Abstract
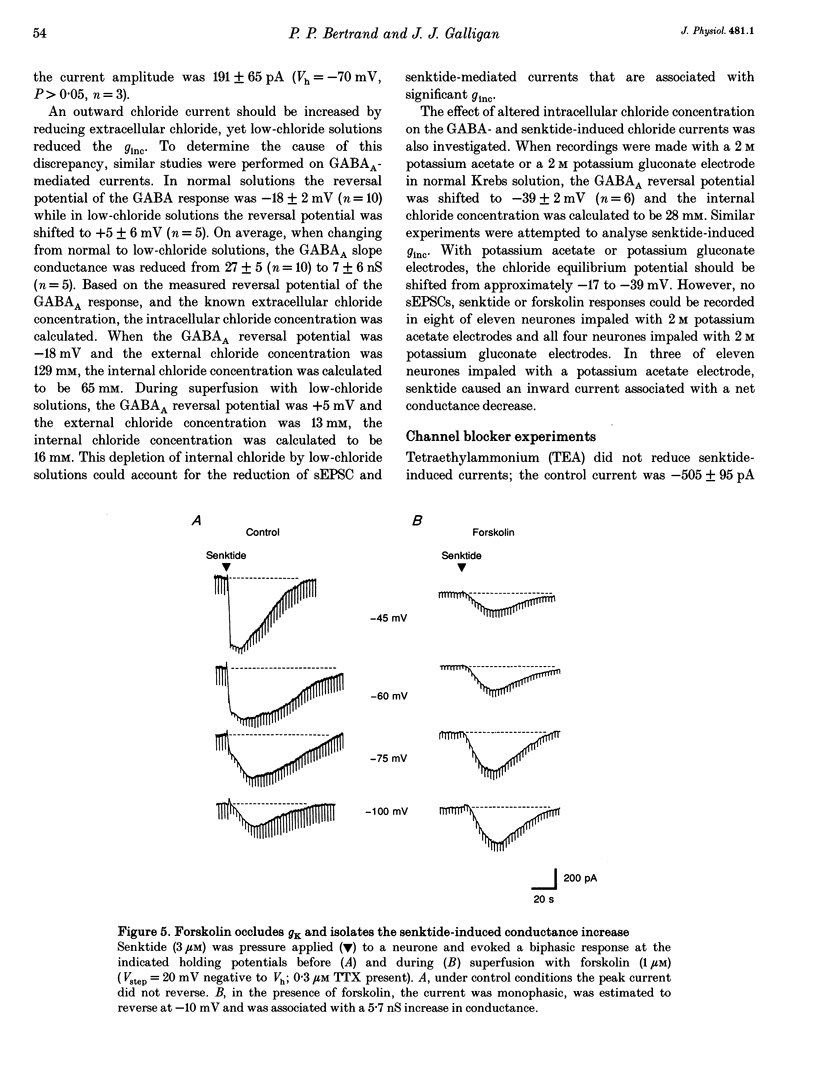
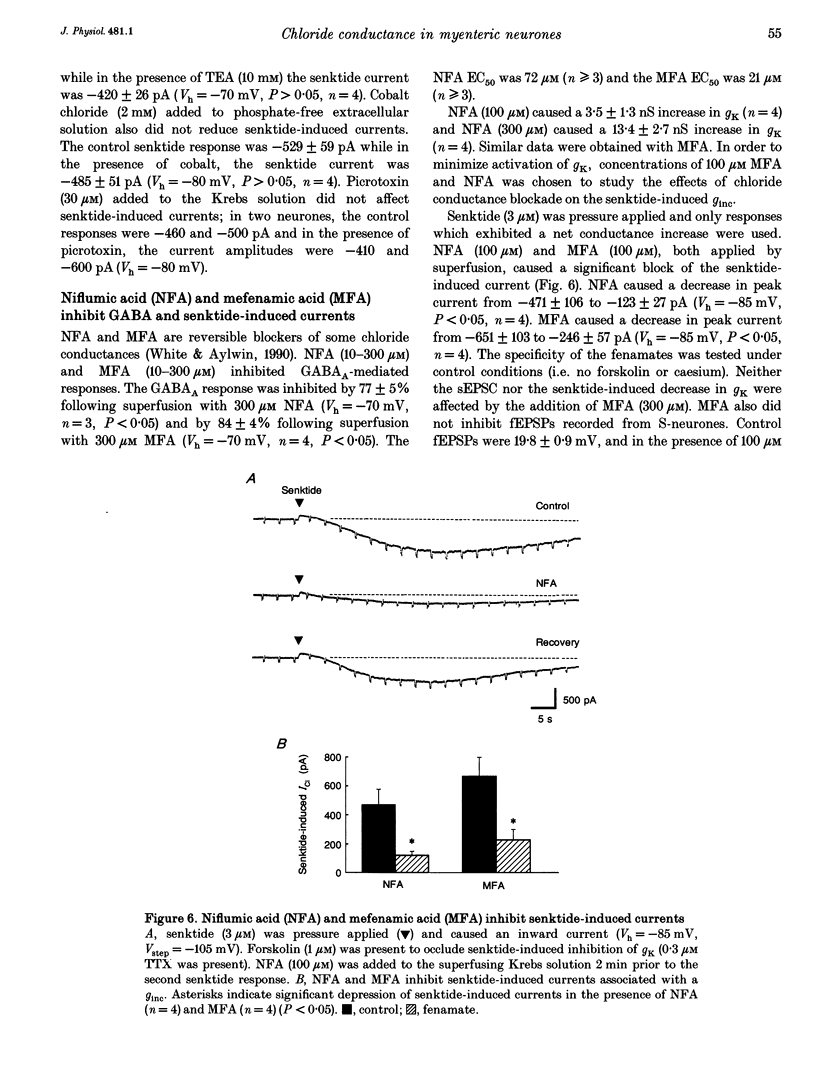
1. Single electrode voltage clamp recordings were obtained from myenteric neurones of guinea-pig ileum in vitro. Slow excitatory postsynaptic currents (sEPSCs) were elicited by focal stimulation of interganglionic nerve strands in twenty-four of thirty neurones more than 30 min after impalement. In seventeen of twenty-four neurones, sEPSCs were associated with a conductance decrease and reversed polarity at -96 +/- 3 mV (near the reversal potential for potassium, EK); this response was due to inhibition of resting potassium conductance, gK. In seven of twenty-four neurones, there was either no net conductance change or a biphasic conductance change during the sEPSC; a reversal potential for peak currents could not be determined. 2. Application of senktide (3 microM), a neurokinin-3 receptor agonist, caused an inward current in forty-one of fifty-three neurones more than 30 min after impalement. In twenty of forty-one neurones, senktide-induced currents were due to inhibition of resting gK. In eleven of forty-one neurones there was either no net conductance change or a biphasic conductance change; a reversal potential for peak currents could not be determined. In ten out of forty-one neurones, senktide-induced currents were associated with a conductance increase (ginc); the estimated reversal potential was -17 +/- 3 mV. 3. Application of forskolin (1 microM) caused an inward current that occluded the decrease in gK caused by senktide and the sEPSC. In neurones in which sESPCs and senktide responses were associated with an unclear or biphasic conductance change, forskolin did not reduce the peak current and residual currents were usually associated with a ginc. 4. In neurones in which senktide-induced currents were associated with a ginc, reducing extracellular Cl- to 13 mM reduced senktide-induced currents by 79%. Reducing extracellular Na+, or adding tetraethylammonium (TEA, 50 mM), cobalt (2 mM) or picrotoxin (30 microM) did not change senktide-induced currents. The chloride transport/channel blockers niflumic acid and mefenamic acid (both at 100 microM) blocked senktide-induced currents. It was concluded that senktide increases chloride conductance (gCl). 5. Chord conductance measurements made between -70 and -90 mV during sEPSCs were used to determine the contribution of an increase in gCl to sEPSCs. These measurements indicated that the peak sEPSC is composed of a 90% decrease in gK and a 10% increase in gCl. Similar data were obtained from measurements made during senktide responses.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Nishimura T., Tokimasa T. Calcium-dependent chloride current in neurones of the rabbit pelvic parasympathetic ganglia. J Physiol. 1990 Mar;422:303–320. doi: 10.1113/jphysiol.1990.sp017985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidan L. V., Zholos A. V., Shuba M. F., Wood J. D. Patch-clamp recording in myenteric neurons of guinea pig small intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G1074–G1078. doi: 10.1152/ajpgi.1992.262.6.G1074. [DOI] [PubMed] [Google Scholar]
- Bertrand P. P., Galligan J. J. Alfaxalone, pentobarbital and diazepam potentiate gamma-aminobutyric acid-induced depolarizations in single myenteric neurons of guinea pig intestine. J Pharmacol Exp Ther. 1992 Aug;262(2):677–682. [PubMed] [Google Scholar]
- Cherubini E., North R. A. Actions of gamma-aminobutyric acid on neurones of guinea-pig myenteric plexus. Br J Pharmacol. 1984 May;82(1):93–100. doi: 10.1111/j.1476-5381.1984.tb16445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin J. L., Motais R. Inhibition of anion permeability by amphiphilic compounds in human red cell: evidence for an interaction of niflumic acid with the band 3 protein. J Membr Biol. 1979 Apr 20;46(2):125–153. doi: 10.1007/BF01961377. [DOI] [PubMed] [Google Scholar]
- Farrugia G., Rae J. L., Szurszewski J. H. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. J Physiol. 1993 Aug;468:297–310. doi: 10.1113/jphysiol.1993.sp019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan J. J., Campbell B. G., Kavanaugh M. P., Weber E., North R. A. 1,3-Di(2-tolyl)guanidine blocks nicotinic response in guinea pig myenteric neurons. J Pharmacol Exp Ther. 1989 Oct;251(1):169–174. [PubMed] [Google Scholar]
- Galligan J. J., Tatsumi H., Shen K. Z., Surprenant A., North R. A. Cation current activated by hyperpolarization (IH) in guinea pig enteric neurons. Am J Physiol. 1990 Dec;259(6 Pt 1):G966–G972. doi: 10.1152/ajpgi.1990.259.6.G966. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Tokimasa T., North R. A. Effects of three mammalian tachykinins on single enteric neurons. Neurosci Lett. 1987 Nov 23;82(2):167–171. doi: 10.1016/0304-3940(87)90123-6. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard S., Watson S. P., Maggio J. E., Too H. P., Watling K. J. Pharmacological analysis of [3H]-senktide binding to NK3 tachykinin receptors in guinea-pig ileum longitudinal muscle-myenteric plexus and cerebral cortex membranes. Br J Pharmacol. 1990 Apr;99(4):767–773. doi: 10.1111/j.1476-5381.1990.tb13004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H., Dahlem D., Englert H. C., Lang H. J. Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 1990 Jul 30;268(1):79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- Hanani M., Burnstock G. The actions of substance P and serotonin on myenteric neurons in tissue culture. Brain Res. 1985 Dec 9;358(1-2):276–281. doi: 10.1016/0006-8993(85)90971-0. [DOI] [PubMed] [Google Scholar]
- Hanani M., Chorev M., Gilon C., Selinger Z. The actions of receptor-selective substance P analogs on myenteric neurons: an electrophysiological investigation. Eur J Pharmacol. 1988 Aug 24;153(2-3):247–253. doi: 10.1016/0014-2999(88)90612-7. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. Does substance P mediate slow synaptic excitation within the myenteric plexus? Nature. 1978 Jul 27;274(5669):387–388. doi: 10.1038/274387a0. [DOI] [PubMed] [Google Scholar]
- Katayama Y., North R. A., Williams J. T. The action of substance P on neurons of the myenteric plexus of the guinea-pig small intestine. Proc R Soc Lond B Biol Sci. 1979 Nov 30;206(1163):191–208. doi: 10.1098/rspb.1979.0101. [DOI] [PubMed] [Google Scholar]
- Mawe G. M. Intracellular recording from neurones of the guinea-pig gall-bladder. J Physiol. 1990 Oct;429:323–338. doi: 10.1113/jphysiol.1990.sp018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Katayama Y. Substance P inhibits activation of calcium-dependent potassium conductances in guinea-pig myenteric neurones. J Physiol. 1992 Feb;447:293–308. doi: 10.1113/jphysiol.1992.sp019003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth P. R., Palmer J. M., Wood J. D., Zafirov D. H. Effects of forskolin on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol. 1986 Jul;376:439–450. doi: 10.1113/jphysiol.1986.sp016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Tokimasa T. Persistent calcium-sensitive potassium current and the resting properties of guinea-pig myenteric neurones. J Physiol. 1987 May;386:333–353. doi: 10.1113/jphysiol.1987.sp016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Wood J. D., Zafirov D. H. Transduction of aminergic and peptidergic signals in enteric neurones of the guinea-pig. J Physiol. 1987 Jun;387:371–383. doi: 10.1113/jphysiol.1987.sp016578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Shen K. Z., Surprenant A. Common ionic mechanisms of excitation by substance P and other transmitters in guinea-pig submucosal neurones. J Physiol. 1993 Mar;462:483–501. doi: 10.1113/jphysiol.1993.sp019565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts M. J., Henke D. C., Boucher R. C. Diphenylamine-2-carboxylate (DPC) inhibits both Cl- conductance and cyclooxygenase of canine tracheal epithelium. Pflugers Arch. 1990 Feb;415(5):611–616. doi: 10.1007/BF02583514. [DOI] [PubMed] [Google Scholar]
- Surprenant A., North R. A., Katayama Y. Observations on the actions of substance P and [D-Arg1,D-Pro2,D-Trp7,9,Leu11)substance P on single neurons of the guinea pig submucous plexus. Neuroscience. 1987 Jan;20(1):189–199. doi: 10.1016/0306-4522(87)90011-x. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Costa M., Schimerlik M., North R. A. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990 May;10(5):1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl- channels in Xenopus oocytes. Mol Pharmacol. 1990 May;37(5):720–724. [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Slow synaptic excitation mediated by serotonin in Auerbach's plexus. Nature. 1978 Dec 21;276(5690):836–837. doi: 10.1038/276836a0. [DOI] [PubMed] [Google Scholar]
- Zafirov D. H., Palmer J. M., Nemeth P. R., Wood J. D. Bombesin, gastrin releasing peptide and vasoactive intestinal peptide excite myenteric neurons. Eur J Pharmacol. 1985 Sep 10;115(1):103–107. doi: 10.1016/0014-2999(85)90590-4. [DOI] [PubMed] [Google Scholar]



