Abstract
In stressful environments, invasive plants acclimate more efficiently than native plants and hybridization mainly contributes to this process. We examined changes in the morphological characteristics, photosynthetic characteristics, and antioxidant capacity of Sphagneticola trilobata and its hybrids in a low-light environment to explore their invasiveness, with Sphagneticola calendulacea serving as the control. The morphological plasticity of S. trilobata was not dominant, the maximal photochemical efficiency of PSII, actual quantum yield of PSII, and electron transport rate of PSII increased and nonphotochemical quenching decreased, while S. calendulacea and the hybrid produced opposite results. S. trilobata showed fewer spots stained for reactive oxygen species in tissues, with an increase in superoxide dismutase activity. Although S. trilobata is a heliophilous plant, we found that the shade tolerance of S. trilobata and the hybrid were stronger than that of S. calendulacea, which may be one important mechanism of invasion.
Keywords: growth strategy, hybridization, low light, photosynthesis, plant invasion
Highlights
Shading promoted the plant height but decreased the stem length of Sphagneticola trilobata
S. calendulacea has the strong photosynthetic capacity and antioxidant activity in the shade
Shade tolerance of the hybrid is more efficient than that of S. calendulacea
Introduction
The rate of biological invasion has increased exponentially over time and is expected to continue to increase in the coming decades due to rapid globalization (Seebens et al. 2017, 2018, 2021). Invasive alien plants invade new areas through natural or artificial processes from their original distribution areas. These invasive species not only reduce the local biodiversity and destroy the ecological community structure (Walker and Steffen 1997, Ju et al. 2016, Dong et al. 2017) but also cause huge economic losses to the invaded areas (Mack et al. 2010, Branco et al. 2015, Funk et al. 2016). Light intensity regulates plant morphogenesis, substance metabolism, and gene expression, which may affect the process of plant growth (Franklin and Whitelam 2005, Wada et al. 2010, Kim et al. 2011). In shaded urban green belts and under forests where little sunlight penetrates, light is one of the main limiting factors for shade-grown plants. In a low-light environment, plant photosynthetic performance may be reduced, thus affecting their growth and performance (Dong et al. 2019, Yang et al. 2020). Studies have shown that alien invasive plants better adapt to stressful environments (Siemann and Rogers 2001, Lee 2002). Lantana camara, which is native to tropical America, successfully invades by increasing the leaf area, leaf biomass, and leaf area index to adapt to weak light (Carrión-Tacuri et al. 2011). Mikania micrantha maintains high photosynthetic efficiency and synthesizes more growth regulators to promote the elongation of the main stem (Liang et al. 2022). Under low light, Eupatorium adenophorum Spreng. reduces the root biomass to supplement the supporting structure and reduces the leaf area index to weaken self-shading (Wang and Feng 2004). Robinia pseudoacacia, one of the most serious alien invasive plants in Italy, captures light as much as possible by reducing leaf thickness and increasing the specific leaf area (SLA), which is beneficial for adapting to low light in the understory (Granata et al. 2020). Therefore, invasive plants adapt to low light by adjusting their morphological indices and biomass allocation patterns to maximize light acquisition, improve photosynthetic efficiency, and promote their growth.
Sphagneticola trilobata is a perennial herb of the Asteraceae family that is native to South and Central America. It was introduced into China in the 1970s and escaped into the wild. It spreads and reproduces through stolons to form a patchy monodominant community, that seriously threatens the local ecosystem and species diversity (Wu et al. 2005). Therefore, it is listed as one of the ‘100 most harmful invasive alien species in the world’ (Lowe et al. 2000). The main reasons for the successful invasion of S. trilobata are as follows: (1) it has a strong vegetative reproductive capacity (clonal growth) (Wu et al. 2005), (2) it secretes allelochemicals that inhibit the growth of surrounding plants and affect the structure of the soil microbial community and soil texture (Zhang et al. 2013, Sun et al. 2020a), and (3) it has a stronger CO2-fixation capacity, wider effective photosynthetic radiation range, and higher light quantum-utilization efficiency than that of S. calendulacea (Song et al. 2010, Li et al. 2016). Studies have shown that S. trilobata has a higher photosynthetic nitrogen-utilization efficiency, stronger competitiveness, and higher plasticity at highly fluctuating water levels of 15 cm than S. calendulacea, and is more likely to invade wetlands (Javed et al. 2020). Compared to the native congener S. calendulacea, the stem of S. trilobata exhibits stronger tolerance and compensation at low temperatures, which are conducive to normal growth and asexual reproduction throughout the year (Cai et al. 2021). Under simulated extreme high-temperature conditions, the net photosynthetic rate of S. trilobata was significantly higher than that of S. calendulacea, indicating strong tolerance (Song et al. 2017). However, in a water-deficient and nitrogen-enriched environment, S. trilobata was less competitive than S. calendulacea (Azeem et al. 2022).
In 2013, a study identified a natural hybrid between S. trilobata and a native species of the same genus, S. calendulacea. The net photosynthetic rate, stomatal conductance, and transpiration rate of the hybrid were significantly higher than those of S. calendulacea, and its growth potential was similar to that of S. trilobata (Wu et al. 2013). Previous studies have shown that the competitive ability of the hybrid was comparable to that of S. trilobata, which was more sensitive to nitrogen deposition (Ni et al. 2014), and its high-temperature tolerance and drought resistance were between the two parents, which were stronger than those of the native species (Song et al. 2017, Zhang et al. 2020a). Hybridization promotes adaptive evolution mainly through evolutionary novelty, fixed heterosis, genetic variation, and dumping genetic load (Ellstrand and Schierenbeck 2000). Natural hybridization plays an important role in the process of biological invasion (Ellstrand and Schierenbeck 2000, Schierenbeck and Ellstrand 2009, Wu et al. 2013). For example, the hybrid of Typha latifolia and Typha angustifolia L. is more suitable to grow in environments with frequent disturbance and wetland ecosystems than their parents, and its litter plays a more important role in the invasion process than the hybrid (Farrer and Goldberg 2009, Olson et al. 2009, Larkin et al. 2012). The hybrid of the invasive plant Xanthium strumarium and the native plant Xanthium sibiricum as well as the hybrid of the invasive plant Corymbia torelliana have strong growth and reproductive abilities, which further strengthen their invasion (Dickinson et al. 2012, Xun et al. 2017). Therefore, monitoring and evaluating the invasiveness and adaptability of the hybrid of S. calendulacea and S. trilobata under adverse conditions is particularly important for the study of invasion ecology.
S. trilobata is a heliophile (Wu and Hu 2004), but many studies have shown that it exhibits a certain shade tolerance and the risk of spreading to shaded areas (Yi et al. 2014, Li et al. 2016, Zhang et al. 2022a). It has also been reported that hybridization is invasive through gene introgression (Ellstrand and Schierenbeck 2000, Xun et al. 2017). However, the performance ability of the hybrid in a low-light environment is not clear. Based on current relevant studies, we explored the shade tolerance mechanism of S. trilobata and whether the hybrid threatens the survival of S. calendulacea under low-light conditions to further promote the invasion of S. trilobata. We used S. calendulacea as the control plant, selected S. trilobata, and the hybrid as the research objects, and analyzed the morphological changes and physiological differences in the three Sphagneticola species through artificial shading treatment. We discussed the response strategy of S. trilobata and the hybrid to a low-light environment to further provide a theoretical basis for the prediction and control of the suitable habitat of S. trilobata and the hybrid.
Materials and methods
Materials and experimental design
Whole plants of S. trilobata, S. calendulacea, and their hybrid were collected from South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China (23°10'N, 113°21'E). Plant materials were propagated through asexual propagation. After defoliation, the stem segments of two stem nodes were cut from the middle stem and then propagated into the water in an incubator [irradiance of 100–120 μmol(photon) m–2 s–1, culture temperature of 28/25°C day/night]. When rooting and growing 3–4 pairs of leaves (approximately 3 weeks), the healthy seedlings were transplanted to the flowerpot (12.2 × 11.2 × 9.5 cm), with one plant in each pot. The cultivation substrate was a mixture of local soil and potting soil (Wing Fung Horticulture, Guangzhou, China) with a volume of 1:1. When the length of the stem reached 20–30 cm, the plants were used for the experiments.
The experiment was conducted at the test site of the College of Life Sciences of South China Normal University from September to November 2021 (the average temperature in September and October in Guangzhou was 32 and 23°C, respectively, and the average relative humidity was 77%, and the average day length was 12 h). Two light environments were established: natural full light and low light (covered with a layer of black nylon net; the nylon net is used for 6-pin encryption). A photosynthesis light quantum meter (TES-1339P, Taiwan) was used to measure the number of photons received at a fixed horizontal position above the plant. The photosynthetic photon flux density (PPFD) of full light was 880 μmol m–2 s–1 and that of low light was 180 μmol m–2 s–1. In addition, the photosynthesis light quantum meter was used to measure the number of photons received at the position of the leaf in three Sphagneticola species. The ratio of the value and PPFD in the two light environments was reported as the relative irradiance above the leaf.
The three Sphagneticola species were exposed to the two light environments for 60 d, with 30 pots for each condition. Sufficient water was added daily and 10 g L–1 fertilizer (250 ml) was applied twice weekly. The compound fertilizer (Jiangsu Huachang Chemical Co. Ltd., China) contained 15% N, 15% P2O5, and 15% K2O.
Determination of morphological and growth indices
On the first day of treatment, the main stem length, stem diameter, leaf length, and leaf width of the plants were measured, and the number of branches and leaf pairs was calculated. Then, the aforementioned indicators were measured every 15 d.
After 60 d of low-light treatment, the whole plant was harvested and washed with tap water. Afterward, the roots, stems, and leaves of each plant were separated, placed in envelopes, and dried in an oven at 75°C for 72 h. The dry masses of roots, stems, and leaves were weighed and recorded.
ImageJ software (ImageJ, National Institutes of Health, USA) was used to calculate the leaf area of fresh mature leaves by setting the unit length. SLA was measured by calculating the ratio of the leaf area to the dry mass of leaves. The measurement was replicated five times.
Chlorophyll (Chl) fluorescence parameters
of plants were measured using a chlorophyll fluorescence imaging system (CF imager, Technical Ltd., Colchester, UK). After 60 d of different light treatments, the third pair of leaves was cut off, and the fresh round leaves were fully dark-adapted for 30 min and then placed in the support IMAG-MAX/L. Five circular test target areas (AOIs, areas of interest) were selected for each plant, and the data listed below were measured. The relevant Chl fluorescence parameters include Fv/Fm, the maximal photochemical efficiency of PSII; ΦPSII, the actual quantum yield of PSII; NPQ, nonphotochemical quenching; and ETR, the electron transport rate of PSII. These parameters were calculated using the methods reported by Gray et al. (1997) and Schreiber et al. (1995), and Genty et al. (1989).
Chemical localization and detection of H2O2 and superoxide anions (O2·–) in tissue
Reactive oxygen species (H2O2 and O2·–) were detected with in situ histochemical staining procedures using 3,3-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT). After light treatment for 60 d, the third pair of leaves was quickly immersed in phosphate acid buffer solution (pH 7.0) containing 0.5 mg ml–1 DAB or phosphate buffer (50 mM, pH 6.4) supplemented with 10 mM sodium azide and 0.1% NBT, vacuum pumped for 20 min until the leaves sank to the bottom without bubbles, and then placed in the dark at room temperature and incubated for 8–12 h. When brown spots appeared on the leaves, they were bleached with boiling 80% ethanol to remove Chl. Finally, the dyeing of leaves was observed and recorded with an iPhone XR camera (Apple, California, USA) (Zhang et al. 2011).
Determination of antioxidant enzyme activity
Leaves (0.1 g of the third or fourth pair of leaves) were homo-genized on ice with a mortar and pestle in 50 mM phosphate buffer (1.5 ml) containing 0.1 mm EDTA, 0.1% Triton X-100, and 2% PVP. The grinding fluid was centrifuged at 12,000 × g for 10 min at 4°C. The supernatant was collected as the enzyme extract for the determination of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), and peroxidase (POD, EC 1.11.1.7) activities. Five replicates of each measurement were performed.
SOD activity was determined using the NBT-illumination method (Giannopolitis and Ries 1977). One unit of SOD activity was defined as the amount of enzyme that resulted in 50% suppression of the photochemical reduction of NBT. In this experiment, the reaction mixture contained 0.1 mL of the enzyme extract, 50 mM phosphate buffer (1.7 mL, pH 7.8), 130 mM methionine (0.3 mL), 0.75 mM NBT (0.3 mL), 0.1 mM EDTA-Na2 (0.3 mL), and 0.02 mM riboflavin (0.3 mL) in a 3 mL volume. The enzyme solution in the positive and negative controls was replaced with phosphate buffer. The reaction was conducted under light (PPFD = 4,500 μmol m–2 s–1) for 15 min. However, the negative control was placed in the dark. Subsequently, the absorbance was determined immediately at 560 nm using a UV-2450 spectrophotometer (Shimadzu, Tokyo, Japan).
POD activity was evaluated using the guaiacol method described by Chance and Maehly (1955). The change in optical density at 470 nm per min was 0.01 as an enzyme activity unit (U). The reaction mixture contained 0.1 mL of the enzyme extract, 50 mM phosphate buffer (1.875 mL, pH 7.0), 30 mM H2O2 (1 mL), and 2-methoxyphenol (0.025 mL) in a quartz cuvette. The absorbance was immediately measured nine times (20 s each time) at 470 nm using a UV-2450 spectrophotometer (Shimadzu, Tokyo, Japan). The activity of POD was calculated per fresh mass.
CAT activity was determined using UV spectrophotometry as described by Chance and Maehly (1955) by measuring the decrease in absorbance at 240 nm for 1 min due to H2O2 consumption, and a reduction of A240 by 0.01 within 1 min was regarded as an enzyme activity unit (U). The reaction mixture contained 0.1 mL of the enzyme extract and 30 mM H2O2 (2.9 mL). After 13 s of incubation, the absorbance was recorded nine times (20 s each time) at 240 nm using a UV-2450 spectrophotometer (Shimadzu, Tokyo, Japan). The activity of CAT was calculated per fresh mass.
Statistical analysis
All results are presented as the means ± standard errors (SE). Significant differences were analyzed using IBM SPSS Statistics 19.0 software (IBM, NY, USA). Two-way analysis of variance (ANOVA) with plant species and light intensity as the grouping factors was used to compare all experimental parameters with Duncan's multiple comparison test. One-way ANOVA was performed to examine differences in traits among the three Sphagneticola species under full and low light. A standardized major axis (SMA) regression analysis was conducted to describe the relationship between each possible pairwise combination of traits. All variables were deemed normed following log transformation. On log-log axes, the SMA regression describes the best-fit scaling relationships between pairs of traits (Warton et al. 2006). SMA was performed using SMATR software (Falster et al. 2006). The figures were constructed using Origin 2018 (OriginLab, Northampton, MA, USA) and Adobe Photoshop CC 2014 (Adobe Systems Inc., USA). Significance levels for all statistical models were set to P<0.05.
Results
Phenotypic characteristics
Under low light, the main stem length, leaf number, and branch number of the three Sphagneticola species increased with the growth process, but the increases were different. With the extension of treatment time, the main stem length of S. calendulacea and the hybrid treated with low light increased rapidly, which was significantly different from the full-light control group, while the growth of S. trilobata was slower (Fig. 1S, supplement).
On the 60th day, the main stem lengths of S. calendulacea and the hybrid were longer under low light than those under full light, increasing by 56 and 50%, respectively, while that of S. trilobata was shorter than that under full light, decreasing by 34% (Figs. 1A; 2S, supplement). A significant difference in the stem diameter of S. trilobata was not observed, but the stem diameters of both S. calendulacea and the hybrid increased significantly under low light (Fig. 1B). The effect of the species on the stem diameter was significant (Table 1).
Fig. 1. Phenotypic characteristics of the three Sphagneticola species at 60 d of cultivation under low light. The main stem length (A), main stem diameter (B), leaf pairs (C), branch number (D), leaf length (E), and leaf width (F) of the three Sphagneticola species under full and low light. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05, **P<0.01, and ***P<0.001) according to one-way analysis of variance (ANOVA).
Table 1. Results from the two-way ANOVA of the effects of interactions between light intensity and three Sphagneticola species on all functional traits. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05, **P<0.01, and ***P<0.001) according to two-way analysis of variance (ANOVA). η2 quantifies the effects of different factors.
| Index/sources | Light intensity (L) | Species (S) | L × S | ||||||
| F | P | η 2 | F | P | η 2 | F | P | η 2 | |
| Stem length | 12.71 | ** | 0.51 | 23.19 | *** | 0.80 | 19.65 | *** | 0.77 |
| Stem diameter | 0.38 | ns | 0.03 | 8.67 | ** | 0.59 | 3.75 | ns | 0.39 |
| Number of leaves | 1.00 | ns | 0.08 | 0.75 | ns | 0.11 | 6.25 | * | 0.51 |
| Number of branches | 16.66 | *** | 0.56 | 33.59 | *** | 0.84 | 1.25 | ns | 0.16 |
| Length of leaves | 106.13 | *** | 0.90 | 37.24 | *** | 0.86 | 8.00 | ** | 0.57 |
| Width of leaves | 9.19 | ** | 0.43 | 2.91 | ns | 0.33 | 22.23 | *** | 0.79 |
| Plant height | 44.96 | *** | 0.79 | 59.32 | *** | 0.91 | 6.58 | * | 0.52 |
| Relative irradiance above leaf | 22.22 | *** | 0.65 | 17.98 | *** | 0.75 | 1.60 | ns | 0.21 |
| SLA | 105.59 | *** | 0.90 | 10.16 | ** | 0.63 | 14.03 | *** | 0.70 |
| Root biomass | 45.64 | *** | 0.79 | 10.27 | ** | 0.63 | 4.86 | * | 0.45 |
| Stem biomass | 6.05 | * | 0.34 | 0.79 | ns | 0.12 | 0.43 | ns | 0.07 |
| Leaf biomass | 10.88 | ** | 0.48 | 71.66 | *** | 0.92 | 7.56 | ** | 0.56 |
| Total biomass | 40.27 | *** | 0.77 | 21.67 | *** | 0.78 | 0.89 | ns | 0.13 |
| Root mass ratio | 103.32 | *** | 0.90 | 27.50 | *** | 0.82 | 10.93 | ** | 0.65 |
| Stem mass ratio | 3.60 | ns | 0.23 | 83.34 | *** | 0.93 | 0.53 | ns | 0.08 |
| Leaf mass ratio | 39.68 | *** | 0.77 | 162.96 | *** | 0.96 | 0.56 | ns | 0.09 |
| Root/shoot | 105.34 | *** | 0.90 | 28.53 | *** | 0.83 | 11.85 | *** | 0.66 |
| Fv/Fm | 46.25 | *** | 0.79 | 22.35 | *** | 0.79 | 80.71 | *** | 0.93 |
| NPQ | 0.03 | ns | 0.00 | 59.44 | *** | 0.91 | 25.95 | *** | 0.81 |
| ΦPSII | 53.34 | *** | 0.82 | 158.52 | *** | 0.96 | 101.75 | *** | 0.94 |
| ETR | 53.34 | *** | 0.82 | 158.52 | *** | 0.96 | 101.75 | *** | 0.94 |
| SOD | 3.43 | ns | 0.22 | 92.74 | *** | 0.94 | 13.89 | *** | 0.70 |
| POD | 1.60 | ns | 0.12 | 18.48 | *** | 0.76 | 8.41 | ** | 0.58 |
| CAT | 0.32 | ns | 0.03 | 32.59 | *** | 0.85 | 12.51 | *** | 0.68 |
In the two light environments, the increasing rates of leaf pairs of the three Sphagneticola species were similar, and the difference in branch number increased over time. An obvious tendency for the increase in the branch number of the three Sphagneticola species was not detected in the first 15 d. Compared to the low-light treatment, the three Sphagneticola species grew more branches under full light after 15 d, while the branch number of the hybrid under low light increased rapidly after 45 d (Fig. 1S).
On the 60th day of low-light treatment, the branch number and leaf pairs of S. trilobata decreased significantly by 25 and 14%, respectively, compared to the full-light group (Fig. 1C,D). The ranges of variations in leaf length and leaf width of the hybrid were between those of the parents under low light. However, under low light, the range of increases in the leaf length of S. trilobata was smaller, at 20%, and the leaf width decreased significantly (Fig. 1E,F).
Plant height and specific leaf area (SLA)
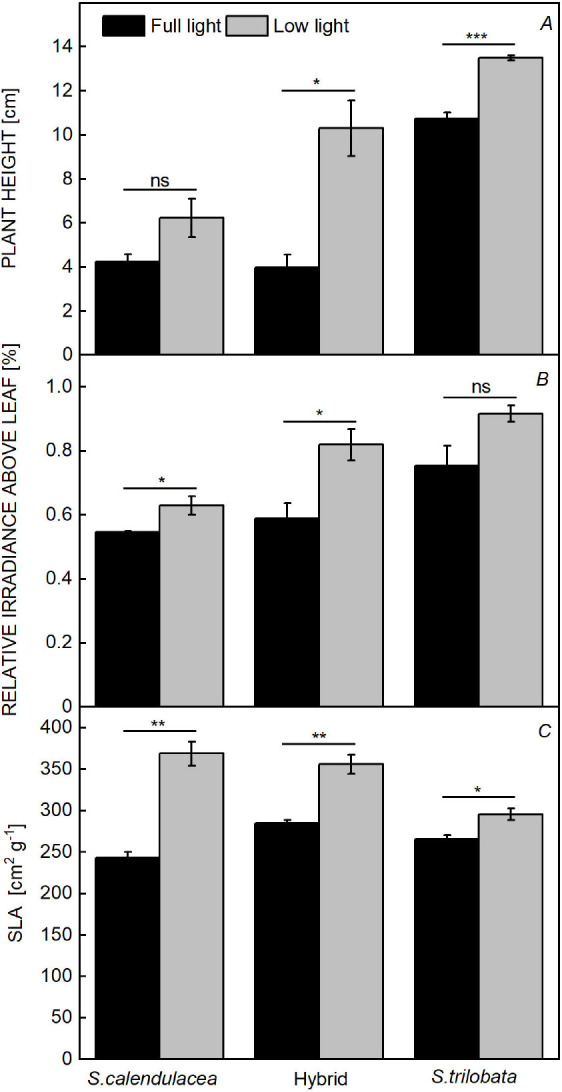
Both species and light intensity significantly affected plant height, the relative irradiance above leaf, and SLA (Table 1). In both light environments, the plant height of S. trilobata and the hybrid were significantly and positively correlated with the relative irradiance (Table 1S, supplement). Compared to full light, the vertical plant height of S. trilobata and the hybrid increased markedly under low light (Figs. 2A; 3S, supplement). Under low light, the relative irradiance of S. trilobata remained high, and did not change significantly, but the values of S. calendulacea and the hybrid remarkably improved (Fig. 2B). When the relative irradiance was the same, the plants of S. trilobata and the hybrid grown under low light were taller than that under full light (Fig. 4S, supplement). As a leaf shading index, SLA is generally greater when the light is weaker. Correspondingly, the SLA of the three Sphagneticola species increased significantly after low-light treatment, with the largest increase of 52% observed for S. calendulacea and the smallest increase of 12% observed for S. trilobata (Fig. 2C).
Fig. 2. Phenotype and relative irradiance of the three Sphagneticola species at 60 d of cultivation under low light. The plant height (A), relative irradiance above leaf (B), and specific leaf area (SLA) (C) of the three Sphagneticola species under full and low light. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05, **P<0.01, and ***P<0.001) according to one-way analysis of variance (ANOVA).
Biomass allocation
In different light environments, the distribution pattern of the biomass of each organ of S. trilobata was leaf > stem > root, while that of S. calendulacea and the hybrid was stem > leaf > root. The light intensity significantly affected the change in stem biomass (Table 1). Under low light, the leaf biomass of S. trilobata decreased by 24.8%. The root biomass of the hybrid decreased by 61%, and the root and leaf biomass of S. calendulacea decreased by 65 and 19%, respectively. After low-light treatment, the total biomass of S. trilobata, S. calendulacea, and their hybrid decreased by 20, 17, and 16%, respectively. However, under both light conditions, the total biomass of S. trilobata was higher than that of the other two species (Table 2).
Table 2. The plant biomass of the three Sphagneticola species at 60 d of cultivation under full and low light. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05, **P<0.01, and ***P<0.001) according to two-way analysis of variance (ANOVA).
| Index/species and treatment | S. calendulacea | Hybrid | S. trilobata | |||||||||
| Full light | Low light | F | P | Full light | Low light | F | P | Full light | Low light | F | P | |
| Root biomass [g] | 0.51 ± 0.05 | 0.18 ± 0.02 | 34.15 | ** | 0.84 ± 0.04 | 0.33 ± 0.05 | 60.66 | *** | 0.43 ± 0.09 | 0.29 ± 0.07 | 1.44 | ns |
| Stem biomass [g] | 6.60 ± 0.31 | 5.78 ± 0.03 | 6.83 | ns | 6.80 ± 0.40 | 5.15 ± 0.46 | 7.35 | ns | 5.90 ± 0.44 | 5.15 ± 1.02 | 0.45 | ns |
| Leaf biomass [g] | 4.02 ± 0.06 | 3.25 ± 0.09 | 49.90 | ** | 3.86 ± 0.13 | 4.20 ± 0.31 | 1.05 | ns | 7.85 ± 0.16 | 5.90 ± 0.61 | 9.57 | * |
| Total biomass [g] | 11.13 ± 0.23 | 9.20 ± 0.06 | 67.97 | *** | 11.49 ± 0.26 | 9.68 ± 0.19 | 32.36 | ** | 14.18 ± 0.58 | 11.34 ± 0.76 | 8.79 | * |
Further analysis of the ratio of plant organs to total biomass showed that under low light, the root biomass ratio of S. calendulacea and the hybrid decreased by 56 and 58%, the leaf biomass ratio increased by 19 and 26%, and the root/shoot decreased by 58 and 60%, respectively. The stem biomass ratio of the hybrid was reduced by 8%. Obvious differences in the aforementioned indices were not detected in S. trilobata. Under full light and low light, the leaf biomass ratio of S. trilobata was the largest among the three Sphagneticola species, the root biomass ratio of the hybrid was the largest, and the stem biomass ratio of S. calendulacea was the largest. The three Sphagneticola species showed different resource allocations and growth adaptations (Table 2).
Chlorophyll fluorescence parameters
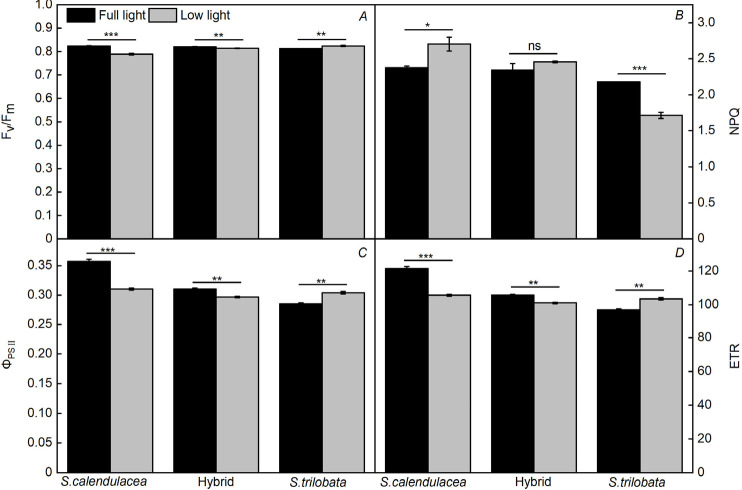
Chl fluorescence parameters effectively reflect the internal photosystem performance of plants in stressful environments and have been used as indicators of plant stress tolerance (Chen et al. 2006). After low-light treatment, the Fv/Fm, ΦPSII, and ETR of the same Sphagneticola species showed the same trends. These three indices increased noticeably in S. trilobata and remained at a high level under low light, while significant decreases were observed in the hybrid and S. calendulacea. S. calendulacea showed a greater decreasing trend (Fig. 3A,C,D). Under low light, the NPQ of S. trilobata was lower than that of the full-light control group, and the NPQ of S. calendulacea increased significantly, while their hybrid showed no remarkable difference (Fig. 3B). The change in NPQ was strongly related to the species (Table 1).
Fig. 3. Chlorophyll fluorescence parameters of the three Sphagneticola species at 60 d of cultivation under low light. The maximal photochemical efficiency of PSII (Fv/Fm) (A), nonphotochemical quenching (NPQ) (B), actual quantum yield of PSII (ΦPSII) (C), and electron transport rate of PSII (ETR) (D) of the three Sphagneticola species under full and low light. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05, **P<0.01, and ***P<0.001) according to one-way analysis of variance (ANOVA).
O2·– and H2O2 tissue localization
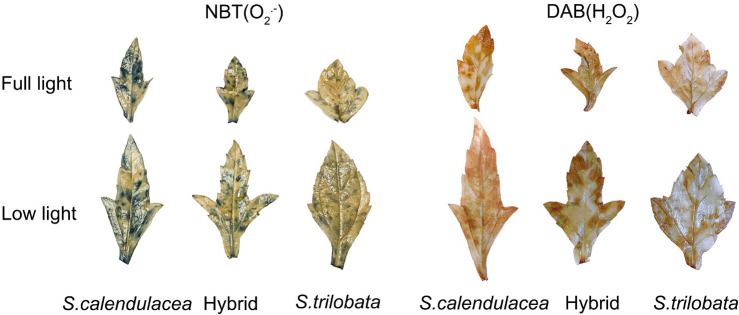
Under low light, the stained spots in the three Sphagneticola species were greater than those under full light. Under low and full light, the leaves of S. calendulacea contained a large number of indigo spots (NBT) and brown spots (DAB) that were deep colored, indicating that more O2·– and H2O2 accumulated, respectively, in the leaves. After low-light treatment, the stained spots on the hybrid were slightly less intense than those of S. calendulacea, while the stained spots on S. trilobata were significantly less intense than those of the other two species, indicating lower accumulation of O2·– and H2O2 (Fig. 4).
Fig. 4. Tissue localization of reactive oxygen species in the three Sphagneticola species at 60 d of cultivation under low light. O2·– was detected using nitroblue tetrazolium (NBT) staining and H2O2 was detected using diaminobenzidine (DAB) staining in leaves of the three Sphagneticola species under full and low light.
Antioxidant enzymes
CAT eliminates H2O2 and reduces the tissue damage caused by reactive oxygen species (Bashir et al. 2019). Comparing the interaction between light intensity and species, species was the main factor affecting the activity of antioxidant enzymes (Table 1). Upon low-light treatment, CAT activity in S. calendulacea increased by 11.8% and that of the hybrid decreased by 8.8%, but low-light stress did not affect CAT activity in S. trilobata (Fig. 5C). SOD is the key enzyme scavenging O2·– (Bowler et al. 1992), and its activity in S. trilobata was markedly elevated by 12% under low light. In contrast, SOD activity in S. calendulacea was slightly lower than that in the control group (Fig. 5A). POD activity in S. calendulacea was also significantly reduced by 26.7% under low light, while S. trilobata and the hybrid showed no significant changes (Fig. 5B). SMA showed that the slopes of SOD and POD activities under low light were greater than those under full light, indicating that compared with full light, low light had a stronger promoting effect on SOD and POD activities than other factors in the same condition. In addition, the effect of the relative irradiance on increasing SOD and POD activities was stronger than that of plant height. The result for CAT activity differed from these results, showing that full light and plant height had greater promoting effect on CAT activity (Table 2S, supplement).
Fig. 5. The antioxidant enzyme activities in the three Sphagneticola species at 60 d of cultivation under low light. Superoxide dismutase (SOD) (A), peroxidase (POD) (B), and catalase (CAT) (C) of the three Sphagneticola species under full and low light. The data are shown as the means ± SE of five biological replicates. Asterisks indicate different significant differences (*P<0.05 and **P<0.01) according to one-way analysis of variance (ANOVA).

Discussion
S. trilobata optimizes resource allocation and reduces changes in morphological characteristics under low light
Most alien invasive plants have a stronger competitiveness than that of native plant species. In heterogeneous environments, their adaptability is improved by changing their morphological and photosynthetic characteristics and self-regulatory mechanisms (Bassett et al. 2011, Cai et al. 2020, Sun et al. 2020b). Two-way ANOVA indicated that the morphological characteristics of the three Sphagneticola species exhibited significant differences in response to light intensity and species. Light intensity and species both exerted independent and interactive effects, and the mechanism underlying their effects was complex (Table 1). Givnish (1988) proposed that in a low-light environment, plants try to maintain the maximum ability to absorb light quanta and improve light energy-utilization efficiency; on the other hand, they maintain life activities by reducing respiration and energy consumption. After low-light treatment, S. trilobata adopted different growth strategies. Compared to full light, the main stem length, main stem diameter, leaf pairs, and leaf width of the hybrid and S. calendulacea increased significantly under low light, while the main stem length, leaf width, leaf number, and branch number of S. trilobata decreased noticeably (Fig. 1). Stem length was negatively correlated with plant height and relative irradiance, and the inhibitory effect of relative irradiance was stronger (Table 2S). In a low-light environment, S. trilobata grew to reach a higher position at the expense of main stem growth (Fig. 2A), consistent with previous research on Chrysanthemum grandiflorum (Lei et al. 2015). Phytohormones are essential intrinsic regulators of plant morphological changes, among which gibberellin (GA) is one of the important factors promoting stem growth (Santner et al. 2009, Kurepin and Pharis 2014). Low light usually leads to the elongation of stems (Tan et al. 2018, Liang et al. 2022). In contrast, the stem length of S. trilobata decreased, possibly because low light reduced the content of GA in S. trilobata or its sensitivity to GA (Panda et al. 2022). Fewer leaf pairs and branch numbers reduce the consumption of organic matter and funnel energy to other processes to ensure sufficient resources for the adaptation to low-light stress (Jha et al. 2008, Chen et al. 2017). According to previous studies, an increase in SLA leads to the thinning and enlargement of leaves, which is conducive to light penetrating the leaf epidermis, improving the light capture ability of the leaves, accumulating more light assimilates, and enhancing competitiveness (Li and Bao 2005, Houter and Pons 2012, Liu et al. 2016). SLA was significantly negatively correlated with relative irradiance (Table 2S). The relative irradiance above the leaf of S. trilobata was significantly higher than that of the other two species, and the SLA was naturally lower but significantly higher than that obtained under full light (Fig. 2B,C). Plants adapt to different light conditions by changing their external shape and plasticity (Zhou et al. 2017, Coverdale and Agrawal 2021). The overall morphology of S. trilobata decreased, while that of the other two Sphagneticola species increased. The morphological characteristics of the hybrid are between those of the two parents, with a bias toward S. calendulacea. As a creeping plant, the vertical height of S. trilobata is higher than that of S. calendulacea under low light, and it intercepts more light when it grows to higher altitudes. However, S. calendulacea in the lower position is likely to be in a lower light-density environment due to the shade formed by S. trilobata, and thus the living environment of S. calendulacea is more difficult.
Generally, plants with large biomasses have relatively large coverage in ecosystems or natural habitats, occupying the advantage of living space (Zhang and Welker 1996). Invasive plants often maintain large biomass to obtain competitive advantages under stress (Liu et al. 2015, Zhang et al. 2020b). Under low light, the biomass of the three Sphagneticola species decreased, but the total biomass of S. trilobata was the largest. In terms of the ratio of each organ to the total biomass, the three species tended to increase the leaf biomass ratio. Among them, the largest leaf biomass ratio was observed for S. trilobata, and a significant difference was not observed under the two light conditions, consistent with the research of Chen et al. (2018). Nevertheless, the root biomass ratio of the hybrid was the largest, and the stem biomass ratio of S. calendulacea was the largest (Table 2). Seedlings of shade-tolerant tree species support growth by enlarging the investment in leaves at the expense of roots, while non-shade-tolerant species do not employ this adaptation (Naumburg et al. 2001, Lusk 2004). Similarly, in terms of morphological structure, S. trilobata focuses more on the growth of leaves and reduces investment in underground organs, which is the adaptation strategy of S. trilobata to low light. This finding is also consistent with the hypothesis proposed by Poorter et al. (2012) that under stressful environments, plants allocate resources to the organs responsible for obtaining the most limited resources (Wang et al. 2016, Soda et al. 2017), which is related to changes in the distribution pattern of phytohormones (Hedden and Thomas 2012, Sugiura et al. 2016). S. trilobata may have evolved a phytohormone-dependent adaptation strategy by sensing and integrating environmental cues through hormone signaling in response to low light (Jiang et al. 2021).
S. trilobata has a strong photosynthetic capacity and active oxygen-scavenging capacity under low light
Chl fluorescence is a probe of photosynthesis that accurately reflects the effect of shading on photosynthesis (Hallik et al. 2012). Fv/Fm is the efficiency of capturing excitation energy in the photochemically open reaction centers (PSII). It is an important index used to measure the degree of photoinhibition, with a normal value of approximately 0.8, which decreases significantly under stress (Zhang 1999, Rascher et al. 2000, Griebeler et al. 2021). The Fv/Fm values of the three Sphagneticola species under full light were all greater than 0.8, indicating that the plant grew well under natural conditions. After low-light treatment, the Fv/Fm of S. calendulacea and the hybrid decreased significantly, indicating that the two plant species experienced low-light stress. The Fv/Fm of S. calendulacea was lesser than 0.8, indicating that it was seriously stressed; it might damage the photosynthetic structures, inhibit photosynthetic electron transfer, and lead to a decrease in photosynthetic capacity (Ojeda-Pérez et al. 2017). In contrast, the Fv/Fm of S. trilobata increased (Fig. 3A), suggesting that low light led to an increase in the openness of PSII reaction centers. The larger the NPQ, the greater the ability of plants to convert excess light energy into heat dissipation (Müller et al. 2001, Li et al. 2018). Under low light, the NPQ of S. trilobata decreased significantly (Fig. 3B), and the proportion of leaves used for heat dissipation decreased, suggesting that it tended to activate photochemical reactions and promoted the accumulation of carbon assimilation. This result is consistent with the research findings reported by Zhang et al. (2022b) for the shade-tolerant species Panax notoginseng. The Fv/Fm, ΦPSII, and ETR of S. trilobata showed the same trends (Fig. 3A,C,D), consistent with the research results for Fritillaria cirrhosa adapted to shade (Li and Chen 2008).
Both biotic and abiotic stresses induce the production of a large number of reactive oxygen species. O2·– is not only an important reactive oxygen species in plants but also the main source of the formation of other reactive oxygen species (Bowler et al. 1992, Møller 2001). O2·– stimulates the activity of antioxidant enzymes (Wagner 1995). SOD catalyzes the conversion of O2·– to H2O2 with relatively low cytotoxicity, which may play a central role in the antioxidant protective mechanism of cells and improve stress resistance (Bowler et al. 1992, Zandalinas et al. 2017, Liu et al. 2018). In addition, POD and CAT are the main enzymes that scavenge H2O2 in cells (Bashir et al. 2019). Under low-light stress, S. trilobata accumulated O2·– and its SOD activity increased significantly, thereby catalyzing the production of H2O2. However, POD and CAT activities did not change markedly, and H2O2 accumulation was not serious, showing that S. trilobata suffered less of low-light stress. S. trilobata was the species with advantages in terms of antioxidant capacity (Table 1). The CAT activity in the hybrid decreased significantly, the CAT activity in S. calendulacea apparently increased, and its SOD and POD activities decreased significantly (Fig. 4). The stained spots in the two species were serious (Fig. 5), indicating that a large amount of reactive oxygen species accumulated, the antioxidant system was severely damaged, and the capacity to scavenge reactive oxygen species was seriously weakened. A study of Trollius chinensis Bunge showed that exposure to shade for 40 d caused weak light stress and SOD activity decreased continuously, shade cultivation for 80 d promoted growth and increased SOD activity, and shade cultivation for 120 d was not conducive to growth, and POD activity decreased (Lü et al. 2013). Studies of Jasmine suggested that shading promoted an increase in SOD and POD activities and a decrease in CAT activity (Deng et al. 2012). Moreover, after heavy shading (10% of full light), the SOD activity in Bruguiera gymnorrhiza showed no significant change, CAT activity decreased, POD activity increased, and the plant could still maintain the normal operation of the antioxidant enzyme system (Zhu et al. 2022). Therefore, the changing trend of antioxidant enzymes caused by shading is closely related to the degree of shading and shading time, and significant differences have been observed between different species.
In summary, S. trilobata has a high ΦPSII and the ETR (Fig. 3C,D) revealed a higher photosynthetic capacity under low light. According to the results for Fv/Fm, H2O2 and O2·– localization (Figs. 3A, 4), S. trilobata suffered less stress and increased the activity of antioxidant enzymes (Fig. 5), indicating that its ability to scavenge reactive oxygen species was enhanced and its antioxidant capacity became stronger. Compared to S. calendulacea and the hybrid, the morphological change of S. trilobata was reduced, showing weak morphological plasticity. Therefore, it invested more energy in physiological activities and showed stronger physiological plasticity, which is different from prior research showing that the adaptation of invasive species to light is based on morphological changes (Wang and Feng 2004, Wang et al. 2018). In contrast to the plasticity of morphological traits, the plasticity of physiological traits provides a faster, more reversible, and lower carbon consumption response for plants (Bradshaw 1965, Grime and Mackey 2002, Hou et al. 2015). Yamashita et al. (2002) postulated that plants with high physiological plasticity and moderate shade tolerance are most likely to become invasive species. Gruntman et al. (2017, 2020) also indicated that a physiological shade adaptation is more adaptive than investing in morphological traits because morphological traits require carbon allocation. Therefore, the greater plasticity of the physiological shade tolerance response is selected in the more mature population rather than in the invasive frontier population (Gruntman et al. 2020). Strong physiological plasticity plays an important role in the invasion of S. trilobata in a low-light environment.
Rhymer and Simberloff (1996) found that hybridization between alien and native plant species is one of the most important causes of habitat degradation of native species. As shown in the present study, the phenotypic plasticity of the hybrid was between the two parents, which was biased toward S. calendulacea, but its shade tolerance was better than that of S. calendulacea. This finding illustrated that the hybrid would further threaten the existence of S. calendulacea in a low-light environment, which is consistent with the study by Li et al. (2016). Wu et al. (2013) also discovered that the rapid expansion of S. trilobata in recent decades has substantially decreased the population of S. calendulacea. Therefore, S. trilobata can invade the low-light environment, which is inseparable from its ability to not only reasonably allocate resources and improve its resistance but also crowd out local plants and become a dominant population by relying on the strong stress resistance of the hybrid offspring.
Conclusions
After low-light treatment, compared to S. calendulacea, S. trilobata showed a strong photosynthetic capacity, low oxidative stress, a certain shade tolerance, and further invasion of the living space and resources of S. calendulacea. The shade tolerance of the hybrid is between the two parents, which is better than that of S. calendulacea and plays an important role in the invasion process. Therefore, the existence of the hybrid should not be ignored while monitoring S. calendulacea and preventing it from invading shaded areas.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (31870374 and 32171493).
Abbreviations
- ANOVA
analysis of variance
- CAT
catalase
- DAB
3,3-diaminobenzidine
- ETR
electron transport rate of PSII
- Fv/Fm
maximal photochemical efficiency of PSII
- GA
gibberellin
- NBT
nitroblue tetrazolium
- NPQ
nonphotochemical quenching
- POD
peroxidase
- SLA
specific leaf area
- SMA
standardized major axis
- SOD
superoxide dismutase
- ΦPSII
actual quantum yield of PSII
Supplementary Materials
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Azeem A., Sun J., Javed Q. et al. : Water deficiency with nitrogen enrichment makes Wedelia trilobata to become weak competitor under competition. – Int. J. Environ. Sci. Technol. 19: 319-326, 2022. 10.1007/s13762-020-03115-y [DOI] [Google Scholar]
- Bashir W., Anwar S., Zhao Q. et al. : Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. – Ecotox. Environ. Safe. 175: 90-101, 2019. 10.1016/j.ecoenv.2019.03.042 [DOI] [PubMed] [Google Scholar]
- Bassett I.E., Paynter Q., Beggs J.R.: Effect of artificial shading on growth and competitiveness of Alternanthera philoxeroides (alligator weed). – New Zeal. J. Agr. Res. 54: 251-260, 2011. 10.1080/00288233.2011.599396 [DOI] [Google Scholar]
- Bowler C., Montagu M., Inze D.: Superoxide dismutase and stress tolerance. – Annu. Rev. Plant Biol. 43: 83-116, 1992. 10.1146/annurev.pp.43.060192.000503 [DOI] [Google Scholar]
- Bradshaw A.D.: Evolutionary significance of phenotypic plasticity in plants. – Adv. Genet. 13: 115-155, 1965. 10.1016/S0065-2660(08)60048-6 [DOI] [Google Scholar]
- Branco S., Videira N., Branco M., Paiva M.R.: A review of invasive alien species impacts on eucalypt stands and citrus orchards ecosystem services: towards an integrated management approach. – J. Environ. Manage. 149: 17-26, 2015. 10.1016/j.jenvman.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Cai M.L., Ding W.Q., Zhai J.J. et al. : Photosynthetic compensation of nonleaf organ stems of the invasive species Sphagneticola trilobata (L.) Pruski at low temperature. – Photosynth. Res. 149: 121-134, 2021. 10.1007/s11120-020-00748-5 [DOI] [PubMed] [Google Scholar]
- Cai M.L., Zhang Q.L., Zhang J.J. et al. : Comparative physiological and transcriptomic analyses of photosynthesis in Sphagneticola calendulacea (L.) Pruski and Sphagneticola trilobata (L.) Pruski. – Sci. Rep.-UK 10: 17810, 2020. 10.1038/s41598-020-74289-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión-Tacuri J., Rubio-Casal A.E., de Cires A. et al. : Lantana camara L.: a weed with great light-acclimation capacity. – Photosynthetica 49: 321-329, 2011. 10.1007/s11099-011-0039-6 [DOI] [Google Scholar]
- Chance B., Maehly A.C.: Assay of catalases and peroxidases. – Method. Enzymol. 2: 764-775, 1955. 10.1016/S0076-6879(55)02300-8 [DOI] [PubMed] [Google Scholar]
- Chen J.M., Yu X.P., Cheng J.A.: [The application of chlorophyll fluorescence kinetics in the study of physiological responses of plants to environmental stresses.] – Acta Agric. Zhejiang. 18: 51-55, 2006. [In Chinese] 10.3969/j.issn.1004-1524.2006.01.012 [DOI] [Google Scholar]
- Chen W., Wang J.H., Zhu H. et al. : [Effects of shade and nutrient level on plant growth, module biomass allocation and PSII functions of Wedelia trilobata.] – Ecol. Sci. 37: 160-167, 2018. [In Chinese] 10.14108/j.cnki.1008-8873.2018.04.020 [DOI] [Google Scholar]
- Chen X.M., Liao Y., Zhang Y.K. et al. : [Effects of shading on growth and response characteristics of photosynthesis in Xanthostemon chrysanthus.] – Forestry Environ. Sci. 33: 77-80, 2017. [In Chinese] 10.3969/j.issn.1006-4427.2017.02.014 [DOI] [Google Scholar]
- Coverdale T.C., Agrawal A.A.: Evolution of shade tolerance is associated with attenuation of shade avoidance and reduced phenotypic plasticity in North American milkweeds. – Am. J. Bot. 108: 1705-1715, 2021. 10.1002/ajb2.1732 [DOI] [PubMed] [Google Scholar]
- Deng Y., Shao Q., Li C. et al. : Differential responses of double petal and multi petal jasmine to shading: II. Morphology, anatomy and physiology. – Sci. Hortic.-Amsterdam 144: 19-28, 2012. 10.1016/j.scienta.2012.06.031 [DOI] [PubMed] [Google Scholar]
- Dickinson G.R., Lee D.J., Wallace H.M.: The influence of pre- and post-zygotic barriers on interspecific Corymbia hybridization. – Ann. Bot.-London 109: 1215-1226, 2012. 10.1093/aob/mcs050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B., Yang H., Liu H. et al. : Effects of shading stress on grain number, yield, and photosynthesis during early reproductive growth in wheat. – Crop Sci. 59: 363-378, 2019. 10.2135/cropsci2018.06.0396 [DOI] [Google Scholar]
- Dong L.J., Yang J.X., Yu H.W., He W.M.: Dissecting Solidago canadensis – soil feedback in its real invasion. – Ecol. Evol. 7: 2307-2315, 2017. 10.1002/ece3.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand N.C., Schierenbeck K.A.: Hybridization as a stimulus for the evolution of invasiveness in plants? – P. Natl. Acad. Sci. USA 97: 7043-7050, 2000. 10.1073/pnas.97.13.7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falster D.S., Warton D.I., Wright I.J.: User's guide to SMATR: standardized major axis tests and routines, version 2.0, 2006. http://bio.mq.edu.au/research/groups/comparative/SMATR/SMATR_users_guide.pdf [Google Scholar]
- Farrer E.C., Goldberg D.E.: Litter drives ecosystem and plant community changes in cattail invasion. – Ecol. Appl. 19: 398-412, 2009. 10.1890/08-0485.1 [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Whitelam G.C.: Phytochromes and shade-avoidance responses in plants. – Ann. Bot.-London 96: 169-175, 2005. 10.1093/aob/mci165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk J.L., Standish R.J., Stock W.D., Valladares F.: Plant functional traits of dominant native and invasive species in Mediterranean-climate ecosystems. – Ecology 97: 75-83, 2016. 10.1890/15-0974.1 [DOI] [PubMed] [Google Scholar]
- Genty B., Briantais J.-M., Baker N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. – BBA-Gen. Subjects 990: 87-92, 1989. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Giannopolitis C.N., Ries S.K.: Superoxide dismutases: I. Occurrence in higher plants. – Plant Physiol. 59: 309-314, 1977. 10.1104/pp.59.2.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish T.J.: Adaptation to sun and shade: a whole-plant perspective. – Funct. Plant Biol. 15: 63-92, 1988. 10.1071/PP9880063 [DOI] [Google Scholar]
- Granata M.U., Bracco F., Catoni R.: Phenotypic plasticity of two invasive alien plant species inside a deciduous forest in a strict nature reserve in Italy. – J. Sustain. For. 39: 346-364, 2020. 10.1080/10549811.2019.1670678 [DOI] [Google Scholar]
- Gray G.R., Chauvin L.P., Sarhan F., Huner N.P.A.: Cold acclimation and freezing tolerance (a complex interaction of light and temperature). – Plant Physiol. 114: 467-474, 1997. 10.1104/pp.114.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebeler A.M., Araujo M.M., Barbosa F.M. et al. : Morphophysiological responses of forest seedling species subjected to different water regimes. – J. Forestry Res. 32: 2099-2110, 2021. 10.1007/s11676-020-01200-z [DOI] [Google Scholar]
- Grime J.P., Mackey J.M.L.: The role of plasticity in resource capture by plants. – Evol. Ecol. 16: 299-307, 2002. 10.1023/A:1019640813676 [DOI] [Google Scholar]
- Gruntman M., Segev U., Glauser G., Tielbörger K.: Evolution of plant defences along an invasion chronosequence: defence is lost due to enemy release – but not forever. – J. Ecol. 105: 255-264, 2017. 10.1111/1365-2745.12660 [DOI] [Google Scholar]
- Gruntman M., Segev U., Tielbörger K.: Shade-induced plasticity in invasive Impatiens glandulifera populations. – Weed Res. 60: 16-25, 2020. 10.1111/wre.12394 [DOI] [Google Scholar]
- Hallik L., Niinemets Ü., Kull O.: Photosynthetic acclimation to light in woody and herbaceous species: a comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. – Plant Biol. 14: 88-99, 2012. 10.1111/j.1438-8677.2011.00472.x [DOI] [PubMed] [Google Scholar]
- Hedden P., Thomas S.G.: Gibberellin biosynthesis and its regulation. – Biochem. J. 444: 11-25, 2012. 10.1042/BJ20120245 [DOI] [PubMed] [Google Scholar]
- Hou Y.P., Peng S.L., Lin Z.G. et al. : Fast-growing and poorly shade-tolerant invasive species may exhibit higher physiological but not morphological plasticity compared with noninvasive species. – Biol. Invasions 17: 1555-1567, 2015. 10.1007/s10530-014-0815-x [DOI] [Google Scholar]
- Houter N.C., Pons T.L.: Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. – Oecologia 169: 33-45, 2012. 10.1007/s00442-011-2175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed Q., Sun J., Azeem A. et al. : Competitive ability and plasticity of Wedelia trilobata (L.) under wetland hydrological variations. – Sci. Rep.-UK 10: 9431, 2020. 10.1038/s41598-020-66385-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P., Norsworthy J.K., Riley M.B. et al. : Acclimation of Palmer amaranth (Amaranthus palmeri) to shading. – Weed Sci. 56: 729-734, 2008. 10.1614/WS-07-203.1 [DOI] [Google Scholar]
- Jiang Y., Ding X., Wang J. et al. : Decreased low-light regulates plant morphogenesis through the manipulation of hormone biosynthesis in Solanum lycopersicum. – Environ. Exp. Bot. 185: 104409, 2021. 10.1016/j.envexpbot.2021.104409 [DOI] [Google Scholar]
- Ju R.T., Chen Y.Y., Gao L., Li B.: The extended phenology of Spartina invasion alters a native herbivorous insect's abundance and diet in a Chinese salt marsh. – Biol. Invasions 18: 2229-2236, 2016. 10.1007/s10530-015-0981-5 [DOI] [Google Scholar]
- Kim S.J., Yu D.J., Kim T.C., Lee H.J.: Growth and photosynthetic characteristics of blueberry (Vaccinium corymbosum cv. Bluecrop) under various shade levels. – Sci. Hortic.-Amsterdam 129: 486-492, 2011. 10.1016/j.scienta.2011.04.022 [DOI] [Google Scholar]
- Kurepin L.V., Pharis R.P.: Light signaling and the phytohormonal regulation of shoot growth. – Plant Sci. 229: 280-289, 2014. 10.1016/j.plantsci.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Larkin D.J., Freyman M.J., Lishawa S.C. et al. : Mechanisms of dominance by the invasive hybrid cattail Typha × glauca. – Biol. Invasions 14: 65-77, 2012. 10.1007/s10530-011-0059-y [DOI] [Google Scholar]
- Lee C.E.: Evolutionary genetics of invasive species. – Trends Ecol. Evol. 17: 386-391, 2002. 10.1016/S0169-5347(02)02554-5 [DOI] [Google Scholar]
- Lei Y., Li Q.W., Li W.G. et al. : [Physiological characteristics of adaptability for two ground-cover chrysanthemum cultivars with shading.] – J. Zhejiang A&F Univ. 32: 708-715, 2015. [In Chinese] 10.11833/j.issn.2095-0756.2015.05.008 [DOI] [Google Scholar]
- Li F.L., Bao W.K.: [Responses of the morphological and anatomical structure of the plant leaf to environmental change.] – Chin. Bull. Bot. 22: 118-127, 2005. [In Chinese] [Google Scholar]
- Li R.J., Chen X.Z., Yue C.L. et al. : [Effects of drought stress on the photosynthetic characteristics of Viburnum japonicum seedlings.] – Acta Ecol. Sin. 38: 2041-2047, 2018. [In Chinese] 10.5846/stxb201702240306 [DOI] [Google Scholar]
- Li T., Huang L.X., Yi L. et al. : Comparative analysis of growth and physiological traits between the natural hybrid Sphagneticola trilobata × calendulacea and its parental species. – Nord. J. Bot. 34: 219-227, 2016. 10.1111/njb.00910 [DOI] [Google Scholar]
- Li X.W., Chen S.L.: [Effect of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Fritillaria cirrhosa.] – Acta Ecol. Sin. 28: 3438-3446, 2008. [In Chinese] https://en.cnki.com.cn/Article_en/CJFDTOTAL-STXB200807057.htm [Google Scholar]
- Liang H.L., Zheng Y.P., Jiang C.Y. et al. : [Stem elongation characteristics of Mikania micrantha and its physiological basis under low light condition.] – J. Trop. Subtrop. Bot. 30: 70-78, 2022. [In Chinese] 10.11926/jtsb.4393 [DOI] [Google Scholar]
- Liu G., Siemann E., Gao Y., Peng S.: Nutrient addition amplifies salinity-dependent differences in competitive ability of invasive and native vines. – Biol. Invasions 17: 3479-3490, 2015. 10.1007/s10530-015-0972-6 [DOI] [Google Scholar]
- Liu T., Hu X., Zhang J. et al. : H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. – BMC Plant. Biol. 18: 34, 2018. 10.1186/s12870-018-1254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Dawson W., Prati D. et al. : Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? – Ann. Bot.-London 118: 1329-1336, 2016. 10.1093/aob/mcw180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S., Browne M., Boudjelas S. et al. : 100 of the world's worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group, Auckland 2000. [Google Scholar]
- Lü J.H., Li Y.F, Wang X. et al. : [Impact of shading on growth, development and physiological characteristics of Trollius chinensis Bunge.] – Sci. Agr. Sin. 46: 1772-1780, 2013. [In Chinese] 10.3864/j.issn.0578-1752.2013.09.004 [DOI] [Google Scholar]
- Lusk C.H.: Leaf area and growth of juvenile temperate evergreens in low light: species of contrasting shade tolerance change rank during ontogeny. – Funct. Ecol. 18: 820-828, 2004. 10.1111/j.0269-8463.2004.00897.x [DOI] [Google Scholar]
- Mack R.N., Simberloff D., Lonsdale W.M. et al. : Biotic invasions: causes, epidemiology, global consequences, and control. – Ecol. Appl. 10: 689-710, 2010. 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2 [DOI] [Google Scholar]
- Møller I.M.: Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. – Annu. Rev. Plant Phys. 52: 561-591, 2001. 10.1146/annurev.arplant.52.1.561 [DOI] [PubMed] [Google Scholar]
- Müller P., Li X.P., Niyogi K.K.: Nonphotochemical quenching. A response to excess light energy. – Plant Physiol. 125: 1558-1566, 2001. 10.1104/pp.125.4.1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumburg E., Ellsworth D.S., Pearcy R.W.: Crown carbon gain and elevated [CO2] responses of understorey saplings with differing allometry and architecture. – Funct. Ecol. 15: 263-273, 2001. 10.1046/j.1365-2435.2001.00518.x [DOI] [Google Scholar]
- Ni G., Zhao P., Wu W. et al. : A hybrid of the invasive plant Sphagneticola trilobata has similar competitive ability but different response to nitrogen deposition compared to parent. – Ecol. Res. 29: 331-339, 2014. 10.1007/s11284-014-1130-9 [DOI] [Google Scholar]
- Ojeda-Pérez Z.Z., Jiménez-Bremont J.F., Delgado-Sánchez P.: Continuous high and low temperature induced a decrease of photosynthetic activity and changes in the diurnal fluctuations of organic acids in Opuntia streptacantha. – PLoS ONE 12: e0186540, 2017. 10.1371/journal.pone.0186540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A., Paul J., Freeland J.R.: Habitat preferences of cattail species and hybrids (Typha spp.) in eastern Canada. – Aquat. Bot. 91: 67-70, 2009. 10.1016/j.aquabot.2009.02.003 [DOI] [Google Scholar]
- Panda D., Mohanty S., Das S. et al. : The role of phytochrome-mediated gibberellic acid signaling in the modulation of seed germination under low light stress in rice (O. sativa L.). –Physiol. Mol. Biol. Pla. 28: 585-605, 2022. 10.1007/s12298-022-01167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H., Niklas K.J., Reich P.B. et al. : Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. – New Phytol. 193: 30-50, 2012. 10.1111/j.1469-8137.2011.03952.x [DOI] [PubMed] [Google Scholar]
- Rascher U., Liebig M., Lüttge U.: Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. – Plant Cell Environ. 23: 1397-1405, 2000. 10.1046/j.1365-3040.2000.00650.x [DOI] [Google Scholar]
- Rhymer J.M., Simberloff D.: Extinction by hybridization and introgression. – Annu. Rev. Ecol. Syst. 27: 83-109, 1996. 10.1146/annurev.ecolsys.27.1.83 [DOI] [Google Scholar]
- Santner A., Calderon-Villalobos L.I., Estelle M.: Plant hormones are versatile chemical regulators of plant growth. – Nat. Chem. Biol. 5: 301-307, 2009. 10.1038/nchembio.165 [DOI] [PubMed] [Google Scholar]
- Schierenbeck K.A., Ellstrand N.C.: Hybridization and the evolution of invasiveness in plants and other organisms. – Biol. Invasions 11: 1093-1105, 2009. 10.1007/s10530-008-9388-x [DOI] [Google Scholar]
- Schreiber U., Bilger W., Neubauer C.: Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. – In: Schulze E.D., Caldwell M.M. (ed.): Ecophysiology of Photosynthesis. Pp. 49-70. Springer, Berlin-Heidelberg: 1995. 10.1007/978-3-642-79354-7_3 [DOI] [Google Scholar]
- Seebens H., Bacher S., Blackburn T.M. et al. : Projecting the continental accumulation of alien species through to 2050. – Glob. Change Biol. 27: 970-982, 2021. 10.1111/gcb.15333 [DOI] [PubMed] [Google Scholar]
- Seebens H., Blackburn T.M., Dyer E.E. et al. : No saturation in the accumulation of alien species worldwide. – Nat. Commun. 8: 14435, 2017. 10.1038/ncomms14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebens H., Blackburn T.M., Dyer E.E., Essl F.: Global rise in emerging alien species results from increased accessibility of new source pools. – P. Natl. Acad. Sci. USA 115: E2264-E2273, 2018. 10.1073/pnas.1719429115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann E., Rogers W.E.: Genetic differences in growth of an invasive tree species. – Ecol. Lett. 4: 514-518, 2001. 10.1046/j.1461-0248.2001.00274.x [DOI] [Google Scholar]
- Soda N., Ephrath J.E., Dag A. et al. : Root growth dynamics of olive (Olea europaea L.) affected by irrigation induced salinity. – Plant Soil 411: 305-318, 2017. 10.1007/s11104-016-3032-9 [DOI] [Google Scholar]
- Song L.Y., Li C.H., Peng S.L.: Elevated CO2 increases energy-use efficiency of invasive Wedelia trilobata over its indigenous congener. – Biol. Invasions 12: 1221-1230, 2010. 10.1007/s10530-009-9541-1 [DOI] [Google Scholar]
- Song L.Y., Liu Z.D., Li X.N. et al. : [Eco-physiological responses of Wedelia trilobata, W. chinensis and their hybrid to simulated extreme high temperature.] – Ecol. Environ. Sci. 26: 183-188, 2017. [In Chinese] 10.16258/j.cnki.1674-5906.2017.02.001 [DOI] [Google Scholar]
- Sugiura D., Kojima M., Sakakibara H.: Phytohormonal regulation of biomass allocation and morphological and physiological traits of leaves in response to environmental changes in Polygonum cuspidatum. – Front. Plant Sci. 7: 1189, 2016. 10.3389/fpls.2016.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Ou Y., Ou Q. et al. : The invasive potential of a hybrid species: insights from soil chemical properties and soil microbial communities. – J. Plant Ecol. 13: 20-26, 2020a. 10.1093/jpe/rtz050 [DOI] [Google Scholar]
- Sun J., Javed Q., Azeem A. et al. : Fluctuated water depth with high nutrient concentrations promote the invasiveness of Wedelia trilobata in Wetland. – Ecol. Evol. 10: 832-842, 2020b. 10.1002/ece3.5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X.F., Guo X., Guo W.F. et al. : Invasive Rhus typhina invests more in height growth and traits associated with light acquisition than do native and noninvasive alien shrub species. – Trees 32: 1103-1112, 2018. 10.1007/s00468-018-1698-8 [DOI] [Google Scholar]
- Wada K.C., Kondo H., Takeno K.: Obligatory short-day plant, Perilla frutescens var. crispa can flower in response to low-intensity light stress under long-day conditions. – Physiol. Plantarum 138: 339-345, 2010. 10.1111/j.1399-3054.2009.01337.x [DOI] [PubMed] [Google Scholar]
- Wagner A.M.: A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. – FEBS Lett. 368: 339-342, 1995. 10.1016/0014-5793(95)00688-6 [DOI] [PubMed] [Google Scholar]
- Walker B., Steffen W.: An overview of the implications of global change for natural and managed terrestrial ecosystems. – Conserv. Ecol. 1: 2, 1997. https://www.ecologyandsociety.org/vol1/iss2/art2/ [Google Scholar]
- Wang J.F., Feng Y.L.: [The effect of light intensity on biomass allocation, leaf morphology and relative growth rate of two invasive plants.] – Chin. J. Plant Ecol. 28: 781-786, 2004. [In Chinese] 10.3321/j.issn:1005-264X.2004.06.006 [DOI] [Google Scholar]
- Wang L.L., Wang B., Shang N. et al. : Effects of experimental defoliation on resource allocation using integrated physiological units in the andromonoecious Camptotheca acuminata. – S. Afr. J. Bot. 104: 47-54, 2016. 10.1016/j.sajb.2015.11.007 [DOI] [Google Scholar]
- Wang T., Hu J., Wang R. et al. : Tolerance and resistance facilitate the invasion success of Alternanthera philoxeroides in disturbed habitats: A reconsideration of the disturbance hypothesis in the light of phenotypic variation. – Environ. Exp. Bot. 153: 135-142, 2018. 10.1016/j.envexpbot.2018.05.011 [DOI] [Google Scholar]
- Warton D.I., Wright I.J., Falster D.S., Westoby M.: Bivariate line-fitting methods for allometry. – Biol. Rev. 81: 259-291, 2006. 10.1017/S1464793106007007 [DOI] [PubMed] [Google Scholar]
- Wu W., Zhou R.C., Ni G.Y. et al. : Is a new invasive herb emerging? Molecular confirmation and preliminary evaluation of natural hybridization between the invasive Sphagneticola trilobata (Asteraceae) and its native congener S. calendulacea in South China. – Biol. Invasions 15: 75-88, 2013. 10.1007/s10530-012-0269-y [DOI] [Google Scholar]
- Wu Y.Q., Hu Y.J., Chen J.N.: [Reproductive characteristics of alien plant Wedelia trilobata.] – Acta Sci. Natur. Univ. Sunyatseni 44: 93-96, 2005. [In Chinese] 10.3321/j.issn:0529-6579.2005.06.025 [DOI] [Google Scholar]
- Wu Y.Q., Hu Y.J.: [Researches on photosynthetic characteristics of exotic plants Wedelia trilobata, Pharbitis nil and Ipomoea cairica.] – Acta Ecol. Sin. 24: 2334-2339, 2004. [In Chinese] 10.3321/j.issn:1000-0933.2004.10.037 [DOI] [Google Scholar]
- Xun Z.F., Bai L., Qu B. et al. : [Effect of nitrogen treatments on growth of the invasive plant Xanthium strumarium, the native plant Xanthium sibiricum, and their reciprocal crosses.] – Acta Pratacult. Sin. 26: 51-61, 2017. [In Chinese] 10.11686/cyxb2016217 [DOI] [Google Scholar]
- Yamashita N., Koike N., Ishida A.: Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. – Plant Cell Environ. 25: 1341-1356, 2002. 10.1046/j.1365-3040.2002.00907.x [DOI] [Google Scholar]
- Yang H., Dong B., Wang Y. et al. : Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. – Sci. Rep.-UK 10: 14353, 2020. 10.1038/s41598-020-71268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Hu X.Y., Wei X. et al. : [Effects of shade on the leaf microstructure and chloroplast ultrastructure of the invasive Wedelia trilobata, the native W. chinensis and their hybrid.] – Guihaia 34: 19-26+3, 2014. [In Chinese] 10.3969/j.issn.1000-3142.2014.01.005 [DOI] [Google Scholar]
- Zandalinas S.I., Balfagón D., Arbona V., Gómez-Cadenas A.: Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. – Front. Plant Sci. 8: 953, 2017. 10.3389/fpls.2017.00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., He W.J., Zhou Y.X. et al. : [Responses of alien plant Wedelia trilobata to simulated salt stress.] – Jiangsu Agr. Sci. 48: 105-111, 2020b. [In Chinese] 10.15889/j.issn.1002-1302.2020.04.017 [DOI] [Google Scholar]
- Zhang C.Y., Zhang Z.J., Huang R.F. et al. : [Comparative analysis of vitamin C contents and anti-oxidization in rice varieties.] – J. Agr. Sci. Technol. 13: 6-11, 2011. [In Chinese] 10.3969/j.issn.1008-0864.2011.06.02 [DOI] [Google Scholar]
- Zhang J.J., Cai M.L., Chen L.H. et al. : Photosynthetic physiological and ecological responses of the invasive Sphagneticola trilobata and the native Sphagneticola calendulacea to experimental shading. – Manag. Biol. Invasions 13: 274-287, 2022a. 10.3391/mbi.2022.13.2.02 [DOI] [Google Scholar]
- Zhang J.Y., Kuang S.B., Cun Z. et al. : Transcriptome and physiology analysis reveal key players of the shade-tolerant species Panax notoginseng in photosynthetic performance under both high and low light regimes. – J. Plant Interact. 17: 371-389, 2022b. 10.1080/17429145.2022.2041118 [DOI] [Google Scholar]
- Zhang Q.L., Chen G.X., Huang J.D., Peng C.L.: Comparison of the ability to control water loss in the detached leaves of Wedelia trilobata, Wedelia chinensis, and their hybrid. – Plants-Basel 9: 1227, 2020a. 10.3390/plants9091227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.R.: [A discussion on chlorophyll fluorescence kinetics parameters and their significance.] – Chin. Bull. Bot. 16: 444-448, 1999. [In Chinese] 10.3969/j.issn.1674-3466.1999.04.021 [DOI] [Google Scholar]
- Zhang Y., Welker J.M.: Tibetan alpine tundra responses to simulated changes in climate: aboveground biomass and community responses. – Arct. Antarct. Alp. Res. 28: 203-209, 1996. 10.2307/1551761 [DOI] [Google Scholar]
- Zhang Z.H., Hu B.Q., Hu G.: Assessment of allelopathic potential of Wedelia trilobata on the germination, seedling growth and chlorophyll content of rape. – Adv. Material. Res. 807-809: 719-722, 2013. 10.4028/www.scientific.net/AMR.807-809.719 [DOI] [Google Scholar]
- Zhou Y., Huang L., Wei X. et al. : Physiological, morphological, and anatomical changes in Rhododendron agastum in response to shading. – Plant Growth Regul. 81: 23-30, 2017. 10.1007/s10725-016-0181-z [DOI] [Google Scholar]
- Zhu Y.M., Li T., Jing Y.H. et al. : [Responses of seedlings of eight mangrove species to light intensity in leaf total soluble protein content and activities of antioxidant enzymes.] – Guihaia 10: 1-14, 2022. [In Chinese] http://www.guihaia-journal.com/ch/reader/download_new_edit_content.aspx?file_no=202203250000004&edit_id=20220704105850001&flag=2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.