Abstract
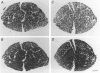
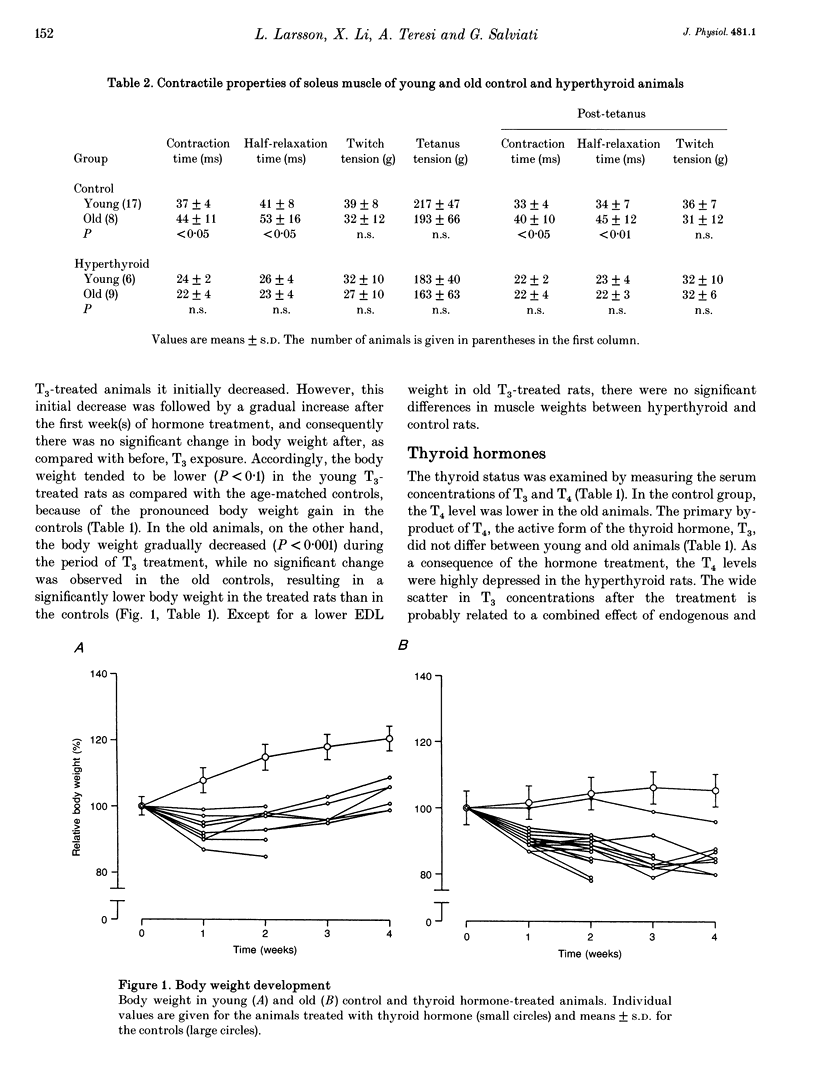
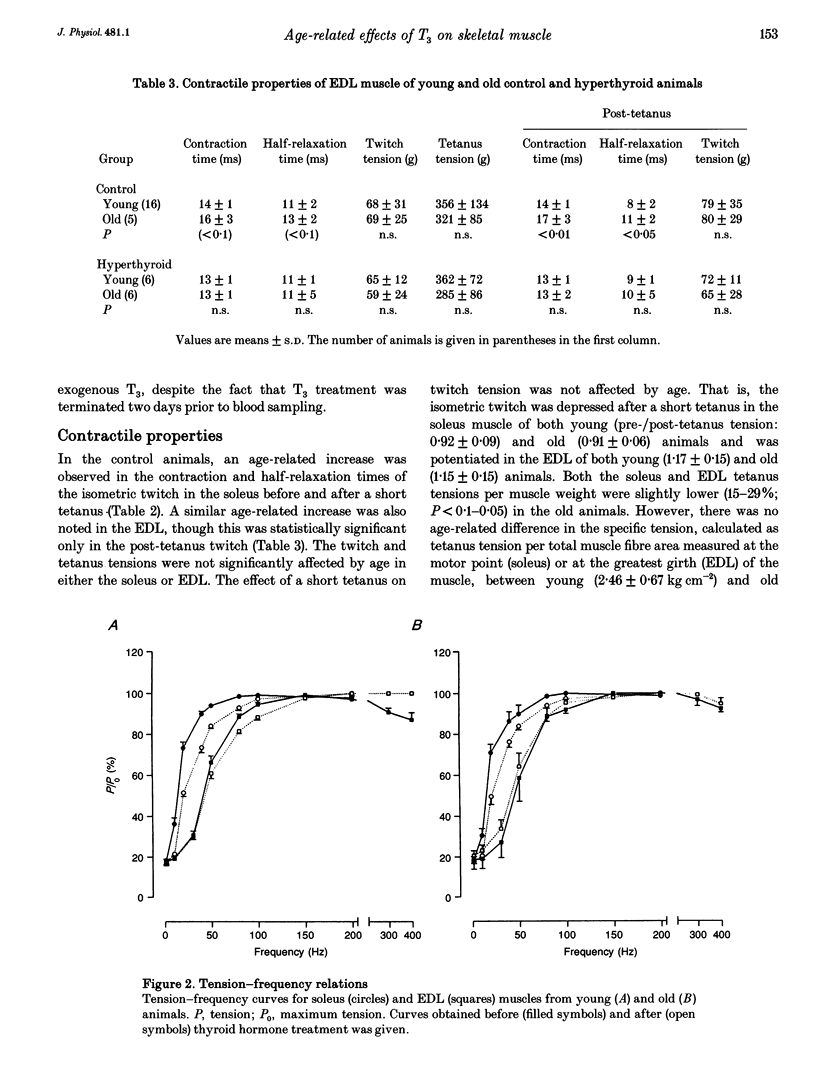
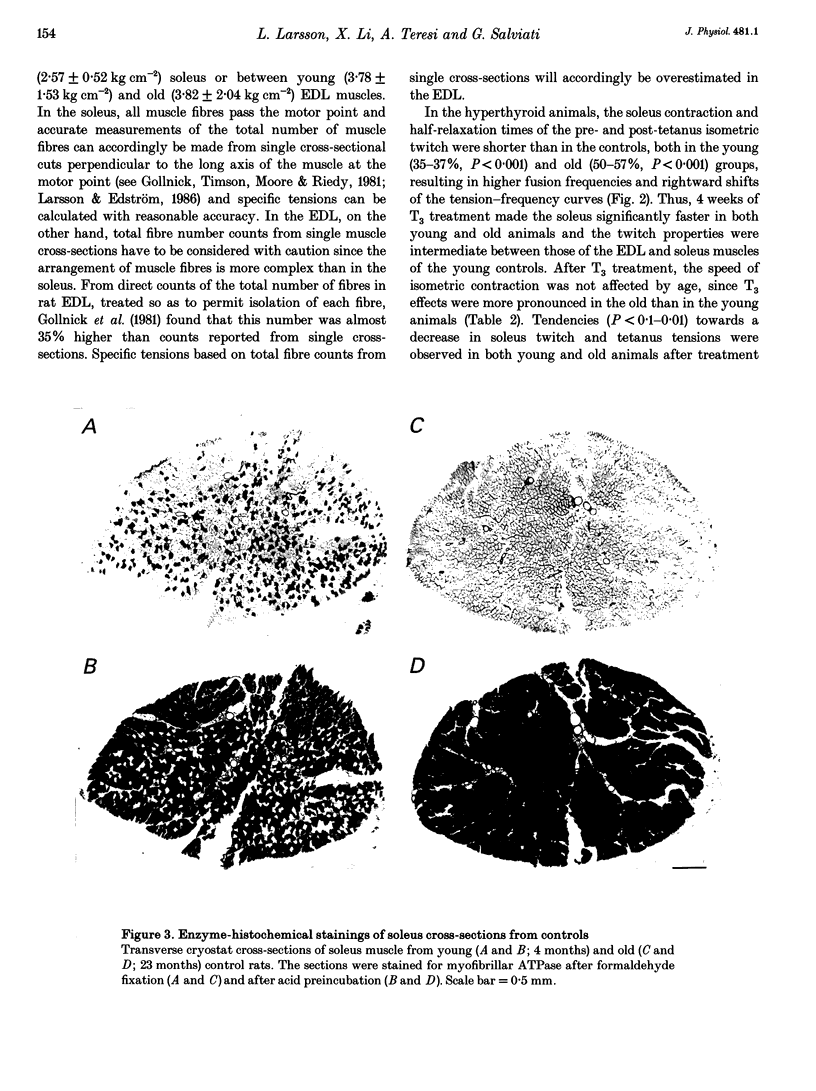
1. The effects of 4 weeks of thyroid hormone treatment on contractile, enzyme-histochemical and morphometric properties and on the myosin isoform composition were compared in the slow-twitch soleus and the fast-twitch extensor digitorum longus (EDL) muscle in young (3-6 months) and old (20-24 months) male rats. 2. In the soleus of untreated controls, contraction and half-relaxation times of the isometric twitch increased by 19-32% with age. The change in contractile properties was paralleled by an age-related increase in the proportions of type I fibres and type I myosin heavy chain (MHC) and slow myosin light chain (MLC) isoforms. 3. In the EDL of controls, contraction and half-relaxation times were significantly prolonged (21-38%) in the post-tetanus twitch in the old animals. No significant age-related changes were observed in enzyme-histochemical fibre-type proportions, although the number of fibres expressing both type IIA and IIB MHCs and of fibres expressing slow MLC isoforms was increased in the old animals. 4. Serum 3,5,3',5'-tetraiodothyronine (T4) levels were lower (34%) in the old animals, but the primary byproduct of T4, 3,5,3'-triiodothyronine (T3), did not differ between young and old animals. 5. The effects of 4 weeks of thyroid hormone treatment were highly muscle specific, and were more pronounced in soleus than in EDL, irrespective of animal age. In the soleus, this treatment shortened the contraction and half-relaxation times by 35-57% and decreased the number of type I fibres by 66-77% in both young and old animals. In EDL, thyroid hormone treatment significantly shortened the contraction time by 24%, but the change was restricted to the old animals. 6. In conclusion, the ability of skeletal muscle to respond to thyroid hormone treatment was not impaired in old age and the age-related changes in speed of contraction and enzyme-histochemical properties and myosin isoform compositions were diminished after thyroid hormone treatment in both the soleus and EDL.
Full text
PDF


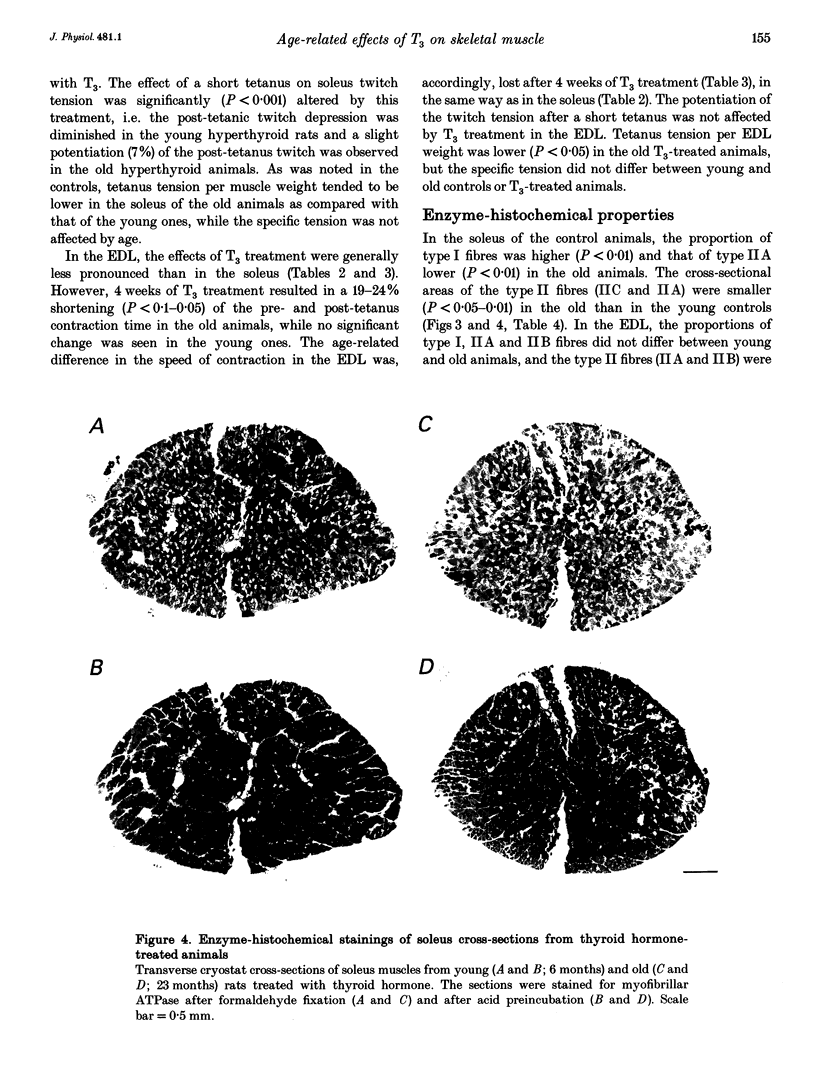
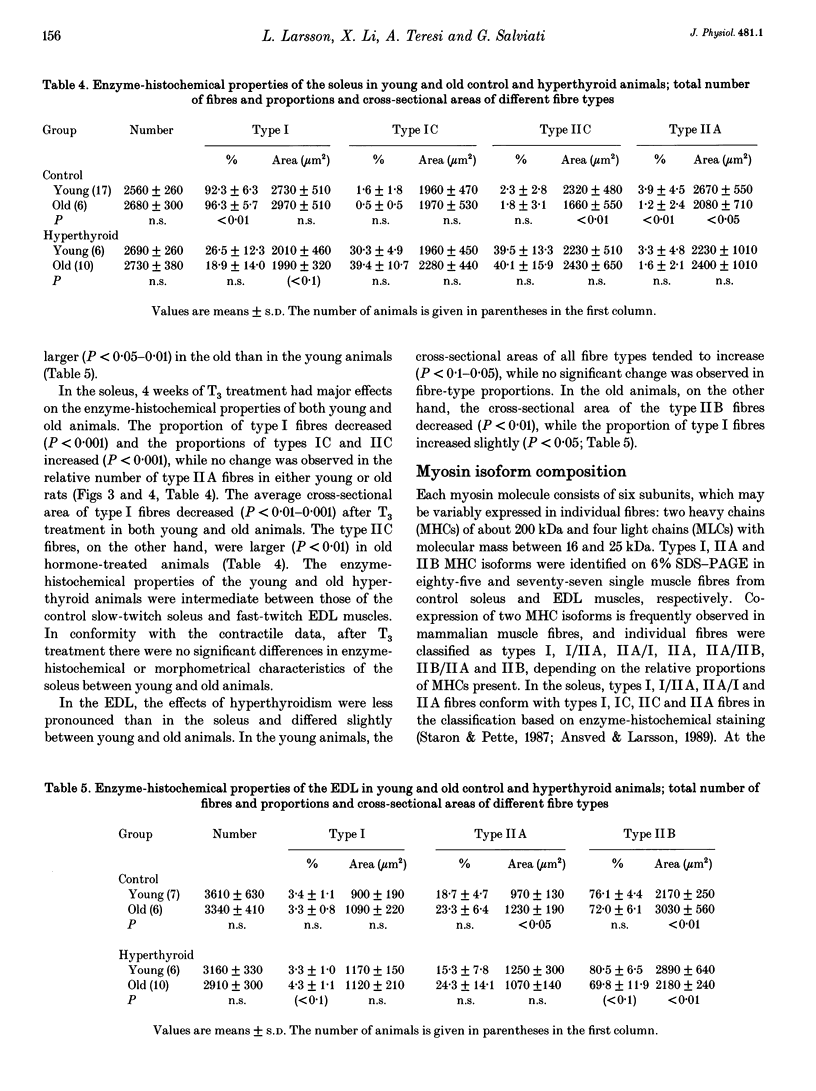
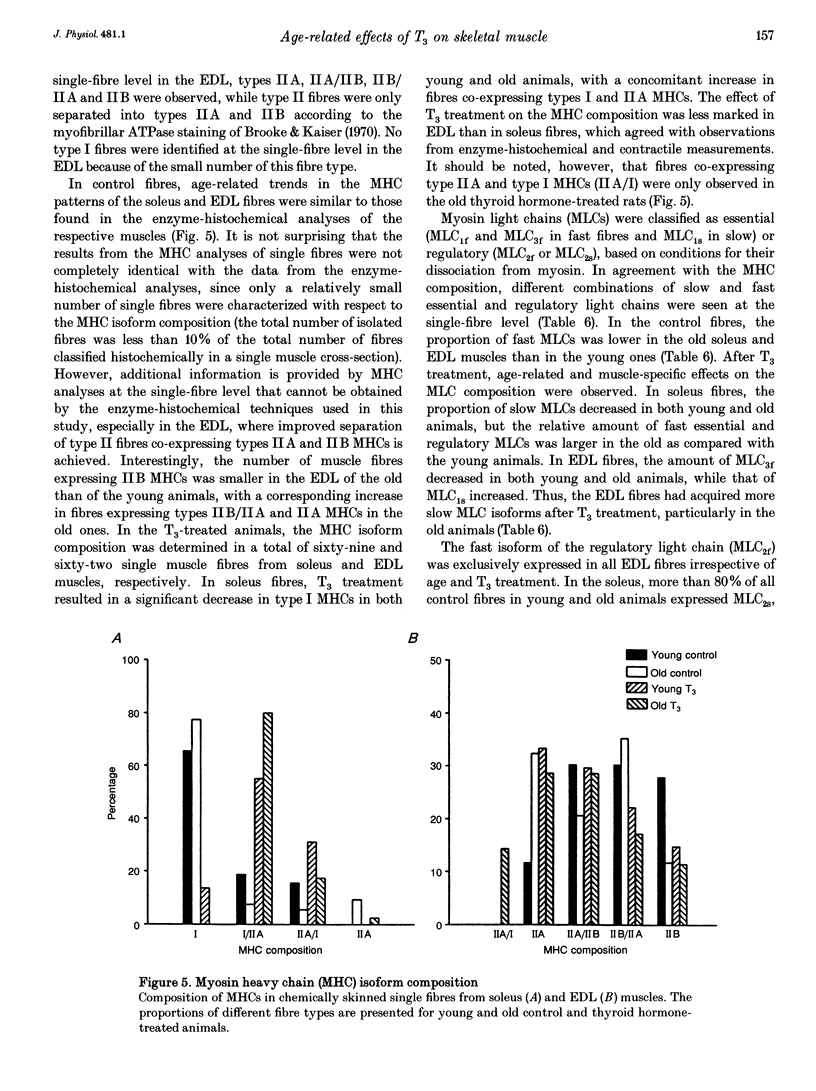
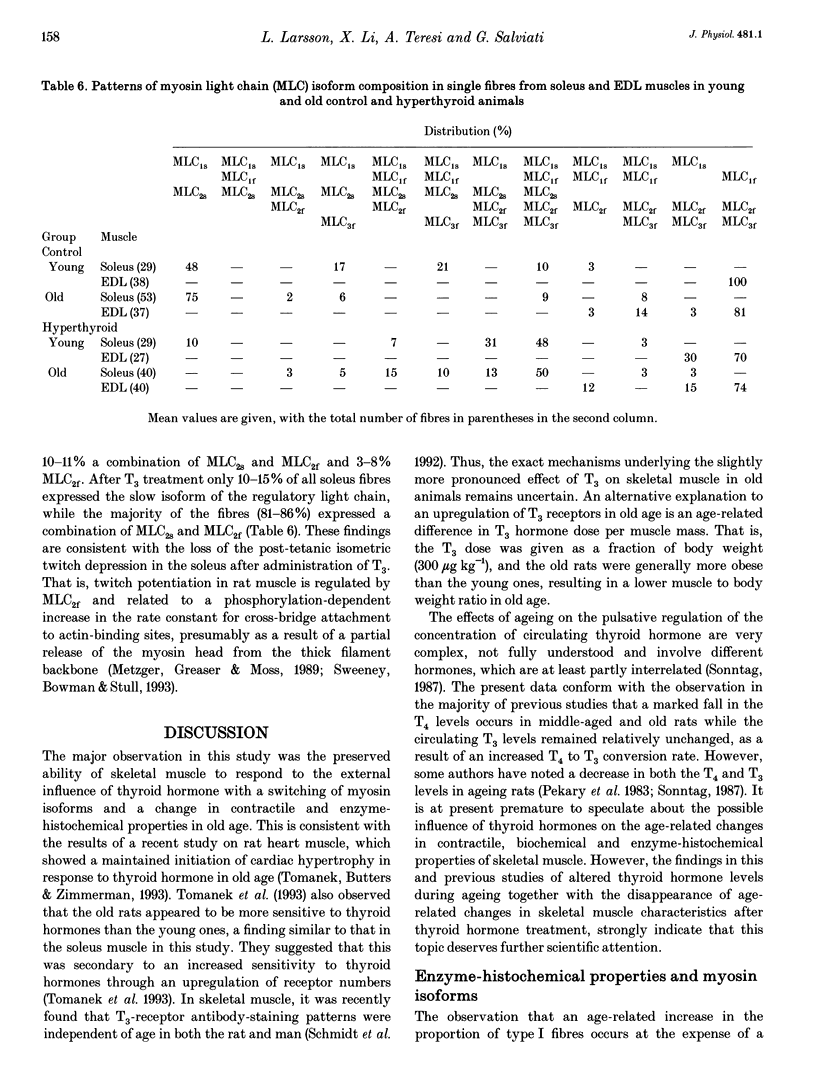



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansved T., Larsson L. Effects of ageing on enzyme-histochemical, morphometrical and contractile properties of the soleus muscle in the rat. J Neurol Sci. 1989 Oct;93(1):105–124. doi: 10.1016/0022-510x(89)90165-2. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Carnac G., Albagli-Curiel O., Vandromme M., Pinset C., Montarras D., Laudet V., Bonnieu A. 3,5,3'-Triiodothyronine positively regulates both MyoD1 gene transcription and terminal differentiation in C2 myoblasts. Mol Endocrinol. 1992 Aug;6(8):1185–1194. doi: 10.1210/mend.6.8.1406697. [DOI] [PubMed] [Google Scholar]
- Clark K. I., White T. P. Neuromuscular adaptations to cross-reinnervation in 12- and 29-mo-old Fischer 344 rats. Am J Physiol. 1991 Jan;260(1 Pt 1):C96–103. doi: 10.1152/ajpcell.1991.260.1.C96. [DOI] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Diffee G. M., Haddad F., Herrick R. E., Baldwin K. M. Control of myosin heavy chain expression: interaction of hypothyroidism and hindlimb suspension. Am J Physiol. 1991 Dec;261(6 Pt 1):C1099–C1106. doi: 10.1152/ajpcell.1991.261.6.C1099. [DOI] [PubMed] [Google Scholar]
- Edström L., Larsson L. Effects of age on contractile and enzyme-histochemical properties of fast- and slow-twitch single motor units in the rat. J Physiol. 1987 Nov;392:129–145. doi: 10.1113/jphysiol.1987.sp016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein D. I., Andrianakis P., Luff A. R., Walker D. Effects of thyroidectomy on development of skeletal muscle in fetal sheep. Am J Physiol. 1991 Nov;261(5 Pt 2):R1300–R1306. doi: 10.1152/ajpregu.1991.261.5.R1300. [DOI] [PubMed] [Google Scholar]
- Fitzsimons D. P., Herrick R. E., Baldwin K. M. Isomyosin distributions in rodent muscles: effects of altered thyroid state. J Appl Physiol (1985) 1990 Jul;69(1):321–327. doi: 10.1152/jappl.1990.69.1.321. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Timson B. F., Moore R. L., Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol Respir Environ Exerc Physiol. 1981 May;50(5):936–943. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990 Feb;38(2):257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Biral D., Campione M., Schiaffino S. An age-related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol Scand. 1993 Feb;147(2):227–234. doi: 10.1111/j.1748-1716.1993.tb09493.x. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci. 1986 Nov;76(1):69–89. doi: 10.1016/0022-510x(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L., Moss R. L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993 Dec;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Salviati G. Effects of age on calcium transport activity of sarcoplasmic reticulum in fast- and slow-twitch rat muscle fibres. J Physiol. 1989 Dec;419:253–264. doi: 10.1113/jphysiol.1989.sp017872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Greaser M. L., Moss R. L. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989 May;93(5):855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol C. J., Bruce D. S. Effect of hyperthyroidism on the contractile and histochemical properties of fast and slow twitch skeletal muscle in the rat. Pflugers Arch. 1981 Apr;390(1):73–79. doi: 10.1007/BF00582715. [DOI] [PubMed] [Google Scholar]
- Nwoye L., Mommaerts W. F., Simpson D. R., Seraydarian K., Marusich M. Evidence for a direct action of thyroid hormone in specifying muscle properties. Am J Physiol. 1982 Mar;242(3):R401–R408. doi: 10.1152/ajpregu.1982.242.3.R401. [DOI] [PubMed] [Google Scholar]
- Pekary A. E., Hershman J. M., Sugawara M., Gieschen K. I., Sogol P. B., Reed A. W., Pardridge W. M., Walfish P. G. Preferential release of triiodothyronine: an intrathyroidal adaptation to reduced serum thyroxine in aging rats. J Gerontol. 1983 Nov;38(6):653–659. doi: 10.1093/geronj/38.6.653. [DOI] [PubMed] [Google Scholar]
- Salviati G., Sorenson M. M., Eastwood A. B. Calcium accumulation by the sarcoplasmic reticulum in two populations of chemically skinned human muscle fibers. Effects of calcium and cyclic AMP. J Gen Physiol. 1982 Apr;79(4):603–632. doi: 10.1085/jgp.79.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Volpe P. Ca2+ release from sarcoplasmic reticulum of skinned fast- and slow-twitch muscle fibers. Am J Physiol. 1988 Mar;254(3 Pt 1):C459–C465. doi: 10.1152/ajpcell.1988.254.3.C459. [DOI] [PubMed] [Google Scholar]
- Salviati G., Zeviani M., Betto R., Nacamulli D., Busnardo B. Effects of thyroid hormones on the biochemical specialization of human muscle fibers. Muscle Nerve. 1985 Jun;8(5):363–371. doi: 10.1002/mus.880080504. [DOI] [PubMed] [Google Scholar]
- Schmidt E. D., Schmidt E. D., van der Gaag R., Ganpat R., Broersma L., de Boer P. A., Moorman A. F., Lamers W. H., Wiersinga W. M., Koornneef L. Distribution of the nuclear thyroid-hormone receptor in extraocular and skeletal muscles. J Endocrinol. 1992 Apr;133(1):67–74. doi: 10.1677/joe.0.1330067. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochem J. 1987 May 1;243(3):687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T., Matoba H., Miyata H., Kawai Y., Murakami N. Myosin heavy chain isoform transition in ageing fast and slow muscles of the rat. Acta Physiol Scand. 1992 Apr;144(4):419–423. doi: 10.1111/j.1748-1716.1992.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Sweeney H. L., Bowman B. F., Stull J. T. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993 May;264(5 Pt 1):C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J., Butters C. A., Zimmerman M. B. Initiation of cardiac hypertrophy in response to thyroxine is not limited by age. Am J Physiol. 1993 Apr;264(4 Pt 2):H1041–H1047. doi: 10.1152/ajpheart.1993.264.4.H1041. [DOI] [PubMed] [Google Scholar]
- Wentworth B. M., Donoghue M., Engert J. C., Berglund E. B., Rosenthal N. Paired MyoD-binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1242–1246. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Toutant M., Butler-Browne G. S., Watkins S. C. Hereditary pituitary dwarfism in mice affects skeletal and cardiac myosin isozyme transitions differently. J Cell Biol. 1985 Aug;101(2):603–609. doi: 10.1083/jcb.101.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]