Abstract
This study aimed to determine the photosynthetic performance and differences in chlorophyll fluorescence (ChlF) parameters between Eulophia dentata and its companion species Bletilla formosana and Saccharum spontaneum when subjected to different photosynthetic photon flux density (PPFDs). Leaf surfaces were then illuminated with 50, 100 (low PPFDs), 300, 500, 800 (moderate PPFDs); 1,000; 1,500; and 2,000 (high PPFDs) μmol m–2·s–1, and the ChlF parameters were measured during the whole process. Increasing nonphotochemical quenching of ChlF and decreasing potential quantum efficiency of PSII, actual quantum efficiency of PSII, and quantum efficiency ratio of PSII in dark recovery from 0–60 min were observed in all leaves. A significant and negative relationship was detected between energy-dependent quenching (qE) and photoinhibition percent in three species under specific PPFD conditions, whereas a significant and positive relationship was detected between photoinhibitory quenching (qI) and photoinhibition percent. The qE and qI can be easily measured in the field and provide useful ecological indexes for E. dentata species restoration, habitat creation, and monitoring.
Keywords: Bletilla, chlorophyll fluorescence, Eulophia, light intensity, photoinhibition, Saccharum
Highlights
E. dentata and S. spontaneum adapt their photosynthesis to high and moderate PPFDs
NPQ can be divided into photoprotection (qE, qZ, qT) and photoinhibition (qI)
The photoprotection mode of E. dentata and S. spontaneum is dominated by qE mediation
Introduction
Eulophia dentata Ames, a critically endangered plant in Taiwan, is endemic there. It grows on sandy banks along the rivers and is distributed from 0–250 m in Miaoli and Yilan, Central Taiwan (24°17'34.6"N 120°50'02.1"E). This wild population is substantially threatened as a result of habitat losses from a forest fire, extreme weather, and severe flooding in the Zhuolan Grand Canyon, Miaoli, causing a sharp decline in its population. Only two native habitat units were found 80 years ago, and the number of plants of this species is now approximately 250 (Editorial Committee of the Red List of Taiwan Plants 2017). Narrow distribution and vulnerability to disturbance make their native habitat conservation to be extremely urgent. Alternatively, in vitro techniques, including seed germination, micropropagation, meristem culture, and callus culture have been undertaken for E. dentata plant propagation, germplasm preservation, and restoration in Taiwan (Chang et al. 2014).
E. dentata plants commonly grow together with Bletilla formosana and Saccharum spontaneum in the current sampling area (Fig. 1S, supplement). We also observed that B. formosana grew along ecotones between forests and fields that do not have graminoid competition, while E. dentata grew in the habitat of S. spontaneum plants, leading to E. dentata plants receiving partial shade in the open field. We assume that E. dentata plants were approximately 50% shaded by tall S. spontaneum grass plants, therefore exhibiting shade tolerance. Saccharum spontaneum L., a perennial rhizomatous tall grass of the family Poaceae, is known to invade abandoned and pastoral lands in many tropical countries and is often planted in riverbeds in Taiwan to suppress dust in the area. In addition to grass vegetation studies, S. spontaneum attracts attention for its potential in the ecological restoration and stabilization of barren fly ash dumps (Pandey et al. 2015). Recently, the optimized pretreatment of S. spontaneum biomass with enzymatic saccharification has been developed to convert it into an ethanol biofuel through a consolidated bioprocess (Vaid et al. 2021). In addition, Li et al. (2021) reported the polyploid characteristics of dehydration-responsive element binding proteins (DREBs) and functions relative to the photosynthesis of S. spontaneum and plant development during drought stress. Bletilla is a common perennial herb that has been used in folk and traditional medicine for the treatment of bleeding, colds, esophagitis, erosive gastritis, and burns (Lin et al. 2016). Bletilla formosana (Hayata) Schltr. is the only member of the genus found in Taiwan on mountain slopes up to 2,200 m altitude, and has been utilized as Chinese medicinal and ornamental material (Wu et al. 2010). B. formosana is currently widely distributed throughout Taiwan and is important for the sustainable development of the orchid industry in conserving the biodiversity of precious orchid genetic resources.
Ecophysiological studies require knowledge of the photosynthetic rates of plants under different environmental conditions, particularly of a broad range of light intensity (photosynthetic photon flux density, PPFD). A photosynthetic light response can be used to assess the ability to capture light and understand the optimal ambient PPFD conditions of plants. Ecophysiological responses to excess sunlight vary among E. dentata, B. formosana, and S. spontaneum, and may significantly affect the survival rate and spatial distribution of E. dentata plants. In full sun-exposed habitats, leaves often absorb considerably more photons than can be utilized, the excess absorbed energy often resulting in reducing the photochemical efficiency of PSII (ΦPSII). Photoinhibition of photosynthesis occurs when PPFD exceeds the capacity/activity of the photosynthetic electron transport chain in the chloroplast, leading to the inactivation of and damage to PSII (Orekhova et al. 2021); in addition, at high temperatures, plants absorb excess light energy and inhibit photosynthesis (Colom et al. 2003, Kalapchieva et al. 2019). Furthermore, excessively high irradiance may result in photoinhibition, which is characterized by a loss of PSII activity and a light-dependent reduction, thus requiring the dissipation of excess excitation energy (Portela et al. 2019). Application of the ChlF fluorescence-based methods allows us to obtain information about the functions of the photosynthetic apparatus, and fluorescence is often used in physiological studies to investigate plant's response to various environmental stresses in controlled environments and the field (Kałużewicz et al. 2018). Unadapted temperature ranges have impacts on the physiological activities of plants, especially in high temperature/high light and low temperature/high light ranges, when the radiant energy captured by the photosynthetic pigment of the plant exceeds the carbon fixation rates; here, the rate of utilization of absorbed energy is low, and excess energy is generated. Dissipation in other ways may cause damage to photosynthetic organs, and this excess energy is often eliminated by loss as heat, through the well-known xanthophyll cycle (Tüffers et al. 1999, Adams et al. 2004). Thus, nonphotochemical quenching (NPQ) plays an important role in photoprotection because it quenches excess energy and dissipates it safely as heat (Murchie and Niyogi 2011). Moreover, plants exposed to high light also show decreases or adjustments in leaf photosynthetic pigment content, providing an important photoprotective mechanism (Souza et al. 2017).
Considering the limited number of E. dentata individuals at the sampling site, chlorophyll fluorescence (ChlF) was used to analyze the characteristics of photosynthesis in E. dentata plants grown under various light environments. ChlF measurements have been proposed to evaluate the conditions of plants in ecological systems and have been successfully applied in the physiological profiling of invasive plant species for ecological restoration (Bussotti et al. 2020, Pandey et al. 2020); further, many related parameters, such as energy-dependent quenching (qE), photoinhibitory quenching (qI), and zeaxanthin-dependent quenching (qZ) have been used to detect the partitioning of light energy to alternative dissipative mechanisms (Guidi et al. 2019). The parameters derived from fluorescence kinetics, obtained using PAM-fluorescence methods, reflect the photosynthetic potential and potential for photochemical dissipation, and also demonstrate the percentage of PSII that is open and its effectiveness in capturing photon energy by light-harvesting complex (LHC) II and the subsequent transfer of quanta (Moya et al. 2019). However, no study has described its ecophysiological response under controlled irradiation conditions, and the function of the photosynthetic apparatus has not yet been examined for the occurrence of ChlF indicators in E. dentata and its B. formosana and S. spontaneum companion species under field conditions to explain the development and distribution of E. dentata. Various PPFD levels may be applied for ChlF measurements to determine the actual state of the photosynthetic apparatus and the photoreceptor involved in E. dentata leaves. Efforts to gain an understanding of the photosynthetic characteristics of E. dentata leaves could benefit field cultivation management. Therefore, it is urgent to regulate, prioritize for management, and monitor this nationally endangered species for potentially threatened eradication. In the present study, we analyzed ChlF parameters in E. dentata, B. formosana, and S. spontaneum, growing in the field, to understand whether they can acclimate to intense light conditions and have higher physiological plasticity toward PPFD and shade tolerance. The mechanisms of capture, transfer, and dissipation of excitation energy were studied, through ChlF measurements in E. dentata, B. formosana, and S. spontaneum leaves in response to varying PPFD, and to check if E. dentata leaves exhibit strong PPFD adjustments in photosynthesis. Our study of E. dentata not only recognizes its ecological distinctness but highlights its critical conservation status. In addition, our research shows that the relationships of ChlF indices can be used for ecophysiological research in E. dentata, and that these parameters can be considered selection indices for examining the growth of E. dentata species grown under artificial light illumination.
Materials and methods
Plant materials and light treatments
We searched for and monitored E. dentata plants growing in Zhuolan Grand Canyon, Miaoli (24°17'34.6"N, 120°50'02.1"E), for the past three years, and discovered only 15 when we revisited the habitat site in April 2021. We selected five individuals of E. dentata plants, 20–30 cm tall, five plants of B. formosana, 30–50 cm tall, and five plants of S. spontaneum, 40–300 cm tall, within the riparian lands at Miaoli, Taiwan (Fig. 1S). These plants were studied in the field, and vegetation and habitat types were recorded on the sites from July to August 2021. The climate there is humid subtropical, with a mean annual rainfall of 1,000 mm, a mean annual air temperature of 26.5°C, and mean PPFDs of 1,000–1,500 μmol m–2 s–1, as recorded from January to December 2012–2022 (Fig. 2S, supplement). The upper leaves of the above-mentioned three species were selected for measuring ChlF parameters, and the light environment was 60–80% of the largest PPFD in the growing area.
Determination of ChlF parameters under fixed light intensity
Measurements were taken from September to October 2021. Five plants of each species per light treatment were used for ChlF measurements, and one upper fully open leaf per plant was used. Measurements were initially made on the dark-adapted leaf, after which the leaf surface was illuminated with 50, 100, 300, 500, 800; 1,000; 1,500; and 2,000 μmol m–2 s–1 PPFD using a portable fluorescence photosynthesis analyzer (MINI-PAM-II, Heinz Walz, Effeltrich, Germany). Overnight dark-adapted plants were exposed to light stepwise from low to high levels of PPFD, and ChlF parameters were measured during 60 min of irradiation and dark adaptation for 30 min. One data point was recorded at each 2-min interval over a 90-min period, followed by calculating the parameters listed below.
The potential and actual quantum efficiency of PSII and electron transport rate
The potential quantum efficiency of PSII (Fv/Fm) is calculated as (Fm – F0)/Fm, and the actual PSII efficiency (ΔF/Fm') is the effective quantum yield of linear electron flux through PSII, which is used to express the ability of PSII to perform photochemistry (Demmig-Adams et al. 1996). Values of the minimal (F0) and maximal ChlF (Fm) of dark-adapted samples were determined using modulated irradiation of a weak light-emitting diode beam (measuring light) and saturating pulse, respectively. Fm' is the maximal level of fluorescence during illumination as determined by applying a saturating flash. The photochemical ΦPSII was calculated as (Fm' – Ft)/Fm', where Ft is the steady-state level of fluorescence excited by actinic light of the applied PPFD levels (Maxwell and Johnson 2000). Furthermore, the degree of photoinhibition is calculated as the relative value of Fv/Fm after 30 min of dark adaptation, where the Fv/Fm value of the same leaves before illumination was taken as 100%. The apparent rate of the photosynthetic electron transport rate (ETR) of PSII was obtained as ETR = ΔF/Fm' × PPFD × 0.5 × α, where factor 0.5 implies equal excitation of both PSII and PSI; α is leaf absorption, and we used the mean ‘default’ value of 0.84 for green leaves (Björkman and Demmig-Adams 1995). The following effective quantum yields were measured using the instant light-response curve program. From these data, several parameters were computed based on modulated fluorescence kinetics (see below).
Nonphotochemical quenching and its components
The NPQ coefficient and its components were calculated as NPQ = (Fm – Fm')/Fm' (Weng et al. 2011). Energy-dependent quenching (qE) as NPQ is largely the dominant high-energy form, which is calculated as (FmD2 – Fm60')/Fm60' (Johnson and Ruban 2011). However, photoinhibitory quenching (qI) is NPQ due to decreased CO2 fixation, which is calculated as (Fm – FmD30)/Fm60' (Müller et al. 2001). In addition, the part after the reaction of qE is (qZ + qT), and is calculated as (FmD30 – FmD2)/Fm60' (Nilkens et al. 2010). The Fm60' is the maximum fluorescence value of leaves at 60 min of light exposure. Both FmD2 and FmD30 are the Fm values measured at 2 and 30 min, respectively, after dark recovery (Wang et al. 2022). Measurements were recorded with WinControl-3 software (Heinz Walz).
Statistical analysis
All PPFD treatments were arranged in a completely randomized design, and all ChlF parameters were subjected to a single-factor analysis of variance (ANOVA) to determine whether a significant difference level of p≤0.05 using PASW (Statistical Product and Service Solutions) Statistics 18 software (PASW 18, IBM, USA) existed between different treatments. Five leaves (one leaf per plant) were measured in each PPFD treatment (for a total of five replicates), and data from each leaf represented one replicate in the statistical analyses. Regression analyses were used to examine relationships among qE, qI, and photoinhibition (in %). Model datasets were based on at least five leaves at each PPFD level. Several models were tested, including the linear regression model, which was selected for the interpretation of the relationships between ChlF parameters and PPFD. All models were evaluated for the goodness of fit by the graphical analysis of residuals and by computing correlation coefficients at a significance level of p≤0.05 for ChlF parameters, and the linear regression model performance was considered to be the most suitable.
Results
Time-course changes in the light induction of ETR, NPQ, Fv/Fm, and ΦPSII
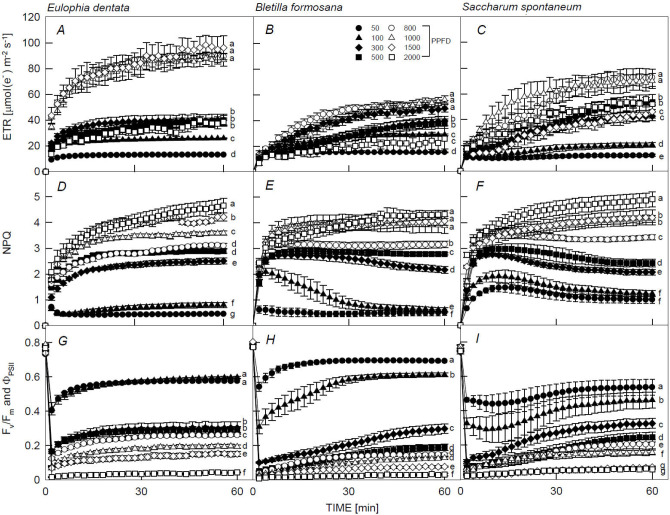
The ETR values of E. dentata plants under 800; 1,000; and 1,500 μmol m–2 s–1 were significantly higher than the other PPFD treatments; ETR suddenly increased at the beginning of a short illumination time (2 min) and reached its peak at 60 min as time increased (Fig. 1A). Similar trends were observed in the ETR of B. formosana (Fig. 1B) and S. spontaneum (Fig. 1C) plants, but ETR values under 800; 1,000; and 1,500 μmol m–2 s–1 treatments at 60 min were remarkably lower [< 70 μmol(electron) m–2 s–1] compared to E. dentata under the same light conditions [> 80 μmol(electron) m–2 s–1]. This indicates that high PPFD limited B. formosana and S. spontaneum leaf growth and development, but that E. dentata plants could be grown under a specific and optimal light intensity and be adapted to less than 1,500 μmol m–2 s–1. However, all the tested plants were intolerant to 2,000 μmol(photon) m–2 s–1. In addition, the ETR values of all the three species, used here, under the 50 μmol m–2 s–1 remained low [< 10 μmol(electron) m–2 s–1] as exposure time increased, and no reduction of electron transport was detectable at longer illumination times compared to the other PPFD treatments, which gradually increased thereafter. Moreover, Fig. 1D–F shows the NPQ values of the three species sharply increasing and continuing to linearly increase until 60 min under 1,000; 1,500; and 2,000 μmol(photon) m–2 s–1. These values were significantly higher (> 3.5) than those under the other PPFD treatments (< 3), suggesting that these tested plants suffered high PPFD stress and that an optimal NPQ could be maintained in these three species exposed to less than 1,000 μmol m–2 s–1. Notably, NPQ values for B. formosana under 100 and 300 μmol m–2 s–1 and S. spontaneum leaves under PPFD of 50, 100, 300, and 500 μmol m–2 s–1 peaked (ranging 2–3) in the first 2–5 min, and then dropped remarkably thereafter during photoinhibition processes (Fig. 1E,F). Lastly, the slow phases of both Fv/Fm and ΦPSII of the three species were found to increase slowly with prolonged illumination after the first 2 min under all the PPFD treatments (Fig. 1F–H). Almost no contribution of the slow phase was detectable, and the maximum amplitude was reached after 60 min of illumination. Significantly higher Fv/Fm and ΦPSII levels were detected in all the tested plants under 50 and 100 μmol m–2 s–1 (ranged 0.42–0.65) compared to other PPFD treatments, in particular, the 2,000 μmol m–2 s–1 treatment, where Fv/Fm and ΦPSII values were close to 0. This indicated that increasing the light led to a reduction in the Fv/Fm and ΦPSII values in the three species, especially those subjected to high PPFD treatments [1,000; 1,500; and 2,000 μmol m–2 s–1], where the values dropped below 0.2.
Fig. 1. Time-course variations in the electron transport rate (ETR), nonphotochemical quenching (NPQ), and Fv/Fm and PSII efficiency (ΦPSII) of Eulophia dentata (A,D,G), Bletilla formosana (B,E,H), and Saccharum spontaneum (C,F,I), respectively. Measurements were made at 25°C under 50, 100, 300, 500, 800, 1,000; 1,500; and 2,000 μmol m–2 s–1 PPFD during the 60 min of light induction. Each point represents the mean of five leaves, and data are means ± standard errors. Different letters indicate significant differences in Tukey's HSD analyses at eight illuminations (P<0.05).
Responses of ΔF/Fm' and Fv/Fm during 30 min of darkness after ceasing illumination
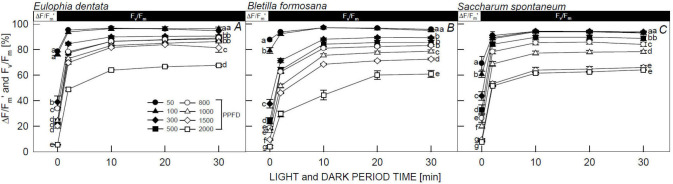
Both ΔF/Fm' and Fv/Fm [%] values of the three species in all PPFD treatments rapidly increased right after stopping illumination during the dark period; this was followed by a gradual increase and then no change after 20 min of darkness. Both ΔF/Fm' and Fv/Fm [%] levels of E. dentata (Fig. 2A), B. formosana (Fig. 2B), and S. spontaneum (Fig. 2C) in the 50 and 100 μmol m–2 s–1 recovered to 90–99% after 2 min of darkness (D2) and thereafter. This exhibited significantly higher photoprotection compared to samples under 800; 1,000; 1,500; and 2,000 μmol m–2 s–1 treatments, with 60–80% recovery after 30 min of darkness (D30), indicating that these plants could be grown under low PPFD conditions. However, in 300, 500, 800; 1,000; 1,500; and 2,000 μmol m–2 s–1, both the ΔF/Fm' and Fv/Fm [%] of B. formosana plants (Fig. 2B) recovered to 28.8–66.6% at D2. This was significantly lower in comparison to E. dentata (Fig. 2A) and S. spontaneum (Fig. 2C), which were 45.6–81.5% at D2, suggesting that the photosynthetic system recovery abilities of E. dentata and S. spontaneum were higher than those of B. formosana.
Fig. 2. Responses of the relative values of actual PSII efficiency (ΔF/Fm') and potential quantum efficiency of PSII (Fv/Fm value of the same leaves before illumination being 100%) in Eulophia dentata (A), Bletilla formosana (B), and Saccharum spontaneum (C). These ChlF parameters were obtained at artificial illumination of 60 min (L60) and a subsequent dark recovery for 2 min (D2), 10 min (D10), 20 min (D20), and 30 min (D30) periods under 50, 100, 300, 500, 800, 1,000; 1,500; and 2,000 μmol m–2 s–1 PPFD at 25°C. Each point represents the mean of five leaves, and data are means ± standard errors. Different letters indicate significant differences in Tukey's HSD analyses at eight illuminations (P<0.05).
The fractions of NPQ under PPFD treatments and after 30 min of darkness
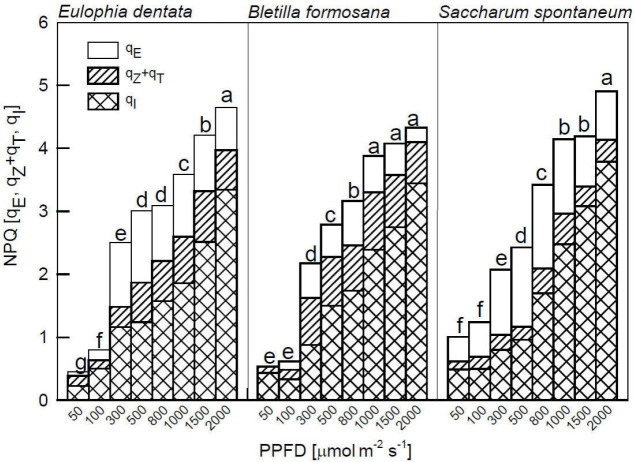
All the three species, used in this study, in all PPFD treatments substantially increased their NPQ values, and their increases seemed unlikely to be related to photoprotection (qE and qZ + qT), but rather linked to photoinhibition (qI), and qI values which increased as PPFD increased from 100 to 2,000 μmol m–2 s–1 (Fig. 3). In other words, the qE and qZ + qT values of three species did not contribute significantly to NPQ in all PPFD treatments. All leaves had significantly higher NPQ levels (ranging 3–5) in 800; 1,000; 1,500; and 2,000 μmol m–2 s–1 compared to those below 500 μmol m–2 s–1 (< 2.7).
Fig. 3. Composition of nonphotochemical quenching in qE, qZ + qT, and qI fractions of Eulophia dentata, Bletilla formosana, and Saccharum spontaneum. Measurements were made at 25°C under 50, 100, 300, 500, 800, 1,000; 1,500; and 2,000 μmol m–2 s–1 PPFD for 60 min and after a dark period for 30 min. Different letters indicate significant differences in Tukey's HSD analyses at eight illuminations (P<0.05). Each datum represents the mean of five leaves. qE – energy-dependent quenching; qI – photoinhibition quenching; qT – state transition quenching; qZ – zeaxanthin-dependent quenching.
Relations of photoinhibition to qE and qI values under PPFD treatments for 60 min
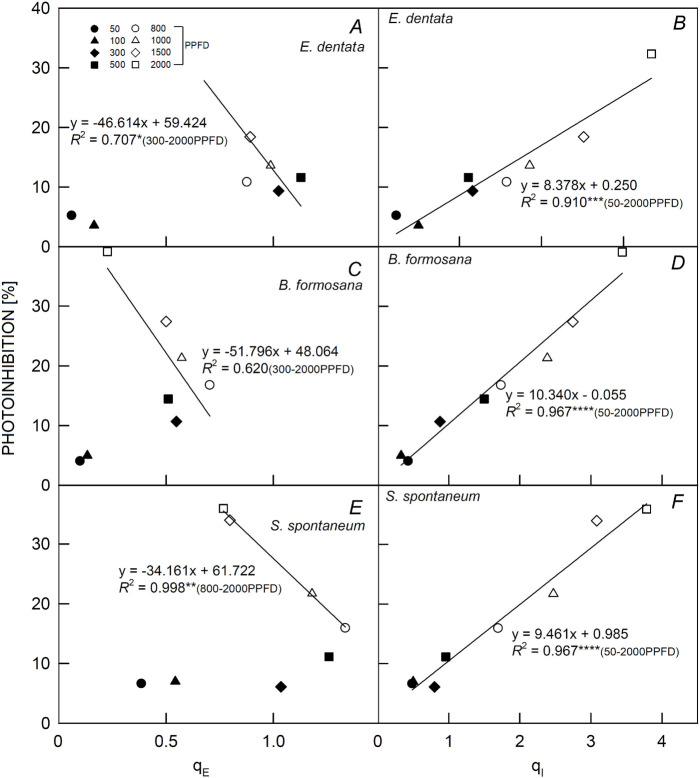
A significant and negative correlation with the r2 value of 0.707 (p<0.05) was detected in the degree of photoinhibition and qE value of E. dentata plants under PPFDs above 100 μmol m–2 s–1 (Fig. 4A), implying that E. dentata plants were more sensitive to PPFD conditions above 100 μmol m–2 s–1, and there might not be a need to adjust the qE value of E. dentata plants under 50 and 100 μmol m–2 s–1. Furthermore, significant and highly negative relationships were also detected between the degree of photoinhibition and qE with an r2 value of 0.993 (p<0.01) in S. spontaneum plants subjected to PPFDs higher than 500 μmol m–2 s–1 (Fig. 4E). In addition, the degree of photoinhibition in E. dentata plants was lower than that in S. spontaneum plants (Fig. 4A,E). Nevertheless, significant and highly positive correlations were observed between the degree of photoinhibition and qI values of E. dentata, B. formosana, and S. spontaneum plants under all PPFD treatments, with r2 values of 0.910 (p<0.001), 0.967 (p<0.0001), and 0.967 (p<0.0001), respectively (Fig. 4B,D,F). The increased % of photoinhibition led to increases in qI values and decreases in qE values due to photoinhibition and greater energy dissipation.
Fig. 4. Relationships between photoinhibition [%] and energy-dependent quenching (qE) and photoinhibition quenching (qI) of Eulophia dentata (A,B), Bletilla formosana (C,D), and Saccharum spontaneum (E,F). Measurements were made at 25°C under 50, 100, 300, 500, 800, 1,000; 1,500; and 2,000 μmol m–2 s–1 PPFD for 60 min. Each symbol represents the average of five leaves from one plant, and five plants were randomly selected for each light treatment. Each ChlF index was calculated using different leaf data (n = 8) from the model's validation datasets. The determination coefficient (r2) and significance of the regression are shown (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Discussion
Photosynthetic light responses were used to assess the ability of plants, selected for this study, to capture light and understand their optimal PPFD habitat conditions; in particular, we examined the interactive effect of PPFD on the growth and development of E. dentata. Measuring ChlF yields gives specific information about photochemical efficiency and heat dissipation (Papageorgiou and Govindjee 2014), and the exposure of plant leaves to illuminations that exceed photosynthetic capacity leads to photoinhibition of the electron transport system (see Demmig-Adams et al. 2006). Measurements were obtained initially after dark adaptation, after which overnight dark-adapted leaves were exposed to 50, 100 (low PPFDs), 300, 500, 800 (moderate PPFDs); 1,000; 1,500; and 2,000 (high PPFDs) μmol m–2 s–1 for 0–60 min of light and 30 min of the dark. Generally, the ETR values of the three species increased as PPFDs increased, except for leaves exposed to 2,000 μmol m–2 s–1. As PPFDs increased from 50 to 2,000 μmol m–2 s–1, increasing NPQ and decreasing Fv/Fm, ΦPSII, ΔF/Fm', and the degree of photoinhibition from 0–60 min were observed in all leaves, indicating that thermal energy dissipation took place in antennae as PPFDs increased. All three species, used here, had low photosynthetic rates and needed to dissipate excess energy, in high light, to protect themselves; thus, all three species exhibited high downregulation of PSII efficiency (cf. Zulfugarov et al. 2007). A similar pattern on Fv/Fm and ΦPSII of four invasive plant species has been found in north India (Pandey et al. 2020). Plants have developed numerous adaptive systems, including morphological and physiological modifications in defense against PPFD stress. When plants were exposed to high PPFD conditions, excess PSII energy increased and led to increases in NPQ values and decreases in Fv/Fm, ΦPSII, ΔF/Fm', and the degree of photoinhibition due to greater energy dissipation. Notably, the NPQ of E. dentata and S. spontaneum leaves were relatively higher than that of B. formosana leaves under 1,500 and 2,000 μmol m–2 s–1 at 60 min, indicating that E. dentata and S. spontaneum had more nonphotochemical quenching with greater damage from photooxidation compared to that in B. formosana. Therefore, E. dentata and S. spontaneum plants appear to be adapted to less than 1,500 μmol m–2 s–1, whereas B. formosana plants are adapted to less than 800 μmol m–2 s–1. Generally, the suitable temperature for photosynthesis of C3-type plants (E. dentata and B. formosana) is ~25°C, while that of C4-type (S. spontaneum) plants is ~35°C. When the plants stay in an appropriate temperature range, the efficiency of PSII rises as temperature increases (Pastenes and Horton 1996). In this study, carried out from September to October 2021, the average temperature was between 23.9–26.6°C and the high temperature was between 32.6–33.9°C, which was not high enough for the plants to reach photoinhibition. The reason for the higher PSII performance of S. spontaneum may lead to the higher adaptation temperature range of C4-type plants.
Lower ETR and NPQ but higher Fv/Fm and ΦPSII at low PPFDs were detected in all the three species, used in this study, compared to moderate and high PPFDs, suggesting that these three species adapt well favoring low PPFDs. The tested plants appeared to be sensitive to high PPFDs, which caused serious photoinhibition and photodamage. Furthermore, both the percent changes in ΔF/Fm, and Fv/Fm recovered faster in E. dentata and S. spontaneum than that in B. formosana leaves at moderate and high PPFD conditions from D2 to D20 (Fig. 2). However, under treatment of darkness until reaching D30, all the three species, used here, seemed to adapt to low PPFDs with higher percent changes in ΔF/Fm' and in Fv/Fm for photoprotection, indicating that protective mechanisms in all the three species with these higher values might prevent their leaves from suffering an excessive reduction in PSII acceptors, avoid excessive energy absorption, and respond with higher PSII photochemical efficiencies. The effect of 800 μmol m–2 s–1 in B. formosana was overcome and plants underwent adaptive changes in physiology after 30 min of the dark period, quickly making up for the damage caused by the PPFD stress. ETR is a parameter to evaluate PSII efficiency, absorbing light, and the relative rate of electron transport through PSII. Thus, the elevated percent changes in ETR, ΔF/Fm, and Fv/Fm of E. dentata and S. spontaneum leaves may help plants avoid high PPFD damage from excess energy. We suggest that the capacity and involvement of photoprotective mechanisms might vary seasonally, which may protect E. dentata and S. spontaneum from direct radiation.
During the 60-min light induction experiments, not only were all Fv/Fm and ΦPSII values of the three species lesser than 0.8 (Fig. 1), but qI values were higher than other parameters (Fig. 3), indicating that there was photoinhibition and that high PPFD conditions were not suitable for the growth of the plants in this study. High PPFD decreases the ability of photosynthetic systems to utilize incident photons, thus leading to photoinhibition and reduced quantum yields in photochemistry and ChlF (Dewir et al. 2015). Low Fv/Fm and ΦPSII can be interpreted as resulting from changes in reaction centers to quenching by excess light or depression after exposure to high PPFDs, which causes suppression of the electron transfer chain (Wong et al. 2012). These decreases in Fv/Fm and ΦPSII reflect the increased thermal dissipation of excess excitation energy before it reaches the reaction centers. Plants adapt their photosynthesis in response to prevailing light irradiances, and the sensitivity of photosynthesis to PPFDs was found to vary among the tested plants. We found relatively higher qI values in S. spontaneum plants than that in E. dentata and B. formosana plants under 1,500 and 2,000 μmol m–2 s–1 treatments (Fig. 3), indicating that S. spontaneum plants tend to drive photosynthetic ETR to quench energy, even when photoinhibition occurs there, when there is excess light. Although the photosynthetic system of the three species, examined in this paper, was dominated by qI during photoinhibition, the photosynthetic system of E. dentata and S. spontaneum plants had higher qE levels with more productive and greater photoprotective ability compared to that of B. formosana plants under moderate and high PPFD conditions. These results suggest that the photosynthetic system recovery of E. dentata and S. spontaneum is higher than that in B. formosana (Fig. 2). The observed light stress tolerance may be directly linked to the coordinated response of ETR, NPQ, qE, qI, Fv/Fm, and ΦPSII, and could help in creating better future control methods to alleviate predicted adverse effects of global warming. This ability could prove to be important in PPFD stress tolerance because the net photodissipative capacity of these plant leaves could further increase under unfavorable conditions. These indicators respond to changes in PPFDs, and the combined analysis of these indicators provides accurate estimates of changes in the photosynthetic flux of the plant canopies and is much more significant for E. dentata, B. formosana, and S. spontaneum species diversity. An optimal strategy for regulation, by PPFD, is expected to help us in designing growth chambers and greenhouse light environments for growing E. dentata plants. The knowledge of these changes would also enable the development of models for planning optimal processing times for different growth and development stages to match the specific needs of PPFDs, and for selecting the most suitable plant species composition and structure of stands to minimize the impact of stressful environments and climate change on these species. However, these data would still reflect the physiological attributes that contribute to our perception of plant ecophysiology and their subsequent growth and development in open fields.
NPQ is associated with xanthophyll cycle-dependent energy quenching (see Demmig-Adams et al. 2006) and photoinhibition, leading to S. spontaneum plants showing more tolerance than E. dentata and B. formosana. Further, B. formosana plants had a lower qE and the xanthophyll cycle also maintained the same proportion; the qE is associated with the violaxanthin to zeaxanthin transformation and thermal dissipation, and is a fast-activated and rapidly reversible component, while qI and qT are associated with conformational changes in LHC and PSII structure (see e.g., Giudici 2019). The increase in NPQ of the xanthophyll cycle is caused by the change in the structure of the PSII antenna system (i.e., in the change in the rate of dissipation of heat from excess light energy). After the completion of the reaction in the subunit PsbS, zeaxanthin is combined with the PsbS protein to dissipate H+s, and qT (phosphorylation shift-dependent quenching) shows the phosphorylation shift of LHCII between PSII and PSI (Malnoë 2018). Higher NPQ is a mechanism to protect plants from photoinhibition and photooxidation damages (Malnoë 2018). The higher the qE value, the stronger the photoprotection mechanism. The fastest and most important component of NPQ is qE, whereas the slowest reaction component of NPQ is qI, which is related to photoinhibition or slow reversible recovery of the PSII reaction center. Stress decreases the ability of photosynthetic systems to utilize incident photons, thus leading to photoinhibition and reduced quantum yields in photochemistry and ChlF. Conversely, under low PPFDs that limit photosynthesis, zeaxanthin is converted to violaxanthin, and the reverse reaction occurs at the high PPFDs that exceed the level of light that can be consumed by photochemistry (Demmig-Adams et al. 2020). More work needs to be done to explore the photosynthetic mechanisms of NPQ, its exact location in the peripheral antenna of PSII, and its regulation and synergy with other quenching components (Malnoë 2018).
The susceptibility of photosynthesis to photoinhibition strongly depends on PPFDs. The larger the qE is, the lower the % of photoinhibition is, but at a higher qI (Fig. 4). Higher % of photoinhibition was detected under high PPFD conditions with higher qE and qI values compared to moderate and low PPFD conditions. The slope of qE, related to % of photoinhibition, shows the photoprotection effect of qE in all the tested plants. Significant and negative relationships were detected between qE and % of photoinhibition in E. dentata and S. spontaneum plants under specific PPFD conditions, but significant and positive relationships were detected between qI and % of photoinhibition in all three species under all PPFD conditions, indicating the differences in adjusting the path of energy flow between qE and qI. The path of energy flow to qI was used mainly for photoinhibition at this stage, but our data show that all our samples may remain photochemically active and able to maintain lower qI under low illumination. Further, E. dentata and S. spontaneum plants may be adjusting the path of energy flow absorption using qE due to a photoprotective mechanism. In this study, plants were grown in the field and had high photoprotection at high PPFD conditions, with 60–80% PPFD in the habitat area, and these plants may adjust their path of energy flow using nonphotochemical quenching. PSII was destabilized in the field-collected samples of all the plants used in this study experiencing high PPFDs, which may have resulted in a physical separation between LHCII and PSII reaction centers leading to an increase in Fv/Fm, ΦPSII, and NPQ at different rates (cf. Makarenko et al. 2016). The susceptibility of photosynthesis to photoinhibition strongly depends on the PPFDs to which the plant is exposed during growth (Rosa-Manzano et al. 2015). During photosynthesis, changes in photoinhibition were mainly affected by qI and qE, followed by photoprotection. As % of photoinhibition and qI levels increased, plants must have increased their qE to cope with the negative effects of high light. Simple evaluations of photosynthesis can be made and relationships between the quenching of Chl a excited state (by heat) and photosynthetic efficiency can be estimated with these photosynthesis parameters, as they are highly sensitive indicators and provide quick means for identifying the physiological condition of plants (cf. Wang et al. 2022). Therefore, qE and qI can be used as an indicator of photoprotection and photoinhibition, respectively.
Our results are useful in efforts to predict the photosynthetic responses to light in E. dentata, B. formosana, and S. spontaneum, and are expected to provide a theoretical basis for afforestation in E. dentata plantations using native species. The habitats of E. dentata in the subtropics undergo greater PPFD changes and plants are less likely to experience high PPFDs. In field cultivation, choosing a suitable region or using artificial shading must be considered to avoid photoinhibition resulting from exposure to high PPFD. All of the above contribute to the moderation of the distribution of absorbed excitation energy in PSII and the better maintenance of the normal operations of the photosystem. These ChlF parameters could be used for the rapid monitoring and early detection of high or low PPFDs suitable for future regeneration. For instance, the impacts of changing qE or qI in E. dentata over time are affected by PPFD applications, and the balance between high or low PPFDs in the tested plants is crucial for determining the steady-state level of qE or qI. This balance can ameliorate high or low PPFDs and can be used as a substitute technology for the regeneration of E. dentata. Since qE or qI can be easily measured in the field, these values provide a useful ecological index for E. dentata restoration, habitat creation, and construction monitoring.
Conclusions
PAM chlorophyll fluorometry was used to monitor PSII efficiency in plant leaves under various PPFD for the abundance of E. dentata, B. formosana, and S. spontaneum plants growing in a subtropical region. Plants at a given PPFD showed variable ChlF values after 0–60 min of light, followed by 30 min of darkness. These plants displayed different capacities for protective mechanisms for avoiding damage to their photosynthetic apparatus when acclimating to various PPFD conditions. ETR values in E. dentata plants were elevated under 800; 1,000; and 1,500 μmol m–2 s–1. Higher NPQ levels in E. dentata and S. spontaneum plants were detected under 2,000 μmol m–2 s–1 compared to those in B. formosana plants that had higher Fv/Fm and ΦPSII levels than the E. dentata and S. spontaneum plants under 50 and 100 μmol m–2 s–1. These results suggest that E. dentata and S. spontaneum plants adapt their photosynthesis to high and moderate PPFDs, whereas B. formosana species acclimates to moderate to low PPFDs. Moreover, qI increased and qE decreased as % of photoinhibition increased, and higher % of photoinhibition was observed in B. formosana and S. spontaneum plants compared to E. dentata plants under high PPFDs. Our results indicate that plants acclimate to dynamic changes in light conditions, and thus can be used to study photosynthetic productivity and provide for ecophysiological research in E. dentata. Both qI and qE are useful for predicting changes in the performance and distribution of E. dentata plants and reflect the physiological attributes that contribute to our perception of E. dentata growth in the field.
Acknowledgements
This work was partially supported by the Taiwan Government Department for Endemic Species Research Institute.
Abbreviations
- ChlF
chlorophyll fluorescence
- ETR
electron transport rate
- F0
minimal ChlF
- Fm
maximal ChlF
- Fv/Fm
maximal quantum efficiency of PSII for dark-adapted states
- NPQ
nonphotochemical quenching
- qE
energy-dependent quenching
- qI
photoinhibition quenching
- qT
state transition quenching
- qZ
zeaxanthin-dependent quenching
- ΔF/Fm'
actual PSII efficiency
- ΦPSII
effective quantum yield of PSII for light-adapted states
Supplementary Materials
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adams W.W. III, Zarter C.R., Ebbert V., Demmig-Adams V.: Photoprotective strategies of overwintering evergreens. – BioScience 54: 41-49, 2004. 10.1641/0006-3568(2004)054[0041:PSOOE]2.0.CO;2 [DOI] [Google Scholar]
- Björkman O., Demmig-Adams B.: Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. – In: Schulze E.D., Caldwell M.M. (ed.): Ecophysiology of Photosynthesis. Pp. 17-47. Springer, Berlin-Heidelberg: 1995. 10.1007/978-3-642-79354-7_2 [DOI] [Google Scholar]
- Bussotti F., Gerosa G., Digrado A., Pollastrini M.: Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. – Ecol. Indic. 108: 105686, 2020. 10.1016/j.ecolind.2019.105686 [DOI] [Google Scholar]
- Chang L.H., Liu C.H., Li. C.Y.: [Asymbiotic germination and plant regeneration of Eulophia dentate Ames, a critically endangered plant in Taiwan.] – Taiwan J. Biodivers. 16: 241-251, 2014. [Google Scholar]
- Colom M.R., Pini Prato E., Giannini R.: Chlorophyll fluorescence and photosynthetic response to light in 1-year-old needles during spring and early summer in Pinus leucodermis. – Trees-Struct. Funct. 17: 207-210, 2003. https://www.airitilibrary.com/Publication/alDetailedMesh?docid=20766971-201407-201408290002-201408290002-241-251 [Google Scholar]
- Demmig-Adams B., Adams W.W. III, Barker D.H. et al. : Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. – Physiol. Plantarum 98: 253-264, 1996. 10.1034/j.1399-3054.1996.980206.x [DOI] [Google Scholar]
- Demmig-Adams B., Adams W.W. III, Mattoo A.K.: Photoprotection, Photoinhibition, Gene Regulation, and Environment. Pp. 382. Springer, Dordrech: 2006. 10.1007/1-4020-3579-9 [DOI] [Google Scholar]
- Demmig-Adams B., Stewart J.J., López-Pozo M. et al. : Zeaxanthin, a molecule for photoprotection in many different environments. – Molecules 25: 5825, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewir Y.H., El-Mahrouk M.E.S., Al-Shmgani H.S. et al. : Photosynthetic and biochemical characterization of in vitro-derived African violet (Saintpaulia ionantha H. Wendl) plants to ex vitro conditions. – J. Plant Interact. 10: 101-108, 2015. 10.1080/17429145.2015.1018967 [DOI] [Google Scholar]
- Editorial Committee of the Red List of Taiwan Plants: The Red List of Vascular Plants of Taiwan, 2017. Pp. 198. Endemic Species Research Institute, Forestry Bureau, Council of Agriculture, Executive Yuan and Taiwan Society of Plant Systematics, 2017. [Google Scholar]
- Giudici G.N.M.: Photoinhibition of primary photosynthetic processes in hydrated Polytrichum commune: analysis of non-photochemical quenching affecting species resistance. – Czech Polar Rep. 9: 160-169, 2019. 10.5817/CPR2019-2-14 [DOI] [Google Scholar]
- Guidi L., Lo Piccolo E., Landi M.: Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species? – Front. Plant Sci. 10: 174, 2019. 10.3389/fpls.2019.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.P., Ruban A.V.: Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH. – J. Biol. Chem. 286: 19973-19981, 2011. 10.1074/jbc.M111.237255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapchieva S., Topalova E., Petkova V.: Morphological, physiological and productivity response in garden pea genotypes during high temperature stress. – Genetika 51: 417-428, 2019. 10.2298/GENSR1902417K [DOI] [Google Scholar]
- Kałużewicz A., Bączek-Kwinta R., Krzesiński W. et al. : Effect of biostimulants on chlorophyll fluorescence parameters of broccoli (Brassica oleracea var. italica) under drought stress and rewatering. – Acta Sci. Pol.-Hortoru. 17: 97-106, 2018. 10.24326/asphc.2018.1.9 [DOI] [Google Scholar]
- Li Z., Wang G., Liu X. et al. : Genome-wide identification and expression profiling of DREB genes in Saccharum spontaneum. – BMC Genomics 22: 456, 2021. 10.1186/s12864-021-07799-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.W., Hwang T.L., Chen F.A. et al. : Chemical constituents of the rhizomes of Bletilla formosana and their potential anti-inflammatory activity. – J. Nat. Prod. 79: 1911-1921, 2016. 10.1021/acs.jnatprod.6b00118 [DOI] [PubMed] [Google Scholar]
- Makarenko M.S., Kozel N.V., Usatov A.V. et al. : A state of PSI and PSII photochemistry of sunflower yellow-green plastome mutant. – J. Biol. Sci. 16: 193-198, 2016. 10.3844/ojbsci.2016.193.198 [DOI] [Google Scholar]
- Malnoë A.: Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. – Environ. Exp. Bot. 154: 123-133, 2018. [Google Scholar]
- Maxwell K., Johnson G.N.: Chlorophyll fluorescence – a practical guide. – J. Exp. Bot. 51: 659-668, 2000. 10.1093/jexbot/51.345.659 [DOI] [PubMed] [Google Scholar]
- Moya I., Loayza H., López M.L. et al. : Canopy chlorophyll fluorescence applied to stress detection using an easy to build micro-lidar. – Photosynth. Res. 142: 1-15, 2019. 10.1007/s11120-019-00642-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie E.H., Niyogi K.K.: Manipulation of photoprotection to improve plant photosynthesis. – Plant Physiol. 155: 86-92, 2011. 10.1104/pp.110.168831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Li X.P., Niyogi K.K.: Non-photochemical quenching. A response to excess light energy. – Plant. Physiol. 125: 1558-1566, 2001. 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M., Kress E., Lambrev P. et al. : Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. – BBA-Biomembranes 1797: 466-475, 2010. 10.1016/j.bbabio.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Orekhova A., Barták M., Casanova-Katny A., Hájek J.: Resistance of Antarctic moss Sanionia uncinata to photoinhibition: chlorophyll fluorescence analysis of samples from the western and eastern coasts of the Antarctic Peninsula. – Plant Biol. 23: 653-663, 2021. 10.1111/plb.13270 [DOI] [PubMed] [Google Scholar]
- Pandey V.C., Bajpai O., Pandey D.N., Singh N.: Saccharum spontaneum: an underutilized tall grass for revegetation and restoration programs. – Genet. Resour. Crop Evol. 62: 443-450, 2015. 10.1007/s10722-014-0208-0 [DOI] [Google Scholar]
- Pandey V.C., Sahu N., Singh D.P.: Physiological profiling of invasive plant species for ecological restoration of fly ash deposits. – Urban For. Urban Gree. 54: 126773, 2020. 10.1016/j.ufug.2020.126773 [DOI] [Google Scholar]
- Papageorgiou G.C., Govindjee: The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: definitions, timelines, viewpoints, open questions. – In: Demmig-Adams B., Garab G., Adams W.W. III, Govindjee (ed.): Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Pp. 1-44. Springer, Dordrecht: 2014. 10.1007/978-94-017-9032-1_1 [DOI] [Google Scholar]
- Pastenes C., Horton P.: Effect of high temperature on photosynthesis in beans. (I. Oxygen evolution and chlorophyll fluorescence) – Plant Physiol. 112: 1245-1251, 1996. 10.1104/pp.112.3.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela F.C.S., Macieira B.P.B., Zanetti L.V. et al. : How does Cariniana estrellensis respond to different irradiance levels? – J. Forestry Res. 30: 31-44, 2019. 10.1007/s11676-017-0578-1 [DOI] [Google Scholar]
- Rosa-Manzano E., Andrade J.L., García-Mendoza E. et al. : Photoprotection related to xanthophyll cycle pigments in epiphytic orchids acclimated at different light microenvironments in two tropical dry forests of the Yucatan Peninsula, Mexico. – Planta 242: 1425-1438, 2015. 10.1007/s00425-015-2383-4 [DOI] [PubMed] [Google Scholar]
- Souza C.S.C.R, Santos V.A.H.F., Ferreira M.J., Gonçalves J.F.C.: [Biomass, growth and ecophysiological responses of young plants of Bertholletia excelsa Bonpl. subjected to different levels of irradiance.] – Cienc. Florest. 27: 557-569, 2017. [In Portuguese] 10.5902/1980509827736 [DOI] [Google Scholar]
- Tüffers A.V., Naid G., von Willert D.J.: The contribution of leaf angle to photoprotection in the mangroves Avicennia marina (FORSSK.) VIERH. and Bruguiera gymnorrhiza (L.) LAM. under field conditions in South Africa. – Flora 194: 267-275, 1999. 10.1016/S0367-2530(17)30912-X [DOI] [Google Scholar]
- Vaid S., Sharma S., Bajaj B.K.: Chemo-enzymatic approaches for consolidated bioconversion of Saccharum spontaneum biomass to ethanol-biofuel. – Bioresource Technol. 329: 124898, 2021. 10.1016/j.biortech.2021.124898 [DOI] [PubMed] [Google Scholar]
- Wang C.W., Wong S.L., Liao T.S. et al. : Photosynthesis in response to salinity and submergence in two Rhizophoraceae mangroves adapted to different tidal elevations. – Tree Physiol. 42: 1016-1028, 2022. 10.1093/treephys/tpab167 [DOI] [PubMed] [Google Scholar]
- Weng J.H., Chen L.F., Jiang C.Y. et al. : A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation, and photoinhibition. – Photosynthetica 49: 361-370, 2011. 10.1007/s11099-011-0046-7 [DOI] [Google Scholar]
- Wong S.L., Chen C.W., Huang H.W., Weng J.H.: Using combined measurements of gas exchange and chlorophyll fluorescence to investigate the photosynthetic light responses of plant species adapted to different light regimes. – Photosynthetica 50: 206-214, 2012. 10.1007/s11099-012-0027-5 [DOI] [Google Scholar]
- Wu T.Y., Chen C.C., Lay H.L.: Study on the components and antioxidant activity of the Bletilla plant in Taiwan. – J. Food Drug Anal. 18: 279-289, 2010. 10.38212/2224-6614.2229 [DOI] [Google Scholar]
- Zulfugarov I.S., Ham O.K., Mishra S.R. et al. : Dependence of reaction center-type energy-dependent quenching on photosystem II antenna size. – BBA-Bioenergetics 1767: 773-780, 2007. 10.1016/j.bbabio.2007.02.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.