Abstract
Introduction:
The risk of dementia in patients with stroke-heart syndrome (SHS) remains unexplored.
Patients and methods:
Retrospective analysis using the TriNetX network, including patients with ischaemic stroke from 2010 to 2020. These patients were categorised into two groups: those with SHS (heart failure, myocardial infarction, ventricular fibrillation, or Takotsubo cardiomyopathy within 30 days post-stroke) and those without SHS. The primary outcome was the 1-year risk of dementia (vascular dementia, dementia in other disease, unspecified dementia, or Alzheimer’s disease). The secondary outcome was the 1-year risk of all-cause death. Cox regression analysis after 1:1 propensity score matching (PSM) was performed to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the outcomes.
Results:
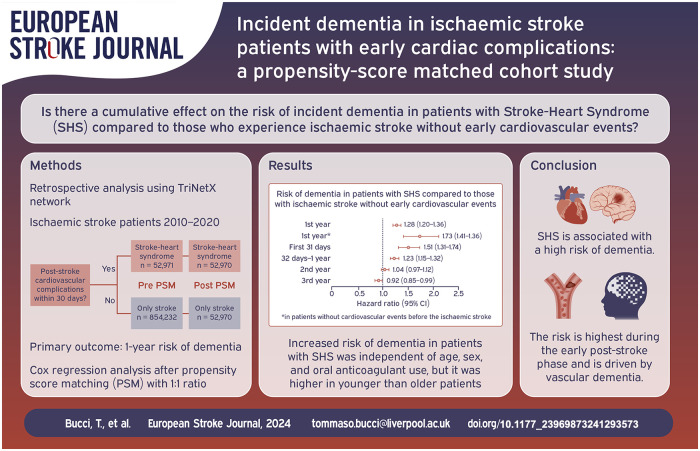
We included 52,971 patients with SHS (66.6 ± 14.6 years, 42.2% females) and 854,232 patients without SHS (64.7 ± 15.4 years, 48.2% females). Following PSM, 52,970 well-balanced patients were considered in each group. Patients with SHS had a higher risk of incident dementia compared to those without SHS (HR 1.28, 95%CI 1.20–1.36). The risk was the highest during the first 31 days of follow-up (HR 1.51, 95%CI 1.31–1.74) and was mainly driven by vascular and mixed forms. The increased risk of dementia in patients with SHS, was independent of oral anticoagulant use, sex and age but it was the highest in those aged <75 years compared to ⩾75 years.
Discussion and conclusion:
SHS is associated with increased risk of dementia. Future studies are needed to develop innovative strategies for preventing complications associated with stroke-heart syndrome and improving the long-term prognosis of these patients.
Keywords: Stroke, cardiovascular events, dementia
Graphical abstract.
Introduction
Patients with ischaemic stroke are at high risk for early cardiovascular complications, which are significantly associated with worsening morbidity and mortality.1–3 Neuronal injury post-stroke triggers the release of substantial amounts of catecholamines and cytokines, leading to a systemic inflammatory response coupled with impaired antioxidant systems that can result in a broad spectrum of cardiac complications.2–5
Stroke-heart syndrome (SHS) encapsulates the early cardiovascular complications following acute ischaemic stroke, characterised by the emergence of new cardiac conditions or the exacerbation of pre-existing cardiac diseases within 30 days of the stroke onset.2,6 It has been reported that approximately 25% of patients with ischaemic stroke develop early cardiovascular complications, with the highest incidence occurring within the first 3 days post-stroke. 2 Recognised risk factors for SHS include advanced age, pre-existing cardiovascular conditions and specific stroke characteristics, such as stroke severity, infarct size and lesion location in the insular cortex. 7 SHS can present with a broad spectrum of cardiovascular complications, ranging from subclinical manifestations like reduced heart rate variability or impaired baroreceptor reflex sensitivity to potentially life-threatening conditions such as new-onset acute myocardial infarction (AMI), heart failure (HF), atrial fibrillation, ventricular fibrillation or flutter (VFF) and Takotsubo cardiomyopathy (TTS). 6 Previous studies have shown that the onset of SHS is associated with 2–3 times the risk of short-term mortality or poor functional outcomes and 1.5–2 times the risk of mortality and major adverse cardiovascular events within 5 years post-stroke, compared to patients without SHS. 8
Recent evidence shows that both ischaemic stroke and cardiovascular events increase the risk of dementia.9–12 However, the potential cumulative effect of ischaemic stroke combined with early cardiovascular complications (SHS), on dementia risks remains unexplored. We hypothesised that ischaemic stroke patients with early cardiovascular complications as part of the SHS are at increased risk of incident dementia. To address this, we assessed the risk of incident dementia in patients with SHS compared to those without SHS in a global federated research database.
Methods
Study design
This study was a retrospective observational analysis carried out using TriNetX, a worldwide federated health research network with access to electronic medical records (EMRs) from various participating healthcare centres. These encompass academic medical centres, specialty physician practices and community hospitals, collectively covering an estimated 300 million individuals worldwide. Within this expansive network, accessible data encompass demographic details, diagnoses recorded using International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-10-CM) codes, as well as medication information coded using Veteran Affairs (VA) Codes. Further details are available online at https://trinetx.com/.
TriNetX is a health research network compliant with the Health Insurance Portability and Accountability Act and the United States (US) federal law that safeguards the privacy and security of healthcare data, including de-identified data as per the de-identification standard of the HIPAA Privacy Rule. To gain access to the data in the TriNetX research network, requests are directed to TriNetX and a data sharing agreement is required. As a federated research network, studies using the TriNetX health research network do not need ethical approval as no patient identifiable information is received. Further information about the data extraction from TriNetX is reported in the Supplemental material.
Cohort
The searches on the TriNetX online research platform were performed on the 14th of September 2024 for individuals aged ⩾18 years who experienced an ischaemic stroke between 1st January 2010 and 31st of December 2020. Based on the development of early cardiovascular complication (AMI, acute HF, VFF or TTS) within 30 days from the stroke, patients were categorised into two groups: patients with SHS and those without SHS (i.e. patients who experienced stroke only) (Supplemental Figure 1). More information about the ICD-10-CM codes utilised for the inclusion and exclusion criteria can be found in Supplemental Table 1.
At the time of the search, 93 participating healthcare organisations, primarily located in the US, had data available for patients who met the study’s inclusion criteria. Any other diagnoses or treatment reported prior to stroke onset were considered the individual’s baseline characteristics. Patients with a prior diagnosis of Alzheimer’s disease, vascular dementia, unspecified dementia, or dementia in other diseases classified elsewhere, as well as those who died within the first 30 days post-ischaemic stroke were excluded.
Outcomes
The primary outcome was the 1-year risk of a composite of Alzheimer’s disease, vascular dementia, unspecified dementia and dementia in other diseases classified elsewhere. The secondary outcome was the 1-year risk of all-cause death. The adverse events of interest were identified via ICD-10-CM codes (Supplemental Table 2).
Statistical analysis
Baseline characteristics of patients with SHS and those without SHS were balanced using logistic regression and propensity score matching (PSM) with a 1:1 ratio. The greedy nearest neighbour method with a caliper of 0.1 pooled standard deviations without replacement was applied. The balance of demographic and clinical variables between groups was evaluated using Absolute Standardised mean Differences (ASD), whit an ASD < 0.1 indicating well matched characteristics. The variables included in the PSM were age, sex, ethnicity, hypertension, diabetes, dyslipidaemia, obesity, chronic kidney disease, sleep apnoea, chronic ischaemic heart diseases, previous ischaemic or haemorragic stroke, chronic heart failure, atrial fibrillation, pulmonary embolism, peripheral artery disease and cardiovascular medications (such as β-blockers, antiarrhythmics, diuretics, lipid lowering agents, antianginals, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, oral anticoagulant (OAC) and antiplatelets). These variables were selected based on their potential association with the cardiovascular risk, supporting our hypothesis that early cardiovascular events in stroke patients may contribute additively to dementia risk. Subsequently, Cox proportional hazard models were used post-PSM to calculate hazard ratios (HRs) and 95% confidence intervals (95%CI) for the risk of defined outcomes in patients with SHS compared to those without SHS. Kaplan-Meier survival curves were constructed for the primary and secondary outcomes to illustrate differences in survival rates among groups. The Log-rank test tests for between-group differences in the probability of developing the outcome of interest at any time point within the study. The index event, marking the start of the observation period, was the 31st day after the ischaemic stroke. Follow-up time was calculated for each patient meeting the index criteria, representing the number of days between the index event and either the end of the analysis window or the patient’s last known data point. Follow-up time was reported as the median, with the interquartile range (IQR) calculated as the difference between the 75th and 25th percentiles of follow-up duration. Patients were censored when they no longer provided data for analysis.
To assess whether the proportional hazards assumption held in the Cox regression models, we applied a Chi-square (χ²) test based on Schoenfeld residuals. More information regarding the performance and interpretation of these test are provided in the Supplemental material. In cases where the proportional hazards assumption in the primary analysis was not met, we divided the 1-year follow-up period into two phases: an early phase (the first 31 days of follow-up) and a late phase (from day 32 day to the end of the first year). We then re-evaluated the risk using Cox regression and retested the proportional hazards assumption for each phase.
The competitive risk analyses were performed utilising the Aalen–Johansen plots to estimate the cumulative incidence of dementia and all-cause death in patients with SHS and those without. Daily cumulative incidence was determined by dividing the total number of new cases by the number of individuals at risk in each day of follow-up.
Sensitivity analyses were conducted to: (i) evaluate the 1-year risk of dementia in SHS patients without cardiovascular events prior the ischaemic stroke (e.g. AMI, HF, VFF and TTS); (ii) determine the 1-year risk for each type of dementia, prior to the ischaemic stroke; (iii) assess the risks of dementia and death at the second and third year after the ischaemic stroke; (iv) assess the 1-year risk of dementia associated with each SHS manifestation; (v) evaluate the 1-year dementia risk within relevant clinical subgroups (age <75 or ⩾75 years, 13 males or females, those on oral anticoagulants (OAC), and those not on OAC); and (vi) account for the presence of a competing risks between dementia and all-cause death.
All analyses were executed within the TriNetX platform, which utilises both R and Python for data analysis. The R Survival library v3.2-3 was used for survival analyses, while propensity risk scores were estimated using logistic regression, implemented via the scikit-learn package in Python version 3.7. TriNetX does not impute or estimate clinical values to fill gaps in a patient’s record. All tests were two-tailed and statistical significance was defined as p-values < 0.05, indicating assuming a Type I error of less than 5% if the null hypothesis is true.
Results
Overall, we included 907,203 patients with ischaemic stroke: 52,971 patients with SHS (mean age 66.6 ± 14.6 years, 42.2% females) and 854,232 patients without SHS (64.7 ± 15.4 years, 48.2% females).
Prior PSM, patients with SHS were slightly older, more likely to be males and had a higher cardiovascular burden compared to those patients without SHS (Table 1). Specifically, patients with SHS had a higher prevalence of cardiovascular risk factors, previous cardiovascular events and were more likely to receive cardiovascular treatments, including OAC and antiplatelets.
Table 1.
Baseline characteristics comparison between patients with SHS and those without SHS, before and after propensity score matching.
| Baseline characteristics | Before propensity score match |
After propensity score match |
||||
|---|---|---|---|---|---|---|
| Patients with SHS | Patients without SHS | ASD | Patients with SHS | Patients without SHS | ASD | |
| n = 52,971 | n = 854,23 | n = 52,970 | n = 52,970 | |||
| Age, years (±SD) | 66.6 ± 14.6 | 64.7 ± 15.4 | 0.128 | 66.6 ± 14.6 | 67.3 ± 14.3 | 0.052 |
| Female, n (%) | 22,358 (42.2) | 411,811 (48.2) | 0.121 | 22,358 (42.2) | 21,690 (40.9) | 0.026 |
| White, n (%) | 31,981 (60.4) | 517,308 (60.6) | 0.004 | 31,981 (60.4) | 32,644 (61.6) | 0.026 |
| Black or African American, n (%) | 9636 (18.2) | 127,826 (15.0) | 0.087 | 9635 (18.2) | 9553 (18.0) | 0.004 |
| Asian, n (%) | 1671 (3.2) | 37,550 (4.4) | 0.065 | 1671 (3.2) | 1622 (3.1) | 0.005 |
| Arterial hypertension, n (%) | 27,321 (51.6) | 347,017 (40.6) | 0.221 | 27,320 (51.6) | 26,454 (49.9) | 0.033 |
| Atrial fibrillation, n (%) | 10,013 (18.9) | 78,203 (9.2) | 0.283 | 10,012 (18.9) | 9843 (18.6) | 0.008 |
| Diabetes mellitus, n (%) | 15,296 (28.9) | 167,270 (19.6) | 0.218 | 15,295 (28.9) | 14,975 (28.3) | 0.013 |
| Chronic kidney disease, n (%) | 10,698 (20.2) | 77,833 (9.1) | 0.317 | 10,697 (20.2) | 10,300 (19.4) | 0.019 |
| Obesity, n (%) | 8052 (15.2) | 84,027 (9.8) | 0.163 | 8052 (15.2) | 7593 (14.3) | 0.024 |
| Dyslipidaemia, n (%) | 20,520 (39.0) | 255,455 (29.9) | 0.187 | 20,520 (38.7) | 19,922 (37.6) | 0.023 |
| Chronic Ischaemic heart disease, n (%) | 18,661 (35.2) | 126,959 (14.9) | 0.484 | 18,660 (35.2) | 19,060 (36.0) | 0.016 |
| Chronic Heart failure, n (%) | ||||||
| Systolic | 4457 (8.4) | 12,652 (1.5) | 0.324 | 4456 (8.4) | 4006 (7.6) | 0.031 |
| Diastolic | 3137 (5.9) | 13,649 (1.6) | 0.229 | 3136 (5.9) | 2934 (5.5) | 0.016 |
| Ischaemic stroke, n (%) | 924 (1.7) | 26,416 (3.1) | 0.088 | 924 (1.7) | 1078 (2.0) | 0.021 |
| Pulmonary embolism, n (%) | 1514 (2.9) | 13,421 (1.6) | 0.088 | 1513 (2.9) | 1442 (2.7) | 0.008 |
| Peripheral vascular disease, n (%) | 5439 (10.3) | 44,517 (5.2) | 0.190 | 5439 (10.3) | 5299 (10.0) | 0.009 |
| Sleep apnoea, n (%) | 5473 (10.3) | 56,145 (6.6) | 0.135 | 5473 (10.3) | 5217 (9.8) | 0.024 |
| Intracerebral haemorrhage, n (%) | 763 (1.4) | 17,746 (2.1) | 0.048 | 763 (1.4) | 660 (1.2) | 0.017 |
| Lipid-lowering drugs, n (%) | 21,525 (40.6) | 255,097 (29.9) | 0.227 | 21,525 (40.6) | 20,789 (39.2) | 0.037 |
| Beta-blockers, n (%) | 23,221 (43.8) | 234,115 (27.4) | 0.348 | 23,220 (43.8) | 22,823 (43.1) | 0.015 |
| Diuretics, n (%) | 20,574 (38.8) | 199,096 (23.3) | 0.340 | 20,573 (38.8) | 20,091 (37.9) | 0.026 |
| Antiarrhythmics, n (%) | 17,084 (32.3) | 180,696 (21.3) | 0.242 | 17,083 (32.3) | 16,254 (30.7) | 0.034 |
| Calcium channel blockers, n (%) | 15,048 (28.4) | 171,287 (20.1) | 0.196 | 15,047 (28.4) | 14,501 (27.4) | 0.023 |
| ACE inhibitors, n (%) | 15,518 (29.3) | 165,424 (19.4) | 0.233 | 15,517 (29.3) | 14,928 (28.2) | 0.025 |
| Angiotensin II inhibitors, n (%) | 8189 (15.5) | 92,904 (10.9) | 0.136 | 8189 (15.5) | 7718 (14.6) | 0.025 |
| Digoxin, n (%) | 2872 (5.4) | 14,925 (1.7) | 0.199 | 2871 (5.4) | 2611 (4.9) | 0.022 |
| Anticoagulant, n (%) | 20,519 (38.7) | 203,138 (23.8) | 0.327 | 20,518 (38.7) | 19,824 (37.4) | 0.027 |
| Antiplatelet, n (%) | 21,427 (40.5) | 239,242 (28.0) | 0.265 | 21,426 (40.4) | 20,726 (39.1) | 0.027 |
ACE: Angiotensin-converting enzyme; ASD: Absolute Standardized mean Difference; SHS: Stroke-Heart Syndrome.
Following PSM, 52,970 patients were matched in each group, resulting in no significant differences between the two groups (Table 1). The median follow-up, after PSM, was 1013 days (IQR 946 days) in SHS patients and 1125 days (IQR 677 days) in patients without SHS.
The number of primary and secondary outcomes recorded during the 1-year follow-up is reported in Table 2. A total of 2027 (3.8%) new cases of dementia were recorded among patients with SHS compared to 1726 (3.3%) cases among those without SHS, HR 1.28, 95%CI 1.20–1.36. Additionally, the number of all-cause deaths recorded was 7636 (14.4%) in the SHS group and 3765 (7.1%) in the group without SHS, HR 2.22, 95%CI 2.14–2.31. Kaplan Meier curves for primary and secondary outcomes are reported in Supplemental Figures 2 and 3.
Table 2.
Risk of primary and secondary outcomes in patients with SHS compared to those without SHS in different time windows after propensity score matching.
| Time windows | Dementia |
All-cause death |
||||||
|---|---|---|---|---|---|---|---|---|
| Patients with SHS (N = 52,970) | Patients without SHS (N = 52,970) | HR (95%CI) | χ2 | Patients with SHS (N = 52,970) | Patients without SHS (N = 52,970) | HR (95%CI) | χ2 | |
| n events (%) | n events (%) | n events (%) | n events (%) | |||||
| First year | 2027 (3.8) | 1726 (3.3) | 1.28 (1.20–1.36) | 17.080 | 7636 (14.4) | 3765 (7.1) | 2.22 (2.14–2.31) | 51.326 |
| First year* | 250 (4.9) / 5126 | 157 (3.1) / 5126 | 1.73 (1.41–2.11) | 11.450 | 735 (14.3) / 5126 | 287 (5.6) / 5126 | 2.77 (2.41–3.17) | 29.854 |
| First 31 days | 489 (0.9) | 332 (0.6) | 1.51 (1.31–1.74) | 0.121 | 2051 (3.9) | 675 (1.3) | 3.13 (2.87–3.41) | 10.234 |
| 32 days – end of the first year | 1742 (3.3) | 1545 (2.9) | 1.23 (1.15–1.32) | 2.551 | 5628 (10.6) | 3037 (5.7) | 2.04 (1.96–2.14) | 16.690 |
| Second year | 1366 (2.6) | 1528 (2.9) | 1.04 (0.97–1.12) | 0.366 | 2810 (5.3) | 2233 (4.2) | 1.48 (1.40–1.56) | 1.707 |
| Third year | 1156 (2.2) | 1509 (2.8) | 0.92 (0.85–0.99) | 0.376 | 2194 (4.1) | 1934 (3.7) | 1.37 (1.29–1.46) | 5.052 |
In each time window, propensity score matching was conducted de novo.
HR: Hazard Ratio; CI: Confidence Interval; SHS: Stroke-Heart Syndrome.
Only in patients without previous cardiovascular events.
A high χ² suggests a greater deviation from the expected values, indicating a potential violation of the proportional hazard assumption. Conversely, a small χ² value indicates that the observed residuals closely match the expected values.
When analysing the proportional hazards assumption for the 1-year risk of primary and secondary outcomes in patients with SHS compared to those without SHS, we found that it was violated for both dementia (χ2 = 17.080, p-value for proportionality < 0.001) and all-cause death (χ2 = 51.326, p-value for proportionality < 0.001) (Table 2). When the follow-up was subdivided, we observed that the risk of dementia was significantly higher during the early phase in patients with SHS compared to those without SHS with no violation of the proportional hazards assumption (HR for early dementia: 1.51, 95%CI 1.31–1.74, χ² = 0.121, p for proportionality = 0.728). During the late phase, patients with SHS still showed a significantly increased risk of dementia compared to those without SHS, but the risk was of a lower magnitude than in the early phase. Again, no violation of the proportional hazards assumption was observed (HR for late dementia: 1.23, 95%CI 1.15–1.32, χ² = 2.551, p for proportionality = 0.110). Conversely, the risk of all-cause death exhibited a significant discrepancy with the expected HR in both the early (HR 3.13, 95%CI 2.87–3.41, χ² = 10.234, p for proportionality = 0.001) and late phases (HR 2.04, 95%CI 1.96–2.14, χ² = 16.690, p for proportionality < 0.001).
Sensitivity analyses
The first sensitivity analysis confirmed the results of the main analysis, even when considering only patients without prior cardiovascular events. In patients with SHS, the 1-year risks of dementia and death were approximately 1.7–2.8 times the risk of those without SHS (Table 2). As for the main analysis, even in this case the proportional hazards assumption was not respected for both the primary and secondary outcomes (Table 2).
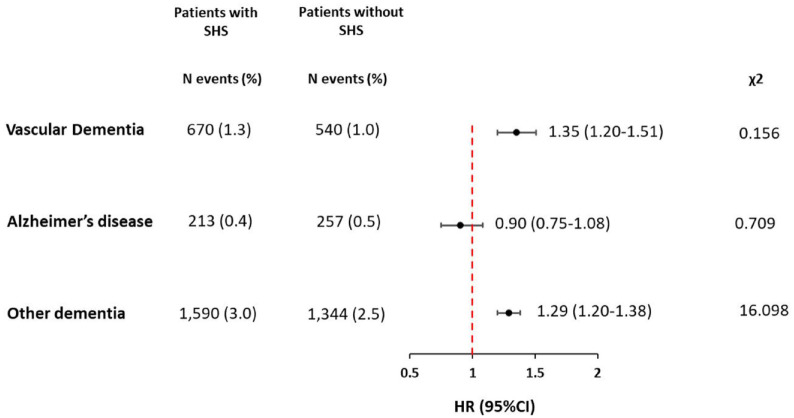
The second sensitivity demonstrated statistically significant differences in the 1-year risk for different types of dementia. In patients with SHS, the highest risk was for vascular and other types of dementia (Unspecified dementia and Dementia in other diseases classified elsewhere), whereas no significant association was found with Alzheimer’s disease (Figure 1). The assessment of the hazard proportionality assumptions showed that it was respected for vascular dementia and Alzheimer’s disease but was violated for other types of dementia (Figure 1).
Figure 1.
One-year risk of different types of dementia in patients with stroke-heart syndrome (n = 52,970) compared to those without stroke-heart syndrome (n = 52,970).
CI: Confidence Interval; HR: Hazard Ratio; N: Number, SHS: Stroke-Heart Syndrome.
Other dementia includes unspecified dementia and dementia in other diseases classified elsewhere.
A high χ² suggests a greater deviation from the expected values, indicating a potential violation of the proportional hazard assumption. Conversely, a small χ² value indicates that the observed residuals closely match the expected values.
The third sensitivity analysis showed that, with extended follow-up, the risk of dementia in patients with SHS decreased by approximately 10% during the second and third years, compared to patients without SHS, eventually becoming non-significant. However, there was no violation of the proportional hazards assumption in either the second or third year (Table 2).
Similarly, the risk of all-cause death decreased by approximately 20% annually, but it remained significantly higher in patients with SHS compared to those without SHS. Although the proportional hazards assumption was not violated during the second year, it became significant again in the third year of follow-up (Table 2).
The fourth sensitivity analysis, aimed at examining the risk of dementia for each specific manifestation of SHS, indicated that an increased risk of dementia was clear in cases involving AMI and HF, while TTS and VFF exhibited only a non-significant trend towards an increased dementia risk (Table 3). Conversely, the risk of all-cause mortality was significantly associated with all manifestations of SHS (Table 3). The proportional hazards assumption was respected for either dementia and all-cause death in patients with TTS or VFF, but it was violated in those with AMI or HF (Table 3).
Table 3.
Risk of primary and secondary outcomes in patients with SHS compared to those without SHS, stratified by the type of cardiovascular events.
| Type of post-stroke cardiovascular | Patients with SHS | Patients without SHS | HR (95%CI) | χ2 |
|---|---|---|---|---|
| (n = number of patients for each group after PSM) | Number of events (%) | Number of events (%) | ||
| AMI (n = 35,966) | ||||
| Dementia | 1301 (3.6) | 1093 (3.0) | 1.28 (1.19–1.39) | 5.928 |
| All-cause death | 4631 (12.9) | 2969 (6.6) | 2.12 (2.02–2.23) | 46.101 |
| HF (n = 21,621) | ||||
| Dementia | 941 (4.4) | 733 (3.4) | 1.44 (1.31–1.59) | 13.330 |
| All-cause death | 4004 (18.5) | 1788 (8.3) | 2.54 (2.40–2.69) | 37.341 |
| VFF (n = 1730) | ||||
| Dementia | 45 (2.6) | 3.8 (2.2) | 1.35 (0.88–2.08) | 1.590 |
| All-cause death | 338 (19.5) | 105 (6.1) | 3.67 (2.95–4.57) | 0.182 |
| TTS (n = 1312) | ||||
| Dementia | 47 (3.6) | 37 (2.8) | 1.92 (0.88–2.08) | 0.372 |
| All-cause death | 165 (12.6) | 58 (4.4) | 3.06 (2.27–4.13) | 18.268 |
AMI: Acute Myocardial Infarction; CI: Confidence Interval; HF: Heart Failure; HR: Hazard Ratio; PSM: Propensity Score Matching; SHS: Stroke-Heart Syndrome; TTS: Takotsubo cardiomyopathy; VFF: Ventricular Flutter-Fibrillation.
A high χ² suggests a greater deviation from the expected values, indicating a potential violation of the proportional hazard assumption. Conversely, a small χ² value indicates that the observed residuals closely match the expected values.
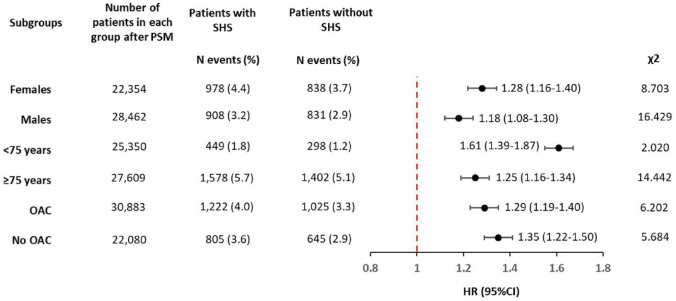
The fifth sensitivity analysis demonstrated that the risk of dementia in patients with SHS compared to those without SHS was consistent across all subgroups analysed, irrespective of age (<75 or ⩾75 years), sex (male or female) and whether OAC were used (Figure 2). The proportional hazards assumption was not respected in most analyses, except for patients aged <75 years, where the risk of dementia was significantly higher compared to those aged ⩾75 years and the proportional hazards assumption was satisfied (Figure 2).
Figure 2.
One-year risk of dementia in patients with stroke-heart syndrome compared to those without stroke-heart syndrome considering different clinically relevant subgroups.
CI: Confidence Interval; HR: Hazard Ratio; PSM: Propensity Score Matching; N: Number; OAC: Oral Anticoagulants, SHS: Stroke-Heart Syndrome.
A high χ² suggests a greater deviation from the expected values, indicating a potential violation of the proportional hazard assumption. Conversely, a small χ² value indicates that the observed residuals closely match the expected values.
Competitive risk analysis
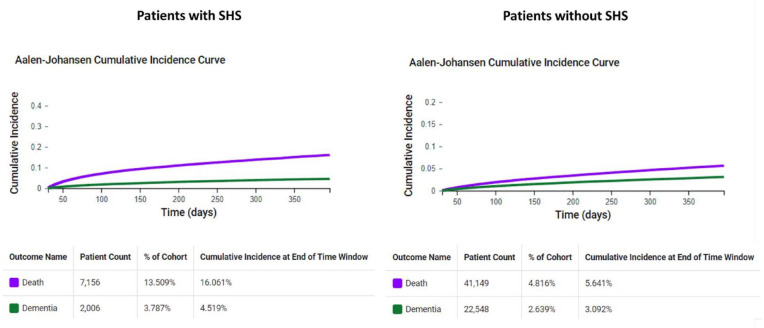
In the analysis of daily cumulative risk for dementia and all-cause death among patients with and without SHS, we observed that the high daily cumulative incidence of all-cause death competes with the risk of developing dementia in both groups (Figure 3). This pattern was particularly pronounced in patients with SHS, who exhibited a daily cumulative incidence of all-cause death at 16.1%, nearly triple that of the 5.6% observed in patients without SHS. These findings suggest that the true risk of dementia in SHS survivors may be higher than the estimates presented in the main analysis.
Figure 3.
One-year Aalen-Johansen cumulative incidence curves for all-cause death and dementia.
SHS: Stroke-Heart Syndrome.
Discussion
In this retrospective, propensity score-matched analysis of a large cohort of patients with ischaemic stroke, we found that (i) patients with SHS had an increased risk of dementia and a higher risk of all-cause death at 1-year of follow-up compared to those without SHS; (ii) the increased risk of dementia was not constant over the time, with the highest risk during the first 31 days after the start of the follow-up; (iii) The increased risk of dementia in patients with SHS was consistent even when considering only patients without a history of cardiovascular events prior to the ischaemic stroke; (iv) The overall increased risk of dementia in patients with SHS was mainly due to vascular or other/mixed forms of dementia rather than Alzheimer’s disease; (v) Both the risk of dementia and all-cause death decreased over time, with the risk of dementia becoming non-significant during the second and third years of follow-up, while the risk of all-cause death remained statistically significant; (vi) All individual components of SHS were associated with a higher risk of both dementia and death, except for cases of TTS and VFF, which only demonstrated a non-significant increase in the risk of dementia. (vii) the increased 1-year risk of dementia observed was regardless of age, sex and OAC use. However, it was higher in those aged <75 years compared to those aged ⩾75 years.
Previous studies have demonstrated that both ischaemic stroke and cardiovascular disease are independently associated with an increased risk of dementia.10,11,12,14–17 In a meta-analysis of 1.9 million patients with prevalent stroke and 1.3 million patients with incident stroke, the authors found that the pooled HR for dementia was 1.69 (95%CI 1.49–1.92) for prevalent stroke and 2.18 (95%CI 1.90–2.50) for incident stroke. 15 A prospective study on 23,572 patients from the US, followed for a median of 6.1 years, demonstrated that in those who experienced incident stroke (2.2%), global cognition declined faster compared to the pre-stroke period. 18 Similarly, a large meta-analysis of 27 studies reported a pooled prevalence of post-stroke dementia of up to 18% at 1 year. 19 Moreover, a community-based cohort of 1301 individuals aged ⩾75 years from Sweden, with a median follow-up of 9 years, showed that HF was associated with an increased risk of dementia (HR 1.84, 95%CI 1.35–2.51) and Alzheimer’s disease (HR 1.80, 95%CI 1.25–2.61). 10 Similar results were reported in patients with AMI, where the risk of dementia was inversely related to the age of AMI onset 20 ; in those with atrial fibrillation, where the risk was highest in individuals who developed this arrhythmia before the age of 65 21 ; and in those who survived cardiac arrest. 22
Ischaemic stroke may cause vascular cognitive impairment and dementia, through cerebral hypoperfusion that results from the acute vascular injury and can be heightened by the pre-existence of asymptomatic brain injuries due to cerebral small vessel disease. 23 Thus, the cerebral hypoperfusion, which can result from both covert cerebrovascular disease and overt brain injury, is likely the primary mechanism leading to cognitive impairment in stroke patients. 24 In this context, dysfunction in the brain-heart axis, associated with post-stroke AMI, HF, or arrhythmias, may impair the cardiac output and worsen cerebral hypoperfusion, contributing to cognitive impairment beyond the effects of brain infarcts.25,26 This hypothesis is supported by our main analysis, which found that the coexistence of both ischaemic stroke and early cardiovascular events was associated with an increased risk of dementia compared to ischaemic stroke alone. Our sensitivity analyses further revealed that this risk was the highest during the early follow-up phase and was primarily driven by vascular and mixed forms rather than Alzheimer’s disease. Additionally, the impact of SHS on dementia risk, was more pronounced in patients without previous cardiovascular events or in those under <75 years, where probably fewer pro-inflammatory confounders were present. We also observed a progressively reduced risk of dementia over the study period, which further support the pivotal role of the acute post-stroke neuronal injury in driving dementia risk, as the association become non-significant during the second and third year of follow-up. However, it should be noted that the declining risk of dementia over the study period may be partially attributable to the high risk of all-cause mortality in patients with SHS, which could have exacerbated the competing risk with dementia in later stages of follow-up and the progression of cardiovascular burden in patients without SHS – due to aging or the development of new cardiovascular risk factors or events – that may have increased the dementia risk over time in this group. Moreover, when hypothesising a direct effect of cerebral and cardiac hypoperfusion on the risk of dementia in patients with SHS, it should be considered that all these clinical conditions share common risk factors, including advanced age, smoking, obesity, hypertension, dyslipidaemia and diabetes. 27 These risk factors have significant pro-atherosclerotic effects, which may contribute not only to ischaemic stroke and post-stroke cardiovascular complications but also to the risk of dementia itself. 28 Myocardial injury in patients with acute ischaemic stroke and high atherosclerotic burden is associated with more extensive white matter lesions and greater global cognitive impairment.29,30 Additionally, in patients with ischaemic stroke and advanced generalised atherosclerosis, autonomic dysregulation may be facilitated.31,32 In this context, it is plausible that patients with SHS are more likely to develop vascular dementia due to the direct impact of vascular events on vulnerable brain tissue due to the preexisting atherosclerotic cerebral vasculopathy.
The high risk of adverse events in patients who develop dementia after ischaemic stroke or cardiovascular events highlights the need of methods to early identify patients at high risk of dementia. Early identification of SHS, through methods such as ECG or prolonged ECG monitoring or serial imaging with echocardiography or cardiac MRI, may help to identify patients at risk of vascular cognitive impairment and dementia. Additionally, dementia risk stratification in patients with SHS could be improved by incorporating brain MRI to detect those with white matter hyperintensities. Previous studies have shown that white matter hyperintensities are highly prevalent in patients with ischaemic stroke, atrial fibrillation, or HF and are associated with global cerebral hypoperfusion and poorer cognitive performance.17,33,34
Currently, no established disease modifying treatment exists for post-stroke dementia and treatments are focused on preventive therapies and risk factor modification. 35 Some evidence suggests symptomatic benefits of acetylcholinesterase inhibitors, memantine, DL-3-n-butylphthalide and nootropics (e.g. cerebrolysin, actovegin and cortexin), which are available for use in various regions.36–38 However, the magnitude of these benefits and the quality of the available evidence are insufficient to support their recommendation for clinical use or to justify changes in practice guidelines at this stage.
Growing evidence has also shown that patients with ischaemic stroke treated with endovascular thrombectomy have better outcomes compared to those treated with thrombolysis or treated with standard medical management. 39 Thus, more research is needed to investigate whether mechanical vascular destruction, by reestablishing cerebral blood flow, can be associated with a lower risk of dementia compared to other treatments. Moreover, no data are available on the potential use of pharmacological or mechanical treatments aimed at supporting cardiac function to improve cerebral perfusion during SHS. Regarding the optimisation of risk factors and comorbidities, this can be addressed through the ABCstroke pathway, an integrative approach to post-stroke management outlined in a position paper by the ESC (European Society of Cardiology) Council on Stroke. 40 This approach is based on three key pillars: (i) avoiding stroke recurrence with optimal antithrombotic strategies; (ii) improving functional and psychological status through routine assessment of post-stroke cognitive and physical impairment, depression and anxiety and (iii) managing cardiovascular risk factors and comorbidities, along with promoting a healthy lifestyle. 41 The benefits of this integrated approach were demonstrated in a prospective cohort of 2513 ischaemic stroke patients from the Athens Stroke Registry followed for a median of 30 months. In this study, full adherence to the ABCstroke pathway was associated with a reduced risk of stroke recurrence (HR: 0.61; 95%CI: 0.37–0.99), major adverse cardiovascular events (HR: 0.59; 95%CI: 0.39–0.88) and death (HR: 0.22; 95%CI: 0.12–0.41), making it a potentially beneficial tool in the context of SHS as well. 42
Strengths and limitations
To the best of our knowledge, this is the first study to investigate the association between the risk of incident dementia and SHS. The study is based on a large contemporary cohort of ischaemic stroke patients and the main results have been validated through several sensitivity analyses.
However, there are also several limitations. The retrospective and observational nature of the study makes it susceptible to selection bias and other unmeasured biases. As TriNetX network relies on administrative data, it may be prone to misclassification and could fail to capture outcomes occurring outside the network. In the PSM, we balanced the two populations based on the prevalence of cardiovascular disease, but not on its severity or specific type. This may have led to residual differences in baseline risk, which could have influenced the risk of incident dementia. Moreover, we focused solely on cardiovascular risk factors and medical treatments, potentially omitting other clinically important variables. Additionally, balancing for intrinsic characteristics of SHS, such as the high prevalence of cardiovascular diseases, may have biased the estimation of dementia risk, making it challenging to generalise the findings to the general population. Only a small subset of patients with ischaemic stroke had comprehensive data on stroke type and severity, limiting our ability to explore the relationship between these factors and the risk of incident dementia. As suggested by the competing risk analysis, the risk of incident dementia in both groups (patients with SHS and those without SHS) may be underestimated due to the high cumulative incidence of all-cause death. No data were available on compliance with medical treatments during the observation period, which prevented us from assessing the impact of vascular secondary prevention on the risk of dementia. The study is also limited by the inability to stratify the analysis according to the use of thrombolytics, or endovascular procedures. Lastly, we did not explore how social determinants of health or insurance-based healthcare systems affect access to healthcare and influence the risk of dementia.
Conclusion
SHS is associated with an increased risk of dementia. Future studies are needed to develop innovative strategies for preventing complications associated with SHS and improving the long-term prognosis of these patients.
Supplemental Material
Supplemental material, sj-pdf-1-eso-10.1177_23969873241293573 for Incident dementia in ischaemic stroke patients with early cardiac complications: A propensity-score matched cohort study by Tommaso Bucci, Sylvia E Choi, Christopher TW Tsang, Kai-Hang Yiu, Benjamin JR Buckley, Pasquale Pignatelli, Jan F Scheitz, Gregory YH Lip and Azmil H Abdul-Rahim in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GYHL is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Anthos. No fees are received personally. He is a National Institute for Health and Care Research (NIHR) Senior Investigator and co-PI of the AFFIRMO project on multimorbidity in AF (grant agreement No 899871), TARGET project on digital twins for personalised management of atrial fibrillation and stroke (grant agreement No 101136244) and ARISTOTELES project on artificial intelligence for management of chronic long term conditions (grant agreement No 101080189), which are all funded by the EU’s Horizon Europe Research & Innovation programme. BJRB has received research funding from BMS/Pfizer and Huawei Europe. All other authors report no relevant disclosures.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: TriNetX is a health research network compliant with the Health Insurance Portability and Accountability Act and the United States (US) federal law which protects the privacy and security of healthcare data, including de-identified data as per the de-identification standard of the HIPAA Privacy Rule. As a federated research network, studies using the TriNetX health research network do not need ethical approval as no patient identifiable information is received.
Informed consent: Informed consent was not required due to the retrospective nature of the study and the absence of identifiable patient information.
Guarantor: TB.
Contributorship: TB, SEC and AHAR conceived the idea, performed the statistical analysis and wrote the first draft of the manuscript, GYHL revised the first draft of the manuscript. All authors reviewed, edited and approved the final version of the manuscript.
ORCID iDs: Tommaso Bucci  https://orcid.org/0000-0003-2895-6234
https://orcid.org/0000-0003-2895-6234
Sylvia E Choi  https://orcid.org/0000-0003-3012-8219
https://orcid.org/0000-0003-3012-8219
Christopher TW Tsang  https://orcid.org/0000-0003-3394-6604
https://orcid.org/0000-0003-3394-6604
Jan F. Scheitz  https://orcid.org/0000-0001-5835-4627
https://orcid.org/0000-0001-5835-4627
Gregory YH Lip  https://orcid.org/0000-0002-7566-1626
https://orcid.org/0000-0002-7566-1626
Azmil H Abdul-Rahim  https://orcid.org/0000-0002-1318-4027
https://orcid.org/0000-0002-1318-4027
Supplemental material: Supplemental material for this article is available online.
References
- 1. Buckley BJR, Harrison SL, Hill A, et al. Stroke-heart syndrome: incidence and clinical outcomes of cardiac complications following stroke. Stroke 2022; 53: 1759–1763. [DOI] [PubMed] [Google Scholar]
- 2. Scheitz JF, Nolte CH, Doehner W, et al. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol 2018; 17: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 3. Sposato LA, Hilz MJ, Aspberg S, et al. Post-stroke cardiovascular complications and neurogenic cardiac injury: JACC state-of-the-art review. J Am Coll Cardiol 2020; 76: 2768–2785. [DOI] [PubMed] [Google Scholar]
- 4. Bucci T, Sagris D, Harrison SL, et al. C-reactive protein levels are associated with early cardiac complications or death in patients with acute ischemic stroke: a propensity-matched analysis of a global federated health from the TriNetX network. Intern Emerg Med 2023; 18: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bucci T, Pastori D, Pignatelli P, et al. Albumin levels and risk of early cardiovascular complications after ischemic stroke: a propensity-matched analysis of a global federated health network. Stroke 2024; 55: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheitz JF, Sposato LA, Schulz-Menger J, et al. Stroke-heart syndrome: recent advances and challenges. J Am Heart Assoc 2022; 11: e026528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prosser J, MacGregor L, Lees KR, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007; 38: 2295–2302. [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Venkat P, Seyfried D, et al. Brain-heart interaction: cardiac complications after stroke. Circ Res 2017; 121: 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rist PM, Chalmers J, Arima H, et al. Baseline cognitive function, recurrent stroke, and risk of dementia in patients with stroke. Stroke 2013; 44: 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu C, Winblad B, Marengoni A, et al. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med 2006; 166: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 11. Sundbøll J, Horváth-Puhó E, Adelborg K, et al. Higher risk of vascular dementia in myocardial infarction survivors. Circulation 2018; 137: 567–577. [DOI] [PubMed] [Google Scholar]
- 12. Weaver NA, Kuijf HJ, Aben HP, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 2021; 20: 448–459. [DOI] [PubMed] [Google Scholar]
- 13. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brain J, Greene L, Tang EYH, et al. Cardiovascular disease, associated risk factors, and risk of dementia: an umbrella review of meta-analyses. Front Epidemiol 2023; 3: 1095236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuźma E, Lourida I, Moore SF, et al. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimer’s Dement 2018; 14: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moroni F, Ammirati E, Hainsworth AH, et al. Association of white matter hyperintensities and cardiovascular disease: the importance of microcirculatory disease. Circ Cardiovasc Imag 2020; 13: e010460. [DOI] [PubMed] [Google Scholar]
- 18. Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015; 314: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craig L, Hoo ZL, Yan TZ, et al. Prevalence of dementia in ischaemic or mixed stroke populations: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2022; 93: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liang J, Li C, Gao D, et al. Association between onset age of coronary heart disease and incident dementia: a prospective cohort study. J Am Heart Assoc 2023; 12: e031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang W, Liang J, Li C, et al. Age at diagnosis of atrial fibrillation and incident dementia. JAMA Netw Open 2023; 6: e2342744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Secher N, Adelborg K, Szentkuti P, et al. Evaluation of neurologic and psychiatric outcomes after hospital discharge among adult survivors of cardiac arrest. JAMA Netw Open 2022; 5: e2213546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilcock D, Jicha G, Blacker D, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement 2021; 17: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–728. [DOI] [PubMed] [Google Scholar]
- 25. Doehner W, Bohm M, Boriani G, et al. Interaction of heart failure and stroke: a clinical consensus statement of the ESC Council on Stroke, the Heart Failure Association (HFA) and the ESC Working Group on Thrombosis. Eur J Heart Fail 2023; 25: 2107–2129. [DOI] [PubMed] [Google Scholar]
- 26. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol 2012; 2012: 367516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordestgaard LT, Christoffersen M, Frikke-Schmidt R. Shared risk factors between dementia and atherosclerotic cardiovascular disease. Int J Mol Sci 2022; 23: 9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iadecola C. Revisiting atherosclerosis and dementia. Nat Neurosci 2020; 23: 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. von Rennenberg R, Siegerink B, Ganeshan R, et al. High-sensitivity cardiac troponin T and severity of cerebral white matter lesions in patients with acute ischemic stroke. J Neurol 2019; 266: 37–45. [DOI] [PubMed] [Google Scholar]
- 30. Broersen LHA, Siegerink B, Sperber PS, et al. High-sensitivity cardiac troponin T and cognitive function in patients with ischemic stroke. Stroke 2020; 51: 1604–1607. [DOI] [PubMed] [Google Scholar]
- 31. Ulleryd MA, Prahl U, Borsbo J, et al. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One 2017; 12: e0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen PL, Kuo TB, Yang CC. Parasympathetic activity correlates with early outcome in patients with large artery atherosclerotic stroke. J Neurol Sci 2012; 314: 57–61. [DOI] [PubMed] [Google Scholar]
- 33. Bernbaum M, Menon BK, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J Cereb Blood Flow Metab 2015; 35: 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williamson W, Lewandowski AJ, Forkert ND, et al. Association of cardiovascular risk factors with MRI indices of cerebrovascular structure and function and white matter hyperintensities in young adults. JAMA 2018; 320: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ip BYM, Ko H, Lam BYK, et al. Current and future treatments of vascular cognitive impairment. Stroke 2024; 55: 822–839. [DOI] [PubMed] [Google Scholar]
- 36. Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol 2007; 6: 782–792. [DOI] [PubMed] [Google Scholar]
- 37. Fan X, Shen W, Wang L, et al. Efficacy and safety of DL-3-n-butylphthalide in the treatment of poststroke cognitive impairment: a systematic review and meta-analysis. Front Pharmacol 2021; 12: 810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alsulaimani RA, Quinn TJ. The efficacy and safety of animal-derived nootropics in cognitive disorders: systematic review and meta-analysis. Cereb Circ Cogn Behav 2021; 2: 100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin Y, Schulze V, Brockmeyer M, et al. Endovascular thrombectomy as a means to improve survival in acute ischemic stroke: a meta-analysis. JAMA Neurol 2019; 76: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lip GYH, Lane DA, Lenarczyk R, et al. Integrated care for optimizing the management of stroke and associated heart disease: a position paper of the European Society of Cardiology Council on Stroke. Eur Heart J 2022; 43: 2442–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lip GYH, Ntaios G. “Novel clinical concepts in thrombosis”: integrated care for stroke management-easy as ABC. Thromb Haemost 2022; 122: 316–319. [DOI] [PubMed] [Google Scholar]
- 42. Sagris D, Lip G, Korompoki E, et al. Adherence to an integrated care pathway for stroke is associated with lower risk of major cardiovascular events: a report from the Athens Stroke Registry. Eur J Intern Med 2024; 122: 61–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-eso-10.1177_23969873241293573 for Incident dementia in ischaemic stroke patients with early cardiac complications: A propensity-score matched cohort study by Tommaso Bucci, Sylvia E Choi, Christopher TW Tsang, Kai-Hang Yiu, Benjamin JR Buckley, Pasquale Pignatelli, Jan F Scheitz, Gregory YH Lip and Azmil H Abdul-Rahim in European Stroke Journal