Abstract
Purpose: To evaluate the use of antivascular endothelial growth factor (anti-VEGF) as treatment for tractional retinal detachments (TRDs) involving the macula. Methods: A case report was evaluated. Results: A 40-year-old man with a medical history notable for congenital heart disease and proliferative diabetic retinopathy presented with decreased vision. Surgery was not feasible for medical reasons, and panretinal photocoagulation was limited by retinal traction and hemorrhage. Despite initial progression of bilateral macula-off, foveal-on TRDs, the patient received intravitreal (IVT) bevacizumab regularly every 8 to 12 weeks. After initial improvement, both eyes remained anatomically stable with attached foveas over more than 1.5 years of follow-up. Conclusions: In cases in which surgery is not possible, macula-involving TRDs with neovascularization can be managed successfully with repeated IVT anti-VEGF injection monotherapy.
Keywords: antivascular endothelial growth factor, surgery, tractional retinal detachment
Introduction
Tractional retinal detachments (TRDs) in the setting of proliferative diabetic retinopathy (PDR) that involve the macula are typically managed surgically.1 –3 Practice patterns have been evolving toward earlier surgical intervention for TRDs because release of the hyaloidal adhesions via vitrectomy can limit progression.2 –7
Although preoperative injections of antivascular endothelial growth factor (anti-VEGF) are common within a week of surgical intervention to reduce intraoperative bleeding, they are not commonly used as primary management strategy for macula-involving TRDs.4,8 –11 Notably, there is the risk for anti-VEGF–associated crunch syndrome, in which rapid neovascular regression and posterior hyaloid contraction and fibrosis can lead to the progression of a TRD and new retinal holes.12,13 The development of new retinal breaks can result in a combined tractional and rhegmatogenous RD, which portends a worse prognosis. The incidence of crunch syndrome in eyes with PDR treated with anti-VEGF has varied in the literature, from 1.5% to 18.4% (most commonly around 5%).13 –18 Crunch syndrome may be more likely in the setting of severe PDR with preexisting fibrotic changes and neovascularization (NV). 13
Although macula-involving TRDs are managed surgically, there have been rare reports of TRDs spontaneously improving without treatment.19 –21 In addition, Lee et al 22 reported 3 eyes in which TRDs showed resolution as a result of favorable crunch syndrome after anti-VEGF injections and another eye without detachment that showed anatomic improvement from release of the traction.
We present a case of bilateral macula-involving, fovea-sparing TRDs, with resolution of vitreous hemorrhage and stability of the TRD, primarily with the use of anti-VEGF therapy.
Case Report
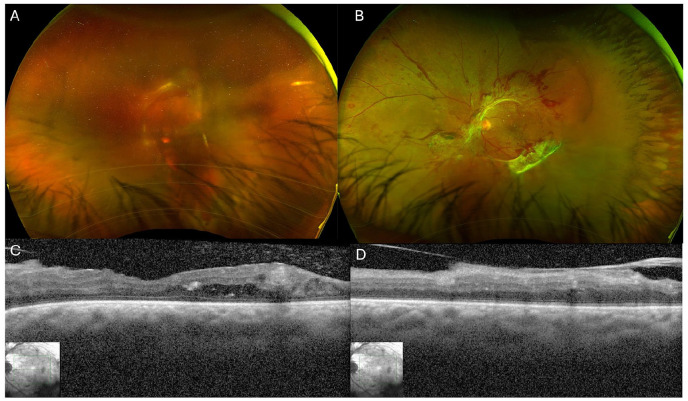
A 40-year-old man presented with decreased vision in both eyes. His medical history included hypertension, hyperlipidemia, sleep apnea, insulin-dependent diabetes, chronic obstructive pulmonary disease, congenital heart disease (D-transposition of the great arteries) for which he had atrial switch repair as a child, sick sinus syndrome with a pacemaker that had been malfunctioning for several years, and coronary artery disease with a history of myocardial infarction. The Snellen best-corrected visual acuity (BCVA) was hand motions (HM) OD and 20/125 OS. A fundus examination showed dense vitreous hemorrhage in the right eye that precluded view of the fundus and PDR with NV at the disc, NV elsewhere, and a fibrovascular membrane at the arcades causing mild macular traction in the left eye, as was also seen on optical coherence tomography (OCT). Figure 1 shows widefield pseudocolor photographs of both eyes and OCT images of the left eye.
Figure 1.
Fundus photographs from the patient’s initial presentation. (A) The right eye shows vitreous hemorrhage with some partially visible fibrovascular proliferation. (B) The left eye shows neovascularization (NV) of the disc, NV elsewhere, hemorrhages, and fibrovascular proliferation along the arcades. Optical coherence tomography (OCT) of the left eye shows an attached fovea with some diabetic macular edema and traction in the superior macula. (C) View through the fovea. (D) View through the superior macula. The OCT image quality of the right eye was poor because of the presence of vitreous hemorrhage and is not shown.
Panretinal photocoagulation (PRP) was performed in the left eye, although the entire treatment could not be completed because of a subhyaloid hemorrhage, traction, and patient intolerance. Given the dense vitreous hemorrhage in the right eye, the patient was taken to the operating room for planned pars plana vitrectomy, laser photocoagulation, and intravitreal (IVT) bevacizumab. Because of systemic concerns, the surgery was performed under monitored anesthesia care with a block. The patient became acutely claustrophobic and hypoxic during surgery; thus, the procedure was stopped just after the core vitrectomy and before laser coagulation could be performed.
At follow-up 1 month later, VA was 20/50 OD and 20/100 OS. A fundus examination showed wolf-jaw configuration TRD in the right eye, although the fovea remained attached (Figure 2, A–B). At this time, the left eye was also found to have a fovea-involving TRD (Figure 3, A–B). IVT bevacizumab was administered in the right eye, and surgery was recommended for the left eye.
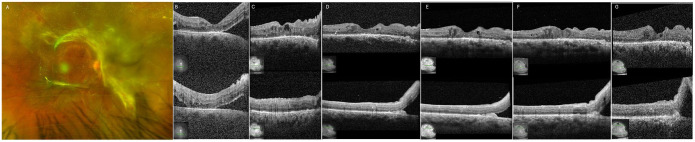
Figure 2.
(A) Fundus photograph of the right eye 1 month after incomplete vitrectomy. (B) Optical coherence tomography (OCT) of the right eye shows improvement 1 month postoperatively, at which time intravitreal (IVT) bevacizumab is given, stabilizing the eye. (C) After being lost to follow-up for 1 year, the patient presented again with reattachment of the fovea, although some traction continued to affect the inferior macula. (D) Approximately 3 months later, the patient received IVT bevacizumab at an outside hospital. Five months after the patient returned after loss to follow-up (2 months after IVT bevacizumab), a focal tractional retinal detachment is seen in the inferior macula but the macula is attached. He has subsequently received regular IVT bevacizumab in the right eye, and his examinations remained stable. OCT 3 months (E), 5 months (F), and 14 months (G) after the patient returned after loss to follow-up.
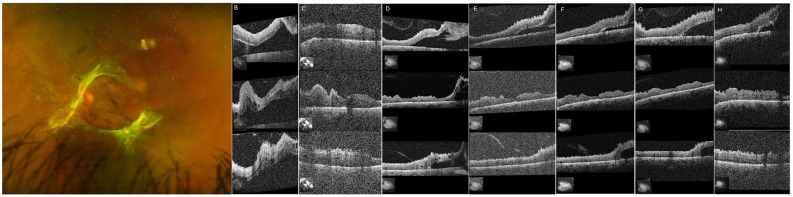
Figure 3.
(A) Fundus photograph of the left eye 1 month after partial panretinal photocoagulation (PRP) of the left eye. (B) One month after partial PRP, optical coherence tomography (OCT) shows improvement, then stability. (C) After being lost to follow-up for 1 year, the patient presented with reattachment of the fovea. (D) About 3 months later, the patient received intravitreal (IVT) bevacizumab at an outside hospital. Five months later (2 months after IVT bevacizumab), the patient presented again, with superior and inferior tractional retinal detachments and an attached fovea. He has subsequently received regular IVT bevacizumab in the left eye, with his examinations remaining stable. OCT 3 months (E), 5 months (F), 7 months (G), and 14 months (H) after the patient returned after loss to follow-up.
The patient attempted to have surgery at this point at an outside hospital; however, this was not possible, and he was lost to follow-up for 1 year. No surgeries, injections, laser treatments, or procedures were performed until the patient presented again 1 year later. At that time, the BCVA was counting fingers (CF) OD and 20/600 OD. Although a fundus examination showed worsened traction in the right eye and a new vitreous hemorrhage, the retina remained attached centrally (Figure 2C). Some improvement in traction was seen in the left eye; however, a TRD with extensive fibrovascular membranes remained, as did NV elsewhere as well as a vitreous hemorrhage (Figure 3C). The recommendation was made to perform a vitrectomy, membrane peeling, endolaser, gas placement, and injection of anti-VEGF under general anesthesia in the right eye followed by the left eye. However, surgery did not proceed because of the anesthesiologist’s concerns regarding the safety of the procedure, despite multiple attempts at various tertiary referral hospitals.
Five months later, the patient’s BCVA was HM OD and 20/100 OS with a stable examination. OCT showed an attached fovea in the right eye with a focal TRD in the inferior macula (Figure 2D) and an attached fovea with superior and inferior traction and TRDs in the left eye (Figure 3D). IVT bevacizumab every 8 to 12 weeks and as needed was initiated, primarily for vitreous hemorrhage and NV, with occasional longer intervals caused by intermittent difficulties with follow-up. Extending the IVT bevacizumab injection intervals resulted in the development of vitreous hemorrhage in both eyes. After the initial improvement in the TRDs, both eyes subsequently remained stable with numerous anti-VEGF injections. The foveas remained attached bilaterally, and the vitreous hemorrhage resolved over the subsequent 1.5 years (Figure 2, E–G and Figure 3, E–H). The patient’s BCVA remained stable at 20/100 OD and fluctuated between 20/160 and CF OS. In addition, the hyaloid remained attached.
Conclusions
We present a case of a patient with bilateral macula-involving, foveal-sparing TRDs that were managed successfully primarily with IVT anti-VEGF injections in the long-term without developing crunch syndrome. In this patient’s case, the management options were limited. There was minimal uptake of PRP given the traction, hemorrhage, and difficulties with tolerance. Specifically, the patient was unable to tolerate in-office laser photocoagulation because of discomfort and positioning. In addition, there was poor laser uptake in the areas of traction, which extended from the arcades into the midperiphery. Multiple attempts at surgery were made but were not possible because of the patient’s high-risk medical comorbidities. There was some release of traction, despite the limited core vitrectomy in the right eye. There was no progression to foveal involvement, despite the high risk for crunch syndrome with NV and the presence of significant traction, and the patient remained stable for more than 1 year.
Diabetic TRDs develop as a downstream effect of retinal ischemia, leading to an upregulation of growth factors, including VEGF, which results in retinal angiogenesis and NV.1,23 The NV then proliferates between the retina and the posterior hyaloid, eventually invading and adhering to the posterior cortical vitreous.1,24,25 Without treatment, a fibrovascular complex forms that may contract and cause traction on the retina.
Previous reports described crunch syndrome, in which anti-VEGF injections may accelerate neovascular regression, fibrosis, and posterior hyaloid contraction. 12 This may induce or exacerbate a TRD or cause a rhegmatogenous component. 13 Crunch syndrome typically manifests 1 to 6 weeks after IVT anti-VEGF and is one of the reasons that TRDs are not classically treated solely with injections. Tan et al 13 found that bevacizumab may be associated with a higher risk for crunch syndrome as well as increased severity of DR and fibrosis. Our case is unique because the safety and efficacy of anti-VEGF injections are shown in a patient with severe PDR and preexisting fibrotic areas. Treatment comprised many bevacizumab injections in both eyes, without development of crunch syndrome. Although this is only a single case, it shows that crunch syndrome is not an inevitable outcome.
Crunch syndrome most commonly has been reported to occur after the initial anti-VEGF injection, most often within 5 days in more than 80% of cases. 13 Thus, it is possible that the lack of crunch syndrome after the initial injection may decrease the risk for its future development. However, there have been no large series supporting this, in particular given that these cases are often taken to surgery.
There have been rare cases of spontaneously improved TRDs.19 –21 In our patient’s case, the traction in the left eye did partially resolve spontaneously for 1 year, at which time he was lost to follow-up and received no treatment. However, the TRD was still present and macula-involving when he presented again. In a cohort of TRDs that spontaneously improved, 19 2 occurred soon after initiation of anti-VEGF injections. These TRDs then spontaneously improved without anti-VEGF injections or other treatment. (Those 2 patients in the cohort were lost to follow-up and returned with improvement of the TRDs.)
There have been reported cases of resolution of TRDs after anti-VEGF injections, termed favorable crunch. In the Lee et al 22 cohort, vitreoretinal traction resolved in 4 eyes with TRDs after anti-VEGF injections, leading to resolution of traction and flattening of the retina in 3 of these eyes. As the authors stated, retinal reattachment in these cases of TRDs without surgery is unusual. In contrast to this cohort of favorable crunch, our patient was initially recommended to have surgery and may have had more severe traction. In addition, our patient received numerous injections while the traction was still present, without retinal flattening, and had no complications.
Factors leading to this patient’s improvement and stability may have included some regression of the NV tethering the vitreous to the retina, allowing for some relaxation. It could also be that the infrequent interval of injections allowed for slower, more controlled regression of the NV that mitigated the crunch; however, this is less likely given that crunch syndrome usually occurs in the setting of the first injection. Changes in the vitreous, including vitreoschisis, have been described in PDR. 26 However, there was no release of the posterior hyaloid face noted on OCT.
In summary, we present a patient with bilateral macular-involving, fovea-sparing TRDs and vitreous hemorrhage treated primarily with repeated bilateral IVT anti-VEGF injections. The patient’s TRDs improved and remained stable for more than 1 year without the development of crunch syndrome. In cases in which surgery may be contraindicated and there are challenges to completing full PRP, anti-VEGF can be a viable monotherapy.
Footnotes
Ethical Approval: This study was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information were performed in a US Health Insurance Portability and Accountability Act–compliant manner.
Statement of Informed Consent: Although the requirement for informed consent was waived given the retrospective nature of the study, the patient provided consent before the study and publication of the case report.
Although the authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article, the following declared financial disclosures: Dr. Patel is a consultant to Regeneron, Dutch Ophthalmic, Genentech, EyePoint Pharmaceuticals, and Alcon Vision. Dr. Kim is a consultant to Ingenia Therapeutics and CureVac AG, receives research support from CureVac AG and Valo Health, and receives grant support from the National Eye Institute (R01EY027739) and US Department of Defense (VR220059).
None of the other authors declared potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding: Dr. Hoyek is supported by the VitreoRetinal Surgery Foundation. Dr. Patel is supported by the Retina Innovation Fund, Massachusetts Eye and Ear, Boston, MA. The funding organizations had no role in the design or conduct of this research.
ORCID iDs: Celine Chaaya  https://orcid.org/0000-0002-1601-885X
https://orcid.org/0000-0002-1601-885X
Nimesh Patel  https://orcid.org/0000-0002-6681-6104
https://orcid.org/0000-0002-6681-6104
References
- 1. Eliott D, Hemeida T. Diabetic traction retinal detachment. Int Ophthalmol Clin. 2009;49(2):153-165. [DOI] [PubMed] [Google Scholar]
- 2. Sokol JT, Schechet SA, Rosen DT, Ferenchak K, Dawood S, Skondra D. Outcomes of vitrectomy for diabetic tractional retinal detachment in Chicago’s county health system. PLoS One. 2019;14:e0220726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan MA, Kuley A, Riemann CD, et al. Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior segment disease. Ophthalmology. 2018;125:423-431. [DOI] [PubMed] [Google Scholar]
- 4. Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian J Ophthalmol. 2018;66:1751-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berrocal MH, Acaba-Berrocal L, Acaba AM. Long-term outcomes of same patient eyes treated with pars plana vitrectomy in one eye and conventional treatment in the other for complications of proliferative diabetic retinopathy. J Clin Med. 2022;11:5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Storey PP, Ter-Zakarian A, Philander SA, et al. Visual and anatomical outcomes after diabetic traction and traction-rhegmatogenous retinal detachment repair. Retina. 2018;38:1913-1919. [DOI] [PubMed] [Google Scholar]
- 7. Shroff CM, Gupta C, Shroff D, Atri N, Gupta P, Dutta R. Bimanual microincision vitreous surgery for severe proliferative diabetic retinopathy: outcome in more than 300 eyes. Retina. 2018;38(suppl 1):S134-S145. [DOI] [PubMed] [Google Scholar]
- 8. Iyer SSR, Regan KA, Burnham JM, Chen CJ. Surgical management of diabetic tractional retinal detachments. Surv Ophthalmol. 2019;64:780-809. [DOI] [PubMed] [Google Scholar]
- 9. Ahn J, Woo SJ, Chung H, Park KH. The effect of adjunctive intravitreal bevacizumab for preventing postvitrectomy hemorrhage in proliferative diabetic retinopathy. Ophthalmology. 2011;118:2218-2226. [DOI] [PubMed] [Google Scholar]
- 10. Al-Kharashi A, Galbinur T, Mandelcorn ED, Muni RH, Nabavi M, Kertes PJ. The adjunctive use of pre-operative intravitreal bevacizumab in the setting of proliferative diabetic retinopathy. Saudi J Ophthalmol. 2016;30:217-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sohn EH, He S, Kim LA, et al. Angiofibrotic response to vascular endothelial growth factor inhibition in diabetic retinal detachment: report no. 1. Arch Ophthalmol. 2012;130:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murali A, Sharma A, Moloney TP. Macula involving tractional retinal detachment after intravitreal ranibizumab – a case of crunch. In Review; 2022. Accessed January 28, 2024. https://www.researchsquare.com/article/rs-1772470/v1
- 13. Tan Y, Fukutomi A, Sun MT, Durkin S, Gilhotra J, Chan WO. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: a review. Surv Ophthalmol. 2021;66:926-932. [DOI] [PubMed] [Google Scholar]
- 14. Torres-Soriano ME, Reyna-Castelán E, Hernández-Rojas M, et al. Tractional retinal detachment after intravitreal injection of bevacizumab in proliferative diabetic retinopathy. Retin Cases Brief Rep. 2009;3:70-73. [DOI] [PubMed] [Google Scholar]
- 15. Arevalo JF, Maia M, Flynn HW, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213-216. [DOI] [PubMed] [Google Scholar]
- 16. Arevalo JF, Sanchez JG, Saldarriaga L, et al. Retinal detachment after bevacizumab. Ophthalmology. 2011;118:2304.e3-2304.e7. [DOI] [PubMed] [Google Scholar]
- 17. Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699-1705. [DOI] [PubMed] [Google Scholar]
- 18. Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927-938. [DOI] [PubMed] [Google Scholar]
- 19. Mahmoudzadeh R, Williamson JE, Salabati M, et al. OCT-based description of spontaneous reattachment of macula-off tractional retinal detachment with significant vision improvement. J Vitreoretin Dis. 2024;8:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kandari FA, Albahlal AA, Algethami RA. Spontaneous resolution of tractional retinal detachment in a type II diabetic patient. Cureus. 2023;15(4):e38010. Accessed January 19, 2024. https://www.cureus.com/articles/121002-spontaneous-resolution-of-tractional-retinal-detachment-in-a-type-ii-diabetic-patient [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tasman W. Retinal detachment secondary to proliferative diabetic retinopathy. Arch Ophthalmol. 1972;87:286-289. [DOI] [PubMed] [Google Scholar]
- 22. Lee IT, Corona ST, Wong TP, Flynn HW, Jr, Wykoff CC. Favorable anti-VEGF crunch syndrome: nonsurgical relief of vitreoretinal traction in eyes with proliferative diabetic retinopathy and tractional retinal detachment. Ophthalmic Surg Lasers Imaging Retina. 2022;53:455-459. [DOI] [PubMed] [Google Scholar]
- 23. Adamis AP, Miller JW, Bernal M-T, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445-450. [DOI] [PubMed] [Google Scholar]
- 24. Kroll P, Rodrigues EB, Hoerle S. Pathogenesis and classification of proliferative diabetic vitreoretinopathy. Ophthalmologica. 2007;221:78-94. [DOI] [PubMed] [Google Scholar]
- 25. Faulborn J, Bowald S. Microproliferations in proliferative diabetic retinopathy and their relationship to the vitreous: corresponding light and electron microscopic studies. Graefes Arch Clin Exp Ophthalmol. 1985;223:130-138. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy: a useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology. 1996;103:323-328. [DOI] [PubMed] [Google Scholar]