Abstract
Systemic glucocorticoid excess causes several adverse metabolic conditions, most notably Cushing’s syndrome. These effects are amplified by the intracellular enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). Here, we determined the less well-characterised effects of glucocorticoid excess, and the contribution of 11β-HSD1 amplification on metabolic rate in mice. Male and female C57BL/6J (wild type, WT) and 11β-HSD1 knockout (11β-HSD1 KO) mice were treated with high-dose corticosterone or a vehicle control for 3 weeks. Indirect calorimetry was conducted during the final week of treatment, with or without fasting, to determine the impact on metabolic rate. We found that corticosterone treatment elevated metabolic rate and promoted carbohydrate utilisation primarily in female WT mice, with effects more pronounced during the light phase. Corticosterone treatment also resulted in greater fat accumulation in female WT mice. Corticosterone induced hyperphagia was identified as a likely causal factor altering the respiratory exchange ratio (RER) but not energy expenditure (EE). Male and female 11β-HSD1 KO mice were protected against these effects. We identify novel metabolic consequences of sustained glucocorticoid excess, identify a key mechanism of hyperphagia, and demonstrate that 11β-HSD1 is required to manifest the full metabolic derangement.
Keywords: 11β-HSD1, glucocorticoid excess, metabolic rate, substrate utilisation
Introduction
Glucocorticoids (GCs) are a class of steroid hormones critical for whole-body energy homeostasis (Tomlinson & Stewart 2001). GCs serve as stress response hormones driving GC receptor-mediated transcription to alter metabolic processes in response to endogenous or exogenous stimuli (Nicolaides et al. 2015). Given their metabolic influence across a range of tissues (Magomedova & Cummins 2016) systemic glucocorticoid levels are tightly governed by the hypothalamus–pituitary–adrenal axis (Ramamoorthy & Cidlowski 2016). Despite this regulatory mechanism, endogenous glucocorticoid excess can occur. However, more commonly, iatrogenic-induced hypercortisolaemia occurs (Barbot et al. 2020). Sustained excess results in metabolic dysfunction, most notably Cushing’s syndrome, which is characterised by a well-documented phenotype in humans and rodents (Lacroix et al. 2015, Lonser et al. 2017). This phenotype, which can be readily induced in mice for experimental purposes (Karatsoreos et al. 2010, Morgan et al. 2014, Uehara et al. 2020, Nishiyama et al. 2022), incorporates numerous metabolic features such as loss of glucose sensitivity, hypertension, hepatic steatosis, myopathy, obesity, and skeletal muscle atrophy (Schakman et al. 2008, Goodwin & Geller 2012, Di Dalmazi et al. 2012, Abraham et al. 2013, Woods et al. 2015, Morgan et al. 2016). Many of these metabolic features of Cushing’s syndrome are underpinned at the pre-receptor level by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (Tomlinson et al. 2002, Morgan et al. 2014). In humans, this involves the conversion of inactive cortisone to active cortisol (11 dehydro-corticosterone and corticosterone in rodents) (Tomlinson & Stewart 2001). Mice with 11β-HSD1 gene deletion (11β-HSD1 KO) and humans born lacking functional 11β-HSD1 are protected from the metabolic consequences of glucocorticoid excess (Tomlinson et al. 2002, Morgan et al. 2014).
Whilst there are many studies documenting the metabolic complications of glucocorticoid excess, there remain deficits in our understanding of impacts upon whole-body metabolic rate and substrate utilisation. These are defined, respectively, by energy expenditure (EE: indirectly calculated using oxygen consumption and carbon dioxide production values) and the respiratory exchange ratio (RER: calculated by dividing carbon dioxide production by oxygen consumption, giving values that allow an estimation of carbohydrate versus fatty acid oxidation in the organism). Normal fluctuations in endogenous glucocorticoid production were reported to have no effect on metabolic rate in mice (Dlugosz et al. 2012) or humans (Jobin et al. 1996). However, elevated EE, oxygen consumption, carbon dioxide production, and altered RER were reported following acute or short-term low-dose exogenous glucocorticoid treatment in humans (Bessey et al. 1984, Chong et al. 1994, Brillon et al. 1995, Tataranni et al. 1996). Often, the effects of acute low-dose glucocorticoid treatment are not formally reported or are incomplete (Bessey et al. 1984, Brillon et al. 1995). Others have reported a decrease in metabolic rate or dispute the direction of altered substrate utilisation (Horber et al. 1991, Gravholt et al. 2002, Short et al. 2004, Radhakutty et al. 2016). Fewer studies report the effects of chronic glucocorticoid administration. Thus, chronic low-dose exogenous glucocorticoid treatment had no effect on EE in humans (Radhakutty et al. 2016), but decreased EE and RER in mice (Poggioli et al. 2013). Patients with Cushing’s syndrome were reported to have normal basal EE for their body mass and composition (Burt et al. 2006). Further research is required to clarify these discrepancies.
Here, we establish the effect of sustained glucocorticoid excess on whole-body metabolic rate and substrate utilisation in male and female mice, and explore what might cause any effects, using an established model of exogenous glucocorticoid excess that is known to replicate the metabolic impacts seen in humans and those with Cushing’s syndrome (Morgan et al. 2014, Fenton et al. 2019). We also determine if any effects are mediated by the enzyme 11β-HSD1.
Materials and methods
Animals and glucocorticoid administration
Male and female C57BL/6J (wild type, WT) mice were purchased from Charles River, UK. All 11β-HSD1 KO mice were homozygous and bred in-house on a C57BL/6J background. Mice were group-housed in sex- and litter-matched cages in groups of 2–4. Cages were kept at a standard temperature (22°C) in a humidity-controlled environment with a uniform 12-h light:darkness cycle. Mice were individually housed during the final 120 h of indirect calorimetry assessment. Throughout, mice were provided with nesting material, with food and water available ad libitum. Mice were given a standard chow diet (EURodent Diet 14%, Labdiet, St. Louis, MO, USA), though the water differed depending on the treatment group. Treatment lasted for 3 weeks and utilised an established protocol known to induce a phenotype typical of sustained glucocorticoid excess (Morgan et al. 2014, Fenton et al. 2019). Drinking water containing 100 mg/L corticosterone (Sigma-Aldrich) or a vehicle control (0.6% ethanol) was given ad libitum. All mice were 10 weeks of age at the start of treatment. Mice were culled immediately upon completion of indirect calorimetry assessment. All animal procedures were conducted in accordance with UK Home Office regulations, the UK Animals (Scientific Procedures) Act 1986, and were approved locally by the University of Birmingham and Nottingham Trent University AWERB committees under the project licence number PP1816482.
Indirect calorimetry
Initial indirect calorimetry was performed during the final week of treatment using a TSE PhenoMaster 8 cage system (TSE Systems, Bad Homburg, Germany). Mice were group-housed for 48 h in the PhenoMaster to acclimatise. Mice were then individually housed for 120 h within the PhenoMaster. The first 24 h of which were to acclimate the mice to isolation, whilst the subsequent 96 h were for undisturbed data collection. Markers of metabolic rate and substrate utilisation (EE and RER) were measured. Food and water intake were also measured. All measurements were recorded in male and female WT (n = 12) and 11β-HSD1 KO mice (n = 5–8). Subsequently, locomotor activity was assessed in female WT mice only (n = 8). Further indirect calorimetry assessment was performed on female WT mice only (n = 8) with food withdrawn during the final 12-h light phase to assess the impact of glucocorticoid excess-induced hyperphagia on the previously described markers of metabolic rate and substrate utilisation.
Statistical analysis
Statistical significance was tested using GraphPad Prism version 9 (GraphPad Software, LLC) as well as CalR (version 1.3) (Mina et al. 2018). Two-way analysis of variances, followed by Tukey’s multiple comparisons test, were used to analyse all indirect calorimetry measures, and physical characteristics, and compare WT and 11β-HSD1 KO mice. Data are presented as mean ± s.e.m. throughout.
Results
Corticosterone treatment induces a Cushing’s phenotype in WT but not 11β-HSD1 KO mice
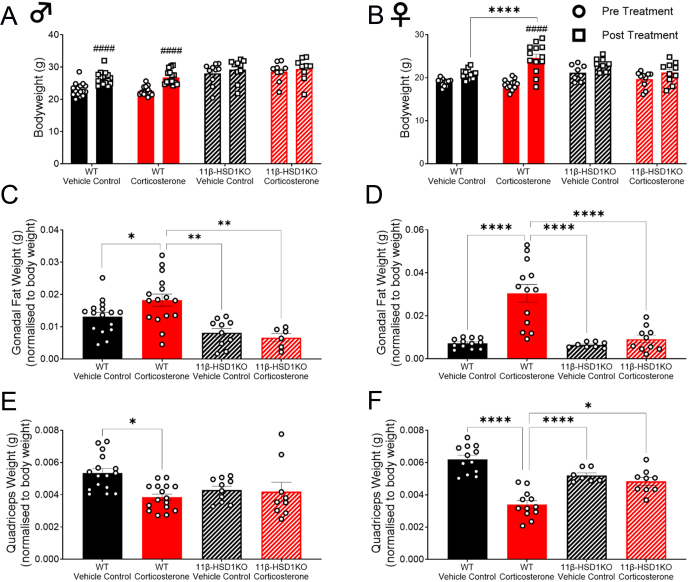
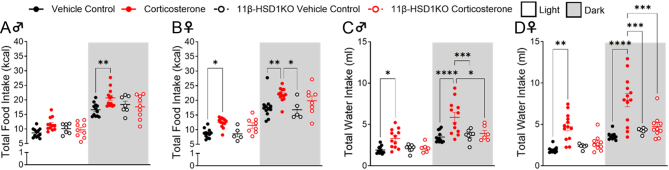
To assess the impact of glucocorticoid excess on metabolic rate and substrate utilisation, we first confirmed the development of the known Cushing’s phenotype in WT mice. As expected, corticosterone treatment caused male and female WT mice to develop these signs (Fig. 1). Increased lipid accumulation (Fig. 1C and D) (Peckett et al. 2011, Gasparini et al. 2016) and muscle atrophy/reduced lean body mass accrual (Fig. 1E and F) (Schakman et al. 2013, Sato et al. 2018, Gasparini et al. 2019) were demonstrated. This occurred despite a significant increase in control male WT body weight and a trend towards increased control female WT bodyweight (P = 0.07) over the duration of the study. Polydipsia (Tataranni et al. 1996) was observed throughout in corticosterone-treated WT mice, whilst hyperphagia (Tataranni et al. 1996) was continuous in females and evident during the dark phase in males (Fig. 2). The Cushingoid phenotype was more prominent in female WT mice, as they exhibited greater body weight and fat accumulation (Fig. 1B and D), as well as both hyperphagia and polydipsia (Fig. 2), compared to corticosterone-treated male mice. Successful knockout of 11β-HSD1, and therefore protection from intracellular glucocorticoid reactivation, was affirmed by the absence of this physical phenotype in male and female 11β-HSD1 KO mice (Morgan et al. 2014) (Fig. 1). Corticosterone-induced polydipsia was also prevented, whilst hyperphagia was attenuated in 11β-HSD1 KO mice (Fig. 2).
Figure 1.
Corticosterone induced a phenotype typical of glucocorticoid excess in WT mice whilst 11β-HSD1 KO prevented it. (A) Male body weight pre- and post-treatment. (B) Female body weight pre- and post-treatment. (C) Male gonadal fat weight normalised to body weight. (D) Female gonadal fat weight normalised to body weight. (E) Male quadriceps weight normalised to body weight. (F) Female quadriceps weight normalised to body weight. Bar graphs are presented as mean ± s.e.m., n = 8–16. *Significant difference between treatments. # Significant pre and post treatment difference *P < 0.05, **P < 0.01, ***P < 0.001, ****/####P < 0.0001.
Figure 2.
Corticosterone treatment increased food and water intake in WT mice whilst 11β-HSD1 KO prevented it. (A) Average male food intake. (B) Average female food intake. (C) Average male water intake. (D) Average female water intake. Scatter plots represent average day and night values and are resented as mean with individual values ± s.e.m., n = 5–12. *Significantly different. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Metabolic rate is elevated in a sex-specific manner by glucocorticoid excess in WT but not 11β-HSD1 KO mice
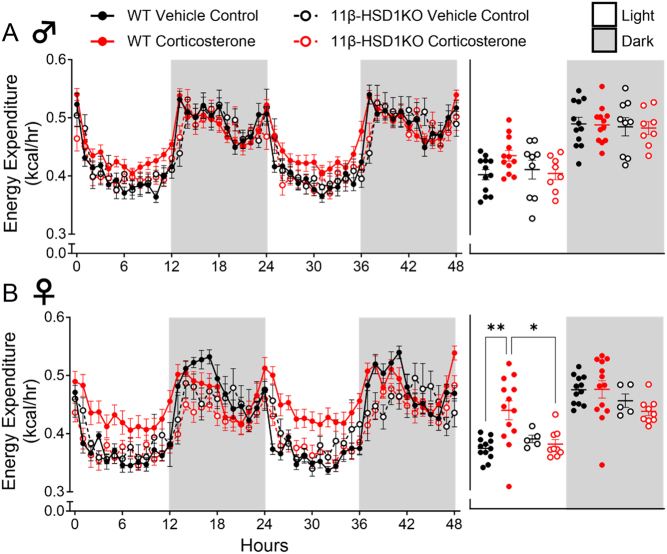
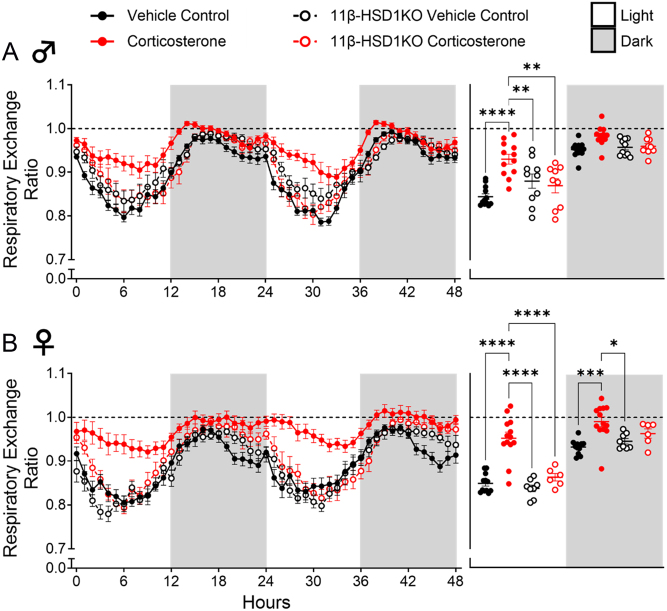
The degree to which glucocorticoid excess impacts mammalian energy metabolism remains unclear (Jimeno & Verhulst 2023). We sought to provide whole-body indirect calorimetric analysis of glucocorticoid excess-exposed WT mice. Corticosterone treatment did not significantly alter EE (Fig. 3A) in male WT mice. However, it did significantly increase the RER during the light phase (Fig. 4A). In female WT mice, corticosterone significantly elevated EE during the light phase (Fig. 3B), and increased RER during the light and darkness phases (Fig. 4B). This suggests increased calorie usage in female mice, which was driven predominantly by increased carbohydrate utilisation, an effect experienced by both females and males. Notably, corticosterone caused average RER to approach or exceed a value of 1.0 during the dark phase in males and females (Fig. 4), effects indicative of an increased rate of de novo lipogenesis (Solinas et al. 2015). Assessment in 11β-HSD1 KO mice revealed further protection against glucocorticoid excess as corticosterone treatment did not elevate EE or change substrate utilisation (RER) in male or female 11β-HSD1 KO mice (Figs. 3 and 4).
Figure 3.
Glucocorticoid excess elevated energy expenditure in female WT mice, whilst 11β-HSD1 KO prevented this. (A) Male energy expenditure. (B) Female energy expenditure. Line graphs represent hourly change and are presented as mean ± s.e.m., n = 12. Scatter plots represent average day and night values and are presented as mean with individual values ± s.e.m., n = 12. *Significantly different. *P < 0.05, **P < 0.01.
Figure 4.
Glucocorticoid excess altered the respiratory exchange ratio in female WT mice, whilst 11β-HSD1 KO prevented this. (A) Male respiratory exchange ratio. (B) Female respiratory exchange ratio. Line graphs represent hourly change and are presented as mean ± s.e.m., n = 12. Scatter plots represent average day and night values and are presented as mean with individual values ± s.e.m., n = 12. *Significantly different. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
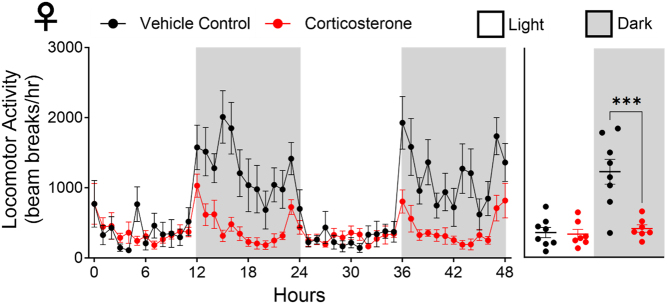
Physical activity was decreased by glucocorticoid excess in female WT mice
Sexual dimorphism is a widely appreciated characteristic of glucocorticoid function, as both androgens and oestrogens can modulate GC transcriptional activity, which can also directly influence endogenous glucocorticoid synthesis (Takahashi et al. 2024). Given our observed increase in female EE, we set out to explore the effects of corticosterone on physical activity, as increased physical activity is known to increase EE (van Baak 1999). This was explored in female WT mice only due to their exaggerated corticosterone responses. Locomotor activity, a definitive measure of physical activity, was assessed. Locomotor activity (Fig. 5) was decreased by corticosterone treatment during the dark phase, resulting in sustained sedentary behaviour across both the light and darkness phases which ultimately revealed a corticosterone-induced disconnect between physical activity and EE.
Figure 5.
Glucocorticoid excess decreased physical activity (locomotor activity) levels in female WT mice. The line graph represents hourly changes and are presented as mean ± s.e.m., n = 8. The scatter plot represents average day and night values and are presented as mean with individual values ± s.e.m., n = 8. *significantly different. ***P < 0.001.
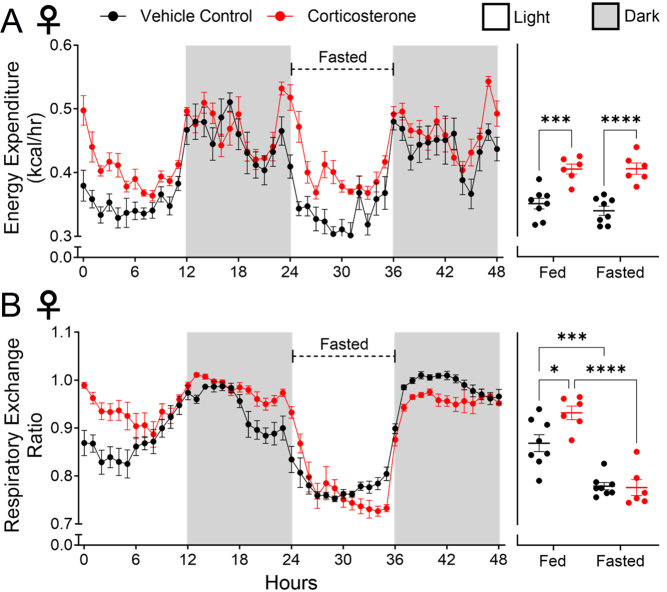
Food withdrawal normalised the respiratory exchange ratio but not energy expenditure in female WT mice
Sustained excess corticosterone exposure is associated with hyperphagia (Sefton et al. 2016). Therefore, increased food intake and the subsequent thermic effect of food were considered possible explanations for both elevated EE and altered RER measured in the female mice (Solinas et al. 2015, Ho 2018). To evaluate this, female WT mice were fasted for a 12-h light phase. Fasting had no effect on EE, which remained elevated and did not differ from the previous fed 12-h light phase (Fig. 6A). This revealed that corticosterone-induced elevations in EE were not an artefact of the hyperphagia it promotes. Fasting, however, reversed the elevation of RER values seen in corticosterone-treated mice (Fig. 6B), suggesting that the corticosterone-treated mice maintained an ability to utilise lipids for fuel when necessary.
Figure 6.
Fasting during the light phase prevented an altered respiratory exchange ratio but did not prevent elevated energy expenditure in female WT mice. (A) Energy expenditure. (B) Respiratory exchange ratio. Line graphs represent hourly change and are presented as mean ± s.e.m., n = 8. Scatter plots represent average day and night values and are presented as mean with individual values ± s.e.m., n = 8. *Significantly different. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Knowledge of the deleterious effects of sustained glucocorticoid excess is well understood but remains incomplete at the level of whole-body physiology. Herein, we demonstrate that glucocorticoid excess elevates metabolic rate (EE) and alters substrate utilisation (RER) in WT mice with greater significance in females than males. Notably, corticosterone suppresses physical activity in female WT mice, whilst light-phase fasting fails to prevent elevated EE. This suggests that neither increased energy of movement nor digestion is responsible. Hyperphagia and lipogenesis likely promote altered RER and are compatible with increased adipose accumulation. RERs exceeding 1.0 are also a signature of lipogenesis (Solinas et al. 2015). Critically, these effects are dependent upon the presence of 11β-HSD1, revealing further protection against glucocorticoid excess from disruption of 11β-HSD1 function.
A strong causal candidate for increased EE is canonical activation of brown adipose tissue non-shivering thermogenesis (Cannon & Nedergaard 2004), which occurs at a tonic level at room temperature in mice (Bastias-Perez et al. 2020). This is unlikely to be responsible, as there is strong evidence that rodents exposed to glucocorticoid excess exhibit profound suppression of the thermogenic protein UCP1 (Poggioli et al. 2013, Doig et al. 2017, Kaikaew et al. 2019). An alternative thermogenic mechanism, futile creatine cycling, is also unlikely to explain our findings, as this process is suppressed in inactive, hyperphagic mice (Hepler et al. 2022). Increased physical activity is another potential explanation (Van Baak 1999) that can be eliminated, as corticosterone-treated WT mice were continuously inactive. Similar to activity, the thermic effect of food requires examination (Tataranni et al. 1996, Solinas et al. 2015, Ho 2018) but is unlikely to be responsible for these measurements, as fasting failed to prevent increased EE. Therefore, increased body weight, driven by increased adiposity, is a likely explanation for GC-induced shifts in female EE. This agrees with the biological interpretation of Newton’s second law, which determines that an animal of greater mass is required to burn more calories to meet increased energy demands (Corrigan et al. 2020). The more significant EE elevation seen in female mice might also be explained by this demand, as their body weight increase was greater than that of male mice.
Elevated RER, close to or exceeding 1.0, indicates that sustained corticosterone exposure forces WT mice to primarily utilise carbohydrates as their fuel source, at the expense of lipid utilisation. It also indicates some mice undergo increased de novo lipogenesis, which can be attributed to glucocorticoid excess and hyperphagia of their 76% carbohydrate-based diet (Magomedova & Cummins 2016, Talal et al. 2021). Increased de novo lipogenesis in female mice, as indicated by a greater RER increase and reported in female mice given a high carbohydrate diet (Low et al. 2018), likely contributes to their greater adipose accrual. Given this, it was hypothesised that hyperphagia of a carbohydrate-based diet was elevating RER. Fasting of mice supported this, as RER decreased towards 0.7, indicating increased lipid utilisation. Therefore, glucocorticoid excess does not directly restrict mice to carbohydrate utilisation, but the glucocorticoid-induced hyperphagia does. Whether different diets, such as those low in carbohydrates, might attenuate the impact of this hyperphagia merits investigation, as a low-carbohydrate diet can aid in the management of Cushing’s syndrome (Dugandzic et al. 2022).
Sex differences in mice treated with corticosterone, in the context of indirect calorimetry, are novel. A greater increase in body weight driven by a greater accumulation of adipose tissue potentially explains the greater EE elevation in female mice. Whilst WT male control mice significantly grew over the course of treatment, and females did not, potentially limiting the relative increase in male body weight and adiposity from corticosterone treatment, it is unlikely this confounded the observed sex differences as WT female control mice in fact trended towards increased body weight over the course of treatment (P = 0.07). Therefore, the cause for this greater body weight and increased adiposity in female mice is more likely due to increased hyperplastic as opposed to hypertrophic expansion of adipose tissue in female mice (Kaikaew et al. 2019). Likely elevated de novo lipogenesis in female mice, as indicated by RER, is another explanation for increased adiposity. Differences might also be caused by increased 5α-reductase expression in male mice, which has the potential to increase corticosterone deactivation and clearance, potentially mitigating the impact of their orally consumed dose (Melcangi et al. 1998, Nixon et al. 2012), but this remains to be established.
The majority of literature related to human metabolic responses to GC excess focusses on acute or low-dose treatment. Of these acute studies, agreement is found with some (Bessey et al. 1984, Chong et al. 1994, Brillon et al. 1995, Tataranni et al. 1996), but not all (Horber et al. 1991, Gravholt et al. 2002, Short et al. 2004, Radhakutty et al. 2016). However, due to the acute nature of these studies, meaningful comparisons are limited. Of the existing chronic studies, no agreement is found. The only existing mouse study, to the authors' knowledge, reported that dexamethasone treatment for 7 weeks decreased both EE and RER (Poggioli et al. 2013). Whereas, in chronic human investigation with rheumatoid arthritis patients, 6 months of 6 mg prednisolone a day had no effect on EE (Radhakutty et al. 2016). Finally, in patients with Cushing’s syndrome, EE remained unaltered (Burt et al. 2006). Reasons for such variability are yet to be determined. However, the use of different synthetic glucocorticoids in the literature might be contributory (Burt et al. 2006, Poggioli et al. 2013, Radhakutty et al. 2016). Differences in indirect calorimetry methodology between the present study and existing mice (Poggioli et al. 2013) and human studies (Burt et al. 2006, Radhakutty et al. 2016) might also be responsible. Additionally, variation might indicate species-specific effects. Finally, as the present study rapidly induced a Cushingoid phenotype, but the other three studies utilised more prolonged glucocorticoid exposure, the length of treatment must also be considered.
This study revealed that 11β-HSD1 KO prevents elevation of EE and altered RER in mice exposed to glucocorticoid excess. Additionally, it attenuates hyperphagia and polydipsia in male and female mice. Interestingly, however, control-treated 11β-HSD1 KO mice did not display comparable growth rates to WT control mice, contrary to existing literature (Morgan et al. 2022). Whilst this is unlikely to have confounded the findings, as 11β-HSD1 KO responded as expected to corticosterone treatment with attenuated lipid accumulation and skeletal muscle atrophy (Morgan et al. 2014), it must be considered. Despite this, and despite being the first to investigate the impact on metabolic rate and substrate utilisation, the findings of the present study complement existing literature reporting 11β-HSD1 mediation of other consequences of glucocorticoid excess in mice and humans (Tomlinson et al. 2002, Morgan et al. 2014, Webster et al. 2021, Othonos et al. 2023). Therefore, altered metabolic rate and substrate utilisation might be consequences of, or causal factors in, other metabolic conditions that are caused by glucocorticoid excess and mediated by 11β-HSD1. As such, they should be assessed as part of the ongoing development of human 11β-HSD1 inhibitors (Othonos et al. 2023, Pofi et al. 2023).
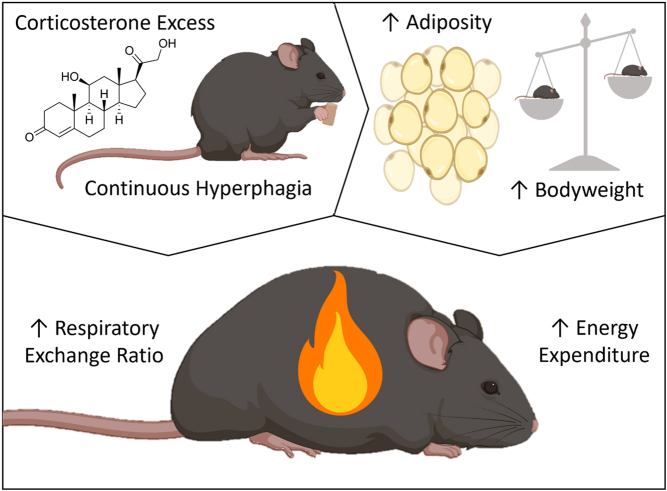
In conclusion, this study identifies novel metabolic consequences of glucocorticoid excess, which include elevated EE and altered RER. Findings also identify greater effects in female mice, with alterations in male mice failing to reach the same scale or significance. Hyperphagia was identified as a causal factor altering RER, whilst increased body weight driven by increased adiposity is theorised to be responsible for elevating EE (Fig. 7). These effects are mediated by 11β-HSD1, with male and female mice receiving protection from 11β-HSD1 KO. Together, these findings provide further insights into the underlying mechanisms and consequences of exogenous glucocorticoid excess.
Figure 7.
Proposed mechanism by which glucocorticoid excess elevates energy expenditure and the respiratory exchange ratio in C57BL/6J mice. Excess corticosterone treatment forces mice into a continuous state of hyperphagia, which, alongside corticosterone excess itself, causes a pronounced increase in adipose tissue. This increases body weight, which likely elevates energy expenditure. Alteration of the respiratory exchange ratio is driven directly by glucocorticoid excess-induced hyperphagia.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
SRH was a recipient of a PhD scholarship funded by the MRC Versus Arthritis Centre for Musculoskeletal Ageing Research, University of Birmingham and University of Nottingham. GGL was supported by a Wellcome Trust Senior Fellowship (104612/Z/14/Z). CLD is supported by Defence Medical Services Research Group funding (23/24.022). MS received funding from the Medical Research Council as part of a Clinical Research Training Fellowship (MR/T008172/1). RSH was supported by Versus Arthritis. Ref: 20843.
Author contributions
SRH, SM, DC, and GGL conceptualised the study. SRH and SH carried out the study and acquisition of data. SRH carried out data analysis. MS and RSH bred and provided 11β-HSD1 KO mice. SRH, SM, CLD, NM, KT, and GGL interpreted the results and contributed to the writing of the manuscript. GGL and KT supervised the study.
References
- Abraham SB Rubino D Sinaii N Ramsey S & Nieman LK. 2013. Cortisol, obesity, and the metabolic syndrome: a cross-sectional study of obese subjects and review of the literature. Obesity (Silver Spring) 21 E105–E117. ( 10.1002/oby.20083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot M Zilio M & Scaroni C. 2020. Cushing’s syndrome: overview of clinical presentation, diagnostic tools and complications. Best Practice and Research. Clinical Endocrinology and Metabolism 34 101380. ( 10.1016/j.beem.2020.101380) [DOI] [PubMed] [Google Scholar]
- Bastias-Perez M Zagmutt S Soler-Vazquez MC Serra D Mera P & Herrero L. 2020. Impact of adaptive thermogenesis in mice on the treatment of obesity. Cells 9. ( 10.3390/cells9020316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessey PQ Watters JM Aoki TT & Wilmore DW. 1984. Combined hormonal infusion simulates the metabolic response to injury. Annals of Surgery 200 264–281. ( 10.1097/00000658-198409000-00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillon DJ Zheng B Campbell RG & Matthews DE. 1995. Effect of cortisol on energy expenditure and amino acid metabolism in humans. American Journal of Physiology 268 E501–E513. ( 10.1152/ajpendo.1995.268.3.E501) [DOI] [PubMed] [Google Scholar]
- Burt MG Gibney J & Ho KKY. 2006. Characterization of the metabolic phenotypes of Cushing’s syndrome and growth hormone deficiency: a study of body composition and energy metabolism. Clinical Endocrinology 64 436–443. ( 10.1111/j.1365-2265.2006.02488.x) [DOI] [PubMed] [Google Scholar]
- Cannon B & Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiological Reviews 84 277–359. ( 10.1152/physrev.00015.2003) [DOI] [PubMed] [Google Scholar]
- Chong PK Jung RT Scrimgeour CM & Rennie MJ. 1994. The effect of pharmacological dosages of glucocorticoids on free living total energy expenditure in man. Clinical Endocrinology 40 577–581. ( 10.1111/j.1365-2265.1994.tb03007.x) [DOI] [PubMed] [Google Scholar]
- Corrigan JK, Ramachandran D, He Y, Palmer CJ, Jurczak MJ, Chen R, Li B, Friedline RH, Kim JK, Ramsey JJ, et al. 2020. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. eLife 9. ( 10.7554/eLife.53560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Dalmazi G Pagotto U Pasquali R & Vicennati V. 2012. Glucocorticoids and type 2 diabetes: from physiology to pathology. Journal of Nutrition and Metabolism 2012 525093. ( 10.1155/2012/525093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz EM Harris BN Saltzman W & Chappell MA. 2012. Glucocorticoids, aerobic physiology, and locomotor behavior in California mice. Physiological and Biochemical Zoology 85 671–683. ( 10.1086/667809) [DOI] [PubMed] [Google Scholar]
- Doig CL Fletcher RS Morgan SA Mccabe EL Larner DP Tomlinson JW Stewart PM Philp A & Lavery GG. 2017. 11beta-HSD1 modulates the set point of brown adipose tissue response to glucocorticoids in male mice. Endocrinology 158 1964–1976. ( 10.1210/en.2016-1722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugandzic MK Pierre-Michel EC & Kalayjian T. 2022. Ketogenic diet initially masks symptoms of hypercortisolism in Cushing’s disease. Metabolites 12. ( 10.3390/metabo12111033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton CG, Webster JM, Martin CS, Fareed S, Wehmeyer C, Mackie H, Jones R, Seabright AP, Lewis JW, Lai YC, et al. 2019. Therapeutic glucocorticoids prevent bone loss but drive muscle wasting when administered in chronic polyarthritis. Arthritis Research and Therapy 21 182. ( 10.1186/s13075-019-1962-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini SJ Weber MC Henneicke H Kim S Zhou H & Seibel MJ. 2016. Continuous corticosterone delivery via the drinking water or pellet implantation: a comparative study in mice. Steroids 116 76–82. ( 10.1016/j.steroids.2016.10.008) [DOI] [PubMed] [Google Scholar]
- Gasparini SJ Swarbrick MM Kim S Thai LJ Henneicke H Cavanagh LL Tu J Weber MC Zhou H & Seibel MJ. 2019. Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia 62 1463–1477. ( 10.1007/s00125-019-4887-0) [DOI] [PubMed] [Google Scholar]
- Goodwin JE & Geller DS. 2012. Glucocorticoid-induced hypertension. Pediatric Nephrology 27 1059–1066. ( 10.1007/s00467-011-1928-4) [DOI] [PubMed] [Google Scholar]
- Gravholt CH Dall R Christiansen JS Moller N & Schmitz O. 2002. Preferential stimulation of abdominal subcutaneous lipolysis after prednisolone exposure in humans. Obesity Research 10 774–781. ( 10.1038/oby.2002.105) [DOI] [PubMed] [Google Scholar]
- Hepler C, Weidemann BJ, Waldeck NJ, Marcheva B, Cedernaes J, Thorne AK, Kobayashi Y, Nozawa R, Newman MV, Gao P, et al. 2022. Time-restricted feeding mitigates obesity through adipocyte thermogenesis. Science 378 276–284. ( 10.1126/science.abl8007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KKY. 2018. Diet-induced thermogenesis: fake friend or foe? Journal of Endocrinology 238 R185–R191. ( 10.1530/JOE-18-0240) [DOI] [PubMed] [Google Scholar]
- Horber FF Marsh HM & Haymond MW. 1991. Differential effects of prednisone and growth hormone on fuel metabolism and insulin antagonism in humans. Diabetes 40 141–149. ( 10.2337/diab.40.1.141) [DOI] [PubMed] [Google Scholar]
- Jimeno B & Verhulst S. 2023. Meta-analysis reveals glucocorticoid levels reflect variation in metabolic rate, not ‘stress’. eLife 12. ( 10.7554/eLife.88205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin N De Jonge L & Garrel DR. 1996. Effects of RU 486 on energy expenditure and meal tolerance in normal men. Journal of the American College of Nutrition 15 283–288. ( 10.1080/07315724.1996.10718599) [DOI] [PubMed] [Google Scholar]
- Kaikaew K Steenbergen J Van Dijk TH Grefhorst A & Visser JA. 2019. Sex difference in corticosterone-induced insulin resistance in mice. Endocrinology 160 2367–2387. ( 10.1210/en.2019-00194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN Bhagat SM Bowles NP Weil ZM Pfaff DW & Mcewen BS. 2010. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151 2117–2127. ( 10.1210/en.2009-1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix A Feelders RA Stratakis CA & Nieman LK. 2015. Cushing’s syndrome. Lancet 386 913–927. ( 10.1016/S0140-6736(1461375-1) [DOI] [PubMed] [Google Scholar]
- Lonser RR Nieman L & Oldfield EH. 2017. Cushing’s disease: pathobiology, diagnosis, and management. Journal of Neurosurgery 126 404–417. ( 10.3171/2016.1.JNS152119) [DOI] [PubMed] [Google Scholar]
- Low WS Cornfield T Charlton CA Tomlinson JW & Hodson L. 2018. Sex differences in hepatic de novo lipogenesis with acute fructose feeding. Nutrients 10. ( 10.3390/nu10091263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magomedova L & Cummins CL. 2016. Glucocorticoids and metabolic control. Handbook of Experimental Pharmacology 233 73–93. ( 10.1007/164_2015_1) [DOI] [PubMed] [Google Scholar]
- Melcangi RC Poletti A Cavarretta I Celotti F Colciago A Magnaghi V Motta M Negri-Cesi P & Martini L. 1998. The 5alpha-reductase in the central nervous system: expression and modes of control. Journal of Steroid Biochemistry and Molecular Biology 65 295–299. ( 10.1016/s0960-0760(9800030-2) [DOI] [PubMed] [Google Scholar]
- Mina AI Leclair RA Leclair KB Cohen DE Lantier L & Banks AS. 2018. CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metabolism 28 656–666.e1. ( 10.1016/j.cmet.2018.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SA Mccabe EL Gathercole LL Hassan-Smith ZK Larner DP Bujalska IJ Stewart PM Tomlinson JW & Lavery GG. 2014. 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proceedings of the National Academy of Sciences of the United States of America 111 E2482–E2491. ( 10.1073/pnas.1323681111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SA Hassan-Smith ZK Doig CL Sherlock M Stewart PM & Lavery GG. 2016. Glucocorticoids and 11beta-HSD1 are major regulators of intramyocellular protein metabolism. Journal of Endocrinology 229 277–286. ( 10.1530/JOE-16-0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SA Gathercole LL Hassan-Smith ZK Tomlinson J Stewart PM & Lavery GG. 2022. 11beta-HSD1 contributes to age-related metabolic decline in male mice. Journal of Endocrinology 255 117–129. ( 10.1530/JOE-22-0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC Kyratzi E Lamprokostopoulou A Chrousos GP & Charmandari E. 2015. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 22 6–19. ( 10.1159/000362736) [DOI] [PubMed] [Google Scholar]
- Nishiyama M Iwasaki Y & Makino S. 2022. Animal models of Cushing’s syndrome. Endocrinology 163. ( 10.1210/endocr/bqac173) [DOI] [PubMed] [Google Scholar]
- Nixon M Upreti R & Andrew R. 2012. 5alpha-Reduced glucocorticoids: a story of natural selection. Journal of Endocrinology 212 111–127. ( 10.1530/JOE-11-0318) [DOI] [PubMed] [Google Scholar]
- Othonos N, Pofi R, Arvaniti A, White S, Bonaventura I, Nikolaou N, Moolla A, Marjot T, Stimson RH, Van Beek AP, et al. 2023. 11beta-HSD1 inhibition in men mitigates prednisolone-induced adverse effects in a proof-of-concept randomised double-blind placebo-controlled trial. Nature Communications 14 1025. ( 10.1038/s41467-023-36541-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckett AJ Wright DC & Riddell MC. 2011. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism: Clinical and Experimental 60 1500–1510. ( 10.1016/j.metabol.2011.06.012) [DOI] [PubMed] [Google Scholar]
- Pofi R Caratti G Ray DW & Tomlinson JW. 2023. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocrine Reviews 44 975–1011. ( 10.1210/endrev/bnad016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli R Ueta CB Drigo RAE Castillo M Fonseca TL & Bianco AC. 2013. Dexamethasone reduces energy expenditure and increases susceptibility to diet-induced obesity in mice. Obesity (Silver Spring) 21 E415–E420. ( 10.1002/oby.20338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakutty A Mangelsdorf BL Drake SM Samocha-Bonet D Heilbronn LK Smith MD Thompson CH & Burt MG. 2016. Effects of prednisolone on energy and fat metabolism in patients with rheumatoid arthritis: tissue-specific insulin resistance with commonly used prednisolone doses. Clinical Endocrinology 85 741–747. ( 10.1111/cen.13138) [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S & Cidlowski JA. 2016. Corticosteroids: mechanisms of action in health and disease. Rheumatic Disease Clinics of North America 42 15–31, vii. ( 10.1016/j.rdc.2015.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato AY Peacock M & Bellido T. 2018. Glucocorticoid excess in bone and muscle. Clinical Reviews in Bone and Mineral Metabolism 16 33–47. ( 10.1007/s12018-018-9242-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakman O Gilson H & Thissen JP. 2008. Mechanisms of glucocorticoid-induced myopathy. Journal of Endocrinology 197 1–10. ( 10.1677/JOE-07-0606) [DOI] [PubMed] [Google Scholar]
- Schakman O Kalista S Barbe C Loumaye A & Thissen JP. 2013. Glucocorticoid-induced skeletal muscle atrophy. International Journal of Biochemistry and Cell Biology 45 2163–2172. ( 10.1016/j.biocel.2013.05.036) [DOI] [PubMed] [Google Scholar]
- Sefton C Harno E Davies A Small H Allen TJ Wray JR Lawrence CB Coll AP & White A. 2016. Elevated hypothalamic glucocorticoid levels are associated with obesity and hyperphagia in male mice. Endocrinology 157 4257–4265. ( 10.1210/en.2016-1571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR Nygren J Bigelow ML & Nair KS. 2004. Effect of short-term prednisone use on blood flow, muscle protein metabolism, and function. Journal of Clinical Endocrinology and Metabolism 89 6198–6207. ( 10.1210/jc.2004-0908) [DOI] [PubMed] [Google Scholar]
- Solinas G Boren J & Dulloo AG. 2015. De novo lipogenesis in metabolic homeostasis: more friend than foe? Molecular Metabolism 4 367–377. ( 10.1016/j.molmet.2015.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Baba T, Christianto A, Yanai S, Lee-Okada HC, Ishiwata K, Nakabayashi K, Hata K, Ishii T, Hasegawa T, et al. 2024. Development of sexual dimorphism of skeletal muscles through the adrenal cortex, caused by androgen-induced global gene suppression. Cell Reports 43 113715. ( 10.1016/j.celrep.2024.113715) [DOI] [PubMed] [Google Scholar]
- Talal S Cease A Farington R Medina HE Rojas J & Harrison J. 2021. High carbohydrate diet ingestion increases post-meal lipid synthesis and drives respiratory exchange ratios above 1. Journal of Experimental Biology 224. ( 10.1242/jeb.240010) [DOI] [PubMed] [Google Scholar]
- Tataranni PA Larson DE Snitker S Young JB Flatt JP & Ravussin E. 1996. Effects of glucocorticoids on energy metabolism and food intake in humans. American Journal of Physiology 271 E317–E325. ( 10.1152/ajpendo.1996.271.2.E317) [DOI] [PubMed] [Google Scholar]
- Tomlinson JW Draper N Mackie J Johnson AP Holder G Wood P & Stewart PM. 2002. Absence of cushingoid phenotype in a patient with Cushing’s disease due to defective cortisone to cortisol conversion. Journal of Clinical Endocrinology and Metabolism 87 57–62. ( 10.1210/jcem.87.1.8189) [DOI] [PubMed] [Google Scholar]
- Tomlinson JW & Stewart PM. 2001. Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase. Best Practice & Research. Clinical Endocrinology & Metabolism 15 61–78. ( 10.1053/beem.2000.0119) [DOI] [PubMed] [Google Scholar]
- Uehara M Yamazaki H Yoshikawa N Kuribara-Souta A & Tanaka H. 2020. Correlation among body composition and metabolic regulation in a male mouse model of Cushing’s syndrome. Endocrine Journal 67 21–30. ( 10.1507/endocrj.EJ19-0205) [DOI] [PubMed] [Google Scholar]
- Van Baak MA. 1999. Physical activity and energy balance. Public Health Nutrition 2 335–339. ( 10.1017/s1368980099000452) [DOI] [PubMed] [Google Scholar]
- Webster JM, Sagmeister MS, Fenton CG, Seabright AP, Lai YC, Jones SW, Filer A, Cooper MS, Lavery GG, Raza K, et al. 2021. Global deletion of 11beta-HSD1 prevents muscle wasting associated with glucocorticoid therapy in polyarthritis. International Journal of Molecular Sciences 22. ( 10.3390/ijms22157828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CP Hazlehurst JM & Tomlinson JW. 2015. Glucocorticoids and non-alcoholic fatty liver disease. Journal of Steroid Biochemistry and Molecular Biology 154 94–103. ( 10.1016/j.jsbmb.2015.07.020) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a