Abstract
Transcatheter aortic valve replacement (TAVR) is considered more effective than surgical aortic valve implantation for patients with a small aortic annulus (SAA), however, the comparative efficacy of different transcatheter heart valves (THVs) remains uncertain. A literature search was performed across databases from their inception until June 2024 to identify eligible randomized controlled trials (RCTs) and propensity-score matched (PSM) studies. Clinical outcomes were evaluated using a random-effects model to pool risk ratios (RRs) with 95 % confidence intervals (CIs). The analysis included 10 studies with 2,960 patients. BEVs were associated with a significantly smaller indexed effective orifice area (MD: −0.18, 95 % CI: −0.27 to −0.10), and a higher transvalvular mean pressure gradient (MD: 5.07, 95 % CI 3.43 to 6.71) than SEVs. The risk for prosthesis-patient mismatch (PPM) (RR = 1.89, 95 % CI: 1.42 to 2.51) and severe PPM (RR = 2.80, 95 % CI: 1.96 to 4.0) was significantly higher for patients receiving BEVs than those receiving SEVs. Although nonsignificant differences were observed between BEVs and SEVs regarding 30-day and 1-year all-cause mortality, 30-day stroke rates, vascular complication, paravalvular leak, and permanent pacemaker implantation (p > 0.05), patients receiving BEVs were associated with a significantly increased risk of 1-year cardiovascular mortality (RR = 1.61, 95 % CI: 1.05 to 2.47) compared to those receiving SEVs. In patients with SAA, BEVs demonstrated worse hemodynamic performance as determined by the higher risk of moderate and severe PPM compared to SEVs. Moreover, the use of BEVs was associated with a higher risk of 1-year cardiovascular mortality.
Keywords: Balloon-expandable valves, Self-expanding valves, Transcatheter aortic valve replacement
1. Introduction
In the United States, the prevalence of aortic stenosis (AS) is around 1.5 million people, from which almost 500,000 people have severe AS [1]. Since the introduction of transcatheter aortic valve implantation (TAVI), it has emerged as an effective substitute for surgery for managing patients with symptomatic severe AS [2], [3]. It is well-established that patients with a small aortic annulus (SAA) have a greater risk of experiencing prosthesis-patient mismatch (PPM), and the presence of PPM has been linked with an increased risk of all-cause mortality [4]. Owing to the lower rates of PPM and superior hemodynamic performance associated with TAVI, patients with SAA may gain greater benefit from TAVI compared to surgical aortic valve replacement [5].
However, considering the uncertainty in the comparative efficacy of different types of transcatheter heart valves (THVs), it is important to assess different valve types for differences in clinical outcomes and hemodynamic performance in patients with SAA. Herein, we designed and conducted a meta-analysis to compare SEVs and BEVs in patients with SAA who underwent TAVI.
2. Methods
This systematic review and meta-analysis followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [6] and the protocol of review was registered with PROSPERO (CRD42024576844).
2.1. Literature search
Authors (IT and MFR) performed a search of PubMed, Cochrane, Clinicaltrials.gov, and Google Scholar independently for randomized controlled trials (RCTs) and observational studies with propensity score matching (PSM). The search strategy included all relevant PubMed entry terms and Medical Subject Headings (MeSH) terms: ((Transcatheter aortic valve implantation) OR (transcatheter aortic valve replacement)) AND ((balloon expandable) OR (SAPIEN*)) OR ((self-expanding) OR (CoreValve) OR (evolutr) OR (portico) OR (acurateneo) OR (Engager)) AND ((small annulus) OR (annular size)). The words were searched in different combinations to retrieve desired results across different databases. Previous meta-analyses were retrieved and carefully studied to identify research gaps and any additional studies. The databases were searched from inception to July 2024. Relevant search strategies for individual databases are recorded in Table S1.
2.2. Study selection criteria and data Extraction
All the articles were imported into Mendeley Desktop 2.112.1 (Mendeley Ltd., Amsterdam, Netherlands), and duplicates were filtered out. Two authors (I.T. and A.M.) independently reviewed the titles and abstracts of the studies and excluded those that didn't meet the inclusion criteria. The full texts of the remaining articles were then evaluated against the eligibility criteria. Any disagreements regarding the data were resolved through discussion, consulting the original article, or seeking the opinion of a third reviewer (M.A.).
The Studies were included if they fulfilled the following eligibility criteria: (i) published RCTs (ii) PSM studies (iii) Patients who went under TAVI with either self-expanding or balloon-expandable valves having SAA (iv) Evaluated at least one of the predetermined hemodynamic or clinical outcomes. The primary outcomes included indexed effective orifice area (iEOA), transvalvular mean/peak pressure gradient, and para-valvular leak. The secondary outcomes included prosthesis-patient mismatch (PPM), severe PPM, 30-day all-cause mortality, 1-year all-cause mortality, 30-day cardiovascular (CV) mortality, 1-year CV mortality, 30-day stroke, pacemaker implantation, major bleeding, any vascular complication, acute kidney injury (AKI), myocardial infarction (MI), and worsening heart failure related hospitalizations (HHF). The studies that were excluded were either observational studies without PSM analysis or ongoing RCTs. Other non-relevant study designs such as case reports and studies with animal subjects were also excluded.
The data for baseline characteristics of included studies was extracted along with the number of events/ the total number of patients for categorical outcomes, while for continuous outcomes, the total number of patients was recorded along with respective mean (standard deviation) were retrieved.
2.3. Risk of bias
The risk of bias was evaluated differently for the two different study designs included. Two authors (AA and MA) independently evaluated the risk of bias using the “Cochrane Risk of Bias tool (RoB 2.0)” and “Risk Of Bias In Non-randomized Studies − of Interventions (ROBINS-I)” for RCTs and PSM studies, respectively. A third author (RA) reviewed the quality assessment and conflicts were resolved after discussion amongst the three authors.
2.4. Data analysis
The statistical analysis was conducted using R version 4.4.1 and ‘meta’ and ‘metasens’ packages were employed to perform meta-analysis. Risk ratios (RR) with corresponding 95 % confidence intervals (CI) were pooled for dichotomous outcomes while for continuous outcomes weighted mean deviations (WMD) with corresponding 95 % CI were calculated. To account for inter-study variations, DerSimonian and Laird random effects models were employed [7]. The Paule-Mandel procedure was used to estimate the heterogeneity variance τ^2 [8]. For each study, the outcome data at the longest follow-up time were used in the pooled analyses and the results were represented graphically as forest plots. The Chi-square test and Higgins' I2 statistic were calculated to assess statistical heterogeneity [9]. Subgroup analyses were performed based on the study design (RCT vs. observational) and valve generation (early, new, both early and new). The early-generation devices included the SAPIEN XT, CoreValve, and Portico, while the newer-generation THVs consisted of the SAPIEN 3, Evolut R/PRO/PRO+, and ACURATE valves [10]. To assess the robustness of the primary analysis, a sensitivity analysis was conducted using the leave-one-out study approach, where each study was removed one at a time from the combined analysis to determine if any individual study had a significant impact on the overall results. A p-value of less than 0.05 indicated statistical significance in all cases.
3. Results
We identified 1,063 potentially relevant studies, of which 642 studies were excluded after screening of titles and abstracts. Full texts of 34 articles were reviewed, and 24 studies did not meet the inclusion criteria. The analysis is primarily based on the results of the shortlisted 10 studies out of which 2 were RCTs [11], [12] and 8 were PSM studies [5], [13], [14], [15], [16], [17], [18], [19]. 1 study [11] reported data for an RCT [20] and an ongoing observational study, however, we only included data for patients who were randomized based on the CHOICE trial with SAA. The PRISMA flow chart (Figure S1) summarizes the selection process.
3.1. Baseline characteristics and bias assessment
This study included a total of 2960 patients with SAA treated with either SEVs (1481 patients) or BEVs (1479 patients). The mean age was 81.8 (±6.4) years for patients receiving BEV and 81.9 (± 6.4) years for those receiving SEVs. The overall percentage of females was 80.7 % for BEVs and 82.3 % for SEVs. Most of the studies included new-generation valves [12], [13], [14], [15], [17], [18], [19], while 2 studies [5], [16] used both, early and new-generation devices, and the CHOICE [11] trial used early-generation devices. SAPIEN XT, SAPIEN XT/3, SAPIEN 3, and SAPIEN 3/3 Ultra were among the BEVs while the SEVs included Symetis ACURATE, CoreValve, Evolut R, Evolut PRO, Evolut PRO+, and Portico. The mean follow-up duration was 2.17 years across 8 studies, excluding CHOICE and Guimarães et al which did not report the follow-up period. Detailed baseline characteristics for analyzed studies and patients are provided in Table 1 and Table S2.
Table 1.
Baseline characteristics of the included studies.
|
Author/trial Name |
Year |
Study design |
Sample size |
Follow-up |
Valve generations |
SAA definition used for inclusion |
Device name |
Age-mean ± SD |
Females-n (%) |
BSA, m2- mean ± SD |
BMI Kg/m2- mean ± SD |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEVs | SEVs | BEVs | SEVs | BEVs | SEVs | BEVs | SEVs | BEVs | SEVs | BEVs | SEVs | ||||||
| Mauri et al. | 2017 | PSM | 92 | 92 | 1-year | new generation | Annulus area < 400 mm2 | SAPIEN 3 | Symetis ACURATE neo | 81.9 ± 5.3 | 82.8 ± 6.5 | 85 (92.4) | 85 (92.4) | 1.71 ± 0.2 | 1.71 ± 0.2 | 26.0 ± 4.7 | 27.3 ± 5.5 |
| Abdel-Wahab (CHOICE) | 2014 | RCT | 43 | 51 | NR | early generation | Mean aortic diameter ≤ 23 mm | SAPIEN XT | CoreValve | 82.0 ± 6.1 | 82.3 ± 6.1 | 37 (86.0) | 47 (92.2) | 1.72 ± 0.15 | 1.73 ± 0.19 | 25.7 ± 4.8 | 26.3 ± 5.6 |
| Guimarães et al. | 2020 | PSM | 52 | 52 | NR | early and new devices | Annular diameter ≤ 21 mm | SAPIEN XT/3 | CoreValve, Evolut R | 80 ± 8 | 80 ± 8 | 80 % | 80 % | 1.7 ± 0.2 | 1.7 ± 0.2 | 27 ± 7 | 27 ± 7 |
| Hase et al. (OCEAN-TAVI) | 2021 | PSM | 69 | 69 | 1 year | new generation | Mean aortic diameter ≤ 23 mm | SAPIEN 3 | Evolut R/Pro | 86 ± 5.3 | 86.3 ± 3.8 | 57 (82.6) | 58 (84.1) | 1.3 (1.3–1.4)* | 1.4 (1.3–1.5)* | 21.4 (19.0–23.3)* | 21.4 (19.5–23.3)* |
| Kornyeva et al. | 2023 | PSM | 192 | 192 | 3 years | new generation | Annular perimeter < 72 mm or aortic annulus area < 400m m2 | SAPIEN 3 | Evolut R/PRO, Acurate neo-2, and Portico THV | 81 ± 6 | 81 ± 7 | 153 (80) | 141 (73 %) | 1.8 ± 0.2 | 1.8 ± 0.2 | 25.96 ± 8.33 | 27.03 ± 8 |
| Okuno et al. (Swiss TAVI) | 2023 | PSM | 171 | 171 | 5 years | early and new devices | Annular area < 430 mm2 | SAPIEN XT/3/3Ultra | CoreValve, Evolut R/PRO/PRO+ | 82.7 ± 6.4 | 82.2 ± 6.2 | 85.40 % | 84.20 % | NR | NR | 26.5 ± 5.6 | 26.9 ± 6.1 |
| Herrmann et al. (SMART Trial) | 2024 | RCT | 361 | 355 | 1 year | new generation | Annulus area of 430 mm2 or less | SAPIEN 3/3 Ultra | Evolut PRO/PRO+/FX | 80.3 ± 6.1 | 80.1 ± 6.3 | 309 (85.6) | 312 (87.9) | 1.8 ± 0.2 | 1.8 ± 0.2 | NR | NR |
| Baudo et al. | 2024 | PSM | 109 | 109 | 2 years | new generation | Annulus area ≤ 430 mm2 | SAPIEN 3/3 Ultra | Evolut PRO/PRO+/FX | 80.5 ± 7.8 | 81.0 ± 7.7 | 83 (76.1) | 91 (83.5) | 1.82 ± 0.27 | 1.80 ± 0.27 | 28.2 ± 6.5 | 28.1 ± 6.9 |
|
Scotti et al. (OPERA-TAVI) |
2024 | PSM | 251 | 251 | 1 year | New generation | aortic annular area < 430 mm2 | SAPIEN Ultra | Evolut PRO/PRO+ | 82.42 (77.97–86.66)* | 82.34 (78.06–86.00)* | 174 (69.3) | 186 (74.1) | NR | NR | 25.78 (23.08–29.38)* | 26.17 (22.92–30.05) |
| Kalogeras et al. | 2023 | PSM | 139 | 139 | 1 year | New generation | Patients treated with an Edwards SAPIEN 3 or Ultra valve ≤ 23 mm or an Evolut PRO/PRO+ ≤26 mm were included in the “small THV” cohort |
SAPIEN S3/Ultra | Evolut PRO/PRO+ | 83 (77–87)* | 83 (78–88)* | 110 (79.1) | 114 (82) | NR | NR | 26.8 (24.4–32)* | 26.6 (23–30.2)* |
BEV: Balloon Expandable Valve; SEV: Self-Expanding Valve; PSM: propensity score matching; RCT: randomized controlled trial; SAA: Small aortic annulus; BSA: body surface area; BMI: body mass index; n: number; NR: not reported.
Data are given as median and interquartile range.
The bias assessment of the included RCTs showed some concerns in the CHOICE trial, primarily due to nonblinding (Figures S2 and S3). Some concerns were observed in 3 PSM studies due to bias in outcomes measurement (Figures S4 and S5).
3.2. Results of meta-analysis
3.2.1. Hemodynamic outcomes
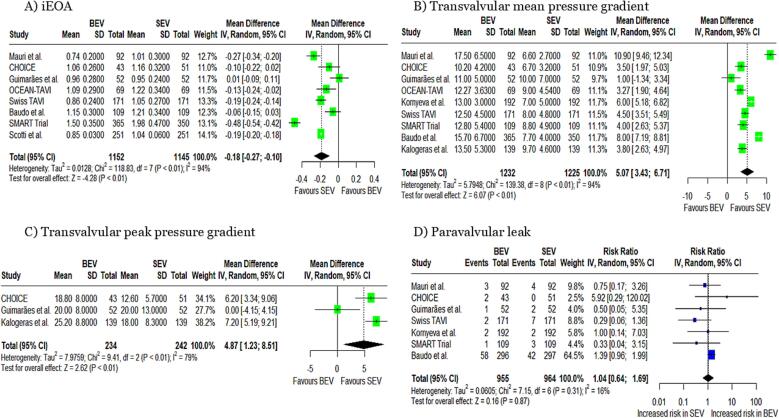
iEOA: The pooled analysis demonstrated that the implantation of BEVs was associated with a significantly smaller iEOA compared to SEVs (MD: −0.18 cm2/m2, 95 % CI: −0.27 to −0.10, p < 0.01, Fig. 1A). The analysis demonstrated significant heterogeneity among the included studies (I2 = 94 %).
Fig. 1.
Forest plots for (A) iEOA, (B) transvalvular mean pressure gradient, (C) transvalvular peak pressure gradient, and (D) paravalvular leak iEOA; indexed effective orifice area.
Transvalvular mean pressure gradient: BEVs were associated with a significantly higher transvalvular mean pressure gradient compared to SEVs (MD: 5.07 mmHg, 95 % CI: 3.43 to 6.71, p < 0.01, Fig. 1B). Significant heterogeneity was observed (I2 = 94 %).
Transvalvular peak pressure gradient: Patients with BEVs experienced a significantly greater peak pressure gradient compared to SEVs (MD: 4.87 mmHg, 95 % CI: 1.23 to 8.51, p < 0.01, Fig. 1C),. Significant heterogeneity was observed (I2 = 79 %).
3.2.2. Clinical outcomes
Paravalvular leak: The pooled analysis demonstrated a non-significant difference in the risk of paravalvular leak between patients undergoing procedures with BEV compared to SEV. (RR: 1.04, 95 % CI: 0.64 to 1.69, p = 0.87, Fig. 1D). The heterogeneity was low, I2 = 16 %.
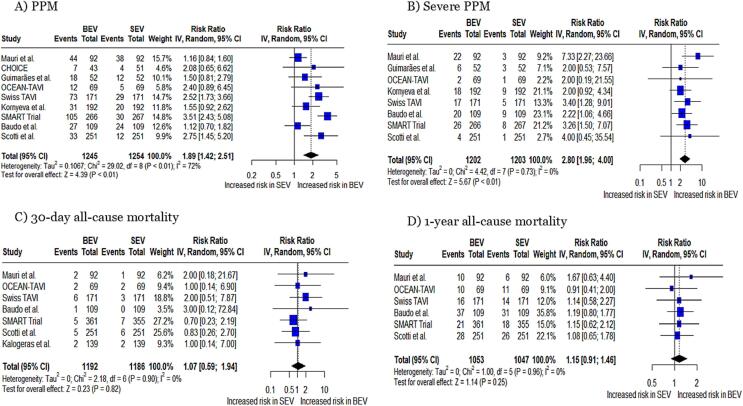
PPM and severe PPM: The pooled analysis demonstrated a significantly higher risk of PPM in patients undergoing TAVR with BEV compared to SEV (RR: 1.89, 95 % CI: 1.42 to 2.51, p < 0.01, Fig. 2A), with a heterogeneity of I2 = 72 %. Moreover, a significantly higher risk of severe PPM was observed in patients undergoing procedures with BEV compared to SEV (RR: 2.80, 95 % CI: 1.96 to 4.0, p < 0.01, Fig. 2B), with no heterogeneity (I2 = 0).
Fig. 2.
Forest plots for (A) PPM, (B) Severe PPM, (C) 30-day all-cause mortality, and (D) 1-year all-cause mortality PPM; patient prosthesis mismatch.
All-cause mortality: Based on the pooled analysis no statistically significant differences were observed for 30-day all-cause (RR: 1.07, 95 % CI: 0.59 to 1.94, p = 0.82, Fig. 2C) and 1-year all-cause mortality (RR: 1.15, 95 % CI: 0.91 to 1.46, p = 0.25, Fig. 2D) between two groups with no heterogeneity (I2 = 0 %).
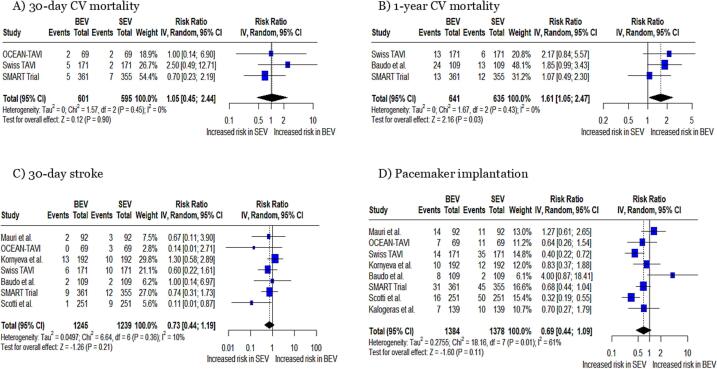
Cardiovascular mortality: No significant difference was observed in 30-day CV mortality (RR: 1.05, 95 % CI: 0.45 to 2.44, p = 0.90 Fig. 3A). 1-year cardiovascular mortality was significantly increased in patients with BEVs compared to those with SEVs (RR: 1.61, 95 % CI: 1.05 to 2.47, p = 0.03, Fig. 3B).
Fig. 3.
Forest plots for (A) 30-day CV mortality, (B) 1-year CV mortality, (C) 30-day stroke, and (D) pacemaker implantation CV; cardiovascular.
Any stroke − 30 days: The pooled analysis demonstrated a nonsignificant difference in risk of the occurrence of stroke at 30 days (RR: 0.73, 95 % CI: 0.44 to 1.19, p = 0.21, Fig. 3C).
Pacemaker implantation: The requirement of pacemaker implantation between the two groups demonstrated a nonsignificant difference between the two groups (RR: 0.69, 95 % CI: 0.44 to 1.09, p = 0.11, Fig. 3D), with a heterogeneity of I2 = 61 %.
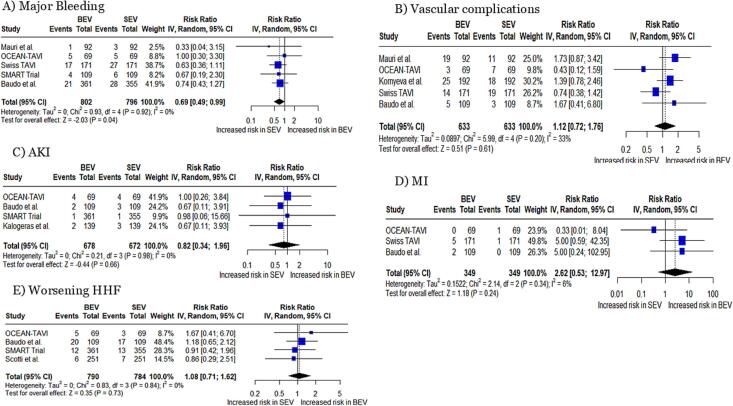
Major bleeding: A significantly decreased risk of major bleeding was observed in BEV as compared to SEV (RR: 0.69, 95 % CI: 0.49 to 0.99, p = 0.04, Fig. 4A), with no heterogeneity (I2 = 0 %).
Fig. 4.
Forest plots for (A) major bleeding, (B) vascular complications, (C) AKI, (D) MI, and (E) Worsening HHF AKI; acute kidney injury, HHF; heart failure-related hospitalizations.
Vascular complications, AKI, MI, and worsening HHF: A non-significant difference was observed in the risk of vascular complications (RR: 1.12, 95 % CI: 0.72 to 1.76, p = 0.61, I2 = 33 %, Fig. 4B), AKI (RR: 0.82, 95 % CI: 0.34 to 1.96, p = 0.66, I2 = 0 %, Fig. 4C), MI (RR: 2.62, 95 % CI: 0.53 to 12.97, p = 0.24, I2 = 6 %, Fig. 4D), and worsening HHF (RR: 1.08, 95 % CI: 0.71 to 1.62, p = 0.73, I2 = 0 %, Fig. 4E), between BEV and SEV.
3.3. Sub-group analysis based on study design
Based on the study design (RCTs vs. PSM studies), there were non-significant subgroup differences for iEOA, transvalvular mean pressure gradient, severe PPM, paravalvular leak, and transvalvular peak pressure gradient (Figures S6-10). However, the analysis for PPM demonstrated significant subgroup interactions based on the study design (FigureS11).
The two study designs (RCTs and PSM studies) demonstrated non-significant subgroup differences for all-cause and CV mortality, pacemaker implantation, any major bleeding, AKI, worsening HHF, and the occurrence of stroke within 30 days (Figures S12-20).
3.4. Sub-group analysis based on valve generation
Sub-group analysis was also performed based on valve generation, dividing them into “new”, “early” and “new and early” generation devices. Studies with “new and early” and “new” generation valves showed comparable results in terms of iEOA, mean pressure gradient, PPM, severe PPM, paravalvular leak, all-cause and CV mortality, stroke at 30 days, major bleeding, vascular complications, MI, and pacemaker implantation (Figures S21-S34). However, a significant subgroup interaction was observed based on valve generation for transvalvular peak pressure gradient (Figures S35).
3.5. Sensitivity analysis
Sensitivity analysis, using the leave-one-out method, was also performed, whereby each study was sequentially removed to assess whether it strongly influenced the overall pooled analysis. Heterogeneity did not reduce significantly in iEOA, transvalvular mean pressure gradient, PPM, pacemaker implantation, and any vascular complication. Upon omitting Swis TAVI and Baudo et al, heterogeneity in paravalvular leak reduced to 0 %. In MI, removing OCEAN TAVI also decreased the heterogeneity to 0 % (Figures S36-39).
4. Discussion
This systematic review and meta-analysis incorporating 10 studies evaluated the clinical outcomes and hemodynamic performance of SEVs versus BEVs in 2,960 patients with SAA. We report that implanting a SEV was linked to a larger iEOA and a lower transvalvular mean pressure gradient, transvalvular peak pressure gradient, lower rates of moderate and severe PPM, and lower one-year CV mortality rates.
Valve management in patients with SAA is a pressing concern and has become an issue of primary clinical attention [21]. Previous studies have revealed that TAVI may be a better approach than surgery in such patients due to the higher iEOA, lower mean aortic gradient, and lower rates of PPM [5], [22]. Although the findings from previous clinical studies advocate for TAVI in patients with SAA, it is not established which type of valve system (SEV or BEV) is superior and also this strategy has not been tested in younger populations with longer lifespans and at lower surgical risk.
Our results were consistent with those of the previous meta-analysis [10] regarding the risk of PPM, severe PPM, 1-year all-cause mortality, iEOA, and transvalvular mean pressure gradient. However, in contrast to our study, the previous analysis found a higher risk of PVL, permanent pacemaker implantation, and 30-day stroke in patients with SEVs, with no difference in cardiac-related mortality between the groups. This difference could be possibly attributed to the non-inclusion of the most recent PSM studies [15], [16], [17], [19] and the SMART trial in the prior meta-analysis. The meta-analysis by Di Pietro et al. [23] also reported a better hemodynamic performance with SEVs when compared with BEVs. Moreover, the meta-analyses comparing TAVI with surgical aortic valve replacement have reported comparable long-term clinical outcomes in low-risk patients [24], [25]. SEV implantation was associated with a significantly larger post-operative iEOA, and significantly reduced risk of PPM and severe PPM in our study. The recognition of BEV implantation as an independent predictor for PPM (3 % with SEV versus 22 % with BEV) in a prior study further corroborates our finding [13].
The transvalvular mean pressure gradient is an important hemodynamic parameter to assess aortic stenosis severity (AS). A high gradient is correlated with severe symptoms and is linked to a worse prognosis. The lower transvalvular mean pressure gradient in SEVs compared to BEVs demonstrated by our analysis reinforces the evidence for improved hemodynamic performance and better clinical outcomes for patients with SEV implantation. It is well-accepted that iEOA is a significant predictor of cardiac mortality rates in patients with PPM following TAVI [26]. The lower risk of 1-year cardiovascular mortality observed in patients with SEVs in our analysis could plausibly be linked to a larger post-operative iEOA and a lower risk of PPM compared to patients with BEVs noted in our study.
Although we identified a lower transvalvular mean pressure gradient, rate of PPM, severe PPM, and risk of 1-year cardiovascular mortality in SEVs, there was a higher risk of major bleeding events in patients with SEVs. The presence of major bleeding events could be negatively associated with survival and poorer clinical outcomes [27]. Moreover, we observed a nonsignificant difference in all-cause mortality between BEVs and SEVs in our analysis, which could be explained by the relatively low sample size and short follow-up periods in most of the included studies to identify a difference in mortality. Head et al. [4] demonstrated that PPM was associated with an increased risk of all-cause and CV mortality over long-term follow-up. Our analysis also reported decreased one-year all-cause mortality and 30-day all-cause mortality with SEV compared to BEV, though these findings did not reach statistical significance. It can be postulated that in studies with long-term follow-ups, this benefit may be more pronounced. The only study included in our analysis having a follow-up duration of 5 years documented no significant difference in all-cause and CV mortality [16].This emphasizes the need for future studies with longer follow-up periods to help us better comprehend the long-term outcomes of SEVs and BEVs in patients with SAA. The LANDMARK RCT compared Myval prosthesis with contemporary THVs (SAPIEN or EVOLUT) in patients with severe AS and demonstrated the non-inferiority of Myval to the contemporary prosthesis [28].
A EuroHeart Survey reported that AS was the most common left-sided valve disease among patients in the Mediterranean and Europe with an incidence of 43.1 % [29]. While in the United States around 0.5 million people are reported to have severe AS [1]. However, the epidemiological data for Asia, Africa and South America is not available [30].
4.1. Clinical Implications and future Directions
In light of evidence from our meta-analysis, we can suggest that SEVs, with their improved hemodynamic performance, reduced risk of PPM and cardiac-related mortality, can lead to enhanced cardiac function and improved survival in patients with smaller annuli. However, the higher risk of major bleeding events necessitates cautious patient selection and management, especially for those with elevated bleeding risk. This emphasizes the significance of individualized treatment plans and close monitoring post-implantation. The limited number of RCTs comparing SEVs and BEVs highlight the need for future high-powered RCTs with long-term follow-ups to support the results of this meta-analysis and to assess if the superior hemodynamic performance of SEVs translates into improved long-term survival benefits. Additionally, further research is needed to develop strategies to mitigate the bleeding risks associated with SEVs.
4.2. Limitations
When interpreting the findings of this meta-analysis, it is important to recognize its limitations. The outcome data were not adjusted based on individual risk profiles, as our analysis did not utilize patient-level data. Though PSM provides balanced groups, there is still a possibility of bias due to unrecognized or unmeasured confounding, an inherent challenge in all observational studies. The definitions used for SAA were not uniform across the studies and the criteria for PPM/severe PPM was inconsistently reported. Significant heterogeneity was observed in some of the outcomes, which is expected in nonrandomized studies and may have reduced the reliability of the results. This stems from the inclusion of observational studies as well as differences in follow‐up durations. The included studies had relatively short follow-ups to identify subtle differences between SEVs and BEVs, and potential survival and hemodynamic differences between the two THVs may become more evident over time with the availability of studies with longer follow-ups. Due to the limited availability of data, we were unable to conduct a subgroup analysis based on the annular design of valves (intra-annular vs supra-annular). RCTs with longer follow-ups are required to assess whether the better hemodynamic performance of SEVs leads to improved hard clinical outcomes like all-cause death. Although our meta-analysis represents current TAVI practice, the mean age was over 80 years. Therefore, the results may not be generalizable to younger patients.
5. Conclusion
This meta-analysis of 2960 patients with SAA undergoing TAVI demonstrated that BEVs had a worse hemodynamic performance as determined by the higher risk of moderate and severe PPM compared to SEVs. Moreover, the use of BEVs was associated with a higher risk of 1-year cardiovascular mortality. Further large-scale RCTs are required to confirm the generalizability of our findings.
Statements and Declarations.
Ethical Approval: No ethical approval was required for the study.
Consent: No consent was needed.
Financial Support
No financial support was received for the study
CRediT authorship contribution statement
Mushood Ahmed: Writing – original draft, Visualization, Validation, Supervision, Resources, Conceptualization. Areeba Ahsan: Writing – original draft, Methodology, Data curation. Shehroze Tabassum: Writing – review & editing, Writing – original draft. Irra Tariq: Writing – original draft, Methodology, Data curation. Eeshal Zulfiqar: Writing – review & editing, Writing – original draft. Mahnoor Farooq Raja: Writing – original draft. Asma Mahmood: Writing – original draft. Raheel Ahmed: Writing – review & editing, Supervision. Farhan Shahid: Writing – review & editing, Supervision, Resources. Syed Khurram M. Gardezi: Supervision, Project administration. Mahboob Alam: Writing – review & editing, Supervision. Rodrigo Bagur: Writing – review & editing, Software. Mamas A. Mamas: Writing – review & editing, Writing – original draft, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101542.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Symptomatic Severe Aortic Stenosis | IntechOpen [Internet]. [cited 2024 Jul 28]. Available from: https://www.intechopen.com/chapters/82317.
- 2.Cribier A., Eltchaninoff H., Bash A., Borenstein N., Tron C., Bauer F., et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 3.Swift S.L., Puehler T., Misso K., Lang S.H., Forbes C., Kleijnen J., et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis: a systematic review and meta-analysis. BMJ Open. 2021;11(12):e054222. doi: 10.1136/bmjopen-2021-054222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Head S.J., Mokhles M.M., Osnabrugge R.L.J., Pibarot P., Mack M.J., Takkenberg J.J.M., et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur Heart J. 2012;33(12):1518–1529. doi: 10.1093/eurheartj/ehs003. [DOI] [PubMed] [Google Scholar]
- 5.Guimarães L., Voisine P., Mohammadi S., Kalavrouzioutis D., Dumont E., Doyle D., et al. Valve hemodynamics following transcatheter or surgical aortic valve replacement in patients with small aortic annulus. Am J Cardiol. 2020;125(6):956–963. doi: 10.1016/j.amjcard.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372) doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Paule R.C., Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand 1977. 1982;87(5):377–385. doi: 10.6028/jres.087.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cochrane Handbook for Systematic Reviews of Interventions | Wiley Online Books [Internet]. [cited 2024 Jul 28]. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119536604.
- 10.Hosseinpour A., Gupta R., Kamalpour J., Hosseinpour H., Chaturvedi A., Agrawal A., et al. Balloon-expandable versus self-expanding transcatheter aortic valve implantation in patients with small aortic annulus: a meta-analysis. Am J Cardiol. 2023;1(204):257–267. doi: 10.1016/j.amjcard.2023.07.100. [DOI] [PubMed] [Google Scholar]
- 11.Abdelghani M., Mankerious N., Allali A., Landt M., Kaur J., Sulimov D.S., et al. Bioprosthetic valve performance after transcatheter aortic valve replacement with self-expanding versus balloon-expandable valves in large versus small aortic valve annuli: insights from the CHOICE Trial and the CHOICE-Extend registry. JACC Cardiovasc Interv. 2018;11(24):2507–2518. doi: 10.1016/j.jcin.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann H.C., Mehran R., Blackman D.J., Bailey S., Möllmann H., Abdel-Wahab M., et al. Self-expanding or balloon-expandable TAVR in patients with a small aortic annulus. N Engl J Med. 2024;390(21):1959–1971. doi: 10.1056/NEJMoa2312573. [DOI] [PubMed] [Google Scholar]
- 13.Mauri V., Kim W.K., Abumayyaleh M., Walther T., Moellmann H., Schaefer U., et al. Short-term outcome and hemodynamic performance of next-generation self-expanding versus balloon-expandable transcatheter aortic valves in patients with small aortic annulus: a multicenter propensity-matched comparison. Circ Cardiovasc Interv. 2017;10(10):e005013. doi: 10.1161/CIRCINTERVENTIONS.117.005013. [DOI] [PubMed] [Google Scholar]
- 14.Hase H., Yoshijima N., Yanagisawa R., Tanaka M., Tsuruta H., Shimizu H., et al. Transcatheter aortic valve replacement with Evolut R versus Sapien 3 in Japanese patients with a small aortic annulus: The OCEAN-TAVI registry. Catheter Cardiovasc Interv off J Soc Card Angiogr Interv. 2021;97(6):E875–E886. doi: 10.1002/ccd.29259. [DOI] [PubMed] [Google Scholar]
- 15.Kornyeva A., Burri M., Lange R., Ruge H. Self-expanding vs. balloon-expandable transcatheter heart valves in small aortic annuli. Front Cardiovasc Med. 2023;3(10):1175246. doi: 10.3389/fcvm.2023.1175246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno T., Tomii D., Lanz J., Heg D., Praz F., Stortecky S., et al. 5-Year outcomes with self-expanding vs balloon-expandable transcatheter aortic valve replacement in patients with small annuli. JACC Cardiovasc Interv. 2023;16(4):429–440. doi: 10.1016/j.jcin.2022.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Baudo M., Sicouri S., Yamashita Y., Ridwan K., Kadri A., Goldman S.M., et al. Improved hemodynamics with self-expanding compared to balloon-expandable transcatheter aortic valve implantation in small annulus patients: a propensity-matched analysis. Am J Cardiol. 2024;15(221):9–18. doi: 10.1016/j.amjcard.2024.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Kalogeras K., Jabbour R.J., Pracon R., Kabir T., Shannon J., Duncan A., et al. Midterm outcomes in patients with aortic stenosis treated with contemporary balloon-expandable and self-expanding valves: does valve size have an impact on outcome? J Am Heart Assoc. 2023;12(11):e028038. doi: 10.1161/JAHA.122.028038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scotti A., Sturla M., Costa G., Saia F., Pilgrim T., Abdel-Wahab M., et al. Evolut PRO and SAPIEN ULTRA performance in small aortic annuli: The OPERA-TAVI registry. JACC Cardiovasc Interv. 2024;17(5):681–692. doi: 10.1016/j.jcin.2024.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Wahab M., Mehilli J., Frerker C., Neumann F.J., Kurz T., Tölg R., et al. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311(15):1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima M., Watanabe Y. Transcatheter aortic valve implantation in small anatomy: patient selection and technical challenges. Interv Cardiol Rev. 2018;13(2):66–68. doi: 10.15420/icr.2017:28:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamioka N., Arita T., Hanyu M., Hayashi M., Watanabe S., Miura S., et al. Valve hemodynamics and clinical outcomes after transcatheter aortic valve replacement for a small aortic annulus. Int Heart J. 2019;60(1):86–92. doi: 10.1536/ihj.17-656. [DOI] [PubMed] [Google Scholar]
- 23.Di Pietro G., Improta R., Bruno F., De Filippo O., Leone P.P., Nebiolo M., et al. Impact of small aortic annuli on the performance of transcatheter aortic valve replacement bioprostheses: an updated meta-analysis of recent studies. Am J Cardiol. 2024;15(229):1–12. doi: 10.1016/j.amjcard.2024.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Di Pietro G., Improta R., De Filippo O., Bruno F., Birtolo L.I., Tocci M., et al. Transcatheter aortic valve replacement in low surgical risk patients: an updated metanalysis of extended follow-up randomized controlled trials. Am J Cardiol. 2024;1(224):56–64. doi: 10.1016/j.amjcard.2024.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Caminiti R., Ielasi A., Vetta G., Parlavecchio A., Rocca D.G.D., Glauber M., et al. Long-term results following transcatheter versus surgical aortic valve replacement in low-risk patients with severe aortic stenosis: a systematic review and meta-analysis of randomized trials. Am J Cardiol. 2024;1(230):6–13. doi: 10.1016/j.amjcard.2024.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Lin Y., Kang B., Wang Z. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardio-Thorac Surg off J Eur Assoc Cardio-Thorac Surg. 2014;45(2):234–240. doi: 10.1093/ejcts/ezt245. [DOI] [PubMed] [Google Scholar]
- 27.Ullah W., Jafar M., Zahid S., Ahmed F., Khan M.Z., Sattar Y., et al. Predictors of in-hospital mortality in patients with end-stage renal disease undergoing transcatheter aortic valve replacement: a nationwide inpatient sample database analysis. Cardiovasc Revascularization Med Mol Interv. 2022;34:63–68. doi: 10.1016/j.carrev.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Baumbach A., van Royen N., Amat-Santos I.J., Hudec M., Bunc M., Ijsselmuiden A., et al. LANDMARK comparison of early outcomes of newer-generation Myval transcatheter heart valve series with contemporary valves (Sapien and Evolut) in real-world individuals with severe symptomatic native aortic stenosis: a randomised non-inferiority trial. Lancet Lond Engl. 2024;403(10445):2695–2708. doi: 10.1016/S0140-6736(24)00821-3. [DOI] [PubMed] [Google Scholar]
- 29.Iung B., Baron G., Butchart E.G., Delahaye F., Gohlke-Bärwolf C., Levang O.W., et al. A prospective survey of patients with valvular heart disease in europe: The euro heart survey on valvular heart disease. Eur Heart J. 2003;24(13):1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 30.Thaden J.J., Nkomo V.T., Enriquez-Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56(6):565–571. doi: 10.1016/j.pcad.2014.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.