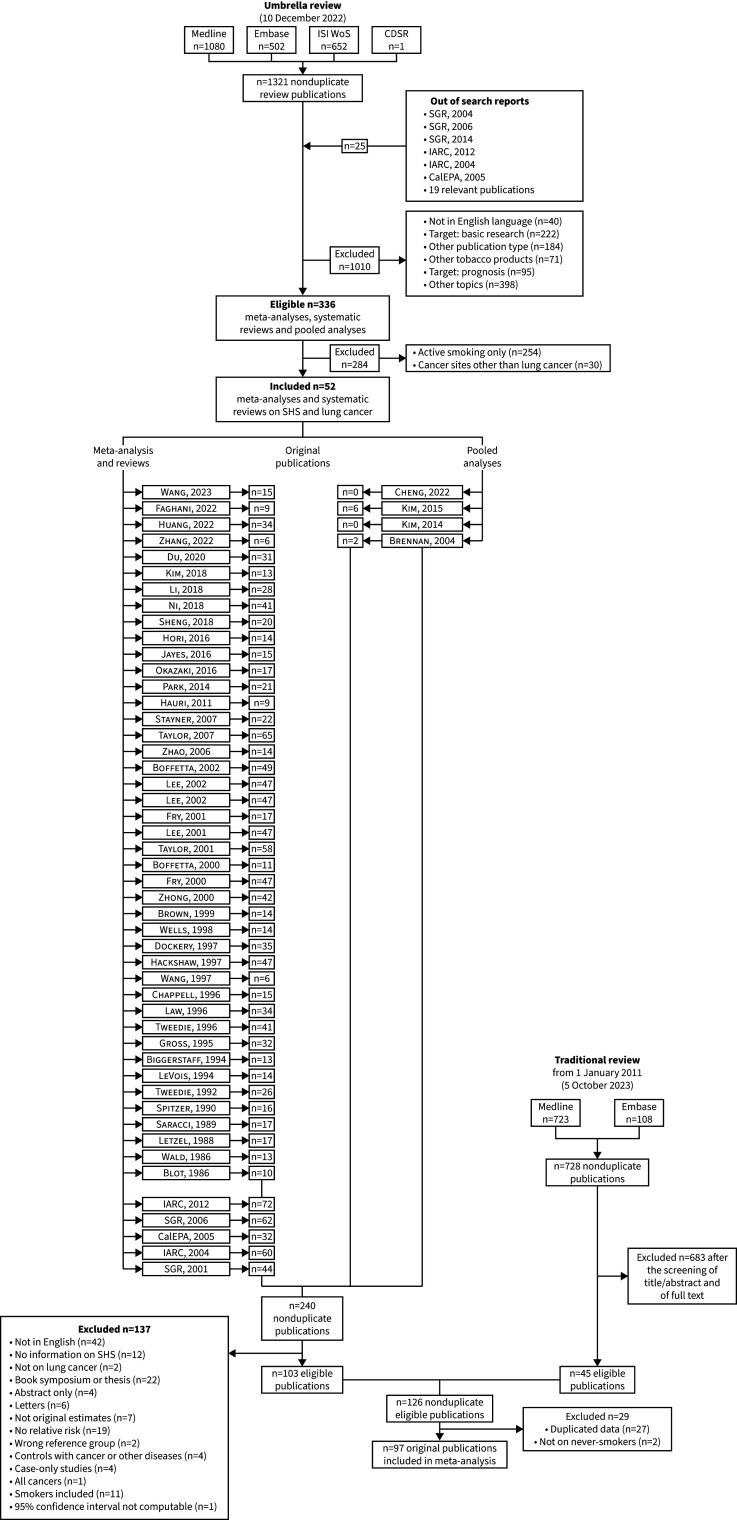
In the first phase of the search strategy, we conducted an umbrella review focusing on active smoking and SHS exposure in relation to the risk of cancer at any site. A thorough search of the scientific literature was carried out across four distinct databases (PubMed/Medline, Embase, Institute for Scientific Information Web of Science and Cochrane Database of Systematic Reviews) to identify all meta-analyses, pooled analyses and systematic reviews investigating the association between smoking or SHS exposure and cancer risk. The initial search was executed on 27 April 2017 [23] and subsequently updated on 14 January 2020 [22] and 12 October 2022. Out of 336 eligible meta-analyses, pooled analyses, systematic reviews and reports, 52 focused on the association between SHS exposure and lung cancer risk (supplementary table S1). From this pool of 52 reports, we systematically extracted all 240 nonduplicate original publications examining the association between SHS exposure and lung cancer risk (figure 1). These articles underwent comprehensive eligibility screening based on their full text, employing the criteria described in the subsequent section, resulting in the inclusion of 103 papers and the exclusion of 137 papers (supplementary table S2).
FIGURE 1.
Flowchart for the selection of the original studies on the association between second-hand (SHS) exposure and lung cancer risk included in the review and meta-analysis. For references cited within the figure, see the supplementary material. CalEPA: California Environmental Protection Agency; CDSR: Cochrane Database of Systematic Reviews; IARC: International Agency for Research on Cancer; ISI WoS: Institute for Scientific Information Web of Science; SGR: Surgeon General Report.
As a subsequent step, we executed a traditional literature search on PubMed/Medline and Embase. The aim was to identify all original studies that addressed the association of interest and that were published in English between 2008 (the year of the conduction of the last and most comprehensive review available on the issue [14]) and 10 May 2023. The search strategy comprised combinations of MeSH (medical subject heading) terms and free text pertaining to lung cancer and SHS (supplementary box S1), yielding a total of 728 nonduplicate original publications. After the exclusion of ineligible publications, the literature update resulted in 45 original articles examining the association between SHS exposure and lung cancer risk.
Eligibility criteria
To be included in the current meta-analysis, studies had to meet the following criteria: 1) be published as an original article in English; 2) be a case–control study (including nested case–control studies or pooled analyses of case–control studies) or a cohort study (including case–cohort studies or pooled analyses of cohort studies); 3) provide information on the association between exposure to SHS and the risk of lung cancer exclusively in the subgroup of never- or nonsmoker adults; 4) either present risk estimates, including risk ratios, odds ratios, hazard ratios or mortality ratios (from now on all expressed as relative risks) for SHS exposure in at least one setting or source or SHS exposure duration (years of exposure), intensity (number of cigarettes per day to which one is exposed) or pack-years (a cumulative measure of SHS exposure), using individuals not exposed to SHS as comparison group, and the corresponding 95% confidence intervals, or provide sufficient information to calculate them. As an exception to the third criterion, if a study was conducted on a population of individuals with a prevalence of smokers lower than 5%, it was included in the analysis even if it did not provide association measures specifically for never- or nonsmokers. There were no restrictions on the assessment of outcomes, whether histologically confirmed or obtained from cancer registries, medical records or death certificates.
Data extraction
For each study deemed eligible, we systematically extracted information concerning the publication (e.g., first author, year of publication and journal), the study (e.g., country in which the study was conducted, study design, calendar period in which the study was conducted and sample size), the setting of SHS exposure (e.g., at home, at workplace or in public places), the source of SHS exposure (e.g., in childhood or from partner), the model used for deriving the relative risk estimates (including adjustment variables), the relative risks with corresponding 95% confidence intervals and, if available, the number of cases and controls (or at-risk subjects/person-years for cohort studies) for the various exposure categories. When necessary, we applied the method described by Hamling et al. [24] to change the reference category or collapse relative risks from two or more categories sharing the same reference group. In cases where relative risks were reported separately for distinct types of lung cancer (e.g., small-cell lung cancer, adenocarcinoma or squamous-cell carcinoma), we used the approach outlined by Rucker et al. [25] to derive a single relative risk for overall lung cancer. In addition, relative risks based on subgroups, such as sex, were extracted when such data were available.
Statistical analysis
Pooled relative risks for the association between SHS exposure and lung cancer risk were calculated taking into account different settings and sources of SHS exposure. These included exposure at home, at workplace, at home or workplace, from a partner, during childhood, and in nonspecified settings. The latter category also included exposures identified through general questions, such as “are you exposed to SHS in at least one setting?” or “have you ever been exposed to SHS in any setting?”. We also performed an overall analysis by considering one risk for each original article. In cases where more than one source or setting of SHS exposure was reported in the same article, we prioritised them as follows: 1) in nonspecified settings with clarification about weekly or daily minimum duration of exposure (in terms of hours per day/week); 2) at home or at workplace; 3) at home (excluding exposure from partner only); 4) from partner; 5) at workplace; and 6) in nonspecified settings without clarification about minimum duration of exposure. Childhood exposure was not included in the overall analysis. This decision was based on the different nature of childhood exposure compared to other SHS exposures, which required separate consideration in stratified analyses. However, as no study focused exclusively on childhood exposure, all studies were included in the overall analysis.
Pooled estimates were derived using random-effects models to account for potential heterogeneity in risk estimates [26]. Between-study heterogeneity was assessed using the χ2 test, while inconsistency was measured using the I2 statistic, which represents the proportion of the total variation that is attributable to between-study variance [27]. We conducted stratified analyses for overall SHS exposure according to different study, population and cancer characteristics (i.e., study design, type of control, end-point, year of publication, presence of adjusting variables, number of cases, study quality, sex, geographic area, income group and lung cancer subtype). Heterogeneity between strata was assessed using the Q-test for subgroup differences, assuming a random-effects model.
To assess publication bias, we examined funnel plots [28] and applied Egger's test for funnel plot asymmetry [29].
Study quality was assessed by two authors (A.L. and I.P.) using the Newcastle–Ottawa Scale (NOS) for case–control and cohort studies [30]. The NOS score ranges from 0 (poor quality) to 9 (good quality) and considers information on three broad categories, as follows: selection (maximum 4 points), comparability (maximum 2 points) and outcome for case–control or exposure for cohort studies (maximum 3 points). In this meta-analysis, high-quality studies were defined as those with NOS scores ≥7. To ensure the completeness and comprehensiveness of our study, no low-quality study was excluded from the meta-analysis.
We evaluated dose–response relationships between SHS exposure variables (i.e., duration, intensity, and pack-years of SHS exposure) and log relative risk of lung cancer, either linear or nonlinear, using one-stage random-effects dose–response models [31]. For each exposure variable, we tested the statistical significance of nonlinear coefficients using the Wald test. If linearity was rejected, nonlinear relationships were modelled using a restricted cubic spline with three knots at fixed percentiles of exposure (10, 50 and 90%) [18, 32]. For each category, the level of exposure was set as the mid-point between the upper and the lower bounds; for open-ended upper categories, the level of exposure was determined as 1.2 times the lower bound [23, 33, 34]. If the number of cases and/or controls in one or more exposure categories was not provided in the original study publication, we estimated the covariance between the log relative risks by considering the total number of cases and/or controls weighted by the average percentage distribution of subjects pooled from all other studies [35].
A 5% significance level was used to determine statistical significance in all analyses.
All statistical analyses were performed using R software version 4.2.2 (R Development Core Team, 2022) and, particularly, the packages “meta” and “dosresmeta” [35, 36].
Results
Study selection and description
A total number of 97 articles were included in the present systematic review and meta-analysis. The main characteristics of the included studies, comprising 71 case–control and 27 cohort studies (one article includes both a case–control study and a cohort study [37]), are detailed in supplementary tables S4 and S5. These studies spanned the years 1983–2022 and together included at least 21 740 lung cancer cases. 67 studies provided an assessment of self-reported SHS exposure at home (including exposure from a partner), 30 for exposure at workplace, 28 for exposure at home or workplace and 24 for exposure in nonspecified settings. In addition, 34 studies reported on the association between SHS exposure during childhood and the risk of lung cancer. The list of publications containing data that were partially excluded from the meta-analysis, along with the corresponding reasons for exclusion, is shown in supplementary table S6. Quality assessments of included case–control and cohort studies are shown in supplementary tables S7 and S8, respectively. Among case–control studies, 15% (11/71) achieved a quality score of 7 or higher on the NOS, compared with 37% (10/27) among cohort studies.
Quantitative data synthesis
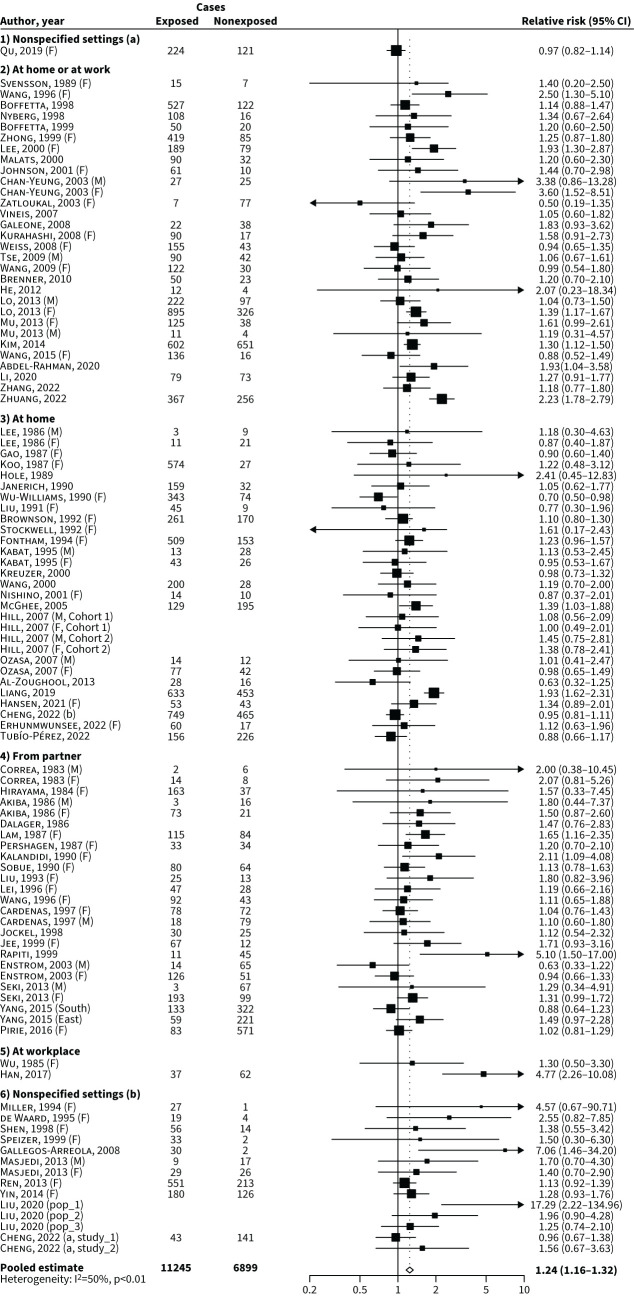
In the overall analysis based on 82 studies, where each article contributed with information from a single source or setting of exposure, the pooled relative risk of lung cancer in never-smokers exposed to SHS compared with never-smokers nonexposed to SHS was 1.24 (95% CI 1.16–1.32; figure 2), significantly higher in case–control studies (relative risk 1.30; 95% CI 1.20–1.41) compared to cohort studies (relative risk 1.07; 95% CI 0.99–1.16; p-value of heterogeneity <0.01).
FIGURE 2.
Forest plot of the overall analysis# and pooled relative risk of lung cancer for never-smokers exposed to second-hand smoke (SHS) compared with nonexposed never-smokers by setting or source of exposure. #: considering one source or setting of exposure for each article. a: including clarifications about weekly or daily minimum duration of exposure; b: without clarifications about weekly or daily minimum duration of exposure; East: Eastern Chinese; F: females; M: males; pop_1: population exposed to low levels of indoor air pollution; pop_2: population exposed to medium levels of indoor air pollution; pop_3: population exposed to high levels of indoor air pollution; South: southern Chinese; study_1: 45 and Up Study; study_2: New South Wales Cancer, Lifestyle and Evaluation of Risk Study. For references cited within the figure, see the supplementary material.
Possible sources of heterogeneity were investigated through stratified analyses (table 1). When examining different settings of SHS exposure, the pooled relative risks for lung cancer were 1.20 (95% CI 1.12–1.28) for SHS exposure at home (supplementary figure S1), 1.38 (95% CI 1.28–1.62) for SHS exposure at the workplace (supplementary figure S2), 1.37 (95% CI 1.22–1.53) for SHS exposure at home or workplace (supplementary figure S3) and 1.27 (95% CI 1.11–1.44) for SHS exposure in nonspecified settings (supplementary figure S4). In addition, the pooled relative risks were 1.18 (95% CI 1.08–1.29) for partner-only exposure (supplementary figure S5) and 1.12 (95% CI 1.00–1.26) for SHS exposure during childhood (supplementary figure S6).
TABLE 1.
Pooled relative risk and corresponding 95% confidence interval of lung cancer for never-smokers exposed to second-hand smoke (SHS) versus nonexposed to SHS, overall and in strata of selected characteristics
| Strata | Number of studies | Pooled relative risk (95% CI) | p-value# | p-value¶ |
|---|---|---|---|---|
| Total | 82 | 1.24 (1.16–1.32) | NA | <0.01 |
| Settings of SHS exposure | ||||
| At home+ | 67 | 1.20 (1.12–1.28) | NA | <0.01 |
| At workplace | 30 | 1.38 (1.28–1.62) | <0.01 | |
| At home or workplace | 28 | 1.37 (1.22–1.53) | <0.01 | |
| Nonspecified | 24 | 1.27 (1.11–1.44) | <0.01 | |
| Specific sources | ||||
| From partner | 41 | 1.18 (1.08–1.29) | NA | <0.01 |
| During childhood | 34 | 1.12 (1.00–1.26) | <0.01 | |
| Sex | ||||
| Male | 15 | 1.15 (0.96–1.37) | 0.66 | 0.67 |
| Female | 51 | 1.20 (1.11–1.29) | 0.04 | |
| Subtypes | ||||
| NSCLC | 26 | 1.43 (1.24–1.65) | NA | <0.01 |
| Adenocarcinoma | 23 | 1.42 (1.21–1.67) | <0.01 | |
| SQCLC | 7 | 1.98 (1.57–2.49) | 0.38 | |
| LCLC | 2 | 1.69 (0.92–3.09) | 0.49 | |
| SCLC | 5 | 1.99 (1.17–3.39) | 0.10 | |
| Geographic area§ | ||||
| Northern and Central America | 19 | 1.12 (1.01–1.24) | 0.02 | 0.52 |
| Europe | 16 | 1.08 (0.97–1.21) | 0.57 | |
| Asia | 42 | 1.33 (1.21–1.46) | <0.01 | |
| Oceania | 2 | 1.05 (0.73–1.51) | 0.30 | |
| Income group## | ||||
| High-income | 57 | 1.19 (1.09–1.29) | 0.23 | <0.01 |
| Middle-income | 25 | 1.31 (1.14–1.51) | <0.01 | |
| Study design | ||||
| Case–control | 57 | 1.30 (1.20–1.41) | <0.01 | <0.01 |
| Cohort | 25 | 1.07 (0.99–1.16) | 0.75 | |
| Type of controls¶¶ | ||||
| Hospital | 15 | 1.30 (1.13–1.49) | 0.87 | <0.01 |
| Population | 35 | 1.28 (1.13–1.45) | <0.01 | |
| End-point++ | ||||
| Incidence | 14 | 1.15 (1.02–1.30) | 0.19 | 0.46 |
| Mortality | 13 | 1.03 (0.93–1.14) | 0.77 | |
| Year of publication | ||||
| <1998 | 26 | 1.20 (1.07–1.35) | 0.85 | 0.38 |
| 1998–2009 | 27 | 1.22 (1.10–1.36) | 0.05 | |
| ≥2010 | 29 | 1.26 (1.13–1.40) | <0.01 | |
| Adjustment | ||||
| Crude | 10 | 1.12 (0.95–1.31) | 0.28 | 0.09 |
| Adjusted | 72 | 1.23 (1.14–1.33) | <0.01 | |
| Number of cases | ||||
| ≤89 | 28 | 1.34 (1.16–1.55) | 0.15 | 0.38 |
| 90–219 | 26 | 1.33 (1.18–1.49) | 0.12 | |
| ≥220 | 28 | 1.17 (1.07–1.28) | <0.01 | |
| Study quality | ||||
| Low (NOS <7) | 62 | 1.25 (1.16–1.35) | 0.68 | <0.01 |
| High (NOS ≥7) | 20 | 1.21 (1.08–1.37) | <0.01 | |
#: p-value for heterogeneity across strata. ¶: p-value for heterogeneity within strata. +: Including exposure from partner. §: No studies from Africa or South America. ##: No studies from low-income countries. ¶¶: Type of controls for case–control studies only. ++: End-point for cohort studies only. LCLC: large-cell lung cancer; NA: not applicable; NOS: Newcastle Ottawa Scale; NSCLC: nonsmall-cell lung cancer; SCLC: small-cell lung cancer; SQCLC: squamous-cell lung cancer.
Significant differences in the association between overall SHS exposure and lung cancer risk were observed by geographic area (relative risks were 1.12 for Northern and Central America, 1.08 for Europe, 1.33 for Asia and 1.05 for Oceania; p=0.02). Stratified analyses based on NOS quality assessment, categorising studies into high- (NOS ≥7) and low- (NOS <7) quality groups, indicated no significant differences. No other statistically significant differences according to study or population characteristics were observed. Subgroup analyses focusing solely on studies with high quality (NOS ≥7) are reported in supplementary table S9.
Regarding nonsmall-cell lung cancer (encompassing adenocarcinoma, squamous-cell lung cancer, large-cell lung cancer and others), the pooled relative risk for SHS exposure was 1.43 (95% CI 1.24–1.65; 26 studies). The pooled relative risk for adenocarcinoma alone was 1.42 (95% CI 1.21–1.65; 23 studies; supplementary figure S7), for squamous-cell carcinoma was 1.98 (95% CI 1.57–2.49; seven studies) and for large-cell lung cancer was 1.69 (95% CI 0.92–3.09; two studies). Compared to never-smokers not exposed to SHS, the pooled relative risk for small-cell lung cancer was 1.99 (95% CI 1.17–3.39; five studies) for never-smokers exposed to SHS.
Publication bias
Evidence of publication bias was absent in the analyses of overall SHS exposure, in the analyses of SHS exposure in specific settings and in the analysis of SHS exposure from partner (supplementary figure S8, panels A–F). However, evidence of publication bias was found for the analysis of SHS exposure during childhood, with a p-value for the Egger's test equal to 0.03 (supplementary figure S8, panel G).
Dose–response analysis
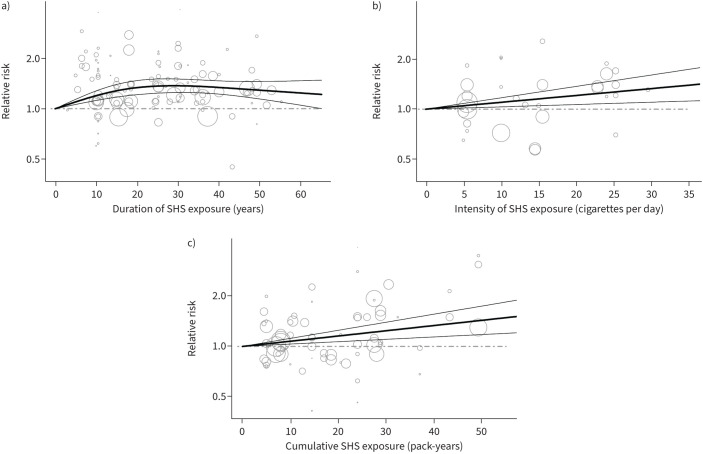
Figure 3 shows the dose–response relationships between duration (panel a), intensity (panel b) and pack-years (panel c) of SHS exposure and the risk of lung cancer. In each dose–response analysis, each article contributed with information from a single source or setting of exposure. A nonlinear increase in lung cancer risk with the duration of SHS exposure was observed (based on 25 studies; figure 3a). Relative risks of lung cancer risk sharply increased with a low number of years of SHS exposure (5 or 10) up to 20 years, with the trend then appearing to decrease for higher numbers of years of exposure. The relative risks were 1.20 (95% CI 1.13–1.28) for 10 years, 1.34 (95% CI 1.22–1.48) for 20 years and 1.34 (95% CI 1.23–1.47) for 40 years of SHS exposure (estimated using the curve functions reported in supplementary box S2). In addition, the risk of lung cancer increased linearly with the intensity of SHS exposure (relative risks: 1.10 (95% CI 1.03–1.17) for 10 cigarettes per day of SHS exposure and 1.21 (95% CI 1.07–1.37) for 20 cigarettes per day of SHS exposure; based on 19 studies; figure 3b). In addition, the risk of lung cancer also increased linearly with pack-years of SHS exposure (relative risks: 1.16 (95% CI 1.07–1.25) for 20 pack-years and 1.34 (95% CI 1.14–1.57) for 40 pack-years of SHS exposure; based on 16 studies; figure 3c).
FIGURE 3.
Relative risk for the dose–response relationships between second-hand smoke (SHS) duration, intensity and pack years, and the risk of lung cancer. a) Years of SHS exposure. b) Cigarettes per day of SHS exposure; c) Pack-years of SHS exposure. Linear model from a random-effects dose–response model (thick solid line); 95% confidence interval of the linear model (thin solid lines); relative risk for nonexposed women (dashed lines); Circles indicate relative risk for various exposure categories in each study included in the analysis. The area of the circle is proportional to the precision (i.e., to the inverse variance) of the relative risk.
Discussion
This systematic review and meta-analysis represents the most up-to-date and comprehensive evidence on the association between SHS exposure and lung cancer risk. It includes 126 eligible articles and uses data from 97 original articles and more than 20 000 lung cancer cases. The results of the meta-analysis showed a statistically significant 24% overall excess risk of lung cancer among individuals who never smoked but were exposed to SHS compared with those who were not exposed. In addition, we found a significant excess risk of lung cancer associated with SHS exposure in all settings and from all sources considered. Dose–response analyses showed that the risk of lung cancer increased with the intensity, duration and pack-years of SHS exposure.
A recent meta-analysis based on the general population of never-smokers, without sex or geographical restriction, but based on only 12 studies, found an excess risk of 25% for lung cancer associated with any type of SHS exposure [38, 39]. Notably, our study, which included eight times the number of articles, strengthens the robustness of these findings.
Regarding the settings of SHS exposure, our analysis identified the highest excess risks for exposure at workplace and exposure at home or at workplace (38 and 37%, respectively). These findings suggest that occupational exposure to SHS plays an important role in the development of lung cancer, likely because of the prolonged and continuous nature of such exposure in certain occupational environments [40]. Workplaces where smoking is permitted may expose individuals to sustained and concentrated exposure, particularly in areas with limited and inadequate ventilation and confined spaces, and thus contribute to a significant increased risk of lung cancer.
A considerable number of studies were conducted in the female population only or presented stratified analyses based on sex, because of the high prevalence of nonsmoking women exposed to SHS, particularly in Asian countries [41], and the relatively high proportion of women among nonsmoking-related lung cancer cases [6]. The possible sex-specific interaction between tobacco carcinogens and lung cancer has been a highly debated topic in the literature [42, 43]. Several biological explanations have been proposed, including molecular and hormonal factors [44, 45], different genetic polymorphisms [46], and differences in lifestyle and behavioural factors related to smoking habits or environmental exposures between men and women [47]. However, recent large studies on active smoking and lung cancer have not supported the hypothesis of a sex-specific interaction [48, 49]. Similarly, our stratified analyses revealed no significant differences in the increased risk of lung cancer associated with SHS exposure between men and women, suggesting no interaction between SHS exposure and sex in the aetiology of lung cancer. The specific results for the relative risk of lung cancer associated with any type of SHS exposure in women are consistent with those of a previous meta-analysis [50].
When examining geographical differences, our analysis revealed a higher increased risk of lung cancer associated with SHS exposure in studies conducted in Asia (which are also the most numerous ones) compared with studies conducted in other continents. In particular, the relative risk of 1.33 found for Asia is lower than those reported in previous meta-analyses of Chinese studies, which ranged from 1.50 to 2.00 [51–53], but is higher compared to a meta-analysis of Japanese studies, which found a relative risk of 1.28 [54]. This difference suggests that China may play an important role in influencing the elevated relative risk associated with Asia. Further research into specific contextual factors within China, such as environmental pollution, cultural influences or the stage of the tobacco epidemic [55], may provide deeper insights into the observed geographical differences.
Our meta-analysis identified significant differences in risk estimates between study designs, with cohort studies showing a lower pooled relative risk compared with case–control studies. One possible explanation for this difference is that the extent of misclassification of SHS exposure may be more pronounced in cohort studies, since they often rely on a single assessment of SHS exposure and thus lack comprehensive information on previous lifetime SHS exposure or repeated assessments during follow-up [56]. Given the relatively high prevalence of SHS exposure in the general population, the misclassification of exposed individuals as nonexposed ones can substantially dilute observed risks [57] and may therefore contribute to the underestimation of the pooled relative risk observed in cohort studies. On the other hand, a different recall bias between cancer cases and healthy subjects is more likely to affect case–control studies than cohort studies. This could lead to an overestimation of the relative risk observed in case–control studies.
In the analysis of histological subtypes, we identified higher risks associated with SHS exposure for small-cell lung cancer and squamous-cell lung cancer compared with adenocarcinoma and large-cell lung cancer. These findings align with those observed for active smoking [58–60] and underline the similar carcinogenic effect of active smoking and SHS [14]. Given that adenocarcinoma is the most common subtype of lung cancer among never-smokers [6, 7], future studies should focus on investigating other potential risk factors to explain the substantial proportion of adenocarcinoma cases not associated with active smoking or SHS exposure.
Our study found a linear dose–response relationship between the intensity of SHS exposure and the risk of lung cancer, showing that exposure to just five cigarettes per day increased the risk of lung cancer by 5%. This finding is consistent with a recent meta-analysis on this topic, which found that the risk of lung cancer increases with greater cigarette exposure [61]. In addition, we found a nonlinear association between the duration of SHS exposure and lung cancer risk, with the relative risk rapidly increasing with a few years of SHS exposure up to 1.37 after 29 years of SHS exposure and then appearing to decrease. This finding is not entirely consistent with previous meta-analyses on the topic, which reported linear trends for SHS exposure duration [62, 63], although they focused on specific settings of exposure and limited numbers of studies. With regard to the nonlinearity of the association, it is possible that individuals who have been exposed to SHS for a long time and have not developed other tobacco-related diseases may be less susceptible to SHS-related harm. In addition, the pattern of risk for long-term exposure to SHS may be influenced by a higher likelihood of exposure misclassification, especially among older individuals, who tend to have longer exposures. Furthermore, the estimates for the highest exposure categories were provided by a limited number of studies, particularly beyond 50 years of SHS exposure, as indicated by the large confidence intervals of these estimates. This suggests that the observed trend may also be due to variability and uncertainty in the data for these high-exposure groups.
Our meta-analysis has several strengths. The innovative methodology used to identify original articles, which involved a combination of umbrella and traditional reviews, allowed the inclusion of data from 126 eligible articles investigating the association between SHS exposure and lung cancer risk. Particularly, the umbrella review technique allows for the systematic identification of all articles included in previous systematic reviews and meta-analyses, thus enabling the inclusion of more studies than a single search string would capture. By updating the literature search with a traditional review, the aim was to identify the most recent studies, while drawing on the extensive work of previous reviews. This approach makes our meta-analysis the most comprehensive one available on this topic, including 75% more studies than the most comprehensive systematic review available in the scientific literature, which included 72 studies [14]. Notably, we identified and included 35 additional articles published before 2009, the year of the search string of such review, that were not included in this systematic review, which significantly increased the scope of our analysis. Although it is possible that some studies may have been overlooked in previous systematic reviews, the increase in the number of studies included in our meta-analysis outweighs any potential loss. In addition, we carried out a rigorous screening process of the publications to avoid data overlap. We also excluded data from original studies that analysed nonsmokers (i.e., never-smokers and former smokers combined) rather than never-smokers only, although the impact on the results was minimal (the inclusion of the two studies on nonsmokers would have resulted in a pooled relative risk for overall SHS exposure of 1.23). Another strength of our analysis is related to the examination of different settings and sources of exposure, which provides a more thorough understanding of the relationship between SHS exposure and lung cancer risk. By examining dose–response relationships, we also provided valuable insights into the different degrees of risk associated with different levels of SHS exposure. Finally, the quality of various studies has been assessed using the NOS, which is the most widely recognised tool for assessing the quality of observational studies [30]. Subgroup analyses on high-quality studies did not reveal differences compared to analyses including all studies. The only exception was for SHS exposure in nonspecified settings, which was not significant in the high-quality studies, though this finding was based on only three studies.
Limitations of this meta-analysis include the presence of heterogeneity between studies, which was observed for almost all sources of exposure. The diversity of study methodologies, definitions of SHS exposure, definition of never-smokers and individual characteristics and background risks may have contributed to this heterogeneity. To account for this heterogeneity, we employed random-effects models, which provide more conservative estimates. Despite this approach, some heterogeneity may persist. We further explored potential sources of heterogeneity by conducting stratified analyses based on factors such as the study design and population characteristics. In addition, the potential misclassification of SHS exposure in the included studies may have affected the precision of our estimates. Particularly, accurate estimation of characteristics of SHS exposure, such as the number of cigarettes passively inhaled per day, can be challenging for participants and thus impact the precision of dose–response analyses. In addition, our analysis did not systematically account for potential confounding factors such as exposure to coal, dust, radon or asbestos. Future studies should consider assessing these factors to provide a more accurate assessment of lung cancer risk associated with SHS exposure.
In conclusion, our meta-analysis provides comprehensive and up-to-date evidence underscoring SHS exposure as a major risk factor for lung cancer development in never-smokers. These findings have significant public health implications and emphasise the need to raise awareness among the general population of the risks associated with SHS exposure, thus encouraging people to adopt voluntary smoking bans, such as smoke-free homes. In addition, policymakers are urged to integrate these research insights into the development, implementation and enforcement of comprehensive smoke-free policies, particularly in workplaces, thereby contributing to an overall reduction of SHS exposure levels and its associated health risks.
Questions for future research
Understanding how cultural attitudes influence exposure to SHS and its associated health effects is essential for the development of comprehensive public health interventions aimed at reducing the burden of lung cancer in nonsmoking populations. In addition, future studies should prioritise the investigation of the interactions between SHS exposure and other risk factors, such as exposure to environmental pollutants, genetic predisposition, occupational exposure and lifestyle factors, to elucidate the substantial proportion of lung adenocarcinoma cases that cannot be attributed to active smoking or SHS exposure alone.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0077-2024.SUPPLEMENT (1.5MB, pdf)
Acknowledgements
We thank Maria Chiara Malevolti and Cosimo Campagni (Institute for Cancer Research, Prevention and Clinical Network (ISPRO), Florence, Italy) for their contributions.
Provenance: Submitted article, peer reviewed.
Conflict of interest: All authors declare that they have no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Support statement: The research leading to these results has received funding from AIRC under MFAG 2021 – ID. 25840 project – P.I. A. Lugo. The work of S. Gallus is partially supported by the Italian League Against Cancer (LILT), Milan. The work of G. Carreras is partially supported by the 2018 Health Research Grant by the Tuscany Region within the project “Attributable Cancer Burden in Tuscany: smoking, environmental and occupational risk factors and evaluation of prevention strategies” (ACAB). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Date last accessed: 8 March 2024. Date last updated: 2024. https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf
- 2.International Agency for Research on Cancer . Tobacco Smoke and Involuntary Smoking. Lyon, World Health Organization, 2004. [Google Scholar]
- 3.US Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004. [Google Scholar]
- 4.Siegel DA, Fedewa SA, Henley SJ,et al. Proportion of never smokers among men and women with lung cancer in 7 US States. JAMA Oncol 2021; 7: 302–304. doi: 10.1001/jamaoncol.2020.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer 2007; 7: 778–790. doi: 10.1038/nrc2190 [DOI] [PubMed] [Google Scholar]
- 6.Dubin S, Griffin D. Lung cancer in non-smokers. Mol Med 2020; 117: 375–379. [PMC free article] [PubMed] [Google Scholar]
- 7.Pirie K, Peto R, Green J,et al. Lung cancer in never smokers in the UK Million Women Study. Int J Cancer 2016; 139: 347–354. doi: 10.1002/ijc.30084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruano-Ravina A, Martin-Gisbert L, Kelsey K,et al. An overview on the relationship between residential radon and lung cancer: what we know and future research. Clin Transl Oncol 2023; 25: 3357–3368. doi: 10.1007/s12094-023-03308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubaux R, Becker-Santos DD, Enfield KS,et al. Arsenic, asbestos and radon: emerging players in lung tumorigenesis. Environ Health 2012; 11: 89. doi: 10.1186/1476-069X-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz SB, Levin SM, Miller A, et al. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American insulator cohort. Am J Respir Crit Care Med 2013; 188: 90–96. doi: 10.1164/rccm.201302-0257OC [DOI] [PubMed] [Google Scholar]
- 11.Gowda SN, DeRoos AJ, Hunt RP,et al. Ambient air pollution and lung cancer risk among never-smokers in the Women's Health Initiative. Environ Epidemiol 2019; 3: e076. doi: 10.1097/EE9.0000000000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lissowska J, Foretova L, Dabek J,et al. Family history and lung cancer risk: international multicentre case-control study in Eastern and Central Europe and meta-analyses. Cancer Causes Control 2010; 21: 1091–1104. doi: 10.1007/s10552-010-9537-2 [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services . The Health Consequences of Involuntary Exposure to Tobacco Smoke. Atlanta, GA, Centers for Disease Control and Prevention, 2006. [Google Scholar]
- 14.International Agency for Research on Cancer . Personal Habits and Indoor Combustions. Lyon, World Health Organization, 2012. [Google Scholar]
- 15.Sheng L, Tu JW, Tian JH,et al. A meta-analysis of the relationship between environmental tobacco smoke and lung cancer risk of nonsmoker in China. Medicine (Baltimore) 2018; 97: e11389. doi: 10.1097/MD.0000000000011389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni X, Xu N, Wang Q. Meta-analysis and systematic review in environmental tobacco smoke risk of female lung cancer by research type. Int J Environ Res Public Health 2018; 15: 1348. doi: 10.3390/ijerph15071348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botteri E, Borroni E, Sloan EK, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol 2020; 115: 1940–1949. doi: 10.14309/ajg.0000000000000803 [DOI] [PubMed] [Google Scholar]
- 18.Lugo A, Peveri G, Bosetti C,et al. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: a comprehensive review and meta-analysis. Eur J Cancer 2018; 104: 117–126. doi: 10.1016/j.ejca.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 19.Malevolti MC, Maci C, Lugo A,et al. Second-hand smoke exposure and cervical cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2023; 149: 14353–14363. doi: 10.1007/s00432-023-04841-9 [DOI] [PubMed] [Google Scholar]
- 20.Possenti I, Scala M, Carreras G,et al. Exposure to secondhand smoke and breast cancer risk in non-smoking women: a comprehensive systematic review and meta-analysis. Br J Cancer 2024; 131: 1116–1125. doi: 10.1038/s41416-024-02732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rota M, Possenti I, Valsassina V,et al. Dose–response association between cigarette smoking and gastric cancer risk: a systematic review and meta-analysis. Gastric Cancer 2024; 27: 197–209. doi: 10.1007/s10120-023-01459-1 [DOI] [PubMed] [Google Scholar]
- 22.Scala M, Bosetti C, Bagnardi V,et al. Dose–response relationships between cigarette smoking and breast cancer risk: a systematic review and meta-analysis. J Epidemiol 2023; 33: 640–648. doi: 10.2188/jea.JE20220206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugo A, Bosetti C, Peveri G,et al. Dose–response relationship between cigarette smoking and site-specific cancer risk: protocol for a systematic review with an original design combining umbrella and traditional reviews. BMJ Open 2017; 7: e018930. doi: 10.1136/bmjopen-2017-018930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamling J, Lee P, Weitkunat R, et al. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 2008; 27: 954–970. doi: 10.1002/sim.3013 [DOI] [PubMed] [Google Scholar]
- 25.Rucker G, Cates CJ, Schwarzer G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res Synth Methods 2017; 8: 392–403. doi: 10.1002/jrsm.1259 [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 28.Peters JL, Sutton AJ, Jones DR,et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008; 61: 991–996. doi: 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O'Connell D,et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Date last accessed: 23 November 2023. Date last updated: 3 May 2021. www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 31.Crippa A, Discacciati A, Bottai M,et al. One-stage dose–response meta-analysis for aggregated data. Stat Methods Med Res 2019; 28: 1579–1596. doi: 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 32.Desquilbet L, Mariotti F. Dose–response analyses using restricted cubic spline functions in public health research. Stat Med 2010; 29: 1037–1057. doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 33.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology 1993; 4: 218–228. doi: 10.1097/00001648-199305000-00005 [DOI] [PubMed] [Google Scholar]
- 34.Bagnardi V, Zambon A, Quatto P, et al. Flexible meta-regression functions for modeling aggregate dose–response data, with an application to alcohol and mortality. Am J Epidemiol 2004; 159: 1077–1086. doi: 10.1093/aje/kwh142 [DOI] [PubMed] [Google Scholar]
- 35.Crippa A, Orsini N. Multivariate dose–response meta-analysis: the dosresmeta R package. J Stat Softw 2016; 72: 1–15. doi: 10.18637/jss.v072.c01 [DOI] [Google Scholar]
- 36.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22: 153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng ES, Weber MF, Steinberg J,et al. Evaluating risk factors for lung cancer among never-smoking individuals using two Australian studies. J Cancer Res Clin Oncol 2022; 148: 2827–2840. doi: 10.1007/s00432-022-04043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Zhang X, Gao Y,et al. Risk factors for the development of lung cancer among never smokers: a systematic review. Cancer Epidemiol 2022; 81: 102274. doi: 10.1016/j.canep.2022.102274 [DOI] [PubMed] [Google Scholar]
- 39.Kim AS, Ko HJ, Kwon JH, et al. Exposure to secondhand smoke and risk of cancer in never smokers: a meta-analysis of epidemiologic studies. Int J Environ Res Public Health 2018; 15: 1981. doi: 10.3390/ijerph15091981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Health and Safety Authority . Report on the health effects of environmental tobacco smoke (ETS) in the workplace. Date last accessed: 2 February 2024. Date last updated: December 2002. www.drugsandalcohol.ie/16566/1/OTC_-_Report_on_Health_Effects_of_Environmental_Tobbaco_Smoke_in_the_workplace.pdf
- 41.World Health Organization . Women's exposure to second-hand smoke: a serious health concern. Date last accessed: 2 February 2024. Date last updated: 6 November 2021. www.who.int/hongkongchina/news/detail/06-11-2012-women-s-exposure-to-second-hand-smoke-a-serious-health-concern
- 42.Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer 2020; 146: 2376–2382. doi: 10.1002/ijc.32716 [DOI] [PubMed] [Google Scholar]
- 43.Yu Y, Liu H, Zheng S,et al. Gender susceptibility for cigarette smoking-attributable lung cancer: a systematic review and meta-analysis. Lung Cancer 2014; 85: 351–360. doi: 10.1016/j.lungcan.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 44.Mollerup S, Ryberg D, Hewer A,et al. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res 1999; 59: 3317–3320. [PubMed] [Google Scholar]
- 45.Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci 2017; 18: 1713. doi: 10.3390/ijms18081713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mollerup S, Berge G, Baera R,et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006; 119: 741–744. doi: 10.1002/ijc.21891 [DOI] [PubMed] [Google Scholar]
- 47.Tanoue LT. Women and lung cancer. Clin Chest Med 2021; 42: 467–482. doi: 10.1016/j.ccm.2021.04.007 [DOI] [PubMed] [Google Scholar]
- 48.De Matteis S, Consonni D, Pesatori AC,et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013; 177: 601–612. doi: 10.1093/aje/kws445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Keeffe LM, Taylor G, Huxley RR,et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open 2018; 8: e021611. doi: 10.1136/bmjopen-2018-021611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Yue N, Shi N,et al. Influencing factors of lung cancer in nonsmoking women: systematic review and meta-analysis. J Public Health (Oxf) 2022; 44: 259–268. doi: 10.1093/pubmed/fdaa254 [DOI] [PubMed] [Google Scholar]
- 51.Wang YT, Hu KR, Zhao J,et al. The association between exposure to second-hand smoke and disease in the Chinese population: a systematic review and meta-analysis. Biomed Environ Sci 2023; 36: 24–37. doi: 10.3967/bes2023.003 [DOI] [PubMed] [Google Scholar]
- 52.Du Y, Cui X, Sidorenkov G,et al. Lung cancer occurrence attributable to passive smoking among never smokers in China: a systematic review and meta-analysis. Transl Lung Cancer Res 2020; 9: 204–217. doi: 10.21037/tlcr.2020.02.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Wang S, Aunan K,et al. Air pollution and lung cancer risks in China–a meta-analysis. Sci Total Environ 2006; 366: 500–513. doi: 10.1016/j.scitotenv.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 54.Hori M, Tanaka H, Wakai K,et al. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 2016; 46: 942–951. doi: 10.1093/jjco/hyw091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thun M, Peto R, Boreham J, et al. Stages of the cigarette epidemic on entering its second century. Tob Control 2012; 21: 96–101. doi: 10.1136/tobaccocontrol-2011-050294 [DOI] [PubMed] [Google Scholar]
- 56.Johnson KC, Hu J, Mao Y, et al. Passive and active smoking and breast cancer risk in Canada, 1994–97. Cancer Causes Control 2000; 11: 211–221. doi: 10.1023/A:1008906105790 [DOI] [PubMed] [Google Scholar]
- 57.Rothman KJ, Greenland S. Modern Epidemiology. 2nd edn. Philadelphia, Lippincott-Raven, 1998. [Google Scholar]
- 58.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 2001; 31: 139–148. doi: 10.1016/S0169-5002(00)00181-1 [DOI] [PubMed] [Google Scholar]
- 59.Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005; 117: 294–299. doi: 10.1002/ijc.21183 [DOI] [PubMed] [Google Scholar]
- 60.Pesch B, Kendzia B, Gustavsson Pet al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer 2012; 131: 1210–1219. doi: 10.1002/ijc.27339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Liu X, Zhang L. The relationship of indoor coal use and environmental tobacco smoke exposure with lung cancer in China: a meta-analysis. J Cancer Res Ther 2018; 14: S7–S13. doi: 10.4103/0973-1482.168965 [DOI] [PubMed] [Google Scholar]
- 62.Stayner L, Bena J, Sasco AJ,et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health 2007; 97: 545–551. doi: 10.2105/AJPH.2004.061275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan P, Buffler PA, Reynolds Pet al. Secondhand smoke exposure in adulthood and risk of lung cancer among never smokers: a pooled analysis of two large studies. Int J Cancer 2004; 109: 125–131. doi: 10.1002/ijc.11682 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0077-2024.SUPPLEMENT (1.5MB, pdf)