Abstract
Objective: Moringa oleifera Lam. (Moringaceae), has traditionally been used for various renal diseases including urolithiasis. Considering the therapeutic and nutritional values, the present study was designed to investigate the antiurolithiatic potential of M. oleifera leaves through in-vitro, in-silico and in-vivo approaches. Methods: Methanolic aqueous extract of M. oleifera. leaves (MoL.Cr) was prepared and screened for phytoconstituents through FTIR and HPLC analysis, while antioxidant potential was determined by DPPH assay. Crystal nucleation, aggregation and growth assays were carried out to ascertain the in-vitro inhibitory effects of MoL.Cr. Molecular docking was performed to analyze the interactions between phytoconstituents and targeted proteins (Glycolate oxidase, Albumin and Tamm-Horsfall). Whereas, ethylene glycol-induced urolithiasis model (1% ammonium chloride +0.75% ethylene glycol) was used for in-vivo study. Presence of alkaloids, phenols, glycosides and flavonoids was confirmed by FTIR and HPLC analysis. Results: MoL.Cr significantly inhibited the CaOx crystal nucleation, aggregation as well as growth and normalized urinary and serum parameters. Histological studies showed that MoL.Cr significantly restored hyperoxaluria-induced irregular epithelial lining, interstitial inflammation and dilated proximal tubules. Conclusions: Thus, M. oleifera demonstrated marked stone inhibiting potential which can be due to its antioxidant, lowering of urinary concentration of stone forming constituents and anti-crystallization effects.
Keywords: Moringa oleifera leaves, urolithiasis, glycolate oxidase, albumin, tamm-horsfall protein
Graphical Abstract.
Introduction
Urolithiasis is a physiological abnormality in which stones are formed in any part of the urinary system including kidney, bladder and ureter. 1 The urinary stone formation includes supersaturation of urine, crystal nucleation, growth, accumulation and translocation to the surface of the renal epithelium. 2 An imbalance between urinary stone promoters (albumin, oxalate and uric acid) and stone-inhibitors (citrate, magnesium, nephrocalcin and urinary prothrombin fragment I) has been suggested as an important factor in the pathogenesis of renal calculi. 3 Kidney stones are classified on the basis of composition; i.e., calcium oxalate, calcium phosphate, uric acid, struvite and cystine stones. There are two main types of calcium oxalate (CaOx) crystals; i.e., calcium oxalate monohydrate (COM) and calcium oxalate dihydrate (COD). Dendritic COM crystals with sharp edges that stick strongly to the renal epithelium and cause damage to epithelial tissue are a common manifestation of hyperoxaluria. The defense against the retention of crystals that spontaneously form in the urine was proposed to include the preferential formation of COD crystals in the urine that prevent them from attaching to the renal tubular epithelium, reduce inflammation and calculogenesis.1,4
Urolithiasis is a medical challenge with a high rate of recurrence. Reports state that 10-12% of individuals in developed nations (10% of men and 3% of women) will experience a urinary stone at some point in their lives. The etiology of this disorder is multifactorial and involves various factors such as lack of physical activity, diet and genetics. 5 In addition to the physicochemical mechanism of stone formation (precipitation, growth and aggregation), the interaction between crystals and renal tubular epithelial cells is an important risk factor in renal stone formation. Supersaturation is also the driving force for crystallization in urine which leads to crystal nucleation. Urinary supersaturation, hyperoxaluria and hypercalciuria produce oxidative stress which may lead to cell apoptosis or necrosis. Thus, resultant cell injury and cell membrane damage cause the up-regulation of crystal-binding molecules (e.g., osteopontin) and enhance the binding ability of crystals to the cell membrane. After binding, crystals translocate into the interstitium and inflammation occurs which may lead to the release of monocyte that causes crystal adhesion or retention and stone formation. 6
Though, various treatment options for the mitigation of urolithiasis developed over the years, variations occur regarding their clinical indications and effectiveness. Currently, urolithiasis treatment focus on reducing the stone recurrence rather than etiologies. 7 Plant-based remedies have been used during the ages to cure renal stones disease with quite beneficial outcomes. Therefore, it is valuable to use medicinal plants as a remedy to treat kidney stones.
One method of molecular modelling is protein and ligand docking. Predicting a ligand’s (small molecule) position and orientation when it binds to an enzyme or protein receptor is the aim of protein–ligand docking. Many pharmaceutical companies have achieved significant advances in computer-aided drug design at several phases of drug development, including lead optimization, hit-to-lead molecule binding affinity enhancement and identification of new targets. 8 In the present study, various proteins such as glycolate oxidase, albumin and Tamm-Horsfall protein were targeted in the present study to evaluate the inhibitory effect of identified phytoconstituents.
Moringa oleifera Lam. is a rapidly growing softwood tree that belongs to the family Moringaceae. M. oleifera is indigenous to Pakistan, Afghanistan, Bangladesh and India, also found all over the world. It is known as a “Miracle tree” due to its nutritional values and “Mothers best friend” for its property to enhance milk production in lactating mothers. In 2007, it was reported that approximately 38% of adults in the USA had used M. oleifera as a natural herbal product for treatment of various ailments in previous years. 9
It has been reported that leaves of M. oleifera have low calories, high concentrations of minerals, natural antioxidants and vitamins. Diuretic, lipid and blood pressure-lowering properties of M.oleifera leaves are attributed to the presence of high polyunsaturated fatty acids and low saturated fatty acids content. Traditionally, it is also used for the improvement of skin and wound healing. 10 It is reported to have antimicrobial, antifungal, antibacterial, anti-inflammatory, antioxidant, antitumor, antifertility, hepatoprotective, antihypertensive, hypocholesterolemic, antiulcer, antipyretic, antidiabetic, anticonvulsant and anti-allergic activities. 11
Various parts of the plant such as dried root bark, 12 dried root wood, 13 fresh bark 14 and fresh pods 15 have already been reported against urolithiasis. Different species of Moringa including Moringa oleifera bark have also been reported for the modulation and inhibition of the crystallization process and crystal morphology of calcium oxalate monohydrate.16,17 Despite the traditional use of M. oleifera leaves against renal disorders, and reported in-vitro crystallization antiurolithiatic potential, 18 no data is available for its use against the treatment of kidney stones. Therefore, the methanolic aqueous extract of M. oleifera leaves, an edible part, was considered for the investigation of effects against urolithiasis by employing various in-vitro crystallization studies, in-vivo and in-silico methods.
Material and Methods
Plant Material
Fresh leaves of Moringa oleifera Lam. were collected from Baghdad-ul-Jadeed campus, IUB and authentictaed by the botanist, Mr. Abdul Hameed. The dried sample was deposited in the herbarium of Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, IUB, Pakistan. Voucher numbers (MO-LE-10-20-170) was issued for future reference.
Preparation of Crude Extract
5 kg leaves of M. oleifera Lam. were washed, cut into small pieces and then soaked in 80% methanolic aqueous solution. After 3 days, the plant material was filtered and soaked again. This procedure was repeated twice. After third filtration, the filtrate was subjected to evaporation to obtain a semi-solid paste. The prepared MoL.Cr was weighed, labelled and stored in freezer for future use.
Chemicals
Analytical grade chemicals such as cystone (Himalaya, India), ammonium chloride (Lahore Pharma, Pakistan), calcium chloride dihydrate, sodium carbonate, sodium chloride, sodium oxalate, ethylene glycol (Merck, Germany), ketamine (Global Pharmaceutical, Pakistan), xylazine (MyLab, Pakistan), sodium acetate trihydrate (Duksan, Korea), hydrochloric acid (BDH, England ), tris base (Fluka, USA) and formalin (Riedel-de Haen, Germany) were used in the study. Colorimetric assay kits were also used for the determination of calcium, creatinine, magnesium, phosphorus, uric acid, urea and total protein (Human Diagnostic Worldwide, Germany) were also used.
Phytochemical Screening
Phytochemical analysis was performed to confirm the presence of secondary metabolites like alkaloids, amino acids, carbohydrates, flavonoids, coumarins, glycosides, phenolic compounds, saponins, tannins and protein in MoL.Cr.19-21
Antioxidant Assay
The antioxidant activity was performed, according to the method followed by Bashir and Gilani (2009) with minor modifications. 22 Using a methanolic solution of DPPH (2, 2 diphenyl-1-picrylhydrazyl), the antioxidant capacity MoL.Cr was assessed and compared with ascorbic acid (standard antioxidant). Various dilutions of MoL.Cr and ascorbic acid (5, 10, 25, 50, 75, 100, 125, and 150 μl/ml) were prepared in order to measure the DPPH free radical scavenging activity. After adding 1 mL of 0.1 mM DPPH solution to 3 mL of each dilution, the solutions were allowed to stand at room temperature for 30 minutes, and the absorbance at 517 nm was measured. The following formula was used to determine antioxidant potential:
AA= Absorbance of control and AB= Absorbance of sample.
FTIR Analysis
IR analysis was carried out using FTIR spectrophotometer (Agilent Cary-630 ATR, USA).
HPLC Analysis
Flavonoids, terpenoids, tannins and phenols were estimated using high-performance liquid chromatography (HPLC) method. During the experiment, standards (50 μg/mL) and MoL.Cr (10 mg/mL) solutions were prepared and left to stand at 4°C. The testing was performed on a Shimadzu LC10-AT VP Liquid Chromatograph with SIL-20A auto-sampler and SPD-10AV UV VIS detector. For separation, a Shim-Pack CLC-ODS (C-18, 25 cm × 4.6 mm, 5 m) was used, which was kept at room temperature. The binary solvent system, consisting of solvent A (water: acetic acid-94:6, pH = 2.2) and solvent B (acetonitrile), was used as the mobile phase, with the following gradient elution: 15 minutes for 85% A: 15% B, 15-30 minutes for 55% A: 45% B, and 30-35 minutes for 0% A: 100% B. The flow rate was recorded; i.e., 1.0 mL/min and absorbance was measured at 280 nm. 23
In-vitro Crystallization Assay
In-vitro crystallization assay (nucleation, aggregation and crystal growth) was carried out in triplicate in compliance with the protocol followed by Jamshed et al (2022) and Mosquera et al (2020) with minor modifications.23,24
Nucleation Assay
In nucleation assay, the effects of MoL.Cr on CaOx crystallization was investigated. For this assay, 5 mM calcium chloride and 7.5 mM sodium oxalate solutions were prepared in a buffer (Tris-HCl, 0.05 M + NaCl, 0.15 M) having pH 6.5. Various dilutions of MoL.Cr and cystone (100-1000 μg/) were prepared in distilled water. 20 μl of each dilution of MoL.Cr and cystone were mixed with 60 μl calcium chloride and then sodium oxalate (60 μl) was added in each dilution. These dilutions were incubated in oven (37°C) for 30 minutes. Then, after cooling the optical density was noted at 630 nm. Percent inhibition of crystal nucleation was calculated from the following formula:
where, OD (test) is the optical density of cystone or MoL.Cr and OD (control) is the optical density of the negative control.
Aggregation Assay
The effects of MoL.Cr and cystone on the aggregation of crystals were studied by preparing the solution of 0.05 M calcium chloride and sodium oxalate, separately. After mixing the solutions, the mixture was heated in the water bath (60°C) for 60 minutes and then incubated overnight (37°C). After drying, 40 mg/50 mL crystals solution was prepared in a buffer (0.05 M Tris-HCl and 0.5 M NaCl) at pH 6.5. 20 μl of each dilution of MoL.Cr and cystone were mixed with 60 μl of CaOx crystals solution, incubated for 30 minutes and then optical density was recorded at 630 nm. Using the same formula as the nucleation assay, the percent inhibition of aggregation was computed.
Crystal Growth Assay
To determine the effects of MoL.Cr and cystone on crystal growth, various dilutions were prepared. 3 g/2 mL of CaOx slurry was made in sodium acetate (0.05 M) buffer at pH 5.7. Calcium chloride (0.004 M) and sodium oxalate (0.004 M) solutions were prepared and then and 1 mL of each solution was mixed with a buffer (0.01 M HCl +0.09 M NaCl) at pH 7.4. 30 μl of CaOx slurry was added to reaction mixture followed by addition of 1 mL each dilution of MoL.Cr and cystone. Then, optical density was measured at 214 nm for 10 minutes. The difference in OD was determined and then by using the formula percent inhibition of crystal growth was calculated.
Molecular Docking Analysis
Molecular docking was performed to explore the interactions between phytoconstituents (quercetin and kaempferol) and targeted proteins. The 3D crystal structures of proteins namely Glycolate oxidase, Albumin and Tamm-Horsfall protein were retrieved from the Protein Data Bank (https://www.rcsb.com) PDB IDs: 2RDT, 4JK4 and 4WRN). 25 Prior to docking analysis, the proteins were prepared using MGL tools, 26 by removing heteroatoms and water molecules followed by the addition of polar hydrogen atoms and kollman charges. After that protein structures were rendered and corrected for missing residues. 27 The docking protocol was validated by first separating the co-crystal ligand from the active pocket of the complex, and then re-docking was performed to validate its accuracy. 28 During the validation procedure it was observed that RMSD values remained less than 2.0 Å, confirming the protocol is validated. After the validation, the 3D structure of quercetin and kaempferol was made by using ChemDraw 3D, 29 to get the most stable arrangement of atoms energy minimization was done. Docking analysis was performed with target protein using AutoDock’s default genetic algorithm as the scoring function. The grid box dimensions were set as x = 25.323920, y = 14.207116, z = 20.050868 for 2RDT, x = 63.837003, y = 23.103888, z = 32.965576 for 4JK4 and x = −0.831054, y = 47.246241, z = 32.470409 for 4WRN. The phytoconstituents were docked within the active pocket of target proteins, 100 different configurations were generated for each protein. The pose possessing most stable configuration with least binding energy was selected and analyzed in 2D and 3D positions using Discovery Studio visualizer version 16 30 to elaborate the interactions formed between synthesized compound and targeted proteins.
Evaluation of In-vivo Antiurolithiatic Potential of MoL.Cr
For the evaluation of antiurolithiatic potential of MoL.Cr, ethylene glycol-induced urolithiasis model was used. The in-vivo study was performed after the approval by Pharmacy Animal Ethics Committee (PAEC) under the registration number PAEC/2020/27.
Animals
Adult male Wistar albino rats (150-270g) and Swiss mice (20-30g) were used in the experiment. The animals were allowed to acclimatize with the experimental conditions for 7 days before starting the experimental procedure.
Sample Size
The sample size or the number of animals in each group was measured by using power analysis method. This method is similar to the method used for calculation of sample size for clinical trials and clinical studies. Simple calculation was carried out manually with the help of formula and for complex calculations power analysis based software was used. 31
Animal Model of Urolithiasis
For the induction of kidney stones, 1% ammonium chloride (AC) and 0.75% ethylene glycol (EG) were administered in drinking water as a lithogenic treatment for the first 5 days and then, only 0.75% EG was given for the next 16 day. 32
After induction, animals were divided randomly into various groups with the same body for 21 days in drinking water. After 21 days, lithogenic treatment was discontinued. Normal control group and intoxicated group were given distilled water (5 mL/kg p.o.) per 24 h. One group was considered as standard group which received cystone (500 mg/kg p.o.) per 24 h, a standard drug. While, other groups were considered as treatment groups and treated with different doses of MoL.Cr; i.e., 100, 300 and 500 mg/kg for the next 14 days.
Urine Collection and Analysis
After the lithogenic treatment at 21st day and after the treatment with MoL.Cr at 35th day animals were placed in the metabolic cages individually for the collection of urine. Fresh 3 h morning urine samples were analyzed for the crystal count. For analysis, 1 mL of each urine sample was centrifuged at 3000 rpm for 5 minutes. After centrifugation, 950 μl of supernatant was removed and remaining portion was analyzed on a neubauer chamber under a light microscope. The number of crystals were counted as described previously. 33 Urinary crystals were semi-quantitatively analyzed using a scoring system; i.e., 0 = approximately no crystals, 1 = few crystals, 2 = several crystals and 3 = many crystals in urine.
After 24 h, urinary pH and urinary volume were determined. Uric acid, calcium, magnesium, phosphorus and total protein levels in urine were determined by using commercially available kits.
Serum Analysis
After the completion of study at 35th day, Animals were anesthetized with 0.2 mL/100 g ketamine/xylazine combination (10:1), intraperitoneally. Then, blood samples were collected through cardiac puncture and retro-orbital techniques. Blood samples were allowed to clot for 15 minutes and then centrifuged at 4000 rpm for 15 minutes. 34 Serums were analyzed for the determination of biochemical parameters; i.e., creatinine and blood urea nitrogen (BUN) by using commercially available kits.
Kidney Histology
At 35th day, animals were sacrificed and one kidney of representative animal from each group was dissected out and preserved in 10% formalin and then histologically analyzed.
Acute Toxicity Assay
Swiss albino mice of either sex (18-30 g) were divided into different groups comprising of five mice each to assess the toxicity of MoL.Cr as described previously. 35 Distilled water (10 mL/kg p.o.) was given to the normal control group. While, different doses of MoL.Cr; i.e., 0.3, 1, 3 and 10 g/kg, respectively were given to the remaining groups. The mice were observed critically for 2 h and then at the interval of 30 minutes for the next 6 h for any type of behavioral changes; i.e., alertness, convulsions, grooming, hyperactivity, salivation, urination, lacrimation, pain response, touch response, corneal reflex, writhing reflex, gripping strength, righting reflex and skin color. Then, mortality rate of animals was observed for the next 48 h.
Statistical Analysis
The values were expressed as Mean ± SEM and results were statistically analyzed by using two-way ANOVA followed by Bonferroni’s post hoc test. The data was analyzed by using Graphpad Prism version 08.
Results
Phytochemical and Antioxidant Screening of MoL.Cr
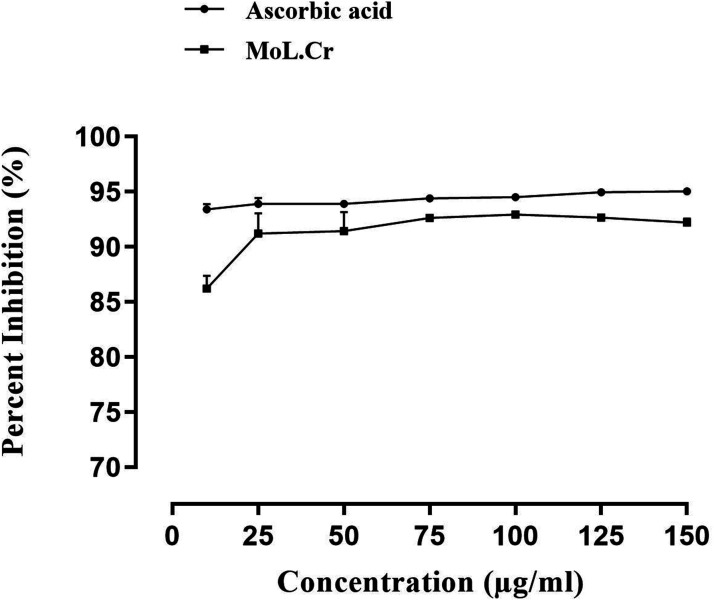
Phytochemical screening revealed the presence of alkaloids, saponins, flavonoids, phenolic contents, glycosides, tannins and coumarins in methanolic aqueous extract of MoL.Cr. Antioxidant assay demonstrated that at the concentration of 150 μg/ml, MoL.Cr showed significant free radical scavenging activity; i.e., 92.19 ± 0.3 and results were comparable to the ascorbic acid (95.04 ± 0.3) Figure 1.
Figure 1.
Antioxidant assay of MoL.Cr.
FTIR and HPLC Analysis
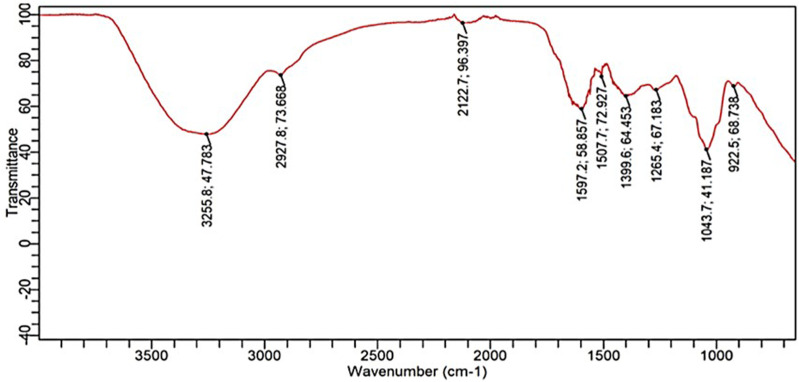
The major functional groups of MoL.Cr were identified and categorized by FTIR results based on transmittance band peak values and spectral features, as shown in Figure 2.
Figure 2.
Representative FTIR spectra of MoL.Cr showing characteristic transmittance peaks of specific functional groups of phytoconstituents.
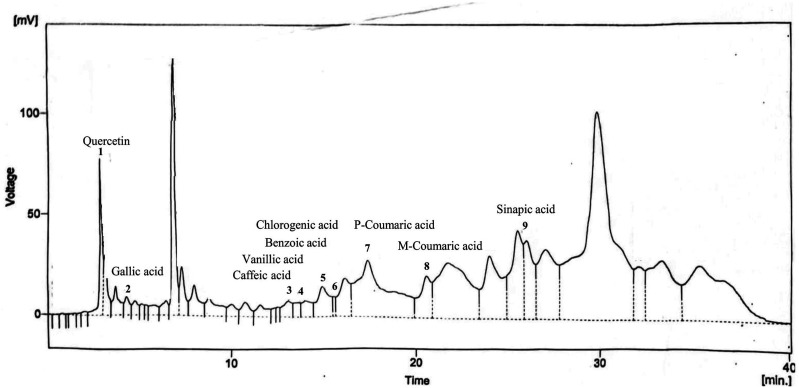
The region from 4000-1800 cm−1 revealed a broad peak at 3255 cm−1, indicating the presence of –OH stretching (hydroxyl or carboxylic acids) and N-H stretching (alkaloids). A minor peak at 2927 cm−1 corresponded to the C-H stretching of the –CH2 group (terpenoids). The fingerprint region (1800-650 cm−1) identified secondary protein structures, phenolic compounds, terpenoids, alkaloids, and carbohydrates. The HPLC analysis of MoL.Cr indicated the presence of quercetin (Rt. 2.85 min), gallic acid (Rt. 4.28), caffeic acid (Rt.12.99), vanillic acid (Rt. 13.55), benzoic acid (Rt.14.82), chlorogenic acid (Rt.15.44), p-coumaric acid (Rt. 17.32). M-coumaric acid (Rt. 20.53) and sinapic acid as shown in Figure 3 and Table 1.
Figure 3.
HPLC chromatogram of MoL.Cr indicating presence of phytochemical compounds.
Table 1.
HPLC Profile of Phytochemical Compounds Detected in the MoL.Cr.
| Phytochemical compounds | Retention time (min) | Area (%) | Concentration (ppm) |
|---|---|---|---|
| Quercetin | 2.85 | 1.7 | 37.76 |
| Gallic acid | 4.28 | 0.4 | 6.18 |
| Caffeic acid | 12.99 | 0.6 | 12.15 |
| Vanillic acid | 13.55 | 0.4 | 10.66 |
| Benzoic acid | 14.82 | 1.6 | 70.59 |
| Chlorogenic acid | 15.44 | 0.2 | 7.83 |
| p-Coumaric acid | 17.32 | 7.7 | 42.99 |
| M-Coumaric acid | 20.53 | 2.1 | 10.89 |
| Sinapic acid | 26.0 | 3.1 | 17.36 |
Molecular Docking Analysis
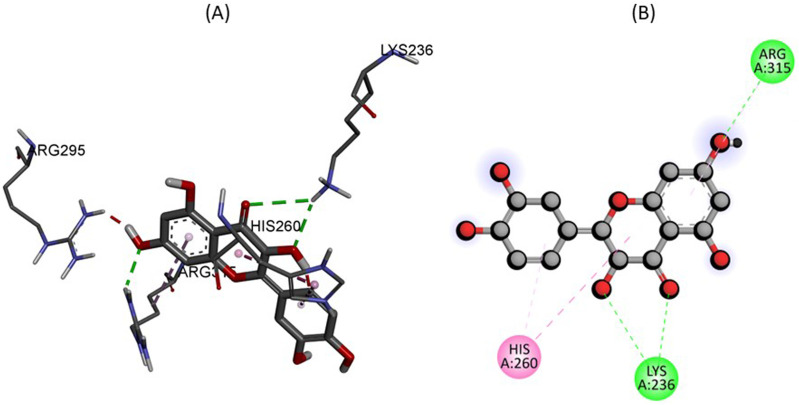
Figure 4 showing 2D and 3D binding interactions between quercetin within the active site of glycolate oxidase. The docking analysis depicts that amino acids residues LYS236 and ARG315 were found to have conventional hydrogen bonds with the quercetin with −9.7 kJ/mol energy as shown in Table 2. Furthermore, amino acid residue HIS260 was found to have pi-pi stacked interaction with aromatic ring of quercetin.
Figure 4.
The predicted 2D and 3D binding mode of Quercetin within the active site of Glycolate oxidase.
Table 2.
Molecular Docking Scores of Compounds With Targeted Protein.
| Compound | Protein | Binding energy kJ/mol | Conventional hydrogen bonds | Hydrophobic interactions |
|---|---|---|---|---|
| Quercetin C15H10O7 | Glycolate oxidase (PDB ID: 2RDT) | −9.7 | LYS236, ARG315 | HIS260 |
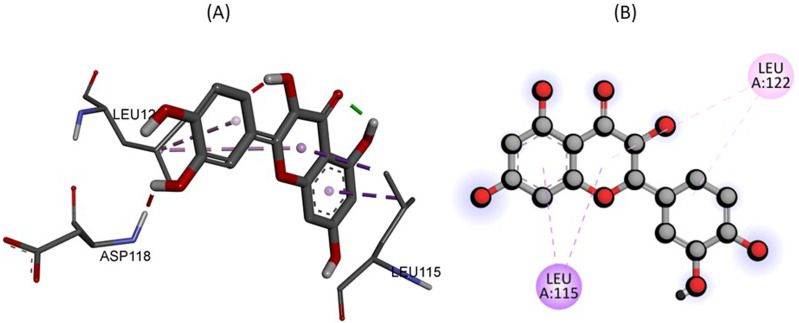
| Quercetin C15H10O7 | Albumin (PDB ID: 4JK4) | −8.2 | No | LEU115, LEU122 |
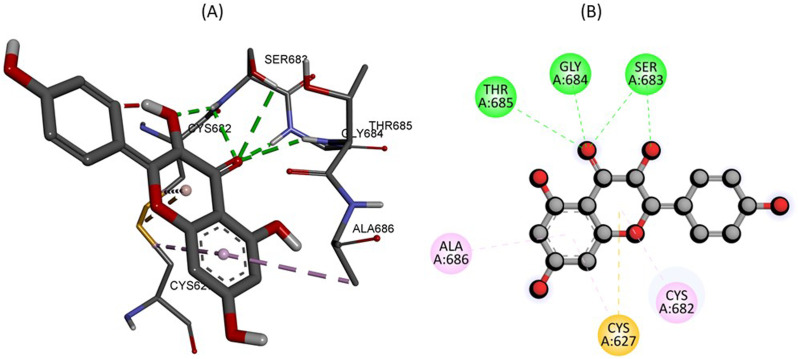
| Kaempferol C15H10O6 | Tamm-horsfall protein (PDB ID: 4WRN) | −8.1 | THR685, GLY684, SER683 |
ALA686, CYS682, CYS627 |
Similarly, Figure 5 describe the 2D and 3D binding interactions between Quercetin within the active pocket of Albumin. The docking study illustrates that amino acid residues LEU115, and LEU122 interact within the active pocket of Albumin, forming pi-sigma and pi-alkyl linkage respectively which potentiate the binding interactions.
Figure 5.
The predicted 2D and 3D binding mode of Quercetin within the active site of Albumin.
In Figure 6, the 2D and 3D binding interactions were retrieved between Kaempferol within the active site of Tamm-Horsfall protein. The docking analysis states that amino acid THR685, GLY684, and SER683 formed 3 conventional hydrogen bonds with kaempferol. While CYS627 interact via pi-Sulphur interaction. Furthermore, the binding interaction was potentiated by amino acid residues ALA686, and CYS682 forming pi-alkyl linkage.
Figure 6.
The predicted 2D and 3D binding mode of Kaempferol within the active site of Tamm-Horsfall protein.
In-vitro Crystallization Assay
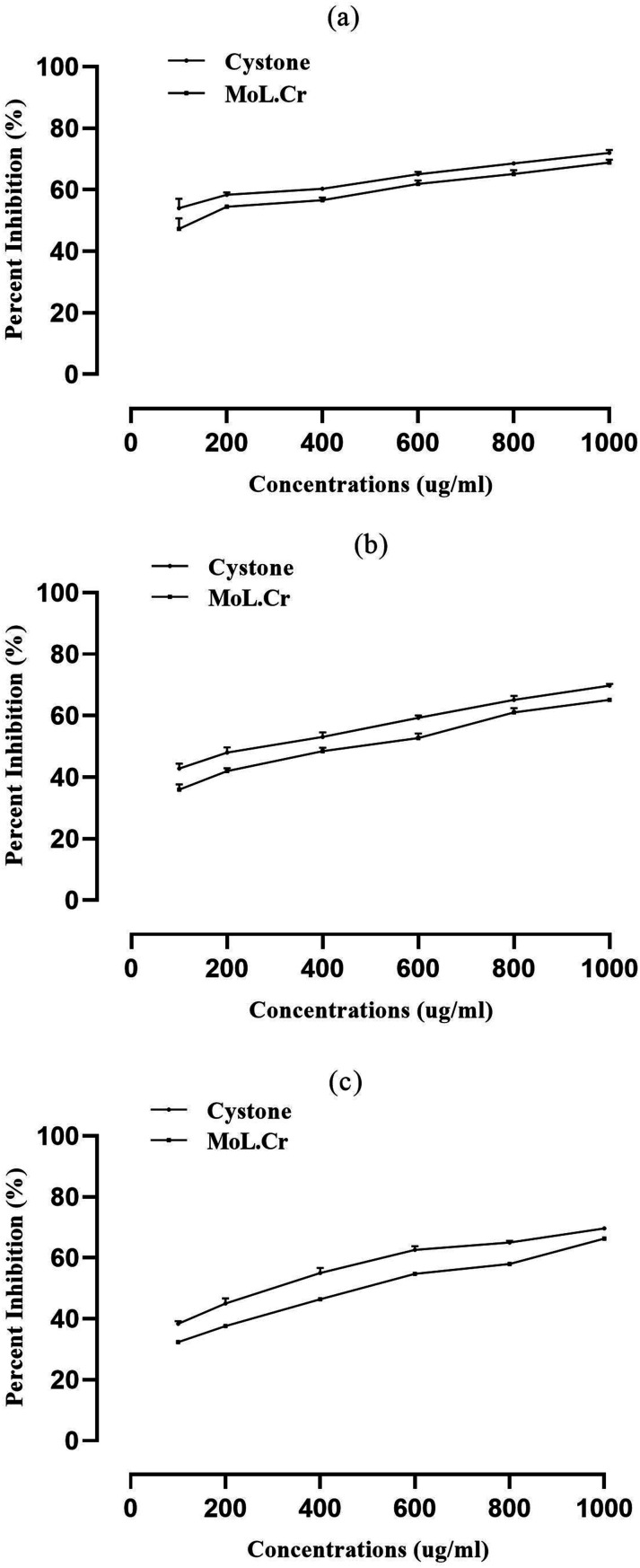
The percent inhibition of nucleation, at highest concentration (1000 μg/ ml) of MoL.Cr was found to be 72 ± 0.92% as similar to cystone; i.e., 68.8 ± 0.93% (Figure 7A). The percent inhibition in aggregation assay for MoL.Cr was 65.2 ± 0.5%; whereas, cystone showed percent inhibition of 69.8 ± 0.60%, at the concentration of 1000 μg/ ml (Figure 7B). The percent reduction of crystal growth in crystal growth assay was calculated to be 66.33 ± 0.3% for 1000 μg/ ml as compared to cystone ; i.e., 69.7 ± 0.33% (Figure 7C).
Figure 7.
Inhibitory potential of MoL.Cr and cystone against calcium oxalate crystals; (A) nucleation assay, (B) aggregation assay and (C) crystal growth assay.
Evaluation of In-vivo Antiurolithiatic Activity
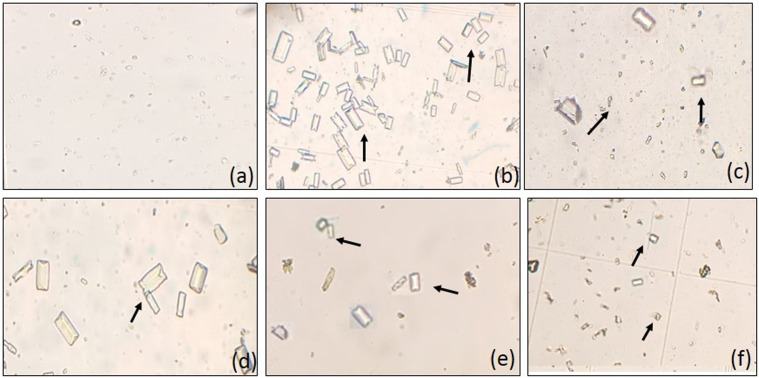
Microscopic analysis of urine showed a significant (P < 0.001) increase in the number and size of crystals in lithogenic rats as compared to the normal control group as depicted in Figure 8. MoL.Cr showed dose-dependent effects and reduced the crystal count significantly (Table 3). According to the scoring system, normal control group showed no crystals, whereas, lithogenic rats showed many crystals. Treatment groups showed significant result at the doses of 100 (several crystals), 300 (many crystals) and 500 mg/kg (few crystals). Lithogenic treatment enhanced the urinary output. While, urine volume of MoL.Cr treated rats was significantly (P < 0.01) less than the untreated group but greater than the normal control group. Lithogenic treatment caused significant (P < 0.001) reduction in the urinary pH of intoxicated rats. MoL.Cr significantly restored the urinary pH dose-dependent and results were comparable to those of cystone (table 3).
Figure 8.
Microscopic images of urinary crystals (magnification 10×), curative model of urolithiasis (A) control, (B) intoxicated, (C) cystone (500 mg/kg), MoL.Cr; (D) 100 mg/kg, (E) 300 mg/kg and (F) 500 mg/kg, arrow(→)showing urinary crystals.
Table 3.
Effects of MoL.Cr and Cystone on Urinary Crystal Count, Urinary Volume and pH in Curative Model of Urolithiasis.
| Treatment | Crystal count/mm3 | Urine volume (ml/100g) | Urinary pH | |||
|---|---|---|---|---|---|---|
| Day 21 | Day 35 | Day 21 | Day 35 | Day 21 | Day 35 | |
| Control group | 10.33 ± 1.2 | 9.2 ± 1.0 | 2.5 ± 0.1 | 2.4 ± 0.1 | 7.15 ± 0.2 | 7.2 ± 0.2 |
| Intoxicated group | 281 ± 5.2### | 274.8 ± 4.4 | 5.3 ± 0.1### | 5.1 ± 0.1 | 5.3 ± 0.2### | 5.5 ± 0.3 |
| MoL.Cr (100 mg/kg) | 276.3 ± 3.6 | 256.5 ± 4.7ns | 4.9 ± 0.2 | 4.7 ± 0.2ns | 4.9 ± 0.2 | 5.4 ± 0.1ns |
| MoL.Cr (300 mg/kg) | 275.8 ± 4.5 | 247.3 ± 8.1** | 5.0 ± 0.1 | 4.4 ± 0.1ns | 5.10 ± 0.3 | 6.3 ± 0.2** |
| MoL.Cr (500 mg/kg) | 276.2 ± 4.6 | 210.8 ± 8.8*** | 5.2 ± 0.1 | 4.4 ± 0.1** | 4.9 ± 0.2 | 7 ± 0.2*** |
| Cystone (500 mg/kg) | 277.5 ± 4.8 | 173.7 ± 4.2*** | 5.1 ± 0.1 | 3.4 ± 0.2*** | 4.9 ± 0.2 | 7.1 ± 0.1*** |
Mean ± SEM; n = 6.
ns: P > 0.05; *: P < 0.05; **: P < 0.01; ***&###: P < 0.001.
(*: Comparison within the groups; i.e., 21st and 35th day and #: comparison of intoxicated group with normal groups at 21st day; One-way ANOVA followed by bonferroni’s post hoc test.
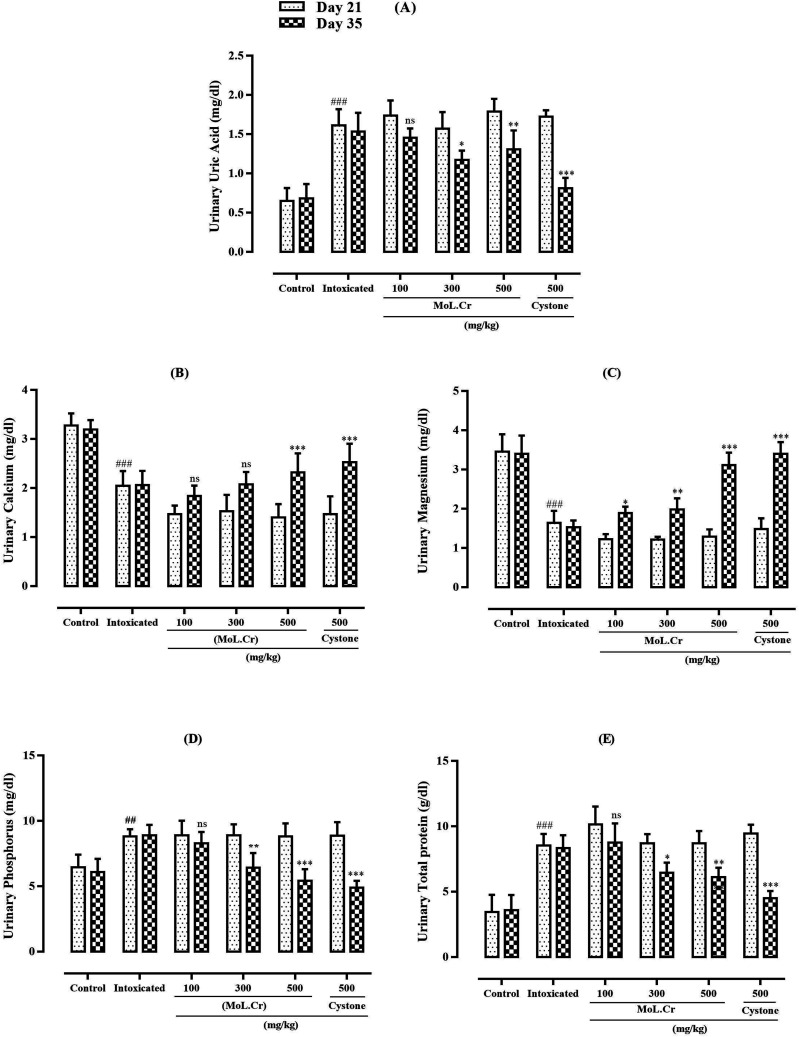
Statistical analysis showed that lithogenic treatment significantly (P < 0.001) enhanced the urinary uric acid, phosphorus and total protein levels as compared to the normal control group. MoL.Cr reduced the urinary uric acid, phosphorus and total protein levels in dose dependent-manners. Urinary calcium and magnesium levels were significantly (P < 0.001) decreased in lithogenic rats at the dose of 500 mg/kg. MoL.Cr elevated urinary calcium and magnesium levels and results were almost comparable to that of cystone (Figure 9).
Figure 9.
The effects of MoL.Cr and cystone on urine parameters (A) Uric acid, (B) Calcium, (C) Magnesium, (D) Phosphorus, (E) Total protein Mean ± SEM; n = 6, ns: P > 0.05; *: P < 0.05; ## &**: P < 0.01; ***&###: P < 0.001. (*: Comparison within the groups; i.e., 21st and 35th day and #: comparison of intoxicated group with normal groups at 21st day; One-way ANOVA followed by Bonferroni’s post hoc test).
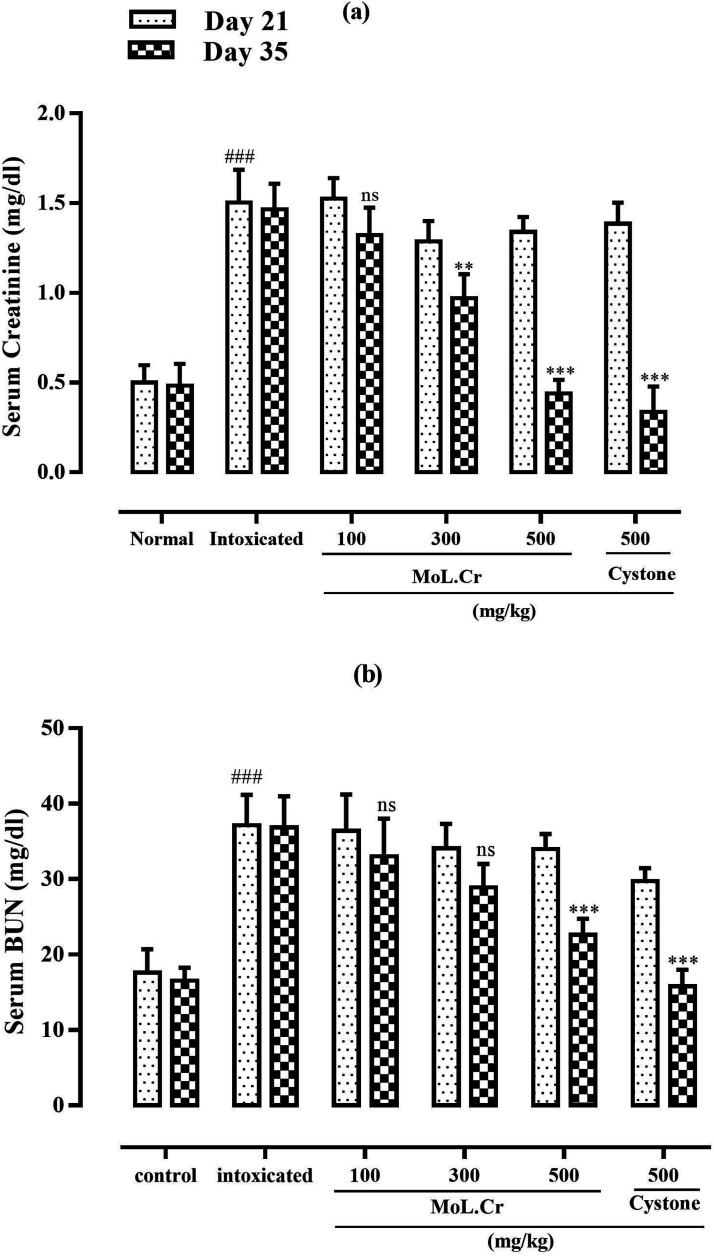
MoL.Cr caused significant reduction of serum creatinine and BUN levels at the doses of 300 (P < 0.01) and 500 mg/kg (P < 0.001) as compared to normal group, which were increased after the lithogenic treatment. Cystone showed same effects at the dose of 500 mg/kg (Figure 10).
Figure 10.
The effects of MoL.Cr and cystone on serum parameters (A) Creatinine, (B) Blood urea nitrogen (BUN). Mean ± SEM; n = 6, ns: P > 0.05; *: P < 0.05; **: P < 0.01; ***&###: P < 0.001. (*: Comparison within the groups; i.e., 21st and 35th day and #: comparison of intoxicated group with normal groups at 21st day; One-way ANOVA followed by bonferroni’s post hoc test).
Histological Examination of Kidney
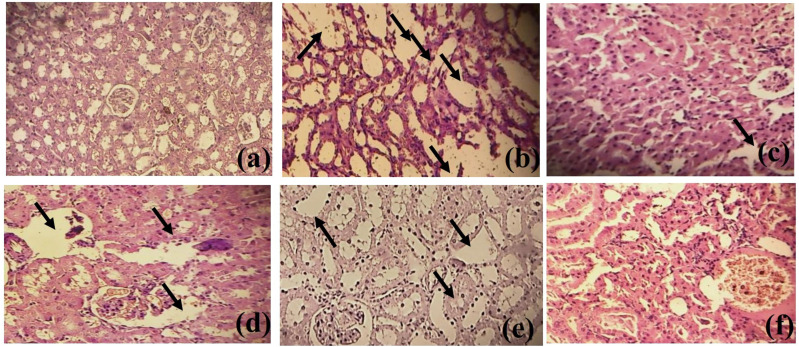
Histological examination showed no nephrotic damage in the normal control group. While, epithelial damage and disarrangement of nephrotic cell membrane were observed in lithogenic rats which were restored in MoL.Cr treated groups. MoL.Cr improved the structure of renal tubular epithelial cells dose-dependently (Figure 11).
Figure 11.
Histology of kidney (A) Normal control group (B) Intoxicated group (C) MoL.Cr; 100 mg/kg (D) MoL.Cr; 300 mg/kg (E) MoL.Cr; 500 mg/kg (F) Cystone; 500 mg/kg in curative model of urolithiasis. ( : epithelium damage and disarrangement of nephrotic cell wall).
: epithelium damage and disarrangement of nephrotic cell wall).
Acute Toxicity Assay
MoL.Cr was found safe upto the dose of 10 g/kg and no mortality, toxicity and behavioral changes were observed after 48 hours.
Discussion
In the current study, the aqueous methanolic extract of M. oleifera Lam. leaves was evaluated to determine its effects against urolithiasis. As a tool for characterizing plant metabolites, FTIR spectroscopy has grown significantly, and it is currently regarded as one of the most accurate, sensitive, quick, non-destructive and affordable methods for identifying functional groups. The functional groups of the pharmacologically active components present in MoL.Cr was identified using FTIR analysis by interpreting the bands obtained in the infrared region, specifically the –OH (polyphenols). HPLC investigation of MoL.Cr showed that leaves of M. oleifera are a rich source of bioactive compounds; i.e., quercetin, gallic acid, caffeic acid, vanillic acid, benzoic acid chlorogenic acid, p-coumaric acid, M-coumaric acid and sinapic acid. Previous studies have also reported the presence of flavonoids and phenolic acid that creates a positive link with its potential as an antiurolithiatic agent. 36 Flavonoids have been reported to diminish experimentally-induced urolithiasis in rats through numerous pathways such as modifying urinary stone-forming composition, reducing renal oxidative stress and inflammatory damage. Terpenes have been known for their spasmolytic, calcium channel blocking, antioxidant and diuretic properties. It has been reported that leaves of M. oleifera possess a thiocarbamate glycoside named niazimicin, reported to possess spasmolytic potential that can be linked to medical expulsive therapy (MET), used for the management of urolithiasis. 37 However, the potential of other phytochemical constituents against urolithiasis activity cannot be neglected.22,23
One of the main enzymes in oxalate production is glycolate oxidase (GOX). Through oxidation, it transforms glycolate into glyoxylate, which is further transformed into oxalate. Current molecular docking study showed that amino acids residues LYS236 and ARG315 were found to have conventional hydrogen bonds with the quercetin and are exclusively involved in the substrate binding and substrate specificity of glycolate oxidase enzymes. Mutations in these amino acids were discovered to diminish their catalytic activity. 38 Docking of Quercetin at the enzyme active site resulted in the lowest energy conformations (−9.7 kJ/mol), indicating the best potential interactions between the ligands and active site residues. Albumin is the most abundant urinary protein that has been detected in the matrix of stones in humans. When exposed to metastable urinary solutions, albumin binds with calcium oxalate (CaOx) and uric acid crystals, and promotes crystal nucleation. Whereas, Tamm-Horsfall protein (THP), also known as uromucoid, is the most extensively investigated urinary macromolecule. THP acts as a first-line defense against kidney stone formation and acts as an effective inhibitor of crystal aggregations. 6 Results showed that quercetin and albumin showed best potential interaction with binding energy −8.2 kJ/mol. Similarly, kaempferol and Tamm-Horsfall protein also showed the strong interaction with binding energy −8.1 kJ/mol. Thus, this work revealed an intriguing breakthrough in the development of novel anti-GOX compounds.
In-vitro assays were carried out to investigate the nucleation, aggregation and growth phases of crystallization. The formation of loose clusters which may grow in size in the supersaturated urine containing ions and macromolecules with the addition of other molecules is the first step towards heterogeneous nucleation. Crystal aggregation, which ultimately results in crystal growth, starts as soon as the nucleus is anchored on the epithelial surface. 23 In in-vitro crystallization assays that involve CaOx crystal aggregation, nucleation and growth, were significantly inhibited by MoL.Cr. These in-vitro tests provide a rapid assessment of the antiurolithiatic, crystal-modifying activity and possible mechanism of actions. However, urolithiasis pathophysiology and biological system are complex, it is difficult and unsafe to extrapolate these in-vitro investigations for the therapeutic benefits. Consequently, the purpose of in-vivo urolithiasis models is to better understand the underlying mechanism of kidney stone formation and explore the potential of MoL.Cr against urolithiasis. After 21 days of lithogenic treatment, relatively large and abundant urinary crystals were found in lithogenic rats as compared to the normal control group. Ethylene glycol increases the urinary oxalate level, which is the major factor that promotes crystallization. In urine, elevated levels of oxalate form the complex with calcium and crystallization occur. 39 MoL.Cr and cystone significantly (P < 0.001) reduced the crystal count in dose-dependent manners and showed almost similar results.
MoL.Cr significantly (P < 0.01) restored the ethylene glycol-induced polyuria in intoxicated rats. Reabsorption of water in kidney decreases due to kidney stones as a result urine volume increases. 34 MoL.Cr and cystone restored the urine volume to a significant level but greater than untreated rats which may be due to the natural diuretic activity. Acidic pH favors the formation of calcium oxalate crystals (CaOx) in urine. Administration of ammonium chloride in lithogenic treatment makes the urine acidic and enhances the deposition of CaOx crystals. Whereas, calcium phosphate crystals are formed in alkaline pH. 40 MoL.Cr and cystone neutralized the urinary pH dose dependently as compared to the intoxicated rats and inhibited the crystal formation.
Urine analysis of lithogenic rats showed significantly increased levels of uric acid as compared to normal control group. Uric acid decreases the solubility of CaOx crystals, binds with glycosaminoglycan and inhibits it stone inhibitory potential. 24 MoL.Cr treatment decreased the urinary uric acid levels and prevented the risk of stone formation and results were comparable to the cystone, the standard drug. Ethylene glycol-induced hyperoxaluria favors the complex formation of oxalate with calcium. After the complex formation, urinary excretion of calcium is decreased. 40 MoL.Cr restored the calcium levels in lithogenic rats, dose-dependently. Magnesium is considered a stone inhibitor as it inhibits the stone formation in the urinary system. Magnesium forms the complex with oxalate and enhance the solubility of CaOx crystals. 41 MoL.Cr is a rich source of magnesium and found to restore magnesium levels in lithogenic rats. Lithogenic treatment showed elevated levels of urinary phosphorus and total protein. Proteinuria indicates a high concentration of protein in urine due to kidney dysfunction. MoL.Cr and cystone reduced the phosphorus and total protein levels, significantly. In previous studies, it is reported that increased gene expression and production of molecules implicated in inflammation and tissue remodeling were triggered by elevated phosphate levels, which formed an optimal situation for crystallization by producing calcium phosphate stones that epitaxially induce CaOx precipitation. 41 Treatment with MoL.Cr reduced phosphorus levels and reduced the risk of renal stones. The results of cystone and MoL.Cr were almost comparable to each other.
Kidney stones decrease the efficiency of glomerulus filtrate and also reduce urine filtration leading to the accumulation of nitrogenous substances such as creatinine and BUN in blood. 24 MoL.Cr and cystone showed pronounced effects and restored these parameters towards normal.
Histological examination of kidney section showed that lithogenic treatment disrupted the epithelial lining, dilated the proximal tubules and increased the interstitial spaces. Significant inflammation was observed in intoxicated rats which may be attributed to the high level of oxalate. MoL.Cr restored the renal tubular integrity in doe-dependent manners and reduced the crystal adhesion and attachment.
Results revealed that administration of MoL.Cr to urolithiatic rats decreased and inhibited the formation of urinary stones. Whereas, mechanism underlying these effects; determination of decreased urinary level of stone forming constituents, isolation and characterization of identified compounds and investigation of isolated compounds against urolithiasis are still unknown.
Conclusions of the Study
The current study provided scientific validation for the traditional claim against kidney stone disease, demonstrating the curative potential of MoL.Cr against urolithiasis. Antiurolithiatic effect of MoL.Cr may be mediated by a confluence of diuretic, antioxidant and crystal inhibitory effects. Additionally, MoL.Cr improved serum and urine biochemistry, providing a safer and more affordable option for the treatment of kidney stone disease.
Acknowledgments
The authors acknowledge HEC (Higher Education Commission), Pakistan for support through NRPU project, Number: 6300, in providing research animals.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Hina Ali https://orcid.org/0000-0002-5474-7922
Ayesha Jamshed https://orcid.org/0000-0001-7321-8754
Syeda Abida Ejaz https://orcid.org/0000-0002-8516-7234
Maria Qadeer https://orcid.org/0000-0001-6022-6405
Mariya Anwaar https://orcid.org/0000-0002-7018-3581
Hafiz Muhammad Farhan Rasheed https://orcid.org/0000-0003-2516-7387
References
- 1.Alomair MK, Alobaid AA, Almajed MAA, et al. Grape seed extract and urolithiasis: protection against oxidative stress and inflammation. Phcog Mag. 2023;19(1). [Google Scholar]
- 2.Liu Y, Liu Q, Wang X, et al. Inhibition of autophagy attenuated ethylene glycol induced crystals deposition and renal injury in a rat model of nephrolithiasis. Kidney Blood Press Res. 2018;43(1):246-255. [DOI] [PubMed] [Google Scholar]
- 3.Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol. 2018;2018(1):3068365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75-88. [DOI] [PubMed] [Google Scholar]
- 5.Raj S, Rajan MSGS, Ramasamy S, et al. An in vitro Anti-Urolithiasis activity of a herbal formulation: Spinacia oleracea L. and Coriandrum sativum L. Clinical Complement Med Pharmacol. 2023;4(1):100124. [Google Scholar]
- 6.Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res Int. 2013;2013:292953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed OM, Ebaid H, El-Nahass E, Ragab M, Alhazza IM. Nephroprotective effect of Pleurotus ostreatus and Agaricus bisporus extracts and carvedilol on ethylene glycol-induced urolithiasis: roles of NF-κB, p53, bcl-2, bax and bak. Biomolecules. 2020;10(9):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mari KR, Muthukrishnan S. Structural characterization and insilico study on Pisonia alba Leaves extract. J Pharmacogn Phytochem. 2018;7(2):681-693. [Google Scholar]
- 9.Posmontier B. The medicinal qualities of Moringa oleifera. Holist Nurs Pract. 2011;25(2):80-87. [DOI] [PubMed] [Google Scholar]
- 10.Meireles D, Gomes J, Lopes L, Hinzmann M, Machado J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: integrative approach on conventional and traditional Asian medicine. Advan Trad Med. 2020;20:495-515. [Google Scholar]
- 11.Paikra BK, Dhongade HKJ, Gidwani B. Phytochemistry and pharmacology of Moringa oleifera Lam. J Pharmacopuncture. 2017;20(3):194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karadi RV, Palkar MB, Gaviraj EN, Gadge NB, Mannur VS, Alagawadi KR. Antiurolithiatic property of Moringa oleifera root bark. Pharm Biol. 2008;46(12):861-865. [Google Scholar]
- 13.Karadi RV, Gadge NB, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105(1-2):306-311. [DOI] [PubMed] [Google Scholar]
- 14.Jameel F, Kumar MCS, Kodancha G, Adarsh B, Udupa AL, Rathnakar UP. Antiurolithiatic activity of aqueous extract of bark of Moringa oleifera (Lam.) in rats. Health. 2010;2(4):352-355. [Google Scholar]
- 15.Jameel F, Kumar MCS, Kodancha P, Benegal A, Udupa AL, Rathnakar UP. Antiurolithiatic activity of aqueous extract of Moringa oleifera (Lam.) pod in rats. Pharmacologyonline. 2010;3:716-721. [Google Scholar]
- 16.Menon S, Al-Saadi AS, Al-Aamri NJ, et al. Inhibition of crystallization of calcium oxalate monohydrate using leaves from different species of Moringa–Experimental and theoretical studies. J Cryst Growth. 2022;598:126859. [Google Scholar]
- 17.Menon S, Shinisha CB, Mamari HKA, et al. Experimental and theoretical studies on the modulation of the crystallization process and crystal morphology of calcium oxalate using Moringa oleifera bark extract. J Mol Struct. 2024;1305:137693. [Google Scholar]
- 18.Banik K, Akshitha S, Poojitha OV, Thulasi C, Vineetha V. Evaluation of antiurolithiatic activity of Moringa leaves by UV spectroscopic method. Asian J Res Pharmaceut Sci. 2020;10(3):141-144. [Google Scholar]
- 19.Agarwal K, Varma R. Radical scavenging ability and biochemical screening of a common Asian vegetable-Raphanus sativus L. Int J Pharmaceut Sci Rev Res. 2014;27(1):127-134. [Google Scholar]
- 20.Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020;8(2):603-608. [Google Scholar]
- 21.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharmaceut Sci. 2011;1(1):98-106. [Google Scholar]
- 22.Bashir S, Gilani AH. Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122(1):106-116. [DOI] [PubMed] [Google Scholar]
- 23.Jamshed A, Jabeen Q. Pharmacological evaluation of Mentha piperita against urolithiasis: an in vitro and in vivo study. Dose Response. 2022;20(1):15593258211073087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosquera DMG, Ortega YH, Quero PC, Martínez RS, Pieters L. Antiurolithiatic activity of Boldoa purpurascens aqueous extract: an in vitro and in vivo study. J Ethnopharmacol. 2020;253:112691. [DOI] [PubMed] [Google Scholar]
- 25.Bank PD. Protein data bank. Nat New Biol. 1971;233:223.20480989 [Google Scholar]
- 26.Huey R, Morris GM, Forli S. Using AutoDock 4 and AutoDock vina with AutoDockTools: a tutorial. The Scripps Research Institute Molecular Graphics Laboratory. 2012;10550(92037):1000. [Google Scholar]
- 27.Fattah TA, Saeed A, Al-Hiari YM, et al. Functionalized furo [3, 2-c] coumarins as anti-proliferative, anti-lipolytic, and anti-inflammatory compounds: synthesis and molecular docking studies. J Mol Struct. 2019;1179:390-400. [Google Scholar]
- 28.Attaullah HM, Ejaz SA, Channar PA, et al. Exploration of newly synthesized azo-thiohydantoins as the potential alkaline phosphatase inhibitors via advanced biochemical characterization and molecular modeling approaches. BMC Chem. 2024;18(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown T. ChemDraw. Sci Teach. 2014;81(2):67. [Google Scholar]
- 30.BIOVIA DS. BIOVIA discovery studio visualizer. Software Version. 2017;20:779. [Google Scholar]
- 31.Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92(1):137-140. [DOI] [PubMed] [Google Scholar]
- 33.Khan A, Bashir S, Khan SR, Gilani AH. Antiurolithic activity of Origanum vulgare is mediated through multiple pathways. BMC Complement Altern Med. 2011;11(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamshed A, Jabeen Q. Prophylactic and curative potential of peppermint oil against calcium oxalate kidney stones. Pak J Pharm Sci. 2021;34(5):1867-1872. [PubMed] [Google Scholar]
- 35.Jabeen Q, Bashir S, Lyoussi B, Gilani AH. Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. J Ethnopharmacol. 2009;122(1):123-130. [DOI] [PubMed] [Google Scholar]
- 36.Saleem A, Saleem M, Akhtar MF, Ashraf Baig MMF, Rasul A. HPLC analysis, cytotoxicity, and safety study of Moringa oleifera Lam.(wild type) leaf extract. J Food Biochem. 2020;44(10):e13400. [DOI] [PubMed] [Google Scholar]
- 37.Gilani AH, Aftab K, Suria A, et al. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother Res. 1994;8(2):87-91. [Google Scholar]
- 38.Shirfule A, Sangamwar A, Khobragade C. Exploring glycolate oxidase (GOX) as an antiurolithic drug target: molecular modeling and in vitro inhibitor study. Int J Biol Macromol. 2011;49(1):62-70. [DOI] [PubMed] [Google Scholar]
- 39.Ali H, Nadeem A, Anwaar M, Jabeen Q. Evaluation of antiurolithiatic potential of Moringa oleifera seed extract. Biomed J Sci Tech Res. 2021;36(5):28889-28895. [Google Scholar]
- 40.Bashir S, Gilani AH. Antiurolithic effect of berberine is mediated through multiple pathways. Eur J Pharmacol. 2011;651(1-3):168-175. [DOI] [PubMed] [Google Scholar]
- 41.Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48(4):1013-1018. [DOI] [PubMed] [Google Scholar]