Abstract
A manipulative experiment with two different water regimes was established to identify the variability of physiological responses to environmental changes in 5-year-old Norway spruce provenances in the Western Carpathians. While variations in the growth responses were detected only between treatments, photosynthetic and biochemical parameters were also differently influenced among provenances. Following drought treatment, an obvious shrinkage of tree stems was observed. In most provenances, drought had a negative effect on leaf gas-exchange parameters and kinetics of chlorophyll a fluorescence. Secondary metabolism was not affected so much with notable differences in concentration of sabinene, o-cimene, and (–)-alpha-terpineol monoterpenes. The most suitable indicators of drought stress were abscisic acid and fluorescence parameters. Seedlings from the highest altitude (1,500 m a.s.l.) responded better to stress conditions than the other populations. Such provenance trials may be a valuable tool in assessing the adaptive potential of spruce populations under changing climate.
Keywords: abscisic acid, drought, monoterpenes, Norway spruce, photosynthesis, provenance
Highlights
Photosynthetic processes in spruce trees were more drought-affected than monoterpenes
ABA and chlorophyll a fluorescence were found as specific severe drought indicators
Seedlings from the highest altitudes maintained the best photosynthetic performance
Introduction
Norway spruce represents the most economically important tree species with a wide range of distribution across Europe. Therefore, it is important to pay considerable attention to research and study of the impacts of climate change on this species. Long-term drought, high temperatures during the growing season, and irregular precipitation distribution have an important negative impact not only on the growth rate of Norway spruce in the subalpine area but also at altitudes below 1,300 m above sea level (Hartl-Meier et al. 2014). Water-limited spruce ecosystems are generally more vulnerable to other stress factors, such as pest attacks, fungi, pollutants, and windstorms (Čermák et al. 2019). Moreover, the populations growing on significantly dry and stony soils show a higher predisposition to pathogens due to carbon starvation (Rehschuh et al. 2017, Tužinský et al. 2017).
One of the challenges of adaptive forest management is to sustain and enhance the stability of Norway's spruce ecosystems in all central parts of Europe (Jamnická et al. 2019). Adaptive strategies may include acclimatization to local conditions and phenotypic plasticity of the populations or change in genotype (frequencies) within the same species due to environmental pressure. Adaptation to drought can be reflected in the variation of key functional traits, including PSII functioning and photosynthetic capacity under water shortage (Bussotti et al. 2015). Epron and Dreyer (1990) noted the susceptibility to photoinhibition is related to the lack of water, which makes it an important adaptation mechanism under the conditions of a changing climate.
Drought is a significant factor affecting the physiological processes in Norway spruce trees, including a complex of negative effects at all levels, starting with the exchange of gases and ending with the suppression of the activity of photosynthetic enzymes (Urban et al. 2017). It also plays an essential role in the activation of protective mechanisms such are stress hormones and stress proteins (Mukarram et al. 2021). Abscisic acid regulates the closing of the stomata and indicates the accumulation of osmotically active compounds, which are essential for protecting cells from further damage (Daszkowska-Golec 2016). Free proline accumulation in leaves with decreasing content of available water means providing an effective mechanism for osmotic regulation, stabilisation of subcellular structures, and cellular adaptation to water stress (Valentovič et al. 2006, Gunes et al. 2008).
After the first event under mild drought, such as stomata closing, investigation of changes in chlorophyll a fluorescence provides a useful tool for understanding photosynthetic metabolism and thus identifying plant performance in their environment or at least reactions of phenotypes to water deficit (Longerberger et al. 2009, Kalaji et al. 2016). While mild drought can enhance photosynthetic processes, intense and long-term drought leads to the decline of photosynthesis through nonstomatal factors. These may result in ATP shortage, and limited RuBP regeneration processes which with stomatal factors of photosynthesis limitations contribute to the decline of CO2 assimilation. Reduction of photosynthesis may cause stressed plants to absorb more light energy, however, they are not able to use it in the fixation of carbon. Downregulation of photosynthesis can lead to many damages of PSII, such as injury of light-harvesting complexes, reaction centers including proteins, especially D1 which affects the activity of electron transport chain or oxygen-evolving complexes (Wang et al. 2018, Shevela et al. 2019). The insight into the functioning of PSII, as the sensitivity of PSII is higher than that of PSI, provides information about the efficiency of the photochemical conversion of radiant energy in PSII during photosynthesis and reflects the efficiency of the entire transfer including changes in photochemical and nonphotochemical quenching (Zivcak et al. 2013, Chen et al. 2018).
In spruce species, also terpenes are an essential part of the spruce defense system and the ability to increase their amounts can be considered an important indicator of tree resistance (e.g., Kopaczyk et al. 2020, Marešová et al. 2022). Synthesis of specialised compounds in the secondary metabolism of spruce may be significantly influenced by water deficit, when the synthesis of these secondary metabolites may change depending on drought intensity, duration, and individual tree genetic predispositions (Holopainen et al. 2018).
The primary objective of the present study was to examine the effects of simulated drought on the primary photosynthesis processes and concerning in Norway spruce (Picea abies L. H. Karst) originating from six different provenances of Western Carpathians, Slovakia. The selected provenances represent different microclimatic conditions with dissimilar precipitation (808–1,279 ml, 30-years' average), altitude (650–1,500 m a.s.l.), and temperature (2.68–6.88°C, 30-years' average). It was hypothesized that provenances from various microclimatic conditions had developed a different adaptive response to ecological conditions. Further, our specific research questions were: (1) Are there certain intra-species differing responses to drought through changes in photosynthetic performance? (2) To what extent does drought affect secondary metabolism? (3) Which provenance is more resistant to drought in terms of the observed physiological traits?
Materials and methods
Plant material
The seedlings of six Norway spruce (Picea abies L. H. Karst) provenances (PV1–PV6) originated within the natural distribution of Norway spruce in Western Carpathians, distributed along an altitudinal gradient from 650 to 1,500 m a.s.l.
Seeds were taken from the gene bank of forest trees of Slovakia (OZ Semenoles, Liptovský Hrádok, Slovakia). They were sown in an experimental research plot of the Mlyňany Arboretum of the Slovak Academy of Sciences, Slovakia, and grown in one plot until the age of five years.
Experimental design
Three weeks before the experiment, similarly homogenous five-year-old spruce seedlings were transported to the Institute of Forest Ecology laboratory to acclimate to the lab conditions. The experiment lasted one month in 2022, from 21 July to 19 August. The daylight was simulated using halogen lamps with gradually increasing light intensity from 150 to 400 μmol(photon) m–2 s–1 for 14 h (from 6:00 to 20:00 h). The absence of light created night settings of 10 h (from 20:00 to 6:00 h). In total, 60 seedlings were randomly separated into two air-conditioned rooms and groups: (1) control group (C) in the first room under fully irrigated conditions for provenances PV1 C–PV6 C (the temperature of 24°C and 70% relative humidity were set up during the day); and (2) drought treatment (D) in the second room without irrigation for provenances PV1 D–PV6 D (the temperature of 28°C and 50% relative humidity were set up during the day). Overall, 60 Norway spruce seedlings were analysed.
| PV | Altitude [m a.s.l.] | Longitude | Latitude | Forest unit | Locality | Annual precipitation [mm] | Precipitation May–September [mm] | Annual temperature [°C] | Temperature May–September [°C] |
| 1 | 650 | 48°46' | 19°24' | Slovenská Ľupča | Pohronský Bukovec | 808 | 441 | 6.88 | 14.52 |
| 2 | 1,335 | 49°14' | 19°13' | Habovka | Zverovka | 936 | 536 | 6.56 | 14.14 |
| 3 | 870 | 49°09' | 19°25' | Liptovská Teplá | Prosečné | 951 | 548 | 6.06 | 13.54 |
| 4 | 1,060 | 48°59' | 19°48' | Malužiná | Tajch | 1,011 | 588 | 4.58 | 11.82 |
| 5 | 1,500 | 48°57' | 19°27' | Partizánska Ľupča | Pod Chabencom | 1,155 | 641 | 3.42 | 10.48 |
| 6 | 1,050 | 49°16' | 19°43' | Habovka | Zadná Kremenná | 1,279 | 723 | 2.68 | 9.56 |
Soil water content
Soil water potential measurements (Ψw [MPa]) were continuously monitored in 1-h intervals in each pot by calibrated gypsum block (Delmhorst Inc., USA) installed at 10-cm depths and stored in data loggers (EMS Brno, Czech Republic). The soil water potential values were in the range of 0.0 up to –1.5 MPa, which were at the lower measurable limit of the equipment.
Diameter variations and extraction of tree water status (ΔW)
Variations of stem diameters were monitored continuously for 20-min intervals using a high-resolution PDS40 SDI sensor (EMS Brno, Czech Republic), which was noninvasively mounted at ca. 15 cm of the tree stem. We used one-hour averages of tree stem diameter to calculate their shrinkage. Diameter variations below the preceding maximum stem diameter were considered tree water deficit or tree water status (ΔW). Measurements started two days after installation. We selected 10 Norway spruce seedlings from the drought-treated group across provenances and 13 from the control group across provenances to calculate average curves. The seedlings whose diameter variation curves were significantly damaged by handling pots and seedlings during measurements were excluded.
Gas exchange
Parameters of gas exchange and water-use efficiency were measured in juvenile needles using a Li-6400XT open gasometric system, with an equipped chamber fitted with a 6400-02B LED light source (LI-COR Inc., Lincoln, NE, USA), for five seedlings per variant and provenance. Inside the chamber, the reference CO2 concentration was 400 μmol mol–1, photosynthetic active radiation was maintained at 1,500 μmol(photon) m–2 s–1, and the system temperature was 24°C. Values of the CO2 photosynthetic rate (PN), transpiration rate (E), and stomatal conductance to water vapour (gs) were recorded immediately after the adaptation of leaves inside the chamber (5–6 min) when the values of CO2 assimilation rate persisted steady (1–2 min).
Fast kinetics of chlorophyll a fluorescence
The performance of the PSII was measured using the portable fluorimeter Handy PEA (Hansatech Instruments, Ltd., United Kingdom). The juvenile needles were adapted to dark conditions for 30 min using the leaf clips. After dark adaptation, they were illuminated by the saturation pulse with high radiation intensity [PAR of 3,500 μmol(photon) m–2 s–1] to enhance Chl a fluorescence for 1 s. Every 10 μs, Chl a fluorescence was detected to attain a polyphasic fluorescence curve with an OJIP shape (Strasser et al. 2004). We determined the basic fluorescence parameters: the basal fluorescence (F0), measured 50 μs after the enlightenment of the saturation pulse, and the maximum quantum yield of the photochemistry of PSII (Fv/Fm), calculated as the ratio between the variable Chl a fluorescence (Fv) and the maximum of the Chl a fluorescence (Fm). Moreover, the photosynthetic performance index based on absorption (PIABS) and the number of active reaction centres (RC/ABS) was quantified. The measurements were done on five seedlings per provenance and variant in two repetitions.
Rapid light curves
The parameters of Chl a fluorescence were determined using a Mini-PAM (Heinz Walz GmbH, Germany). These parameters were measured on juvenile needles of five Norway spruce seedlings per provenance and variant in two repetitions. Rapid light curves (RLC) were automatically measured under the control of the program. The actinic light was applied in eight steps, with increasing intensities from PAR of 38 to 616 μmol(photon) m–2 s–1 and a duration of 10 s. The illumination periods were divided by a 0.8-s saturating pulse from a white halogen lamp [PAR of 2,000–3,000 μmol(photon) m–2 s–1]. The parameters of RLC measurements were determined: (1) the effective quantum yield in the PSII (ΦPSII); (2) coefficients of photochemical quenching (qL and qP) (Zivcak et al. 2013), (3) coefficients of nonphotochemical quenching (qN and NPQ). Mentioned parameters are indicators of the excess energy of the light or heat-dissipated excitation energy in the antenna complexes (Zivcak et al. 2013).
The content of abscisic acid (ABA)
Juvenile needles were collected from the five spruce seedlings per provenance and variant at the endpoint of the experiment and immediately stored in liquid nitrogen at –190°C (five samples per each provenance and variant). The concentration of ABA [pmol g–1(FM)] was detected using two-dimensional (2D) high-performance liquid chromatography (HPLC) at the Institute of Experimental Botany of the Czech Academy of Sciences (Prague, Czech Republic) (e.g., Marešová et al. 2022).
Free proline
Juvenile needles were collected from the five spruce seedlings per provenance and variant at the endpoint of the experiment and immediately placed in a freezer at –20°C. The content of free proline [μmol g–1(FM)] was detected using a ninhydrin-based colorimetric method according to the method of Bates et al. (1973). Samples of 0.5 g of frozen young needles (from five seedlings per variant and provenance) were homogenised with 10 ml of 3% sulfosalicylic acid and filtered. Then, a mixture of 2 ml of filtrate, 2 ml of glacial acetic acid, and 2 ml of acidic ninhydrin was incubated for one hour at 100°C in a water bath. The reaction was terminated on ice, and the created reagent was mixed with 4 ml of toluene for 20 s. The absorbance of the extracted chromophore at 520 nm was determined with the toluene as a reference using a CINTRA spectrometer (GBC Scientific Equipment, Braeside, Victoria, Australia). After all, the proline concentration was determined from a standard concentration curve and recalculated to the fresh mass (FM) of the sample.
Terpene sampling, extraction, and analysis
Samples of juvenile needles obtained at the end of the experiment from five Norway spruce seedlings per provenance and variant were stored at –20°C in a freezer for two weeks. The mortar and the pestle were used to ground 0.2 g of frozen needle samples for 15–20 s with 10 ml of liquid nitrogen. Then, 2.0 ml of n-hexane (99% p.a., Sigma Aldrich, Germany) was added to 0.15 g of sample homogenate in a 20-ml tare glass tube, which was immediately closed. The suspension was mixed for 10 min in an ultrasonic bath at 20°C (Tesla UC 005 AJ1, frequency 50 kHz), following the filtration through filter paper (Filter Discs Grade: 390; 84 g m–2; Munktell). Obtained hexane sample extracts (1 μl of each eluate) were analysed using gas chromatography with mass spectrometry (GC-MS). The GC-MS system consisted of a 7890B GC and a 5977A MS instrument (Agilent Technologies, Palo Alto, CA, USA). The injection mode was splitless with a purge time of 0.35 min and an injector temperature of 250°C. The constant flow rate of a carrier gas helium (He) was 0.5 ml min–1/10 psi. MS system was operated using electron impact ionisation (70 eV) in the scan mode and a mass range of 20–350 m/z. The MS quadrupole temperature was 150°C, the ion source was 250°C, and the interface was 230°C. The used column was HP-INNOWax (30 m × 0.25 mm, film thickness 0.5 μm, Agilent). The GC oven program started at 50°C for 3.0 min, following ramping at a rate of 3°C min–1 to 110°C. Further ramping was continued at a rate of 20°C min–1 to 220°C and it was stopped after 3 min holding at 220°C. Terpenes identification was made by a comparison of their mass profile and their retention times with authentic commercial pure standards [alpha-pinene (AP), camphene (CAM), beta-pinene (BP), limonene (LIM), o-cimene (OCI), terpinolene (TER), sabinene (SAB); (–)-alpha-terpineol (ATEol); 99% p.a., Sigma Aldrich]. Agilent Mass Hunter Qualitative Analysis software B.07.00 and Mass Hunter GC-MS Data Acquisition software were used for the quantification of mass profiles of samples and standard spectra. The extracted ion chromatogram data (93.1 m/z) was used for obtaining the absolute amounts of monoterpenes expressed as a percentage of the GC-MS results. Quantitative data were calculated by the sample mass spectra comparison with the mass spectra of the closest standard (10 μg ml–1) from the calibration curve (1.0–100.0 μg ml–1). Finally, terpene concentration data were recalculated on the sample fresh mass (FM).
Statistical analysis
Data from the measurements of photosynthetic and biochemical parameters were analysed using Kruskal-Wallis tests (K-W test) followed by pairwise Wilcoxon rank sum tests. The analysis was performed in the R (4.1.2) environment using the rstatix (0.7.0) library (R Core Team, Austria). Letters denoting statistically significant differences were created using the multcompView (0.1-8) library. Groups that do not share the same letter are statistically different. The average curves and 95% confidence intervals for Ψsoil and ΔW were calculated in Excel (MS Office). For statistical comparison of their relation, we used Spearman's rank correlation coefficient using Statistica® statistical software (Statsoft, Tulsa, OK, USA).
Results
Changes in soil- and tree-water status
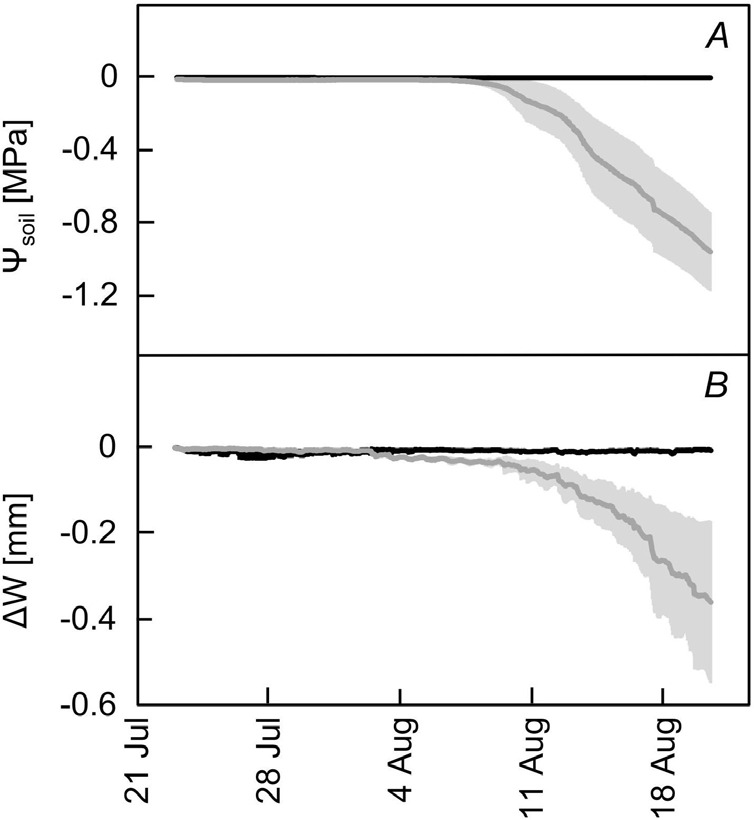
The average soil water potential (Ψsoil, Fig. 1A) in the drought-treated group began to decrease continuously after 31 July until 20 August at noon, when it reached a value of –1.40 MPa. This was accompanied by apparent shrinkage (decrease of ΔW) of tree trunks (Fig. 1B, Spearman's rank correlation r = 0.984). This was highlighted especially after 10 August, during which Ψsoil dropped below –0.8 MPa when shrinkage (ΔW) continuously decreased synchronously with decreasing Ψsoil until 20 August (Spearman's rank correlation r = 0.998), reaching a minimum value of –0.359 mm at noon, which represented 3.1% of trees initial diameter. The values of Ψsoil and ΔW of the control group were around 0.1 during the entire monitoring period.
Fig. 1. The average soil water potential (Ψsoil) of control (black trace) and drought treated (grey trace) groups (A). The average tree water deficit (ΔW) of control variant (black trace) and drought treated (grey trace) groups. Vertical lines represent 95% confidence intervals.
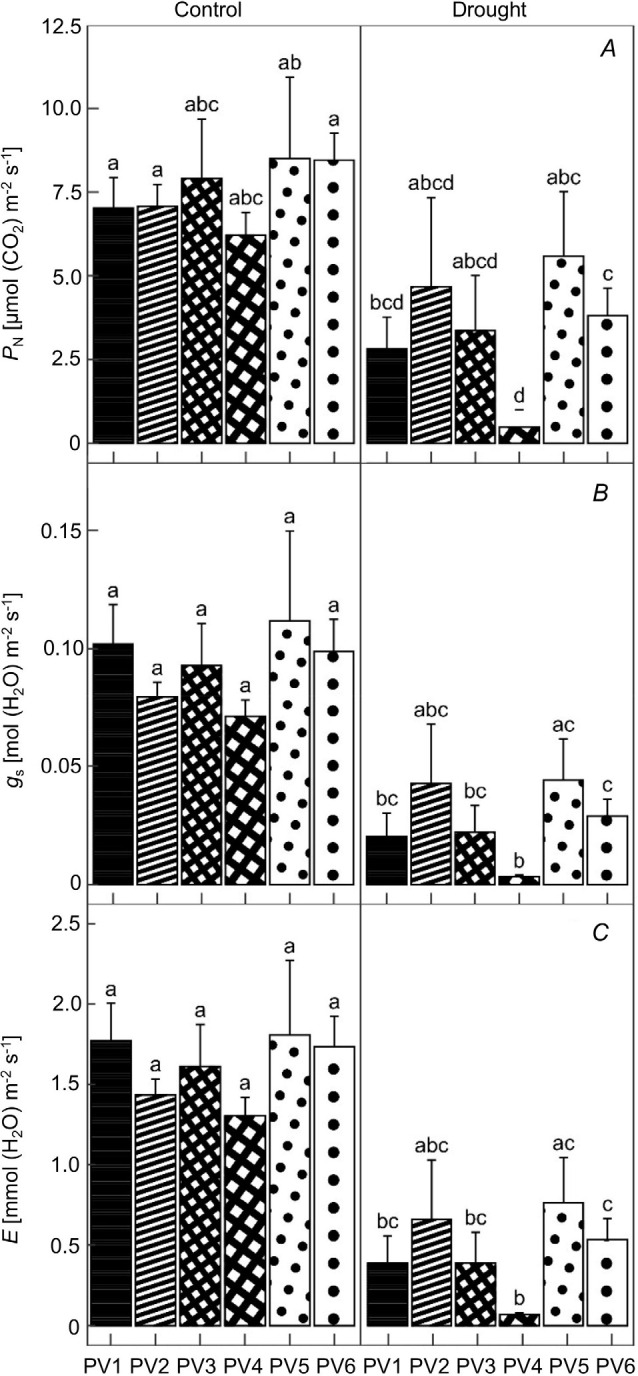
Photosynthetic traits
The gas-exchange parameters were similar in the control group (C variant) in different provenances. The mean values fluctuated around the 7.9 μmol m–2 s–1 for PN (Fig. 2A), 0.09 mol m–2 s–1 for gs (Fig. 2B), and 1.6 mmol m–2 s–1 for E (Fig. 2C) in all provenances. Conditions in the drought variant (D variant) negatively affected these parameters. The net photosynthetic rate significantly decreased in PV1 (about 60%), PV4 (about 90%), and PV6 (54%). Stomatal conductance was significantly lower in PV1, PV3, PV4, and PV6, with the highest decrease in PV4 (about 90%). The lowest transpiration rate was also observed in PV4, whereas PV2 and PV5 stood significantly unaffected. These results point to a different drought response of the examined populations, where the provenances from higher altitudes PV2 (1,335 m a.s.l.) and PV5 (1,500 m a.s.l.) appear to be less affected.
Fig. 2. Quantification of the changes in leaf gas-exchange parameters measured in needles of six Norway spruce provenances. PN – photosynthetic rate [μmol(CO2) m–2 s–1] (A); gs – stomatal conductance to water vapour [mol(H2O) m–2 s–1] (B); and E – transpiration rate [mmol(H2O) m–2 s–1] (C). C – control variant, D – drought variant. PV1–PV6 – the numbering of the provenances. Means ± SD (n = 5). Different small letters represent statistically significant differences among the groups at p<0.05 according to K-W test.
Chl a fluorescence traits
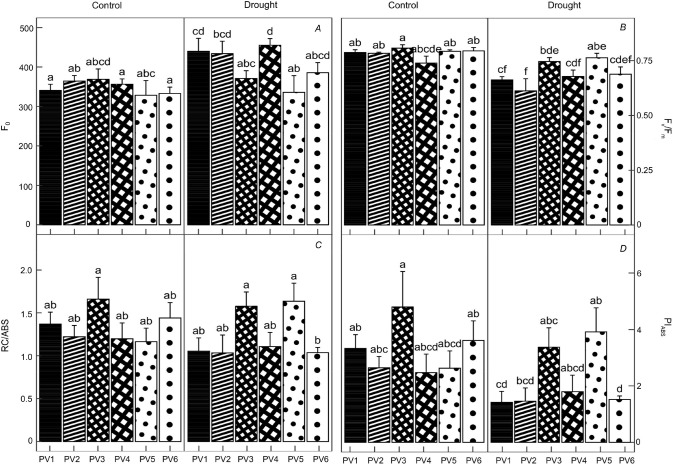
Fast kinetics of chlorophyll a fluorescence was affected by drought, and in several cases, the differences between provenances were proved in the D variant (Fig. 3). The basal fluorescence (F0) significantly increased in provenances PV1 and PV4. Drought-induced reduction of Fv/Fm was observed mainly in PV1, PV2, and PV6. The number of active reaction centres (RC/ABS) was not reduced by drought. Still, the interaction of the provenance and variant significantly influenced RC/ABS, where the differences between provenances PV5 and PV3 on one hand and PV6 on the other hand, were observed in the D variant. In the case of PIABS, provenance PV5 showed higher values, while the other provenances had lower values in the D variant.
Fig. 3. Quantification of the changes in parameters of fast kinetics of chlorophyll a fluorescence measured in needles of six Norway spruce provenances. F0 – basal fluorescence of dark-adapted leaf (A); Fv/Fm – maximum quantum yield of photochemistry in dark-adapted leaf (B); RC/ABS – number of active reaction centres per antenna in PSII based on the absorption (C); PIABS – performance index of photochemical activity based on the absorption (D). C – control variant, D – drought variant. PV1–PV6 – the numbering of the provenances. Means ± SD (n = 5). Different small letters represent statistically significant differences among the groups at p<0.05 according to K-W test.
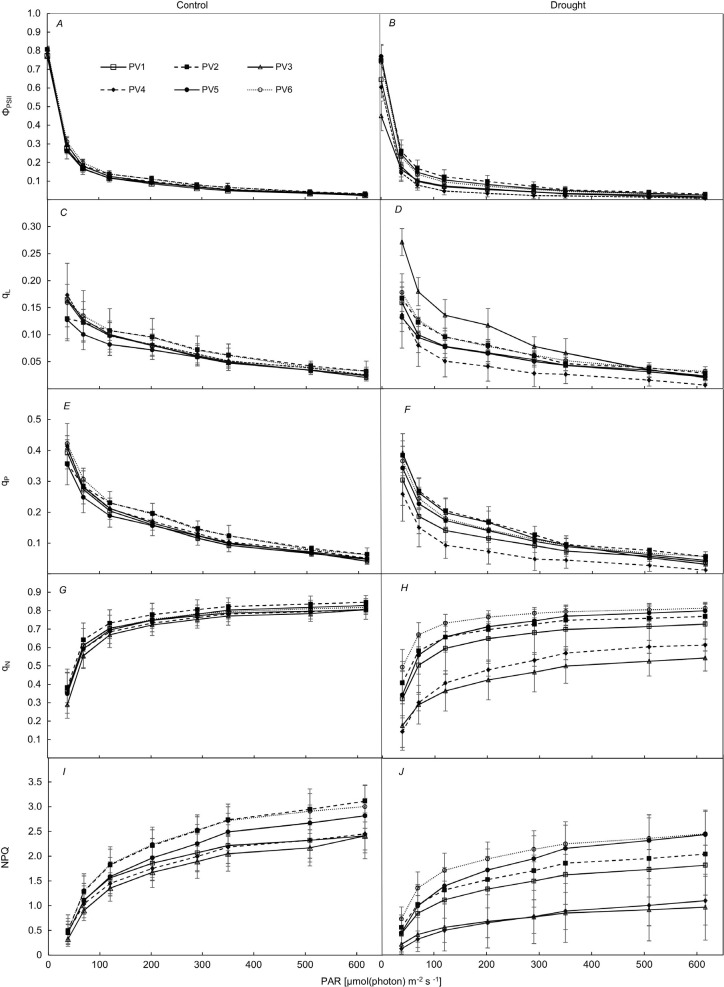
From the analysis of the RLC of the ΦPSII, we found values about 0.78–0.81 for the C variant. The actual efficiency of photochemistry decreased in the D variant for provenances PV1, PV3, and PV4 (Table 1, Fig. 4). In the case of the coefficients of photochemical quenching, PV4 exhibited the lowest values of qP and qL in the D variant and significantly differed from PV2, PV3, PV5, and PV6 provenances in the qP parameter, as well as significantly differed from PV3 in the qL parameter.
Table 1. Statistical differences in parameters of the slow kinetics of chlorophyll a fluorescence among the provenances in different variants. For the quantification of RLCs, the curve values at the startpoints (ΦPSII, qL, qP) and endpoints (qN, NPQ) were used. The data presented are the means ± SD. Different letters indicate significant differences between provenances after Wilcoxon rank sum tests (p<0.05). NPQ – nonphotochemical quenching of fluorescence; qL – coefficient of photochemical quenching based on the ‘Lake’ model; qN – coefficient of nonphotochemical quenching of variable fluorescence; qP – coefficient of photochemical quenching based on the ‘Puddle’ model; ΦPSII – the actual efficiency of PSII photochemistry.
| Provenance | ΦPSII | qL | qP | qN | NPQ |
| Control | |||||
| PV1 | 0.772a | 0.165b | 0.393a | 0.805a | 2.411ab |
| PV2 | 0.807a | 0.130b | 0.357a | 0.846a | 3.111a |
| PV3 | 0.772a | 0.163b | 0.410a | 0.805a | 2.429ab |
| PV4 | 0.742a | 0.173b | 0.414a | 0.806a | 2.221ab |
| PV5 | 0.806a | 0.127b | 0.355a | 0.828a | 2.816a |
| PV6 | 0.809a | 0.159b | 0.422a | 0.818a | 3.002a |
| Drought | |||||
| PV1 | 0.632ab | 0.159b | 0.306ab | 0.728ab | 1.793ab |
| PV2 | 0.749a | 0.168b | 0.385a | 0.770a | 2.015ab |
| PV3 | 0.504b | 0.271a | 0.393a | 0.544b | 0.956b |
| PV4 | 0.641ab | 0.136b | 0.259b | 0.615b | 1.084b |
| PV5 | 0.771a | 0.132b | 0.344ab | 0.800a | 2.406ab |
| PV6 | 0.699a | 0.178b | 0.367a | 0.814a | 1.979ab |
Fig. 4. The rapid light curves of parameters derived from chlorophyll a fluorescence as a function of photosynthetically active radiation (PAR) [μmol(photon) m–2 s–1] measured in needles of six Norway spruce provenances. ΦPSII – the actual efficiency of PSII photochemistry (measured in light-adapted leaf) (A,B); qL – coefficient of photochemical quenching based on ‘Lake’ model (C,D); qP – coefficient of photochemical quenching based on the ‘Puddle’ model (E,F); qN – coefficient of nonphotochemical quenching of variable fluorescence (G,H); NPQ – nonphotochemical quenching of fluorescence (I,J). PV1–PV6 – the numbering of the provenances. Means ± SD (n = 5).
Regarding nonphotochemical quenching, in control variants, the highest values of qN and NPQ were found in provenances from higher altitudes (PV2, PV5, PV6) (Table 1, Fig. 4G–J). In the D variant, the ability to dissipate excess energy by heat significantly declined in provenances PV3 and PV4.
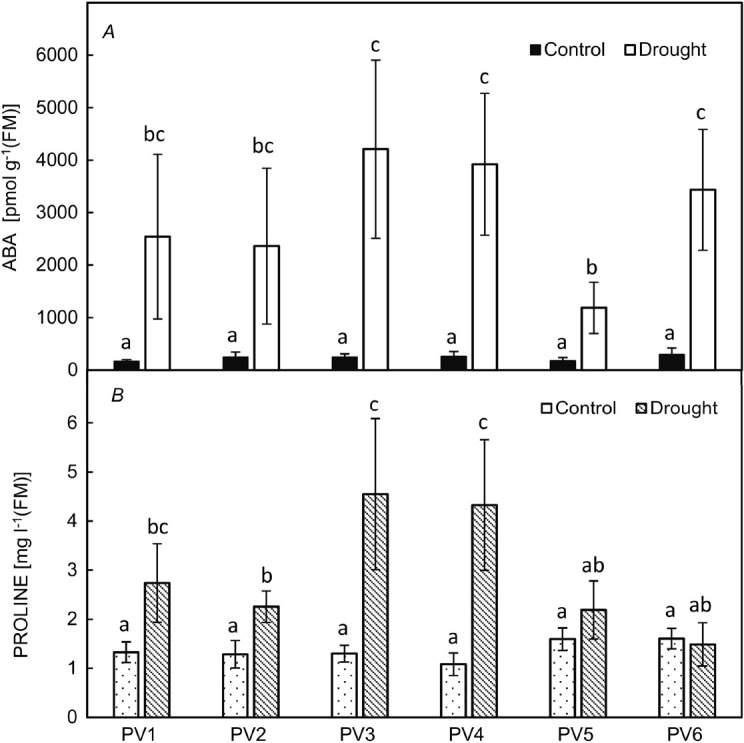
Specialised biochemical compounds: In the evaluation of the concentrations of free proline and ABA, which are directly involved in defensive mechanisms against drought stress in spruce species, we did not observe any significant differences between the provenances under the control conditions (Fig. 5A). However, slightly higher concentrations of free proline were found in the provenances PV5 C and PV6 C from higher and wetter locations (Fig. 5B). In the D variant, the contents of free proline significantly increased in four provenances except for PV5 D and PV6 D. The proline concentration in PV3 D and PV4 D was 3.5-times higher than that in the C variant. Dehydration significantly affected free ABA accumulation in spruce needles of all provenances. However, the lowest concentration of the drought-signalling phytohormone was observed in PV5 D from the highest altitude.
Fig. 5. The concentrations of ABA phytohormone [pmol g–1(FM)] (A) and free proline [mg l–1(FM)] (B) in the spruce needles of six different provenances. PV1–PV6 – the numbering of the provenances. Means ± SD (n = 5). Statistically significant differences between the variants and among the provenances are presented by the small letters (Wilcoxon rank sum tests after K-W test, p<0.05).
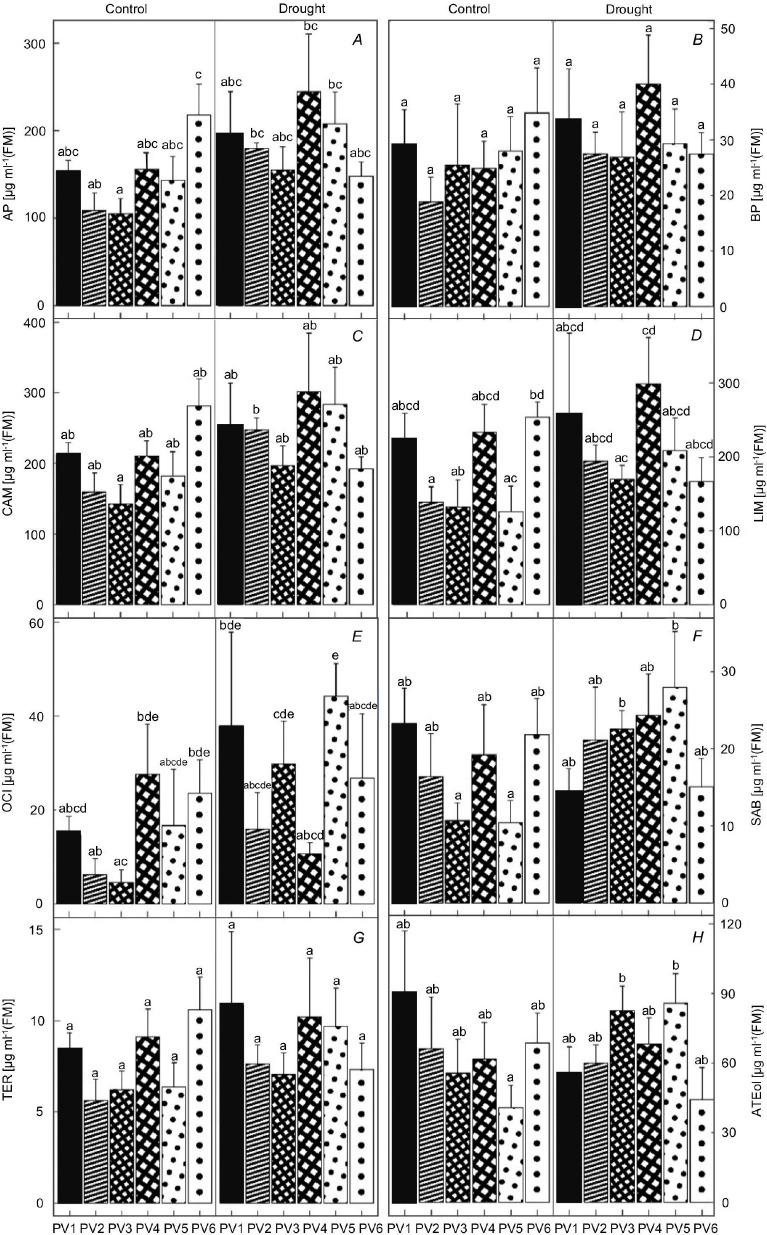
The studied constituents contained in the spruce needles were: AP, BP, CAM, LIM, OCI, TER, SAB, and ATEol (Fig. 6). The total monoterpenes (MTs) showed insignificant changes in the D variant. In the case of individual monoterpenes, from a wide spectrum of evaluated MTs, the result of the Kruskal-Wallis test showed an increase in the concentrations of monoterpene OCI (all of PVs except for PV4 D) and sabinene (SAB) and oxygenated monoterpene ATEol (both significant in PV5 D) between the variants (Fig. 6E,F,H).
Fig. 6. The fluctuation of individual monoterpenes (MTs) concentration [μg ml–1(FM)] in the needles of seedlings of six Norway spruce provenances. AP – alpha-pinene (A); BP – beta-pinene (B); CAM – camphene (C); LIM – limonene (D); OCI – o-cimene (E); SAB – sabinene (F); TER – terpinolene (G); ATEol – (–)-alpha-terpineol (H). C – control variant, D – drought variant. PV1–PV6 – the numbering of the provenances. Means ± SD (n = 5). Different small letters represent statistically significant differences between the groups at p<0.05 according to K-W test.
Discussion
Tree water status (ΔW, e.g., Zweifel et al. 2005, Oberhuber et al. 2015a) is associated with water storage and its intricate traffic with transpiration, transport, and root water uptake (e.g., Betsch et al. 2011, Köcher et al. 2012). In our case, the minimal circadian variations of ΔW seem somewhat in contrast with other reports (Zweifel et al. 2005, Oberhuber et al. 2015a). It could result from relatively moderate daily dynamics of evapotranspiration demands.
Data from drought and irrigated groups suggest a high correlation between ΔW and Ψsoil. It can be a consequence of increasing drought that results in the decreased water potential of stem conducting tissues. According to Offenthaler et al. (2001), spruce xylem water potential showed a linear relation to wood diameter, and during a drought period of 3 weeks, the predawn xylem diameter shrank continuously with decreasing soil water content. Oberhuber et al. (2015b) reported ΔW values ca. –0.5 mm on the radius of mature spruce trees accompanied by minimum needle water potentials in predawn ca. –0.75 MPa and the afternoon –2.35 MPa. Oberhuber et al. (2015a) stated saplings showed a more tensed stem water status and higher sensitivity to environmental conditions than mature trees.
Severe drought restricts stomatal activity and pushes toward carbon starvation. We also found reduced stomatal conductance (gs) and transpiration (E), and photosynthetic rate (PN) in drought-stressed PV1, PV4, and PV6. It could arise from a direct dehydration effect on Rubisco (Rubisco hydrolysis) that scars photosynthesis (Lawlor 2002). Tang et al. (2002) have shown that a combination of stomatal and nonstomatal effects on photosynthesis exists, depending on the extent of drought stress and even if the plants are well hydrated. A significant decline in gas-exchange parameters was observed mainly in the provenances originating from lower elevations. Bigras (2005) did not observe a substantial decrease in the net rate of photosynthesis in two white spruce families till the shoot water pressure declined under –2.0 MPa. However, Marešová et al. (2022) observed a significant decrease in net photosynthetic rate after exposure of Norway spruce seedlings to mild drought. Moreover, in the perennial ryegrass, Dąbrowski et al. (2019) detected a significant decrease in net photosynthetic rate, stomatal conductance, and transpiration rate after 240 h of drought exposure when field water capacity decreased below 50%.
The fast kinetics of Chl a fluorescence indicated the drought-reduced capacity of primary photosynthetic processes. Despite the Fv/Fm ratio being a quite stable parameter, water deficit reduced it close to the disturbance limit of 0.725 (Critchley 2000) in several provenances (PV1, PV2, PV4, and PV6). It represents critical photoinhibition arising from a decreased PSII photochemistry constant rate (caused by damaged PSII reaction centres) and/or augmented dissipation constant rate of excitation. Deteriorated PSII photochemistry increases F0, while improved dissipation declines F0 and Fm (Guidi et al. 2019). Thus, a significantly increased F0 in PV1 and PV4 suggests a lower PSII photochemistry constant rate.
Bigras (2005) also depicted the higher sensitivity of PSII to drought (shoot ΨW ≤ –1 MPa) through parameters of Chl a fluorescence (F0, Fv/Fm, qN, and NPQ) in white spruce. A decreased Fv/Fm ratio is not necessarily linearly related to the number of deactivated reaction centres of PSII (PSII inactivation) as it can also come from charge separation processes such as NPQ (Malnoë 2018). Pukacki and Kamińska-Rożek (2005), in the study on Norway spruce, also observed a significant reduction (77%) in Fv/Fm after a severe drought (shoot ΨW ≤ –2.4 MPa). They also observed a significant decrease in other Chl fluorescence parameters when shoot ΨW decreased below –1.1 MPa. Surprisingly, RC/ABS density showed no significant differences between variants and provenances. This could also be caused by the relatively low sensitivity of parameters to drought stress or by relatively high variability in the response of PSII between tested Norway spruce seedlings and provenances. Moreover, Bussotti et al. (2020) describe RC/ABS as quite insensitive parameter to moderate stress conditions, however, under severe drought, this parameter reflects well damage of RC components. Thus, parameters such as Fv/Fm and PIABS are better markers for drought than RC/ABS (Kalaji et al. 2016, Sousaraei et al. 2021). Bussotti et al. (2020) found a strong correlation between Fv/Fm and PIABS. PIABS is a multifactor parameter involving RC/ABS, Fv/Fm, and the ability of the electron to reach the electron transport chain, which could better reflect the damage of the PSII. However, heat stress can affect it more than drought (Kalaji et al. 2016). Interestingly, parameters of the fast kinetics of fluorescence were negatively affected except for one provenance from the highest altitude (PV5).
By maintaining higher ΦPSII and qP values during water stress, provenances from higher altitudes (PV2, PV5, PV6) exhibited a higher efficiency in electron transport and disposed of a higher proportion of opened reaction centres. Moreover, these provenances showed the higher capacity of heat dissipation of chlorophyll excessive energy (NPQ, qN), which may be related to better photoprotective reaction resulting from the long-term adaptation to extreme mountain conditions and high radiation. NPQ is composed of energy-dependent quenching induced by changes in the proton gradient across the thylakoid membrane, state transition quenching impressed by reversible phosphorylation of the LHC of PSII, and quenching of photoinhibition (Stefanov et al. 2022). This photoprotective mechanism removes excitation energy within chlorophyll complexes and prevents the likelihood of free radicals' formation (Demmig-Adams and Adams 2006).
Conversely, a pronounced decrease of ΦPSII and qP in PV4 suggested that the capability of photochemical conversion and the linear electron flux were the most sensitive to drought in this provenance. Consequently, the decrease of heat dissipation indicated a limiting factor for generating the trans-thylakoid proton gradient in the chloroplast (Ruban and Murchie 2012) and regulating protein PsbS and the modification of violaxanthin to zeaxanthin in the xanthophyll cycle (Murchie and Niyogi 2011). This phenomenon can relate to defective PSI activity (Zivcak et al. 2013). In provenance PV3 from the middle altitudinal range (similarly to PV4), the high number of opened and active reaction centres per antenna in PSII (qP, qL, RC/ABS) was achieved under drought. Nevertheless, a significant decline in heat dissipation ability was also observed, which can damage photosystems during prolonged drought.
Tomášková et al. (2021) traced the PIABS and ΦPSII and revealed higher adaptability for high-mountain Norway spruce ecotypes over their lower-elevation counterparts. This illustrates the photosynthetic apparatus's phenotypic plasticity and the spruce ecotypes' stress resilience. The location of the studied Norway spruce provenances might explain its plasticity to water deficit, and thus, a potential genetic adaptation to drought in higher altitudes with shallow stony soil (e.g., Kmeť et al. 2010, Hlásny et al. 2014, Jamnická et al. 2019, Zlobin et al. 2019).
Zlobin et al. (2019) observed the adaptive mechanisms of white spruce to water deficit, which could be analogous to the well-studied adaptive mechanisms to winter stress indicating the complex protective mechanisms under various stresses in gymnosperms. It is possible that frequent exposure to adverse scenarios inculcates ‘stress memory’ in Picea abies. A similar suggestion was made by Petrik et al. (2022) in Fagus sylvatica. The stress memory could be driven by accrued signalling molecules and transcription factors (Bruce et al. 2007, Avramova 2015). Nonetheless, the modus operandi of stress memory-driven drought tolerance in trees is still poorly understood (Fleta-Soriano and Munné-Bosch 2016, Godwin and Farrona 2020).
Proline is considered one of the most reliable drought biomarkers in Norway spruce (Schiop et al. 2017). The drought-stressed Norway spruce ecotypes generally accumulated more proline than their control counterparts. However, the proline spike was not regular in provenances PV5 and PV6. The same provenances belonged to the wettest and coldest conditions, which can counteract drought. Conversely, proline accumulation was magnified in the drought ecotypes of more moderate environments (average altitude mixed with average precipitation and temperature). Thus, the highest proline content was amassed in PV3 and PV4. It is expected that drought variants of Norway spruce would experience higher osmotic imbalance and proline contents (Wohlfahrt et al. 1998). It seems PV3 and PV4 were the most drought-sensitive among all provenances, given that elevated proline contents suggest maximised ‘osmotic emergency’ and maneuvers for osmotic adjustments to maintain cellular homeostasis (Mukarram et al. 2021, 2023). Proline assists plants in stabilising proteins and membranes, maintaining cytoplasmic pH, and tolerating low water potential during stress (Hayat et al. 2012, Krasensky and Jonak 2012, Mukarram et al. 2022). Proline also serves as an alternative electron donor for PSII if the oxygen-releasing complex is inhibited or dissociated by various stress factors. Its increased accumulation protects against photoinhibition and improves the energy status of plant cells during their regeneration after the end of stress (De Ronde et al. 2004). Although proline biosynthesis was upregulated in PV3 and PV4, it was short to mask the drought-induced damage to the fast (F0, Fv/Fm, PIABS) and slow (ΦPSII, qP, qL, qN, NPQ) kinetics of Chl a fluorescence. Our understanding of the proline-photosynthesis interplay is shared by several other studies with Picea abies during drought stress (Miron and Sumalan 2015, Schiop et al. 2017).
Ψw, gs, and ABA make the perfect triangle in deciding plant tolerance to drought (Hsu et al. 2021, Mukarram et al. 2021). Since stomatal responses are more closely linked to soil moisture content than to leaf water status, they are responding to ‘nonhydraulic’ chemical signals (Yordanov et al. 2000). Drought-induced low Ψw instigates guard cell ABA signalling for stomatal closure to restrict transpiration loss (Schroeder et al. 2001, Chater et al. 2014). Drought-induced ABA concentrations were high in all spruce provenances irrespective of altitude, precipitation, and temperature. It could have resulted from the de novo biosynthesis of ABA in spruce ecotypes (Pashkovskiy et al. 2019). Similar to proline, ABA showed the highest sensitivity in PV3 and PV4 and was negligible in control. This suggests that lower Ψw could be critical in instigating ABA-mediated stomatal closure in Picea abies. The gs was further intensified with the moderate environment that could be ascribed to upregulated ABA signalling (Jamnická et al. 2019, Marešová et al. 2022). This might have reduced the net photosynthetic and transpiration rate, as we observed in PV4 drought ecotypes. It is possible that drought-induced ABA activated SnRK2 (sucrose nonfermenting 1 related protein kinase 2) in spruce provenances (Munemasa et al. 2015, Wu et al. 2019). The SnRK2 might have induced stomatal closure in PV4 through regulating K+ and Ca2+ channels, SnRK2 protein kinase OST1 (OPEN STOMATA 1), and slow anion channels SLAS1 in guard cells (Golldack et al. 2014, Yu et al. 2019, Haas et al. 2021).
The trade-offs between monoterpenes and the physiological fitness of Norway spruce have been earlier correlated with climatic settings and altitude (Huang et al. 2019, Večeřová et al. 2021, Hrivnák et al. 2022). A harsh climate also regulates several other secondary metabolites in other forest trees (Holopainen et al. 2018). In particular, a few studies have reported drought influencing monoterpene biosynthesis and concentration in several spruce species (Madmony et al. 2018, Perreca et al. 2022). As our results showed, the concentrations of monoterpenes were not influenced by water deficit to such an extent. Individual monoterpenes dominated the moderate PV4 except for SAB, OCI, and ATEol. These monoterpenes (SAB, OCI, and ATEol) were maximally expressed in the highest altitude PV5 drought variant. Mullin et al. (2021) found that elevation strongly influences the expression of tree monoterpenes, while others suggested temperature and higher CO2 content as the potential driving force for secondary metabolites alteration (Kivimäenpää et al. 2013). However, we did not notice such a correlation. PV4 and PV6 shared almost similar altitudes but monoterpenes response was the opposite. Additionally, reduced content of monoterpenes was observed only in PV6, however, no trend was found along the altitudinal gradient, e.g., PV1 and PV5 had the contrast origin with altitudinal difference of 850 m and their monoterpene concentrations were almost similar. Thus, although there was variability in monoterpenes accumulation among provenances, no clear pattern was found. Virjamo and Julkunen-Tiitto (2016) suggested that changes in metabolite chemistry could result from the different origins of Norway spruce. Another study (Marešová et al. 2022) suggested monoterpenes are not definite biochemical markers of drought stress, and they probably serve other defensive purposes, primarily.
Conclusion
Our study helps determine the physiological responses of Norway spruce to drought. The most pronounced effect was found in parameters related to photosynthesis and ABA increase. Drought restricted tree physiology, but the intensity was not the same for all spruce provenances, and different intra-species provenance-related responses were observed. Data indicated that the highest altitude provenance (PV5; 1,500 m a.s.l.) was the most drought-tolerant. The provenances originated from the highest altitudes have developed better adaptation mechanisms to adjust to dry conditions during the vegetation season. It may be in connection with harsher marginal mountain/alpine conditions in which they are frequently exposed to high irradiance and physiological drought. Higher temperatures and strong sunlight stimulate transpiration even when the soil is still frozen and the roots do not have access to water. Moreover, assimilatory organs can dispose of more effective protection against damage of PSII, the resistance of photoinhibition, and over-reduction. Thus, future studies would be needed to know more about its molecular mechanism and to what extent stress memory controls drought-induced responses in Norway spruce. Overall, our results highlight Norway spruce seedlings from high elevations as suitable candidates for the development of ‘climate-smart’ forests.
Acknowledgements
This study was supported by the Slovak Research and Development Agency grant no. APVV-16-0306 (Ľ.D. and G.J.), APVV-21-0270 (G.J. and E.P.), and APVV-19-0606 (J.M.); the Slovak Grant Agency for Science - grant no. VEGA 1/0535/20 (M.J., A.S.K., G.J., and H.H.), and by the Doctogrant APP 0306 (H.H.). We thank Anna Kracinová for her invaluable help in plant care and laboratory analyses. We are also grateful to Dr. Petre I. Dobrev from the Institute of Experimental Botany CAS for his help with the methodology of ABA determination in spruce samples.
Abbreviations
- AP
alpha-pinene
- ATEol
(–)-alpha-terpineol
- BP
beta-pinene
- CAM
camphene
- CAR
caryophyllene
- CI
1,8-cineole
- C i
intercellular concentration of CO2
- Chl
chlorophyll
- E
transpiration rate
- F', F
chlorophyll a fluorescence measured on light-adapted (F') or dark-adapted leaf (F)
- F0', F0
basal fluorescence of light-adapted (F0') or dark-adapted leaf (F0)
- Fm', Fm
maximal fluorescence in light (Fm') or dark-adapted leaf (Fm)
- FM
fresh mass
- Fq', Fq
photochemical quenching of fluorescence of light-adapted (Fq') or dark-adapted leaf (Fq)
- Fs'
measured fluorescence of steady-state in light-adapted leaf
- Fv', Fv
variable fluorescence of light-adapted (Fv') or dark-adapted (Fv) leaf
- Fv/Fm
maximum quantum yield of photochemistry in dark-adapted leaf
- g s
stomatal conductance to water vapour
- HUM
humulene
- LIM
limonene
- NPQ
nonphotochemical quenching of fluorescence
- OCI
o-cimene
- PIABS
performance index of photochemical activity based on the absorption
- P N
photosynthetic rate
- qL
coefficient of photochemical quenching based on the ‘Lake’ model
- qN
coefficient of nonphotochemical quenching of variable fluorescence
- qP
coefficient of photochemical quenching based on the ‘Puddle’ model
- RC/ABS
number of active reaction centres per antenna in PSII based on the absorption
- SH
sabinene hydrate
- TER
terpinolene
- ΔW
tree water status
- ΦPSII
the actual efficiency of PSII photochemistry (measured in light-adapted leaf)
- Ψw
soil water potential
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Avramova Z.: Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. – Plant J. 83: 149-159, 2015. 10.1111/tpj.12832 [DOI] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D.: Rapid determination of free proline for water-stress studies. – Plant Soil 39: 205-207, 1973. 10.1007/BF00018060 [DOI] [Google Scholar]
- Betsch P., Bonal D., Breda N. et al. : Drought effects on water relations in beech: The contribution of exchangeable water reservoirs. – Agr. Meteorol. 151: 531-543, 2011. 10.1016/j.agrformet.2010.12.008 [DOI] [Google Scholar]
- Bigras F.J.: Photosynthetic response of white spruce families to drought stress. – New Forest. 29: 135-148, 2005. 10.1007/s11056-005-0245-9 [DOI] [Google Scholar]
- Bruce T.J.A., Matthes M.C., Napier J.A., Pickett J.A.: Stressful “memories” of plants: evidence and possible mechanisms. – Plant Sci. 173: 603-608, 2007. 10.1016/j.plantsci.2007.09.002 [DOI] [Google Scholar]
- Bussotti F., Gerosa G., Digrado A., Pollastrini M.: Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. – Ecol. Indic. 108: 105686, 2020. 10.1016/j.ecolind.2019.105686 [DOI] [Google Scholar]
- Bussotti F., Pollastrini M., Holland V., Brüggemann W.: Functional traits and adaptive capacity of European forests to climate change. – Environ. Exp. Bot. 111: 91-113, 2015. 10.1016/j.envexpbot.2014.11.006 [DOI] [Google Scholar]
- Critchley C.: Photoinhibition. – In: Raghavendra A. (ed.): Photosynthesis – a comprehensive treatise. Pp. 264-273. Cambridge University Press, Cambridge: 2000. https://www.cambridge.org/do/academic/subjects/life-sciences/plant-science/photosynthesis-comprehensive-treatise [Google Scholar]
- Čermák P., Kolář T., Žid T. et al. : Norway spruce responses to drought forcing in area affected by forest decline. – For. Syst. 28: e016, 2019. 10.5424/fs/2019283-14868 [DOI] [Google Scholar]
- Dąbrowski P., Baczewska-Dąbrowska A.H., Kalaji H.M. et al. : Exploration of chlorophyll a fluorescence and plant gas exchange parameters as indicators of drought tolerance in perennial ryegrass. – Sensors-Basel 19: 2736, 2019. 10.3390/s19122736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszkowska-Golec A.: The role of abscisic acid in drought stress: How ABA helps plants to cope with drought stress. – In: Hossain M., Wani S., Bhattacharjee S. et al. (ed.): Drought Stress Tolerance in Plants. Vol. 2. Pp. 123-151. Springer, Cham: 2016. 10.1007/978-3-319-32423-4_5 [DOI] [Google Scholar]
- De Ronde J.A., Cress W.A., Krüger G.H.J. et al. : Photosynthetic response of transgenic soybean plants, containing an Arabidopsis P5CR gene, during heat and drought stress. – J. Plant Physiol. 161: 1211-1224, 2004. 10.1016/j.jplph.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams III W.W.: Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. – New Phytol. 172: 11-21, 2006. 10.1111/j.1469-8137.2006.01835.x [DOI] [PubMed] [Google Scholar]
- Epron D., Dreyer E.: Stomatal and non-stomatal limitation of photosynthesis by leaf water deficits in three oak species: a comparison of gas exchange and chlorophyll a fluorescence data – Ann. For. Sci. 47: 435-450, 1990. 10.1051/forest:19900503 [DOI] [Google Scholar]
- Fleta-Soriano E., Munné-Bosch S.: Stress memory and the inevitable effects of drought: a physiological perspective. – Front. Plant Sci. 7: 143, 2016. 10.3389/fpls.2016.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J., Farrona S.: Plant epigenetic stress memory induced by drought: a physiological and molecular perspective. – In: Spillane C., McKeown P. (ed.): Plant Epigenetics and Epigenomics. Methods in Molecular Biology. Vol. 2093. Pp. 243-259. Humana, New York: 2020. 10.1007/978-1-0716-0179-2_17 [DOI] [PubMed] [Google Scholar]
- Golldack D., Li C., Mohan H., Probst N.: Tolerance to drought and salt stress in plants: unraveling the signalling networks. – Front. Plant Sci. 5: 151, 2014. 10.3389/fpls.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi L., Lo Piccolo E., Landi M.: Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? – Front. Plant Sci. 10: 174, 2019. 10.3389/fpls.2019.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes A., Inal A., Adak M.S. et al. : Effect of drought stress implemented at pre- or post-anthesis stage on some physiological parameters as screening criteria in chickpea cultivars. – Russ. J. Plant Physiol. 55: 59-67, 2008. 10.1134/S102144370801007x [DOI] [Google Scholar]
- Haas J.C., Vergara A., Serrano A.R. et al. : Candidate regulators and target genes of drought stress in needles and roots of Norway spruce. – Tree Physiol. 41: 1230-1246, 2021. 10.1093/treephys/tpaa178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl-Meier C., Zang C., Dittmar C. et al. : Vulnerability of Norway spruce to climate change in mountain forests of the European Alps. – Clim. Res. 60: 119-132, 2014. 10.3354/cr01226 [DOI] [Google Scholar]
- Hayat S., Hayat Q., Alyemeni M.N. et al. : Role of proline under changing environments. – Plant Signal. Behav. 7: 1456-1466, 2012. 10.4161/psb.21949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlásny T., Mátyás C., Seidl R. et al. : Climate change increases the drought risk in Central European Forests: What are the options for adaptation? – Lesn. Cas. For. J. 60: 5-18, 2014. https://web.nlcsk.org/wp-content/uploads/2019/10/21.pdf [Google Scholar]
- Holopainen J.K., Virjamo V., Ghimire R.P. et al. : climate change effects on secondary compounds of forest trees in the northern hemisphere. – Front. Plant Sci. 9: 1445, 2018. 10.3389/fpls.2018.01445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrivnák M., Krajmerová D., Kurjak D. et al. : Differential associations between nucleotide polymorphisms and physiological traits in Norway spruce (Picea abies Karst.) plants under contrasting water regimes. – Forestry 95: 686-697, 2022. 10.1093/forestry/cpac027 [DOI] [Google Scholar]
- Hsu P.-K., Dubeaux G., Takahashi Y., Schroeder J.I.: Signaling mechanisms in abscisic acid-mediated stomatal closure. – Plant J. 105: 307-321, 2021. 10.1111/tpj.15067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Hammerbacher A., Weinhold A. et al. : Eyes on the future – evidence for trade-offs between growth, storage and defense in Norway spruce. – New Phytol. 222: 144-158, 2019. 10.1111/nph.15522 [DOI] [PubMed] [Google Scholar]
- Chater C.C.C., Oliver J., Casson S., Gray J.E.: Putting the brakes on: abscisic acid as a central environmental regulator of stomatal development. – New Phytol. 202: 376-391, 2014. 10.1111/nph.12713 [DOI] [PubMed] [Google Scholar]
- Chen J., Burke J.J., Xin Z.: Chlorophyll fluorescence analysis revealed essential roles of FtsH11 protease in regulation of the adaptive responses of photosynthetic systems to high temperature. – BMC Plant Biol. 18: 11, 2018. 10.1186/s12870-018-1228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnická G., Fleischer P., Konôpková A. et al. : Norway spruce (Picea abies L.) provenances use different physiological strategies to cope with water deficit. – Forests 10: 651, 2019. 10.3390/f10080651 [DOI] [Google Scholar]
- Kalaji H.M., Jajoo A., Oukarroum A. et al. : Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. – Acta Physiol. Plant. 38: 102, 2016. 10.1007/s11738-016-2113-y [DOI] [Google Scholar]
- Kivimäenpää M., Riikonen J., Ahonen V. et al. : Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. – Environ. Exp. Bot. 90: 32-42, 2013. 10.1016/j.envexpbot.2012.11.004 [DOI] [Google Scholar]
- Kmeť J., Ditmarová Ľ., Priwitzer T. et al. : Physiological limits – a possible cause of spruce decline. – Beskydy 3: 55-64, 2010. [Google Scholar]
- Köcher P., Horna V., Leuschner C.: Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. – Tree Physiol. 32: 1021-1032, 2012. 10.1093/treephys/tps049 [DOI] [PubMed] [Google Scholar]
- Kopaczyk J.M., Warguła J., Jelonek T.: The variability of terpenes in conifers under developmental and environmental stimuli. – Environ. Exp. Bot. 180: 104197, 2020. 10.1016/j.envexpbot.2020.104197 [DOI] [Google Scholar]
- Krasensky J., Jonak C.: Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. – J. Exp. Bot. 63: 1593-1608, 2012. 10.1093/jxb/err460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.W.: Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. – Ann. Bot.-London 89: 871-885, 2002. 10.1093/aob/mcf110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longerberger P.S., Smith C.W., Duke S.E., McMichael B.L.: Evaluation of chlorophyll fluorescence as a tool for the identification of drought tolerance in upland cotton. – Euphytica 166: 25-33, 2009. 10.1007/s10681-008-9820-4 [DOI] [Google Scholar]
- Madmony A., Tognetti R., Zamponi L. et al. : Monoterpene responses to interacting effects of drought stress and infection by the fungus Heterobasidion parviporum in two clones of Norway spruce (Picea abies). – Environ. Exp. Bot. 152: 137-148, 2018. 10.1016/j.envexpbot.2018.03.007 [DOI] [Google Scholar]
- Malnoë A.: Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. – Environ. Exp. Bot. 154: 123-133, 2018. 10.1016/j.envexpbot.2018.05.005 [DOI] [Google Scholar]
- Marešová J., Húdoková H., Sarvašová L. et al. : Dynamics of internal isoprenoid metabolites in young Picea abies (Norway spruce) shoots during drought stress conditions in springtime. – Phytochemistry 203: 113414, 2022. 10.1016/j.phytochem.2022.113414 [DOI] [PubMed] [Google Scholar]
- Miron M.S., Sumalan R.L.: Physiological responses of Norway spruce (Picea abies [L.] Karst) seedlings to drought and overheating stress conditions. – J. Hortic. For. Biotechnol. 19: 146-151, 2015. https://www.usab-tm.ro/Journal-HFB/romana/2015/Lucrari%20PDF/Lucrari%20PDF%2019(2)/27Miron%20Marius.pdf [Google Scholar]
- Mukarram M., Choudhary S., Kurjak D. et al. : Drought: Sensing, signalling, effects and tolerance in higher plants. – Physiol. Plantarum 172: 1291-1300, 2021. 10.1111/ppl.13423 [DOI] [PubMed] [Google Scholar]
- Mukarram M., Khan M.M.A., Kujak D. et al. : Silicon nanoparticles (SiNPs) restore photosynthesis and essential oil content by upgrading enzymatic antioxidant metabolism in lemongrass (Cymbopogon flexuosus) under salt stress. – Front. Plant. Sci. 14: 1116769, 2023. 10.3389/fpls.2023.1116769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukarram M., Khan M.M.A., Zehra A. et al. : Suffer or survive: Decoding salt-sensitivity of lemongrass and its implication on essential oil productivity. – Front. Plant. Sci. 13: 903954, 2022. 10.3389/fpls.2022.903954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin M., Klutsch J.G., Cale J.A. et al. : Primary and secondary metabolite profiles of lodgepole pine trees change with elevation, but not with latitude. – J. Chem. Ecol. 47: 280-293, 2021. 10.1007/s10886-021-01249-y [DOI] [PubMed] [Google Scholar]
- Munemasa S., Hauser F., Park J. et al. : Mechanisms of abscisic acid-mediated control of stomatal aperture. – Curr. Opin. Plant Biol. 28: 154-162, 2015. 10.1016/j.pbi.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie E.H., Niyogi K.K.: Manipulation of photoprotection to improve plant photosynthesis. – Plant Physiol. 155: 86-92, 2011. 10.1104/pp.110.168831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W., Hammerle A., Kofler W.: Tree water status and growth of saplings and mature Norway spruce (Picea abies) at a dry distribution limit. – Front. Plant Sci. 6: 703, 2015a. 10.3389/fpls.2015.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W., Kofler W., Schuster R., Wieser G.: Environmental effects on stem water deficit in co-occurring conifers exposed to soil dryness. – Int. J. Biometeorol. 59: 417-426, 2015b. 10.1007/s00484-014-0853-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenthaler I., Hietz P., Richter H.: Wood diameter indicates diurnal and long-term patterns of xylem water potential in Norway spruce. – Trees-Struct. Funct. 15: 215-221, 2001. 10.1007/s004680100090 [DOI] [Google Scholar]
- Pashkovskiy P.P., Vankova R., Zlobin I.E. et al. : Comparative analysis of abscisic acid levels and expression of abscisic acid-related genes in Scots pine and Norway spruce seedlings under water deficit. – Plant Physiol. Bioch. 140: 105-112, 2019. 10.1016/j.plaphy.2019.04.037 [DOI] [PubMed] [Google Scholar]
- Perreca E., Eberl F., Santoro M.V. et al. : Effect of drought and methyl jasmonate treatment on primary and secondary isoprenoid metabolites derived from the MEP pathway in the white spruce Picea glauca. – Int. J. Mol. Sci. 23: 3838, 2022. 10.3390/ijms23073838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik P., Petek-Petrik A., Kurjak D. et al. : Interannual adjustments in stomatal and leaf morphological traits of European beech (Fagus sylvatica L.) demonstrate its climate change acclimation potential. – Plant Biol. 24: 1287-1296, 2022. 10.1111/plb.13401 [DOI] [PubMed] [Google Scholar]
- Pukacki P.M., Kamińska-Rożek E.: Effect of drought stress on chlorophyll a fluorescence and electrical admittance of shoots in Norway spruce seedlings. – Trees-Struct. Funct. 19: 539-544, 2005. 10.1007/s00468-005-0412-9 [DOI] [Google Scholar]
- Rehschuh R., Mette T., Menzel A., Buras A.: Soil properties affect the drought susceptibility of Norway spruce. – Dendrochronologia 45: 81-89, 2017. 10.1016/j.dendro.2017.07.003 [DOI] [Google Scholar]
- Ruban A.V., Murchie E.H.: Assessing the photoprotective effectiveness of non-photochemical chlorophyll fluorescence quenching: A new approach. – BBA-Bioenergetics 1817: 977-982, 2012. 10.1016/j.bbabio.2012.03.026 [DOI] [PubMed] [Google Scholar]
- Shevela D., Ananyev G., Vatland A.K. et al. : ‘Birth defects’ of photosystem II make it highly susceptible to photodamage during chloroplast biogenesis. – Physiol. Plantarum 166: 165-180, 2019. 10.1111/ppl.12932 [DOI] [PubMed] [Google Scholar]
- Schiop S.T., Al Hassan M., Sestras A.F. et al. : Biochemical responses to drought, at the seedling stage, of several Romanian Carpathian populations of Norway spruce (Picea abies L. Karst). – Trees-Struct. Funct. 31: 1479-1490, 2017. 10.1007/s00468-017-1563-1 [DOI] [Google Scholar]
- Schroeder J.I., Kwak J.M., Allen G.J.: Guard cell abscisic acid signalling and engineering drought hardiness in plants. – Nature 410: 327-330, 2001. 10.1038/35066500 [DOI] [PubMed] [Google Scholar]
- Sousaraei N., Mashayekhi K., Mousavizadeh S.J. et al. : Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. – Hortic. Environ. Biotech. 62: 521-535, 2021. 10.1007/S13580-020-00328-5 [DOI] [Google Scholar]
- Stefanov M.A., Rashkov G.D., Apostolova E.L.: Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. – Int. J. Mol. Sci. 23: 3768, 2022. 10.3390/ijms23073768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R.J., Tsimilli-Michael M., Srivastava A.: Analysis of the chlorophyll a fluorescence transient. – In: Papageorgiou G.C., Govindjee (ed.): Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration. Pp. 321-362. Springer, Dordrecht: 2004. 10.1007/978-1-4020-3218-9_12 [DOI] [Google Scholar]
- Tang A.-C., Kawamitsu I., Kanechi M., Boyer J.S.: Photosynthetic oxygen evolution at low water potential in leaf discs lacking an epidermis. – Ann. Bot.-London 89: 861-870, 2002. 10.1093/aob/mcf081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomášková I., Pastierovič F., Krejzková A. et al. : Norway spruce ecotypes distinguished by chlorophyll a fluorescence kinetics. – Acta Physiol. Plant. 43: 24, 2021. 10.1007/s11738-020-03190-1 [DOI] [Google Scholar]
- Tužinský L., Bublinec E., Tužinský M.: Development of soil water regime under spruce stands. – Folia Oecol. 44: 46-53, 2017. 10.1515/foecol-2017-0006 [DOI] [Google Scholar]
- Urban L., Aarrouf J., Bidel L.P.R.: Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. – Front. Plant Sci. 8: 2068, 2017. 10.3389/fpls.2017.02068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentovič P., Luxová M., Kolarovič L., Gašparíková O.: Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. – Plant Soil Environ. 52: 186-191, 2006. 10.17221/3364-PSE [DOI] [Google Scholar]
- Večeřová K., Klem K., Veselá B. et al. : Combined effect of altitude, season and light on the accumulation of extractable terpenes in Norway spruce needles. – Forests 12: 1737, 2021. 10.3390/f12121737 [DOI] [Google Scholar]
- Virjamo V., Julkunen-Tiitto R.: Variation in piperidine alkaloid chemistry of Norway spruce (Picea abies) foliage in diverse geographic origins grown in the same area. – Can. J. Forest Res. 46: 456-460, 2016. 10.1139/cjfr-2015-0388 [DOI] [Google Scholar]
- Wang Z., Li G., Sun H. et al. : Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. – Biol. Open 7: bio035279, 2018. 10.1242/bio.035279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfahrt S., Schmitt V., Wild A.: Investigation on phosphoenol pyruvate carboxylase and proline in damaged and undamaged needles of Picea abies and Abies alba. – Chemosphere 36: 877-881, 1998. 10.1016/S0045-6535(97)10141-2 [DOI] [Google Scholar]
- Wu Q., Wang M., Shen J. et al. : ZmOST1 mediates abscisic acid regulation of guard cell ion channels and drought stress responses. – J. Integr. Plant Biol. 61: 478-491, 2019. 10.1111/jipb.12714 [DOI] [PubMed] [Google Scholar]
- Yordanov Y., Velikova V., Tsonev T.: Plant responses to drought, acclimation and stress tolerance. – Photosynthetica 38: 171-186, 2000. 10.1023/A:1007201411474 [DOI] [Google Scholar]
- Yu D., Wildhagen H., Tylewicz S. et al. : Abscisic acid signalling mediates biomass trade-off and allocation in poplar. – New Phytol. 223: 1192-1203, 2019. 10.1111/nph.15878 [DOI] [PubMed] [Google Scholar]
- Zivcak M., Brestic M., Balatova Z. et al. : Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. – Photosynth. Res. 117: 529-546, 2013. 10.1007/s11120-013-9885-3 [DOI] [PubMed] [Google Scholar]
- Zlobin I.E., Kartashov A.V., Pashkovskiy P.P. et al. : Comparative photosynthetic responses of Norway spruce and Scots pine seedlings to prolonged water deficiency. – J. Photoch. Photobio. B 201: 111659, 2019. 10.1016/j.jphotobiol.2019.111659 [DOI] [PubMed] [Google Scholar]
- Zweifel R., Zimmermann L., Newbery D.M.: Modeling tree water deficit from microclimate: an approach to quantifying drought stress. – Tree Physiol. 25: 147-156, 2005. 10.1093/treephys/25.2.147 [DOI] [PubMed] [Google Scholar]