Abstract
Dinosaurs thrived for over 160 million years in Mesozoic ecosystems, displaying diverse ecological and evolutionary adaptations. Their ecology was shaped by large-scale climatic and biogeographic changes, calling for a ‘deep-time’ macroecological investigation. These factors include temperature fluctuations and the break up of Pangaea, influencing species richness, ecological diversity and biogeographic history. Recent improvements in the dinosaur fossil record have enabled large-scale studies of their responses to tectonic, geographic and climatic shifts. Trends in species diversity, body size and reproductive traits can now be analysed using quantitative approaches like phylogenetic comparative methods, machine learning and Bayesian inference. These patterns sometimes align with, but also deviate from, first-order macroecological rules (e.g. species–area relationship, latitudinal biodiversity gradient, Bergmann’s rule). Accurate reconstructions of palaeobiodiversity and niche partitioning require ongoing taxonomic revisions and detailed anatomical descriptions. Interdisciplinary research combining sedimentology, geochemistry and palaeoclimatology helps uncover the environmental conditions driving dinosaur adaptations. Fieldwork in under-sampled regions, particularly at latitudinal extremes, is crucial for understanding the spatial heterogeneity of dinosaur ecosystems across the planet. Open science initiatives and online databases play a key role in advancing this field, enriching our understanding of deep-time ecological processes, and offering new insights into dinosaur macroecology and its broader implications.
Keywords: dinosaurs, macroecology, macroevolution, Mesozoic, palaeoecology
1. Introduction
For over 160 million years (from approximately 230 to 66 million years ago, Ma), Earth’s terrestrial ecosystems were dominated by dinosaurs [1]. These organisms diversified into a remarkable array of ecological niches, evolving diverse dietary preferences [2] and occupying a broad spectrum of body sizes [3] (figure 1), rivalling other terrestrial vertebrate groups, and posing intriguing questions about the ecology of Mesozoic ecosystems. How did multiple whale-sized terrestrial herbivores coexist in the same ecosystem [10], and how could primary productivity remain stable under such intense browsing pressure [6]? Did several species of giant theropod carnivores share high trophic levels simultaneously [11]? Did dinosaur body size evolve, or their diversity increase, in response to the unique climatic regimes of Mesozoic Earth systems [12]? These and many other ecological questions seem insurmountable due to the vagaries of an incomplete fossil record, which nonetheless reveals a high disparity in diversity and ecologies that evolved over a 160 million-year interval.
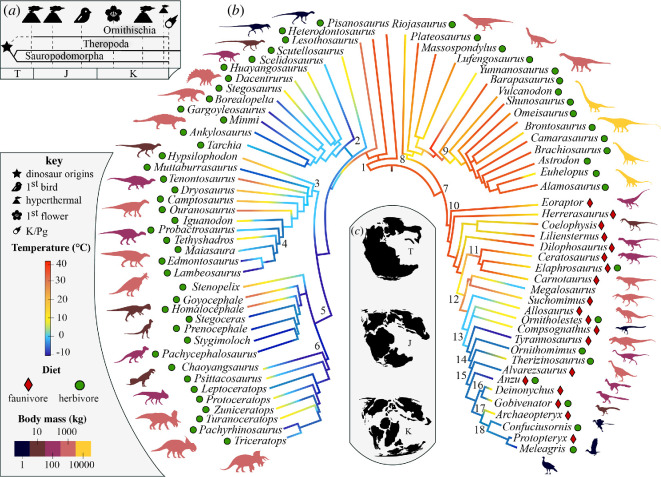
Figure 1.
Evolution of macroecological traits in Dinosauria. Large scale event in dinosaur evolution (a); the origin of dinosaurs (star), hyperthermals (volcano), the earliest fossil Avialae (bird), the earliest fossil angiosperm (flower), the Cretaceous/Palaeogene mass extinction (asteroid). Phylogeny of dinosaurs (b) redrawn from Sereno [2] and adapted to the current consensus and upon which an ancestral state reconstruction of temperature niche (mean annual temperature) after Chiarenza et al. [4] is plotted; Mesozoic palaeogeographies (c) for Triassic (T), Jurassic (J) and Cretaceous (K). Silhouette colours symbolize body mass for each of the taxa represented (after [5]); information on dietary habits are plotted after Barrett [6] and Zanno & Makovicky [7]; numbers represent clades discussed through this study: 1, Ornithischia; 2, Thyreophora; 3, Ornithopoda; 4, Hadrosauroidea; 5, Marginocephalia; 6, Ceratopsia; 7, Saurischia; 8, Sauropodomorpha; 9, Sauropoda; 10, Theropoda; 11, Ceratosauria; 12, Tetanurae; 13, Coelurosauria; 14, Maniraptoriformes; 15, Maniraptora; 16, Deinonychosauria; 17, Avialae; 18, Ornithothoraces. Palaeogeographies modified from original plots via R package ‘mapast’ [8] using plate models by Scotese [9].
While twentieth century palaeontological research focused on large-scale evolutionary processes (macroevolution), increased global data coverage with high sampling in some exceptional areas [13–15] now allows the investigation of the drivers of spatial patterns [16], a ‘deep-time’ macroecological enterprise. Although its origins date back to the nineteenth century [17–19], macroecology is a 35-year-old subdiscipline of ecology focusing on large-scale processes like climate and geography shaping biodiversity patterns. Macroecology, a term coined in 1971 [20], has seen limited application in palaeontology since its scientific formalization [21], often being paired with macroevolution (figure 2). Macroecology and macroevolution are often conflated in the scientific literature due to their interconnectedness, although macroevolution addresses prevalently temporal patterns (e.g. timing of clade diversification), while macroecology emphasizes spatial patterns (responses of biodiversity to broad Earth system interactions and feedback) [22].
Figure 2.
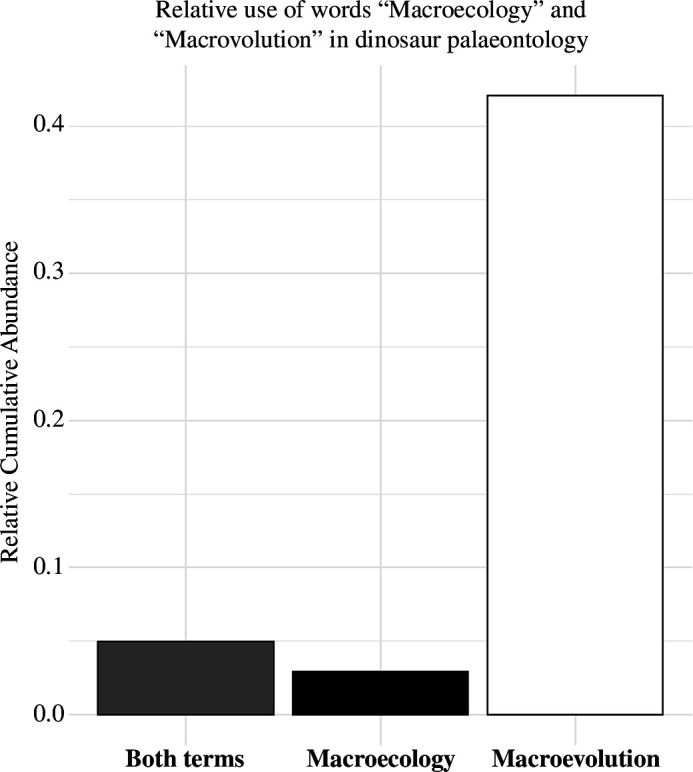
The use of macroecology in dinosaur palaeontology. Relative cumulative abundance of the use of the terms ‘macroecology’ and ‘macroevolution’ in dinosaur palaeontological literature since 1989. Data downloaded from Google Scholar (https://scholar.google.com/) on 05/04/2024.
While macroecology is prevalent in Quaternary palaeoecology [23], it is rarely used alone by dinosaur palaeontologists, despite its first use in this subject dating back to right after its formalization [24]. As a major component of their environments in terms of diversity and biomass [2,25], dinosaurs influenced various ecological processes, affecting other non-dinosaurian species. Their global distribution, extensive temporal range and role in understanding modern birds make them crucial for studying macroecology in deep time, broadening the scope of a science limited by present-day perspectives and an anthropogenically depauperated Quaternary record.
In the last four decades, there has been an exponential increase in dinosaur studies, with new species described at an average rate of two per week [26]. This constant update continuously challenges our understanding of dinosaur palaeobiology and Mesozoic ecosystems. The growing dataset of new taxa, localities and phenotypic diversity has allowed palaeontology to transition from an idiographic (descriptive and data collection) phase to a nomothetic one, in which principles relevant to evolutionary theory, including spatial patterns and their bearing in understanding the effects of large scale (e.g. climate and geography) on biodiversity, are formulated and tested against the empirical record [27].
Here, I outline the main topics investigated in recent years in non-avian dinosaur macroecology, including their evolutionary origins, ecosystem structure, the geographic factors influencing their diversity and trait distribution (e.g. trends in body size, diversity and reproductive traits across different regions and climates; table 1), and recent methodological advances enabling such studies. I then discuss and propose new perspectives for developing a methodologically robust and epistemologically grounded research programme on dinosaur macroecology moving forward.
Table 1.
Summary of main macroecological principles investigated in dinosaur palaeontology with key taxonomic, regional and temporal examples from the literature.
| macroecological principles | description | case studies |
|---|---|---|
| Allen’s rule | in homeothermic animals living in cold environments, a reduced ratio of body surface area to volume helps them retain heat more efficiently (shorter and stouter appendices). | Late Campanian–Early Maastrichtian Hadrosauridae in North America [28]. |
| Bergmann’s rule | homeothermic animals in colder climates are typically larger than those in warmer regions, which helps them conserve heat more effectively. | Late Campanian–Early Maastrichtian Troodontidae [29]; Ornithischia and Theropoda in the Campanian–Maastrichtian of North America [12]. |
| Damuth’s law | inverse relationship between body size and population density: as the size of a species increases, the number of individuals living in a given area decreases. | Maastrichtian of North America, Tyrannosaurus rex [30]. |
| ecological release | population growth and increased diversification result from the relaxation of environmental limiting factors, such as competition, which allows for expansion into new niches and ecomorphological space. | Late Triassic Dinosauria versus non-dinosaur tetrapods (global) [31–34]. |
| island rule (Foster’s rule) | animals isolated on islands often experience changes in body size, with smaller species tending to become larger (gigantism) and larger species tending to become smaller (dwarfism). | Europasaurus, Kimmeridgian (Europe) [35]; titanosaurs in Europe [36,37] and South America [38]; Tethyshadros insularis, Campanian of Europe [39,40]. |
| latitudinal biodiversity gradient (LBG) | species richness peaks in the tropics and declines progressively toward the poles. | Jurassic–Cretaceous Dinosauria (global) [41,42]. |
| thermogeochromic egg variation (TEV) | in birds from cold habitats, especially those with nests exposed to direct sunlight (and coinciding with high latitudes), eggshells are darker to enhance heat absorption; eggshells tend to be lighter (e.g. light blue and green) in the tropics. | modern examples [43], with future potential for investigation in the dinosaur fossil record [44,45]. |
| species–area relationship (SAR) | species richness increases in proportion to the available area, with larger areas supporting greater biodiversity. | Maastrichtian Dinosauria (global) [46]. |
2. Origins and ecosystem structure of dinosaur ecosystems
(a). Competition, ecological release and the rise of dinosaurs
Dinosaurs originated in the Late Triassic, with early Sauropodomorpha and Theropoda appearing around 230 Ma in South America [47]. Earliest ornithischian occurrences come from the Hettangian–Sinemurian (201−193 Ma) of Gondwana [48,49]. Some phylogenetic analyses suggest that Silesauridae might represent early ornithischians, possibly extending the presence of this main dinosaurian subclade and in turn pushing back dinosaur origins to the Middle Triassic [50]. Initially, dinosaurs were small [5], bipedal and less ecologically significant compared with other archosaurs and synapsids [31]. Their diet varied from carnivory to herbivory [51]. It remains unclear how dinosaurs succeeded in becoming the dominant archosaur lineages after the end-Triassic extinction. In terms of species richness (mere count of unique taxa), dinosaurs were less diverse than crurotarsans (crocodile-line archosaurs) in Late Triassic ecosystems [32]. If ecological niches are approximated with ecomorphological diversity, dinosaurs occupied a noticeably reduced ecospace compared with crurotarsans [31]. This ecological imbalance was reversed in the Early Jurassic (from Hettangian to Toarcian), with the ecomorphological diversity of dinosaurs surpassing that of the crurotarsans. A gradual ‘competitive exclusion’ model, with dinosaurs slowly outcompeting other archosaur clades through the Triassic–Jurassic transition, seems unlikely [33,34]. Instead, opportunism in the wake of the end-Triassic extinction as a process for their ecological release at the dawn of the Jurassic is currently favoured [33]. Extrinsic factors such as climate might have acted as a main constraint on dinosaur ecological release. Olsen et al. [52] discussed evidence for volcanically generated cold-pulses interspersed with the longer term volcanic hypsithermal event at the Triassic/Jurassic boundary as a possible extrinsic filter selecting the climatically more adaptable dinosaurs at the expense of more stenothermal non-dinosaurian tetrapods.
Evaluating ecologically relevant traits through functional morphology can reveal how some traits may have conferred advantages during this key transition, highlighting the selective roles operated by various environmental or biotic factors (see García-Girón et al. [53] for an end-Cretaceous analogue). Integrating functional ecology with machine learning, as performed in Foster et al. [54] to test extinction selectivity in invertebrates, can provide new methods to explore hypotheses explaining the rise of dinosaurs.
(b). Dinosaurs and primary productivity
The lower trophic levels of dinosaur ecosystems were composed of plant groups partially not comparable with modern ones. Mesozoic primary productivity included cycadophytes, gymnosperms, ferns, horsetails and ginkgoes, likely representing the main food source for herbivorous dinosaurs [6,55]. Herbivorous dinosaur dietary preferences have been explored via insights from animal nutrition science and comparative physiology on modern herbivores’ digestive tract. As bulk feeders [56], sauropods likely relied on plants providing substantial biomass and rapidly regenerating foliage, like conifers and ginkgoes, over less nutritious ferns and cycads. The distribution of plant biomes influenced herbivorous dinosaur distribution and diversity across different latitudes. Mesozoic conifers, evolving into frost-tolerant specialists, thrived in high-latitude regions, as evidenced by fossil records from polar regions [57]. Their abundance at high latitude suggest they could support the dietary needs of giant dinosaurian foragers at all latitudes, including polar biomes [4]. Angiosperms provided a succulent and poorly chemically defended food source [6,55], and their mid-Cretaceous rise coincides with their radiation and the decline of gymnosperms [58], particularly at lower latitudes [57]. A direct macroevolutionary response in dinosaurs to the appearance of angiosperms has been hypothesized [59], but quantitative assessments have found limited support for this interpretation [60–62]. The rise of angiosperms has been suggested as a cause of sauropod decline, at least in the Northern Hemisphere [60] while the more complex dental batteries of later diverging neornithischians (e.g. hadrosaurs and ceratopsians) might have provided an advantage for angiosperm consumption [59,61]. Detailed microwear [63,64] or isotopic studies [16,65] may shed new light on these clades’ preferential habits in plant consumption.
(c). Co-occurrence and spatial patterns
Collective aggregation or solitary habits in animals affect various aspects of terrestrial ecology, from the extent of occupied areas to migratory behaviour in response to resource consumption and depletion [25,66]. Evidence from ichnology and bonebeds suggests that many dinosaur groups, such as ceratopsians [67,68], ornithopods [69,70], theropods [71,72] and sauropods [73–75], may have been gregarious. This social behaviour likely served as an adaptation for protection against predators, efficient foraging and enhanced breeding success [76]. Parallel trackways with consistent depth, direction and lacking superposition suggest herding behaviour [77], though some may represent solitary dinosaurs moving in the same direction at different times due to geographical constraints [78,79]. Theropod trackways may indicate gregarious behaviour [80–82] in predatory dinosaurs, but the social nature of theropods remains debated [83,84]. Several monospecific bonebeds, previously considered evidence of gregarious aggregations, are more likely predator traps [85]. While body fossils provide useful taxonomic and population composition details, skeletal evidence must be carefully examined within its taphonomic context to accurately interpret dinosaur social behaviour [86].
Spatial distribution of dinosaur ichnofacies from the upper Pliensbachian of Poland, suggest closer association for early ornithischians to the coastlines, co-occurring with small theropods, while larger theropods and sauropomorphs are more abundantly found inland [87]. Brinkman et al. [88] observed an increase in ceratopsid occurrences and a decrease in ankylosaurs and pachycephalosaurs towards the palaeoshoreline, attributed to clade-specific environmental segregation [88,89]. Abelisaurids and carcharodontosaurids in South America share distributions with titanosaur sauropods. In the Western Interior, tyrannosaurid distributions closely parallel those of herbivores, with albertosaurine tyrannosaurids co-occurring with centrosaurine ceratopsids and lambeosaurine hadrosaurids, while tyrannosaurines co-occurred with chasmosaurine ceratopsids and hadrosaurine hadrosaurids [90].
Butler & Barrett [91] used correlative statistics to investigate the environmental preferences of Cretaceous herbivorous dinosaurs based on their spatial distribution. They found that nodosaurid ankylosaurs and hadrosaurids are strongly associated with marine sediments, while ceratopsians, pachycephalosaurs, theropods, sauropods and ankylosaurine are more common in terrestrial sediments. This supports the hypothesis that nodosaurids (although see Arbour et al. [92] for some caveats) and hadrosaurids were more prevalent in coastal or fluvial environments, while marginocephalians and ankylosaurids preferred inland habitats [93]. Quantitative studies of predatory theropod habitat preferences have shown that spinosaurids were predominantly associated with coastal palaeoenvironments, supporting a primarily piscivorous diet [94]. By contrast, Abelisauridae and Carcharodontosauridae are associated with terrestrial habitats, with abelisaurids being more common in inland areas compared with spinosaurids and carcharodontosaurids. Lyson & Longrich [95] used lithologies as proxies for palaeoenvironments to test habitat preferences in North American Maastrichtian dinosaurs. Their findings suggested ceratopsians preferred habitats away from rivers in coastal lowlands, while ornithopods favoured habitats closer to riverine systems. Tyrannosaurus rex, the primary secondary consumer in these terminal Cretaceous assemblages, is associated with both environments, indicating a generalist diet and extensive coexistence with various herbivores.
3. Large-scale patterns in dinosaur macroecology
(a). Geographic influence on dinosaur diversification
The Jurassic radiation and diversification of major dinosaur lineages may have been driven by continental fragmentation resulting from the breakup of Pangaea, which affected the available area for species [96,97] (figure 1c), vicariance and access to landmasses [98]. The species–area relationship (SAR) indicates that species richness scales with increasing available area, an ecological principle relevant to various terrestrial and marine systems [99]. Habitat fragmentation can either limit available area, negatively affecting diversity, or enhance isolation and speciation, thereby promoting diversity. These principles have been integrated into the species-fragmented area relationship (SfAR) [100]. The fragmentation of Pangaea since the Late Triassic, with the Atlantic Ocean spreading and sea level transgression inundating continental crust [101], particularly in the Late Cretaceous (figure 1c), led to isolation and continental endemicity, as seen between Northern Laurasian and Southern Gondwanan faunas [102]. This contrasts with the more uniform and cosmopolitan communities of the Late Triassic to Middle Jurassic [102–104]. Due to its generalization and wide applicability, SAR has been used in dinosaur palaeontology to estimate species numbers during specific intervals and understand the impact of changing terrestrial area extent on diversification. Dinosaur species richness has been modelled to largely track SAR [105] throughout the Mesozoic. However, principles of island biogeography [98] also predict increased species diversity due to enhanced isolation. Results from SAR modelling are susceptible to undersampling, leading to an underestimation of standing diversity in several large areas [46,106]. This underestimation has significant implications for understanding critical changes in diversity due to large-scale processes such as long-term climatic fluctuations and extinction events [106].
(b). Macroecological rules in dinosaur palaeobiology
The impact of environmental agents on biodiversity at the macroscale has been quantified into first-order macroecological rules (table 1), describing features like the variation in species richness and body size within abiotic and biotic domains. At the intersection of area extent and climate are discussions about the latitudinal biodiversity gradient (LBG) and its establishment in deep time [107]. The LBG, characterized by a peak in species richness in the tropics that declines towards the poles, stands as a fundamental pattern in modern biodiversity [108,109]. Explanations for this phenomenon include biological and abiotic drivers, such as gradients in temperature, light, seasonality and differential speciation rates associated with latitude, suggesting climate as the primary driver [108,110–112]. Conversely, some arguments propose the larger land availability at the equator compared with the poles, implying a SAR effect, to explain the LBG [108,113,114].
Deep-time investigations on LBG encompass marine invertebrates [115,116], plants [117], insects, turtles, crocodylomorphs, Cenozoic mammals and non-avian dinosaurs [41,118–121]. Contrary to the modern LBG, Mesozoic dinosaur studies suggest a diversity peak at temperate latitudes, with a ‘relaxed’ LBG, particularly during the Late Cretaceous [41]. Geographic partitioning was recovered between major herbivorous dinosaur taxa, with sauropod diversity peaking at lower palaeolatitudes and ornithischian diversity at higher palaeolatitudes. The combined influence of equable climates and land area likely dictated species distribution in the Mesozoic. Climatic factors influenced sauropod distribution similarly to crocodylomorphs [122,123], with preferences for tropical and subtropical zones [124–126]. These patterns may reflect thermophysiological differences between dinosaur subclades [4]. Despite Mesozoic dinosaur records contradicting a modern-type LBG, fossil bias must be considered. Global fossil data are crucial for understanding LBG’s temporal dynamics, but Mesozoic spatial patterns pose challenges due to the predominantly mid-latitudinal spread of fossil localities [127]. Close et al. [128] highlighted the limited palaeolatitudinal band available for vertebrate fossil sampling (between 30° and 60°). Fossil-bearing localities beyond this range typically yield singleton taxa but show equal sampling coverage independently of the systematic group, particularly at high latitudes [42]. Heterogeneous sample compositions across latitudinal bands [129] may obscure recognizing a modern-style LBG, impeding the estimation of correlations between species richness and environmental proxies. Nevertheless, rare fossiliferous localities from latitudinal extremes and thorough taxonomic sampling provide valuable references for reconstructing past environmental conditions [90,130–133] and biogeographic trends [134]. Understanding these climatic constraints and accounting for sampling artefacts are crucial for elucidating the abiotic drivers that shape latitudinal distribution and affect LBG configuration [110].
Spatial variation in dinosaur body-size is another deep-time macroecological pattern worth investigating. Bergmann’s rule posits that homeotherm animals in colder climates tend to be larger than their counterparts in warmer regions. To verify this rule, Fiorillo [29] took an empirical approach, focusing on troodontid dinosaurs in North America, particularly analysing tooth size as a proxy for body size, finding northern troodontids to be bigger than southern relatives. This method offers valuable insights due to the relatively higher abundance and preservability of dental material compared with other skeletal elements. It is crucial though to consider the taxonomic scale when applying Bergmann’s rule. While this pattern may hold true for closely related populations with similar phenotypic and ecological characteristics, its validity diminishes when applied across ‘higher ranked’ clades (e.g. supraspecific systematic groups). Wilson et al. [12] advocated for phylogenetically informed statistical models to test Bergmann’s rule, finding limited support for a correlation between dinosaur body size and temperature. However, these models, operating above the species level, may overlook intraspecific variation at which the rule is defined. A nuanced interpretation suggests that Bergmann’s rule might better suit lineage-level analyses rather than species-level assessments. Approaches like those by Wilson et al. [12] follow this rationale, highlighting general trends and describing spatially explicit relationships due to the increasing availability of fossil occurrences and palaeoclimate models for the Mesozoic. This approach has the virtue of overcoming the constraints of bias obscuring sub-generic diversity and mitigating it with a phylogenetic inference-guided approach. Studies by Blackburn et al. [135] and Meiri [136] support this perspective, focusing on closely related taxa inhabiting cooler climates, and highlight the future promise of following this example.
Complementary to Bergmann’s rule is Allen’s rule, which states that the body-surface area to volume ratio is minimized in homeothermic animals living in cold environments. Although no quantitative verification of this rule in the Mesozoic dinosaur record has been performed, some relevant observations can be reported. An implication of Allen’s rule is that the surface area of appendages (such as tails, crests or limbs) is reduced to minimize heat dissipation in homeotherms adapted to cold environments. Assuming at least two lineages of dinosaurs as homeotherms (Ornithischia and Theropoda [4]), they would be expected to have shorter, slimmer tails and limbs, conferring a somewhat stockier appearance to their body plan, in cold settings. Fiorillo & Gangloff [28] documented several predominantly juvenile individuals from the Liscomb bonebed in the Prince Creek Formation of Alaska to test the hypothesis that hadrosaurs from these latitudes were seasonal migrants. A corollary of their investigation, which excluded such a hypothesis, was the assessment of similar body proportions (both in juveniles and adults) compared with those of more southern relatives (e.g. Maiasaura [137] and Edmontosaurus [138]). This implied a lack of variation in limb-to-body proportions on a latitudinal gradient, representing an—admittedly loose—but probably the best available to date, test of Allen’s rule in Mesozoic dinosaurs. More complete remains of polar tyrannosauroids [139], hadrosaurs [140,141] and ceratopsians [142] are welcome to further test this hypothesis.
The island rule [143] is a macroecological principle describing how isolated animals on islands often undergo significant changes in body size, with smaller species tending toward gigantism and larger species toward dwarfism (table 1). Initially identified in modern mammals and documented in Quaternary ones, such as Mediterranean dwarf elephants, this pattern has been observed across vertebrate groups, including Mesozoic dinosaurs [32]. Insular dwarfism in non-avian dinosaurs, however, requires careful evidence beyond mere size comparisons with larger relatives, including histological markers of reduced growth rates and osteological signs of maturity (e.g. an external fundamental system [144]) alongside a robust tectonostratigraphic framework confirming insular environments. For instance, insular dwarfism has been well-documented in the sauropod Europasaurus from the Kimmeridgian Lower Saxony Basin of Germany [35]. By contrast, the hadrosauroid Tethyshadros insularis from the Campanian Liburnian Formation of Italy, once thought to be an insular dwarf for its smaller size compared with later diverging hadrosaurids [39], lacks osteohistological maturity signals. Recent revisions of its stratigraphic context [40] challenge the original interpretation, arguing for a less insular palaeoenvironment. These revisions suggest that the small size of Tethyshadros fits within the broader size range of early diverging hadrosauroids, compared with their later, larger relatives (e.g. North American and Asian latest Cretaceous hadrosaurids [145]).
Variation in egg colours and other shell features with latitude (thermogeochromic egg variation, TEV from now on; table 1) has been documented for many clades of birds and has been investigated under a macroevolutionary lens by Wisocki et al. [43]. We now know that the pigmentation in bird eggshells, once believed to be a unique avian trait, actually originated from non-avian dinosaurs [44]. The implications of studying egg colour and shell morphology in the dinosaur fossil record [45] may open new scenarios in investigating the relationships between the environment and these reproductive traits. The morphology and geographic distribution of fossil eggs may offer clues about nesting habits and environmental adaptations in Mesozoic dinosaurs [146]. Pigment analyses of maniraptoran eggs highlights that coloured eggs may have evolved alongside open nesting behaviours and brooding [146], likely serving as camouflage against predators and enabling nesting site recognition, as seen in modern birds. Other clades, like sauropods, built sandy in-filled hole nests (like modern marine turtles) that relied on solar or geothermal heat for incubation [146,147]. Furthermore, the identification of green, blue and speckled eggs may suggest both ecological and thermoregulatory functions in dinosaurs inhabiting different climates, highlights adaptations to different environments as seen in birds today, and a potential adherence to TEV in non-avian dinosaurs.
Damuth’s law relates large body sizes to low population density in land mammals. Marshall et al. [30] applied this principle, using a power law with proxies from palaeobiogeography and palaeobiology to estimate the overall population density of the theropod Tyrannosaurus rex during its approximately 2.5 million-year stratigraphic range. Despite uncertainties in parameters like areal extent and metabolism, they provided a rough estimate: up to 2.5 billion T. rex individuals may have ever lived, with a fossil recovery rate of about 1 per 80 million individuals, or 1 per 16 000 in areas of higher sampling. The remarkable aspect revealed in this study is not the absolute numbers provided, but the uncertainty bracket and extremely low sample size for one of the better-known dinosaurs. This highlights the severe limitations palaeontologists face to investigate large-scale macroecological patterns from even more incomplete palaeobiological samples.
4. Future directions
To shape the future of dinosaur macroecology, we must first address the limitations imposed by an incomplete and patchy vertebrate fossil record, especially in terrestrial environments. This necessitates scrupulous sampling strategies and careful extrapolation of ecological trends and patterns over time and space. To improve our analytical approaches and investigation of patterns in dinosaur biology and palaeoecology throughout the Mesozoic, several key recommendations emerge.
First, improving chronostratigraphic frameworks will help distinguish between contemporaneous and time-averaged assemblages, clarifying species interactions and ecological dynamics. This could involve more refined use of advanced radiometric dating techniques (e.g. tracing zircon-rich crystals in volcanoclastic layers through sedimentary successions [148] or from apatite in fossil bones using laser-ablation coupled with plasma-mass spectrometry [149] for U–Pb dating [148,150]); use of Bayesian age-stratigraphic modelling [151] (for constraining the age of the site from multiple data points) and extensive biostratigraphic and lithostratigraphic correlations. The presence of geochronologists [150,152] in any field enterprise is recommended, as it would enable palaeontologists to construct more accurate timelines of dinosaur communities. Time-averaging is a significant issue affecting palaeoecological chronologies, in which the mixing of skeletal elements from different chronological horizons can distort perceptions of palaeocommunity synecology [153]. The ‘mid-Cretaceous’ Kem Kem Compound Assemblage, with its presumably overcrowded guild of predators—known as ‘Stromer’s riddle’—demonstrates how separating different facies into distinct chronological horizons can clarify similar palaeoecological paradoxes [11,154]. Improved chronostratigraphic frameworks can also elucidate our understanding of multiple sauropod species coexistence in the Morrison Formation [10,15]. Enhancing stratigraphic and spatial contexts through the integration of sequence stratigraphy, sedimentology and taphonomical observations is crucial [10,15,152,155,156].
Second, thorough taxonomy and appropriately based systematics, solidly grounded in up-to-date and carefully considered anatomical characters, are the foundation of any community-level reconstruction and palaeoecological consideration. For instance, taxonomic re-evaluations, like the synonymy of ‘Nanotyrannus’ with Tyrannosaurus rex, affect our understanding of predator–prey dynamics and niche partitioning [157,158] in latest Cretaceous North American ecosystems [159]. Overzealous conservatism in species lumping and uncertainty in species delimitation have likely forced artificially low levels of alpha-diversity in many dinosaur-dominated assemblages. Conversely, ascribing variation to taxonomic distinction that might be ontogenetic would overinflate alpha diversity. Future refinements in species-level identification in dinosaur palaeontology will likely overhaul palaeodiversity in these study systems, restructuring our reconstructions of their palaeoecology.
Third, adopting advanced statistical methods and computational tools to analyse fossil data can quantitatively support inferences on the direct or indirect effects of the abiotic environment on dinosaur ecology [61,62]. Statistical investigations emphasize the significant role of sampling in diversity reconstructions, though further studies are needed to address the influences of sedimentation and fossil accumulation rates [128,160]. Computational methods, such as those developed by Close et al. [128], attempt to mitigate these biases using minimum-spanning trees and subsampling techniques. Approaches that emphasize the uncertainty bracket over absolute metrics are highly valuable, highlighting time intervals [161] or areas [42,106] where the record should be more carefully scrutinized for distortive agents rather than relying on absolute but labile measured signals. Techniques such as machine learning [162–164], network analysis [165] and Bayesian inference [166–169] offer promising avenues for corroborating hypothesis testing in palaeodiversity and macroecology, providing a more robust quantitative framework than ever before. This is similar to how quantitative systematics approaches in the 1970s provided replicable, numerical tools to evolutionary biologists, improving the methods used to reconstruct dinosaur evolutionary trees. Besides reconstructing ancient ecosystems with greater accuracy, these methods can handle large datasets and complex variables, providing more robust models for testing hypotheses on dinosaur ecology and co-evolution with the Earth System [4,13,42,53,54,106,170].
Fourth, large, open-source fossil occurrence databases, such as the Paleobiology Database (paleobiodb.org), along with advances in computational power and analytical techniques, have renewed interest in addressing biases and conducting macroecological studies in deep time. Invertebrate palaeontology [171–174] has pioneered many of these approaches, which have since been applied to vertebrate palaeontology [175–180] and, more recently, dinosaur palaeontology over the last two decades (e.g. [41,42,181]). This wealth of data is crucial for quantifying broad patterns of dinosaur diversity and palaeobiology through time and space. Ensuring the accuracy of stratigraphic and taxonomic data (e.g. [4,129,161]) within these occurrences is essential, and these databases should be seen as evolving systems that benefit from continuous input and refinement. Just as with museum collections, data from online fossil occurrence databases should be carefully vetted and cross-referenced. Researchers are encouraged to apply their own expertise (chronological, regional, taxonomic), as well as collaborate with colleagues with compatible expertise, to ensure that the data they analyse are reliable and robust. Initiatives like the Paleobiology Database should be expanded and further integrated with new digital tools (macrostrat.org for stratigraphic [182], morphobank.org for morphological and gbif.org for biodiversity data) and research platforms/consortia (e.g. Palaeoverse [183]) can streamline these research efforts. A use of database does not just provide ready to access information to scholars, but also improve wider accessibility and replicability of results, other than allowing a wider community to cross-check, verify and implement such sources of information for the benefit of the broader community. Integrating sequence stratigraphy [155,184,185] into palaeontological models may better advance our understanding of how sedimentation rates and facies deposition influence diversity signals, separating chaotic and random mixing of communities to identify only those taxa truly in sympatry that might have been interacting in their original, pre-diagenetic biocoenoses.
Fifth, extensive and targeted fieldwork in under-sampled continents, regions and palaeoenvironments is crucial to observe the heterogeneity in spatial patterns of palaeodiversity. Fieldwork should be strategically planned to cover diverse habitats and geological settings, ensuring a more representative sample of dinosaur life through different Mesozoic intervals.
Lastly, interdisciplinary approaches that integrate sedimentology, geochemistry and palaeoclimatology can offer deeper insights into the environmental contexts in which dinosaurs lived. Understanding the interplay between climate, vegetation and dinosaur communities, with a synergic integration of efforts from vertebrate palaeontologists, palaeobotanists and physical geoscientists (e.g. sedimentologists and stratigraphers) will shed light on their adaptive strategies and responses to environmental changes (e.g. feeding preferences and flexibility, shifting growth rates, thermophysiology, size variation [4,42,186,187]). Advanced analytical techniques, such as stable isotope analysis, biomechanical and palaeoclimate modelling, are essential for understanding the complex interactions between abiotic factors and these traits [188]. By examining patterns of body size, dietary requirements, population density and spatial distribution, palaeontologists can elucidate the niche dynamics and trophic structures of dinosaur-dominated communities in the Mesozoic [13,189,190]. Future research should focus on refining these methods and developing phylogenetically informed statistical methods and spatially explicit models to test macroecological hypotheses [4,12].
5. Conclusions
Studying macroecological principles applied to the dinosaur fossil record enhances our understanding of evolutionary and ecological processes over geological time. Fostering a more collaborative, open science ecosystem will increase public engagement and education about the importance of palaeontological research, garnering support and funding for future investigations. Public interest in dinosaurs is already high, and leveraging this fascination through outreach programmes and citizen science projects can boost resources for addressing key palaeoecological questions. Dinosaur macroevolution was essentially a 160 million-year natural experiment on the effects of dramatic geographic and climatic changes [34,130,146,191], impacting terrestrial ecosystems at multiple levels, which we can only document through palaeontology. By elucidating the dynamics of ancient ecosystems and the factors driving biodiversity patterns, palaeontologists can provide valuable insights into how organisms respond to environmental changes, equally generating broader interest in the scientific community and among the general public. Leveraging this acquisition, deep-time macroecology can direct public fascination with dinosaurs towards understanding the impact of climate change on ecosystem evolution. This is crucial for our modern society and underscores the role of dinosaur palaeontologists in addressing these issues.
This review aims to guide those interested in a richer understanding of ancient terrestrial ecosystems. By addressing the challenges here presented and developing new technologies and methodologies to tackle them, we can advance dinosaur palaeoecology and its broader impact on our knowledge of natural history and societal relevance.
Acknowledgements
The Editors, Paul Barrett and Susannah Maidment are thanked for inviting me to write this review and for handling this manuscript. The Editors and two anonymous reviewers are thanked for greatly improving this manuscript with their suggestions. The palaeoartist Davide Bonadonna (http://www.davidebonadonna.it/) is thanked for the reconstruction in figure 3. Thanks for the feedback on a preliminary version of this work by the London Palaeontology Journal Club, in particular to: Samantha Beeston, Paul Burke, Marco Camaiti, Ryan Felice, Anjali Goswami, Devin Hoffman, Andrew Knapp, Jack McMinn, Cassius Morrison, Bethany Pittard, Lucy Roberts, James Rule, Perth Sethapanichsakul, Lizzy Steell, Wendy Wen. Silhouettes are sourced from http://phylopic.org/ and their creators (Pete Buchholz, Manuel Brea Lueiro, Matt Dempsey, Roberto Diaz Sibaja, Domser, DiBgd, Tasman Dixon, Andrew A. Farke, Scott Hartman, Jaime Headden, Ivan Iofrida, Jagged Fang Designs, Michael Keesey, Martin Kevil, Andrew Knight, Matt Martyniuk, Gareth Monger, Bruno Navarro, Diego Ortega, Remes K, Ortega F, Fierro I, Joger U, Kosma R et al., Iain Reid, Nobu Tamura, Mathew Wedel) are acknowledged.
Figure 3.
Dinosaur ecology in the Nemegt Basin (Upper Cretaceous of Mongolia). A Tarbosaurus is consuming a Deinocheirus carcass, imposing a distortive, first-order taphonomic filter for interpreting the palaeoecology of this community (the hadrosaurid Saurolophus drinking opposite the tyrannosaurid and the sauropod Nemegtosaurus foraging in the background). Reconstruction by Davide Bonadonna.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
This article has no additional data.
Supplementary material is available online [192].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
A.A.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing.
Conflict of interest declaration
I declare having no competing interests.
Funding
The author is funded by a Royal Society Newton International Fellowship (NIF\R1\231802).
References
- 1. Benson RBJ. 2018. Dinosaur macroevolution and macroecology. Annu. Rev. Ecol. Evol. Syst. 49, 379–408. ( 10.1146/annurev-ecolsys-110617-062231) [DOI] [Google Scholar]
- 2. Sereno PC. 1999. The evolution of dinosaurs. Science 284, 2137–2147. ( 10.1126/science.284.5423.2137) [DOI] [PubMed] [Google Scholar]
- 3. Lee MSY, Cau A, Naish D, Dyke GJ. 2014. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science 345, 562–566. ( 10.1126/science.1252243) [DOI] [PubMed] [Google Scholar]
- 4. Chiarenza AA, Cantalapiedra JL, Jones LA, Gamboa S, Galván S, Farnsworth AJ, Valdes PJ, Sotelo G, Varela S. 2024. Early Jurassic origin of avian endothermy and thermophysiological diversity in dinosaurs. Curr. Biol. 34, 2517–2527. ( 10.1016/j.cub.2024.04.051) [DOI] [PubMed] [Google Scholar]
- 5. Benson RBJ, Hunt G, Carrano MT, Campione N. 2018. Cope’s rule and the adaptive landscape of dinosaur body size evolution. Palaeontology 61, 13–48. ( 10.1111/pala.12329) [DOI] [Google Scholar]
- 6. Barrett PM. 2014. Paleobiology of herbivorous dinosaurs. Annu. Rev. Earth Planet. Sci. 42, 207–230. ( 10.1146/annurev-earth-042711-105515) [DOI] [Google Scholar]
- 7. Zanno LE, Makovicky PJ. 2011. Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proc. Natl Acad. Sci. USA 108, 232–237. ( 10.1073/pnas.1011924108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varela S, Rothkugel S. 2018. mapast: combine paleogeography and paleobiodiversity. R package version 0.1. See https://github.com/macroecology/mapast.
- 9. Scotese CR. 2016. PALEOMAP PaleoAtlas for GPlates and the PaleoDataPlotter Program, PALEOMAP Project. See http://www.earthbyte.org/paleomappaleoatlas-for-gplates/.
- 10. Mannion PD, Tschopp E, Whitlock JA. 2021. Anatomy and systematics of the diplodocoid Amphicoelias altus supports high sauropod dinosaur diversity in the Upper Jurassic Morrison Formation of the USA . R. Soc. Open Sci. 8, 210377. ( 10.1098/rsos.210377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiarenza AA, Cau A. 2016. A large abelisaurid (Dinosauria, Theropoda) from Morocco and comments on the Cenomanian theropods from North Africa. PeerJ 4, e1754. ( 10.7717/peerj.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson LN, Gardner JD, Wilson JP, Farnsworth A, Perry ZR, Druckenmiller PS, Erickson GM, Organ CL. 2024. Global latitudinal gradients and the evolution of body size in dinosaurs and mammals. Nat. Commun. 15, 2864. ( 10.1038/s41467-024-46843-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García‐Girón J, Heino J, Alahuhta J, Chiarenza AA, Brusatte SL. 2021. Palaeontology meets metacommunity ecology: the Maastrichtian dinosaur fossil record of North America as a case study. Palaeontology 64, 335–357. ( 10.1111/pala.12526) [DOI] [Google Scholar]
- 14. Cullen TM, Zanno L, Larson DW, Todd E, Currie PJ, Evans DC. 2021. Anatomical, morphometric, and stratigraphic analyses of theropod biodiversity in the Upper Cretaceous (Campanian) Dinosaur Park Formation . Can. J. Earth Sci. 58, 870–884. ( 10.1139/cjes-2020-0145) [DOI] [Google Scholar]
- 15. Maidment SCR. 2023. Diversity through time and space in the Upper Jurassic Morrison Formation, Western U.S.A. J. Vertebr. Paleontol. 43, e2326027. ( 10.1080/02724634.2024.2326027) [DOI] [Google Scholar]
- 16. Cullen TM, Zhang S, Spencer J, Cousens B. 2022. Sr‐O‐C isotope signatures reveal herbivore niche‐partitioning in a Cretaceous ecosystem. Palaeontology 65, e12591. ( 10.1111/pala.12591) [DOI] [Google Scholar]
- 17. von Humboldt A, Bonpland A. 1805. Essai sur la géographie des plantes: accompagné d’un tableau physique des régions équinoxiales, fondé sur des mesures exécutées, depuis le dixième degré de latitude boréale jusqu’au dixième degré de latitude australe, pendant les années 1799, 1800, 1801, 1802 et 1803. Paris, France: Chez Levrault, Schoell et compagnie, libraires. [Google Scholar]
- 18. Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray. ( 10.5962/bhl.title.68064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wallace AR. 1876. The geographical distribution of animals: with a study of the relations of living and extinct faunas as elucidating the past changes of the Earth’s surface. vol. 1. New York, NY: Harper & Brothers. ( 10.5962/bhl.title.46581) [DOI] [Google Scholar]
- 20. Marquet PA. 2009. III.16 Macroecological perspectives on communities and ecosystems. In The Princeton guide to ecology (eds Levin S, Carpenter S, Godfray H, Kinzig A, Loreau M, Losos J, Walker B, Wilcove D), pp. 386–394. Princeton, NJ: Princeton University Press. ( 10.1515/9781400833023.386) [DOI] [Google Scholar]
- 21. Brown JH, Maurer BA. 1989. Macroecology: the division of food and space among species on continents. Science 243, 1145–1150. ( 10.1126/science.243.4895.1145) [DOI] [PubMed] [Google Scholar]
- 22. McGill BJ, et al. 2019. Unifying macroecology and macroevolution to answer fundamental questions about biodiversity. Glob. Ecol. Biogeogr. 28, 1925–1936. ( 10.1111/geb.13020) [DOI] [Google Scholar]
- 23. Lyons SK, Smith FA. 2010. Using a macroecological approach to study geographic range, abundance and body size in the fossil record. Paleontol. Soc. pap. 16, 117–141. ( 10.1017/S1089332600001844) [DOI] [Google Scholar]
- 24. Andrews JH. 1991. Size. In Comparative ecology of microorganisms and macroorganisms (ed. Andrews JH), pp. 100–143. New York, NY: Springer. ( 10.1007/978-1-4612-3074-8_4) [DOI] [Google Scholar]
- 25. Barrett PM, Rayfield EJ. 2006. Ecological and evolutionary implications of dinosaur feeding behaviour. Trends Ecol. Evol 21, 217–224. ( 10.1016/j.tree.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 26. Tennant JP, Chiarenza AA, Baron M. 2018. How has our knowledge of dinosaur diversity through geologic time changed through research history? PeerJ 6, e4417. ( 10.7717/peerj.4417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gould SJ. 1980. The promise of paleobiology as a nomothetic, evolutionary discipline. Paleobiology 6, 96–118. ( 10.1017/S0094837300012537) [DOI] [Google Scholar]
- 28. Fiorillo AR, Gangloff RA. 2001. The caribou migration model for Arctic hadrosaurs (Dinosauria: Ornithischia): a reassessment. Hist. Biol. 15, 323–334. ( 10.1080/0891296021000037327) [DOI] [Google Scholar]
- 29. Fiorillo AR. 2008. On the occurrence of exceptionally large teeth of Troodon (Dinosauria: Saurischia) from the Late Cretaceous of northern Alaska. Palaios 23, 322–328. ( 10.2110/palo.2007.p07-036r) [DOI] [Google Scholar]
- 30. Marshall CR, Latorre DV, Wilson CJ, Frank TM, Magoulick KM, Zimmt JB, Poust AW. 2021. Absolute abundance and preservation rate of Tyrannosaurus rex. Science 372, 284–287. ( 10.1126/science.abc8300) [DOI] [PubMed] [Google Scholar]
- 31. Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. The first 50 Myr of dinosaur evolution: macroevolutionary pattern and morphological disparity. Biol. Lett. 4, 733–736. ( 10.1098/rsbl.2008.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brusatte SL, Benton MJ, Lloyd GT, Ruta M, Wang SC. 2010. Macroevolutionary patterns in the evolutionary radiation of archosaurs (Tetrapoda: Diapsida). Earth Environ. Sci. Trans. R. Soc. Edinb. 101, 367–382. ( 10.1017/S1755691011020056) [DOI] [Google Scholar]
- 33. Brusatte SL, Benton MJ, Ruta M, Lloyd GT. 2008. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488. ( 10.1126/science.1161833) [DOI] [PubMed] [Google Scholar]
- 34. Corecco L, Kohn MJ, Schultz CL. 2024. Triassic climate and the rise of the dinosaur empire in South America. J. South Am. Earth Sci. 142, 104977. ( 10.1016/j.jsames.2024.104977) [DOI] [Google Scholar]
- 35. Martin Sander P, Mateus O, Laven T, Knötschke N. 2006. Bone histology indicates insular dwarfism in a new Late Jurassic sauropod dinosaur. Nature 441, 739–741. ( 10.1038/nature04633) [DOI] [PubMed] [Google Scholar]
- 36. Stein K, Csiki Z, Rogers KC, Weishampel DB, Redelstorff R, Carballido JL, Sander PM. 2010. Small body size and extreme cortical bone remodeling indicate phyletic dwarfism in Magyarosaurus dacus (Sauropoda: Titanosauria). Proc. Natl Acad. Sci. USA 107, 9258–9263. ( 10.1073/pnas.1000781107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Company J. 2011. Bone histology of the titanosaur Lirainosaurus astibiae (Dinosauria: Sauropoda) from the latest Cretaceous of Spain. Nat. Wiss. 98, 67–78. ( 10.1007/s00114-010-0742-3) [DOI] [PubMed] [Google Scholar]
- 38. Navarro BA, et al. 2022. A new nanoid titanosaur (Dinosauria: Sauropoda) from the Upper Cretaceous of Brazil. Ameghiniana 59, 317–355. ( 10.5710/AMGH.25.08.2022.3477) [DOI] [Google Scholar]
- 39. Dalla Vecchia FM. 2009. Tethyshadros insularis, a new hadrosauroid dinosaur (Ornithischia) from the Upper Cretaceous of Italy . J. Vertebr. Paleontol. 29, 1100–1116. ( 10.1671/039.029.0428) [DOI] [Google Scholar]
- 40. Chiarenza AA, Fabbri M, Consorti L, Muscioni M, Evans DC, Cantalapiedra JL, Fanti F. 2021. An Italian dinosaur Lagerstätte reveals the tempo and mode of hadrosauriform body size evolution. Sci. Rep. 11, 23295. ( 10.1038/s41598-021-02490-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mannion PD, Benson RBJ, Upchurch P, Butler RJ, Carrano MT, Barrett PM.. 2012. A temperate palaeodiversity peak in Mesozoic dinosaurs and evidence for Late Cretaceous geographical partitioning. Glob. Ecol. Biogeogr. 21, 898–908. ( 10.1111/j.1466-8238.2011.00735.x) [DOI] [Google Scholar]
- 42. Chiarenza AA, Mannion PD, Farnsworth A, Carrano MT, Varela S. 2022. Climatic constraints on the biogeographic history of Mesozoic dinosaurs. Curr. Biol. 32, 570–585.( 10.1016/j.cub.2021.11.061) [DOI] [PubMed] [Google Scholar]
- 43. Wisocki PA, Kennelly P, Rojas Rivera I, Cassey P, Burkey ML, Hanley D. 2020. The global distribution of avian eggshell colours suggest a thermoregulatory benefit of darker pigmentation. Nat. Ecol. Evol. 4, 148–155. ( 10.1038/s41559-019-1003-2) [DOI] [PubMed] [Google Scholar]
- 44. Wiemann J, Yang TR, Norell MA. 2018. Dinosaur egg colour had a single evolutionary origin. Nature 563, 555–558. ( 10.1038/s41586-018-0646-5) [DOI] [PubMed] [Google Scholar]
- 45. Wiemann J, Yang TR, Sander PN, Schneider M, Engeser M, Kath-Schorr S, Müller CE, Sander PM. 2017. Dinosaur origin of egg color: oviraptors laid blue-green eggs. PeerJ 5, e3706. ( 10.7717/peerj.3706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Loeuff J. 2012. Paleobiogeography and biodiversity of Late Maastrichtian dinosaurs: how many dinosaur species went extinct at the Cretaceous–Tertiary boundary? Bull. Soc. Géol. Fr. 183, 547–559. ( 10.2113/gssgfbull.183.6.547) [DOI] [Google Scholar]
- 47. Ezcurra MD. 2010. A new early dinosaur (Saurischia: Sauropodomorpha) from the Late Triassic of Argentina: a reassessment of dinosaur origin and phylogeny. J. Syst. Palaeontol. 8, 371–425. ( 10.1080/14772019.2010.484650) [DOI] [Google Scholar]
- 48. McPhee B, Bordy E, Sciscio L, Choiniere J. 2017. The sauropodomorph biostratigraphy of the Elliot Formation of Southern Africa: tracking the evolution of Sauropodomorpha across the Triassic–Jurassic boundary. Acta Palaeontol. Pol. 62. ( 10.4202/app.00377.2017) [DOI] [Google Scholar]
- 49. Irmis RB, Parker WG, Nesbitt SJ, Liu J. 2007. Early ornithischian dinosaurs: the Triassic record. Hist. Biol. 19, 3–22. ( 10.1080/08912960600719988) [DOI] [Google Scholar]
- 50. Fonseca AO, Reid IJ, Venner A, Duncan RJ, Garcia MS, Müller RT. 2024. A comprehensive phylogenetic analysis on early ornithischian evolution. J. Syst. Palaeontol. 22, 2346577. ( 10.1080/14772019.2024.2346577) [DOI] [Google Scholar]
- 51. Ballell A, Benton MJ, Rayfield EJ. 2022. Dental form and function in the early feeding diversification of dinosaurs. Sci. Adv. 8, eabq5201. ( 10.1126/sciadv.abq5201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olsen P, et al. 2022. Arctic ice and the ecological rise of the dinosaurs. Sci. Adv. 8, eabo6342. ( 10.1126/sciadv.abo6342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. García-Girón J, Chiarenza AA, Alahuhta J, DeMar DG, Heino J, Mannion PD, Williamson TE, Wilson Mantilla GP, Brusatte SL. 2022. Shifts in food webs and niche stability shaped survivorship and extinction at the end-Cretaceous. Sci. Adv. 8, eadd5040. ( 10.1126/sciadv.add5040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Foster WJ, et al. 2023. How predictable are mass extinction events? R. Soc. Open Sci. 10, 221507. ( 10.1098/rsos.221507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sander PM, Gee C, Hummel J, Clauss M. 2010. Mesozoic plants and dinosaur herbivory. In Plants in Mesozoic time: morphological innovations, phylogeny, ecosystems, pp. 331–359. Bloomington, IN: Indiana University Press. ( 10.5167/uzh-35283) [DOI] [Google Scholar]
- 56. Rogers KC, Wilson J. 2005. The sauropods: evolution and paleobiology. Berkeley, CA: University of California Press. [Google Scholar]
- 57. Peralta-Medina E, Falcon-Lang HJ. 2012. Cretaceous forest composition and productivity inferred from a global fossil wood database. Geology 40, 219–222. ( 10.1130/G32733.1) [DOI] [Google Scholar]
- 58. Benton MJ, Wilf P, Sauquet H. 2022. The angiosperm terrestrial revolution and the origins of modern biodiversity. New Phytol. 233, 2017–2035. ( 10.1111/nph.17822) [DOI] [PubMed] [Google Scholar]
- 59. Bakker RT. 1978. Dinosaur feeding behaviour and the origin of flowering plants. Nature 274, 661–663. ( 10.1038/274661a0) [DOI] [Google Scholar]
- 60. Barrett PM, Willis KJ. 2001. Did dinosaurs invent flowers? Dinosaur–angiosperm coevolution revisited. Biol. Rev. Camb. Philos. Soc. 76, 411–447. ( 10.1017/s1464793101005735) [DOI] [PubMed] [Google Scholar]
- 61. Butler RJ, Barrett PM, Kenrick P, Penn MG. 2009. Diversity patterns amongst herbivorous dinosaurs and plants during the Cretaceous: implications for hypotheses of dinosaur/angiosperm co-evolution. J. Evol. Biol. 22, 446–459. ( 10.1111/j.1420-9101.2008.01680.x) [DOI] [PubMed] [Google Scholar]
- 62. Butler RJ, Barrett PM, Penn MG, Kenrick P. 2010. Testing coevolutionary hypotheses over geological timescales: interactions between Cretaceous dinosaurs and plants. Biol. J. Linn. Soc. 100, 1–15. ( 10.1111/j.1095-8312.2010.01401.x) [DOI] [Google Scholar]
- 63. Melstrom KM, Chiappe LM, Smith ND. 2021. Exceptionally simple, rapidly replaced teeth in sauropod dinosaurs demonstrate a novel evolutionary strategy for herbivory in Late Jurassic ecosystems. BMC Ecol. Evol. 21, 202. ( 10.1186/s12862-021-01932-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ősi A, Barrett PM, Földes T, Tokai R. 2014. Wear pattern, dental function, and jaw mechanism in the Late Cretaceous ankylosaur Hungarosaurus . Anat. Rec. 297, 1165–1180. ( 10.1002/ar.22910) [DOI] [PubMed] [Google Scholar]
- 65. Cullen TM, Longstaffe FJ, Wortmann UG, Huang L, Fanti F, Goodwin MB, Ryan MJ, Evans DC. 2020. Large-scale stable isotope characterization of a Late Cretaceous dinosaur-dominated ecosystem. Geology 48, 546–551. ( 10.1130/G47399.1) [DOI] [Google Scholar]
- 66. Bulai IM, Venturino E. 2017. Shape effects on herd behavior in ecological interacting population models. Math. Comput. Simul. 141, 40–55. ( 10.1016/j.matcom.2017.04.009) [DOI] [Google Scholar]
- 67. Currie PJ, Dodson P. 1984. Mass death of a herd of ceratopsian dinosaurs. In Third Symp. on Mesozoic Terrestrial Ecosystems: short papers, p. 61. Tübingen, Germany: Attempto-Verlag. [Google Scholar]
- 68. Ryan MJ, Russell AP, Eberth DA, Currie PJ. 2001. The taphonomy of a Centrosaurus (Ornithischia: Certopsidae) bone bed from the Dinosaur Park Formation (Upper Campanian), Alberta, Canada, with comments on cranial ontogeny. Palaios 16, 482–506. () [DOI] [Google Scholar]
- 69. Horner JR. 1982. Evidence of colonial nesting and ‘site fidelity’ among ornithischian dinosaurs. Nature 297, 675–676. ( 10.1038/297675a0) [DOI] [Google Scholar]
- 70. Rogers RR. 1990. Taphonomy of three dinosaur bone beds in the Upper Cretaceous Two Medicine Formation of northwestern Montana: evidence for drought-related mortality. Palaios 5, 394. ( 10.2307/3514834) [DOI] [Google Scholar]
- 71. Schwartz HL, Gillette DD. 1994. Geology and taphonomy of the Coelophysis quarry, Upper Triassic Chinle Formation, Ghost Ranch, New Mexico . J. Paleontol. 68, 1118–1130. ( 10.1017/S0022336000026718) [DOI] [Google Scholar]
- 72. Currie PJ. 1998. Possible evidence of gregarious behavior in tyrannosaurids. GAIA 15, 271–277. ( 10.7939/R3348GX03) [DOI] [Google Scholar]
- 73. Jain SL. 1980. The continental Lower Jurassic fauna from the Kota Formation, India. Flagstaff, AZ: L.L. Jacobs, Museum of Northern Arizona Review. [Google Scholar]
- 74. Coria RA. 1994. On a monospecific assemblage of sauropod dinosaurs from patagonia: implications for gregarious behavior. GAIA 10, 209–213. http://www.arca.museus.ul.pt/ArcaSite/obj/gaia/MNHNL-0000277-MG-DOC-web.PDF [Google Scholar]
- 75. Myers TS, Fiorillo AR. 2009. Evidence for gregarious behavior and age segregation in sauropod dinosaurs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 274, 96–104. ( 10.1016/j.palaeo.2009.01.002) [DOI] [Google Scholar]
- 76. Currie PJ, Eberth DA. 2010. On gregarious behavior in Albertosaurus. Can. J. Earth Sci. 47, 1277–1289. ( 10.1139/E10-072) [DOI] [Google Scholar]
- 77. Lockley MG. 1995. Track records. Nat. Hist. 104, 46–51. [Google Scholar]
- 78. Getty PR, Hardy L, Bush AM. 2015. Was the Eubrontes track maker gregarious? Testing the herding hypothesis at Powder Hill Dinosaur Park, Middlefield, Connecticut. Bull. Peabody Mus. Nat. Hist. 56, 95–106. ( 10.3374/014.056.0109) [DOI] [Google Scholar]
- 79. Getty PR, Aucoin C, Fox N, Judge A, Hardy L, Bush AM. 2017. Perennial lakes as an environmental control on theropod movement in the Jurassic of the Hartford Basin. Geosciences 7, 13. ( 10.3390/geosciences7010013) [DOI] [Google Scholar]
- 80. Moreno K, Valais S de, Blanco N, Tomlinson AJ, Jacay J, Calvo JO. 2012. Large theropod dinosaur footprint associations in western Gondwana: behavioural and palaeogeographic implications. Acta Palaeontol. Pol. 57, 73–83. ( 10.4202/app.2010.0119) [DOI] [Google Scholar]
- 81. García-Ortiz E, Pérez-Lorente F. 2014. Palaeoecological inferences about dinosaur gregarious behaviour based on the study of tracksites from La Rioja area in the Cameros Basin (Lower Cretaceous, Spain). J. Iber. Geol. 40, 113–127. ( 10.5209/rev_JIGE.2014.v40.n1.44091) [DOI] [Google Scholar]
- 82. McCrea RT, Buckley LG, Farlow JO, Lockley MG, Currie PJ, Matthews NA, Pemberton SG. 2014. A 'terror of tyrannosaurs': the first trackways of tyrannosaurids and evidence of gregariousness and pathology in Tyrannosauridae. PLoS One 9, e103613. ( 10.1371/journal.pone.0103613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barco JL, Canudo JI, Ruiz-Omeñaca JI. 2006. New data on Therangospodus oncalensis from the Berriasian Fuentesalvo tracksite (Villar del Río, Soria, Spain): an example of gregarious behaviour in theropod dinosaurs . Ichnos 13, 237–248. ( 10.1080/10420940600843682) [DOI] [Google Scholar]
- 84. Roach BT, Brinkman DL. 2007. A reevaluation of cooperative pack hunting and gregariousness in Deinonychus antirrhopus and other nonavian theropod dinosaurs. Bull. Peabody Mus. Nat. Hist. 48, 103–138. ( 10.3374/0079-032X(2007)48[103:AROCPH]2.0.CO;2) [DOI] [Google Scholar]
- 85. Kirkland JI, Simpson EL, DeBLIEUX DD, Madsen SK, Bogner E, Tibert NE. 2016. Depositional constraints on the Lower Cretaceous stikes quarry dinosaur site: Upper Yellow Cat Member, Cedar Mountain Formation, Utah. Palaios 31, 421–439. ( 10.2110/palo.2016.041) [DOI] [Google Scholar]
- 86. Hone DWE, Farke AA, Watabe M, Shigeru S, Tsogtbaatar K. 2014. A new mass mortality of juvenile Protoceratops and size-segregated aggregation behaviour in juvenile non-avian dinosaurs. PLoS One 9, e113306. ( 10.1371/journal.pone.0113306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pacyna G, Ziaja J, Barbacka M, Pieńkowski G, Jarzynka A, Niedzwiedzki G. 2022. Early Jurassic dinosaur-dominated track assemblages, floristic and environmental changes in the Holy Cross mountains region, Poland. Geol. Q 66, 66–29. ( 10.7306/gq.1660) [DOI] [Google Scholar]
- 88. Brinkman DB, Ryan MJ, Eberth DA. 1998. The paleogeographic and stratigraphic distribution of ceratopsids (Ornithischia) in the Upper Judith River Group of western Canada. PALAIOS 13, 160. ( 10.2307/3515487) [DOI] [Google Scholar]
- 89. Lehman TM. 1987. Late Maastrichtian paleoenvironments and dinosaur biogeography in the Western Interior of North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 60, 189–217. ( 10.1016/0031-0182(87)90032-0) [DOI] [Google Scholar]
- 90. Holtz T, Chapman R, Lamanna M. 2004. Mesozoic biogeography of dinosauria. In The Dinosauria, pp. 627–642, 2nd edn. Berkeley, CA. ( 10.1525/california/9780520242098.003.0030) [DOI] [Google Scholar]
- 91. Butler RJ, Barrett PM. 2008. Palaeoenvironmental controls on the distribution of Cretaceous herbivorous dinosaurs. Naturwissenschaften 95, 1027–1032. ( 10.1007/s00114-008-0417-5) [DOI] [PubMed] [Google Scholar]
- 92. Arbour VM, Zanno LE, Gates T. 2016. Ankylosaurian dinosaur palaeoenvironmental associations were influenced by extirpation, sea-level fluctuation, and geodispersal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 449, 289–299. ( 10.1016/j.palaeo.2016.02.033) [DOI] [Google Scholar]
- 93. Horner JR. 1979. Upper Cretaceous dinosaurs from the Bearpaw Shale (marine) of south-central Montana with a checklist of Upper Cretaceous dinosaur remains from marine sediments in North America. J. Paleontol. 53, 566–577. https://www.jstor.org/stable/1303998 [Google Scholar]
- 94. Sales MAF, Lacerda MB, Horn BLD, de Oliveira IAP, Schultz CL. 2016. The “χ” of the matter: testing the relationship between paleoenvironments and three theropod clades. PLoS One 11, e0147031. ( 10.1371/journal.pone.0147031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lyson TR, Longrich NR. 2011. Spatial niche partitioning in dinosaurs from the latest Cretaceous (Maastrichtian) of North America. Proc. R. Soc. B 278, 1158–1164. ( 10.1098/rspb.2010.1444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. O’Donovan C, Meade A, Venditti C. 2018. Dinosaurs reveal the geographical signature of an evolutionary radiation. Nat. Ecol. Evol. 2, 452–458. ( 10.1038/s41559-017-0454-6) [DOI] [PubMed] [Google Scholar]
- 97. Dunhill AM, Bestwick J, Narey H, Sciberras J. 2016. Dinosaur biogeographical structure and Mesozoic continental fragmentation: a network‐based approach. J. Biogeogr. 43, 1691–1704. ( 10.1111/jbi.12766) [DOI] [Google Scholar]
- 98. MacArthur RH, Wilson EO. 2001. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 99. Arrhenius O. 1921. Species and area. J. Ecol. 9, 95. ( 10.2307/2255763) [DOI] [Google Scholar]
- 100. Hanski I, Zurita GA, Bellocq MI, Rybicki J. 2013. Species–fragmented area relationship. Proc. Natl Acad. Sci. USA 110, 12715–12720. ( 10.1073/pnas.1311491110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rowley DB, Markwick PJ. 1992. Haq et al. Eustatic sea level curve: implications for sequestered water volumes. J. Geol. 100, 703–715. ( 10.1086/629623) [DOI] [Google Scholar]
- 102. Bonaparte JF. 1986. History of the terrestrial Cretaceous vertebrates of Gondwana. In Evolucion de los vertebrados mesozoicos, 1986, in iv congreso argentino de paleontología y bioestratigrafía: actas 4, mendoza, 1986. congreso argentino de paleontología y bioestratigrafía mendoza, noviembre 23-27 (ed. Bonaparte JF), pp. 63–95. [Google Scholar]
- 103. Upchurch P, Hunn CA, Norman DB. 2002. An analysis of dinosaurian biogeography: evidence for the existence of vicariance and dispersal patterns caused by geological events. Proc. R. Soc. Lond. B 269, 613–621. ( 10.1098/rspb.2001.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Upchurch P. 1997. The evolutionary history of sauropod dinosaurs. Phil. Trans. R. Soc. Lond. B 349, 365–390. ( 10.1098/rstb.1995.0125) [DOI] [Google Scholar]
- 105. Vavrek MJ. 2016. The fragmentation of Pangaea and Mesozoic terrestrial vertebrate biodiversity. Biol. Lett. 12, 20160528. ( 10.1098/rsbl.2016.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chiarenza AA, Mannion PD, Lunt DJ, Farnsworth A, Jones LA, Kelland SJ, Allison PA. 2019. Ecological niche modelling does not support climatically-driven dinosaur diversity decline before the Cretaceous/Paleogene mass extinction. Nat. Commun. 10, 1091. ( 10.1038/s41467-019-08997-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mannion PD, Upchurch P, Benson RBJ, Goswami A. 2014. The latitudinal biodiversity gradient through deep time. Trends Ecol. Evol. (Amst.) 29, 42–50. ( 10.1016/j.tree.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 108. Willig MR, Kaufman DM, Stevens RD. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309. ( 10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 109. Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 110. Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 111. Field R, et al. 2009. Spatial species‐richness gradients across scales: a meta‐analysis. J. Biogeogr. 36, 132–147. ( 10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 112. Saupe EE. 2023. Explanations for latitudinal diversity gradients must invoke rate variation. Proc. Natl Acad. Sci. USA 120, e2306220120. ( 10.1073/pnas.2306220120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Terborgh J. 1973. On the notion of favorableness in plant ecology. Am. Nat. 107, 481–501. ( 10.1086/282852) [DOI] [Google Scholar]
- 114. Rosenzweig ML. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press. ( 10.1017/CBO9780511623387) [DOI] [Google Scholar]
- 115. Jablonski D, Roy K, Valentine JW. 2006. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106. ( 10.1126/science.1130880) [DOI] [PubMed] [Google Scholar]
- 116. Alroy J. 2008. Dynamics of origination and extinction in the marine fossil record. Proc. Natl Acad. Sci. USA 105, 11536–11542. ( 10.1073/pnas.0802597105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Donoghue MJ. 2008. Colloquium paper: a phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 11549–11555. ( 10.1073/pnas.0801962105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nicholson DB, Holroyd PA, Benson RBJ, Barrett PM. 2015. Climate-mediated diversification of turtles in the Cretaceous. Nat. Commun. 6, 7848. ( 10.1038/ncomms8848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rose PJ, Fox DL, Marcot J, Badgley C.. 2011. Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology 39, 163–166. ( 10.1130/G31099.1) [DOI] [Google Scholar]
- 120. Marcot JD, Fox DL, Niebuhr SR. 2016. Late Cenozoic onset of the latitudinal diversity gradient of North American mammals. Proc. Natl Acad. Sci. USA 113, 7189–7194. ( 10.1073/pnas.1524750113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Archibald SB, Bossert WH, Greenwood DR, Farrell BD.. 2010. Seasonality, the latitudinal gradient of diversity, and eocene insects. Paleobiology 36, 374–398. ( 10.1666/09021.1) [DOI] [Google Scholar]
- 122. Carvalho I de S, de Gasparini ZB, Salgado L, de Vasconcellos FM, Marinho T da S. 2010. Climate’s role in the distribution of the Cretaceous terrestrial Crocodyliformes throughout Gondwana. Palaeogeogr. Palaeoclimatol. Palaeoecol. 297, 252–262. ( 10.1016/j.palaeo.2010.08.003) [DOI] [Google Scholar]
- 123. Mannion PD, Benson RBJ, Carrano MT, Tennant JP, Judd J, Butler RJ. 2015. Climate constrains the evolutionary history and biodiversity of crocodylians. Nat. Commun. 6, 8438. ( 10.1038/ncomms9438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lockley MG, Meyer CA, Hunt AP, Lucas SG. 1994. The distribution of sauropod tracks and trackmakers. Gaia: Revista de Geociencias, Museu Nacional de Historia Natural (Lisbon). GAIA 10, 233–248. http://www.arca.museus.ul.pt/ArcaSite/obj/gaia/MNHNL-0000280-MG-DOC-web.PDF [Google Scholar]
- 125. Mannion PD, Upchurch P. 2010. Completeness metrics and the quality of the sauropodomorph fossil record through geological and historical time. Paleobiology 36, 283–302. ( 10.1666/09008.1) [DOI] [Google Scholar]
- 126. Poropat SF, et al. 2016. New Australian sauropods shed light on Cretaceous dinosaur palaeobiogeography. Sci. Rep. 6, 34467. ( 10.1038/srep34467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Jablonski D, Huang S, Roy K, Valentine JW. 2017. Shaping the latitudinal diversity gradient: new perspectives from a synthesis of paleobiology and biogeography. Am. Nat. 189, 1–12. ( 10.1086/689739) [DOI] [PubMed] [Google Scholar]
- 128. Close RA, Benson RBJ, Upchurch P, Butler RJ. 2017. Controlling for the species–area effect supports constrained long-term Mesozoic terrestrial vertebrate diversification. Nat. Commun. 8, 15381. ( 10.1038/ncomms15381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Maidment SCR, Dean CD, Mansergh RI, Butler RJ. 2021. Deep-time biodiversity patterns and the dinosaurian fossil record of the Late Cretaceous Western Interior, North America. Proc. R. Soc. B 288, 20210692. ( 10.1098/rspb.2021.0692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rees PM, Noto CR, Parrish JM, Parrish JT. 2004. Late Jurassic climates, vegetation, and dinosaur distributions. J. Geol. 112, 643–653. ( 10.1086/424577) [DOI] [Google Scholar]
- 131. Chiarenza AA, Fiorillo AR, Tykoski RS, McCarthy PJ, Flaig PP, Contreras DL. 2020. The first juvenile dromaeosaurid (Dinosauria: Theropoda) from Arctic Alaska. PLoS One 15, e0235078. ( 10.1371/journal.pone.0235078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Godefroit P, Golovneva L, Shchepetov S, Garcia G, Alekseev P. 2009. The last polar dinosaurs: high diversity of latest Cretaceous Arctic dinosaurs in Russia. Nat. Wiss. 96, 495–501. ( 10.1007/s00114-008-0499-0) [DOI] [PubMed] [Google Scholar]
- 133. Amiot R, Golovneva LB, Godefroit P, Goedert J, Garcia G, Lécuyer C, Fourel F, Herman AB, Spicer RA. 2023. High-latitude dinosaur nesting strategies during the latest Cretaceous in north-eastern Russia. Diversity 15, 565. ( 10.3390/d15040565) [DOI] [Google Scholar]
- 134. Darroch SAF, Fraser D, Casey MM. 2021. The preservation potential of terrestrial biogeographic patterns. Proc. Biol. Sci. 288, 20202927. ( 10.1098/rspb.2020.2927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Blackburn TM, Gaston KJ, Loder N. 1999. Geographic gradients in body size: a clarification of Bergmann’s rule. Divers. Distrib. 5, 165–174. ( 10.1046/j.1472-4642.1999.00046.x) [DOI] [Google Scholar]
- 136. Meiri S. 2011. Bergmann’s rule—what’s in a name? Glob. Ecol. Biogeogr. 20, 203–207. ( 10.1111/j.1466-8238.2010.00577.x) [DOI] [Google Scholar]
- 137. Horner JR, Makela R. 1979. Nest of juveniles provides evidence of family structure among dinosaurs. Nature 282, 296–298. ( 10.1038/282296a0) [DOI] [Google Scholar]
- 138. Lambe LM. 1917. A new genus and species of crestless hadrosaur from the Edmonton Formation of Alberta, pp. 65–73, vol. 31. Ottawa: The Ottawa Naturalist. See https://www.biodiversitylibrary.org/item/17603. [Google Scholar]
- 139. Fiorillo AR, Tykoski RS. 2014. A diminutive new tyrannosaur from the top of the world. PLoS One 9, e91287. ( 10.1371/journal.pone.0091287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mori H, Druckenmiller P, Erickson G. 2016. A new Arctic hadrosaurid (Dinosauria: Hadrosauridae) from the Prince Creek Formation (lower Maastrichtian) of northern Alaska. Acta Palaeontol. Pol. 61. ( 10.4202/app.00152.2015) [DOI] [Google Scholar]
- 141. Takasaki R, Fiorillo AR, Tykoski RS, Kobayashi Y. 2020. Re-examination of the cranial osteology of the Arctic Alaskan hadrosaurine with implications for its taxonomic status. PLoS One 15, e0232410. ( 10.1371/journal.pone.0232410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fiorillo AR, Tykoski RS. 2012. A new Maastrichtian species of the centrosaurine ceratopsid Pachyrhinosaurus from the North Slope of Alaska . Acta Palaeontol. Pol. 57, 561–573. ( 10.4202/app.2011.0033) [DOI] [Google Scholar]
- 143. Foster JB. 1964. Evolution of mammals on islands. Nature 202, 234–235. ( 10.1038/202234a0) [DOI] [Google Scholar]
- 144. Hone DWE, Farke AA, Wedel MJ. 2016. Ontogeny and the fossil record: what, if anything, is an adult dinosaur? Biol. Lett. 12, 20150947. ( 10.1098/rsbl.2015.0947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Horner J, Weishampel D, Forster C. 2004. Hadrosauridae. In The Dinosauria, pp. 438–463. Berkeley, CA: University of California Press. ( 10.1525/california/9780520242098.003.0023) [DOI] [Google Scholar]
- 146. Tanaka K, Zelenitsky DK, Therrien F, Kobayashi Y. 2018. Nest substrate reflects incubation style in extant archosaurs with implications for dinosaur nesting habits. Sci. Rep. 8, 3170. ( 10.1038/s41598-018-21386-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Grellet-Tinner G, Fiorelli LE. 2010. A new Argentinean nesting site showing neosauropod dinosaur reproduction in a Cretaceous hydrothermal environment. Nat. Commun. 1, 32. ( 10.1038/ncomms1031) [DOI] [PubMed] [Google Scholar]
- 148. Tucker RT, Roberts EM, Hu Y, Kemp AIS, Salisbury SW. 2013. Detrital zircon age constraints for the Winton Formation, Queensland: contextualizing Australia’s Late Cretaceous dinosaur faunas. Gondwana Res. 24, 767–779. ( 10.1016/j.gr.2012.12.009) [DOI] [Google Scholar]
- 149. Tanabe M, Aoki K, Chiba K, Saneyoshi M, Kodaira S, Nishido H, Mainbayar B, Tsogtbaatar K, Ishigaki S. 2023. Apatite U–Pb dating of dinosaur teeth from the Upper Cretaceous Nemegt Formation in the Gobi Desert, Mongolia: contribution to depositional age constraints. Island Arc 32, e12488. ( 10.1111/iar.12488) [DOI] [Google Scholar]
- 150. Tucker RT, Crowley JL, Mohr MT, Renaut RK, Makovicky PJ, Zanno LE. 2023. Exceptional age constraint on a fossiliferous sedimentary succession preceding the Cretaceous thermal maximum. Geology 51, 962–967. ( 10.1130/G51278.1) [DOI] [Google Scholar]
- 151. Schoene B, Eddy MP, Samperton KM, Keller CB, Keller G, Adatte T, Khadri SFR. 2019. U–Pb constraints on pulsed eruption of the Deccan Traps across the end-Cretaceous mass extinction. Science 363, 862–866. ( 10.1126/science.aau2422) [DOI] [PubMed] [Google Scholar]
- 152. Tucker RT, Suarez CA, Makovicky PJ, Zanno LE. 2022. Paralic sedimentology of the Mussentuchit Member coastal plain, Cedar Mountain Formation, central Utah, USA. J. Sediment. Res. 92, 546–569. ( 10.2110/jsr.2021.028) [DOI] [Google Scholar]
- 153. Walker K, Bambach R. 1971. The significance of fossil assemblages from fine-grained sediments: time-averaged communities. Geol. Soc. Am. Abstr. with Prog. 3, 783–784. [Google Scholar]
- 154. Paterna A, Cau A. 2023. New giant theropod material from the Kem Kem Compound Assemblage (Morocco) with implications on the diversity of the mid-Cretaceous carcharodontosaurids from North Africa. Hist. Biol. 35, 2036–2044. ( 10.1080/08912963.2022.2131406) [DOI] [Google Scholar]
- 155. Fanti F, Cau A, Martinelli A, Contessi M. 2014. Integrating palaeoecology and morphology in theropod diversity estimation: a case from the Aptian-Albian of Tunisia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 410, 39–57. ( 10.1016/j.palaeo.2014.05.033) [DOI] [Google Scholar]
- 156. Tucker RT, Roberts EM, Darlington V, Salisbury SW. 2017. Investigating the stratigraphy and palaeoenvironments for a suite of newly discovered mid-Cretaceous vertebrate fossil-localities in the Winton Formation, Queensland, Australia. Sediment. Geol. 358, 210–229. ( 10.1016/j.sedgeo.2017.05.004) [DOI] [Google Scholar]
- 157. Schroeder KM, Lyons SK, Smith FA. 2021. The influence of juvenile dinosaurs on community structure and diversity. Science 371, 941–944. ( 10.1126/science.abd9220) [DOI] [PubMed] [Google Scholar]
- 158. Benson RBJ, Brown CM, Campione NE, Cullen TM, Evans DC, Zanno LE. 2022. Comment on 'The influence of juvenile dinosaurs on community structure and diversity.' Science 375, eabj5976. ( 10.1126/science.abj5976) [DOI] [PubMed] [Google Scholar]
- 159. Holtz TR. 2021. Theropod guild structure and the tyrannosaurid niche assimilation hypothesis: implications for predatory dinosaur macroecology and ontogeny in later Late Cretaceous Asiamerica . Can. J. Earth Sci. 58, 778–795. ( 10.1139/cjes-2020-0174) [DOI] [Google Scholar]
- 160. Butler RJ, Benson RBJ, Carrano MT, Mannion PD, Upchurch P. 2011. Sea level, dinosaur diversity and sampling biases: investigating the ‘common cause’ hypothesis in the terrestrial realm. Proc. R. Soc. B 278, 1165–1170. ( 10.1098/rspb.2010.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dean CD, Chiarenza AA, Maidment SCR. 2020. Formation binning: a new method for increased temporal resolution in regional studies, applied to the Late Cretaceous dinosaur fossil record of North America. Palaeontology 63, 881–901. ( 10.1111/pala.12492) [DOI] [Google Scholar]
- 162. Zhong SH, Liu Y, Li SZ, Bindeman IN, Cawood PA, Seltmann R, Niu JH, Guo GH, Liu JQ. 2023. A machine learning method for distinguishing detrital zircon provenance. Contrib. Miner. Petrol. 178. ( 10.1007/s00410-023-02017-9) [DOI] [Google Scholar]
- 163. Lallensack JN, Romilio A, Falkingham PL. 2022. A machine learning approach for the discrimination of theropod and ornithischian dinosaur tracks. J. R. Soc. Interface 19, 20220588. ( 10.1098/rsif.2022.0588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wills S, Underwood CJ, Barrett PM. 2023. Machine learning confirms new records of maniraptoran theropods in Middle Jurassic UK microvertebrate faunas . Pap. Palaeontol. 9, e1487. ( 10.1002/spp2.1487) [DOI] [Google Scholar]
- 165. Morueta‐Holme N, et al. 2016. A network approach for inferring species associations from co‐occurrence data. Ecography 39, 1139–1150. ( 10.1111/ecog.01892) [DOI] [Google Scholar]
- 166. Banner KM, Irvine KM, Rodhouse TJ. 2020. The use of Bayesian priors in ecology: the good, the bad and the not great. Methods Ecol. Evol. 11, 882–889. ( 10.1111/2041-210X.13407) [DOI] [Google Scholar]
- 167. Burgener L, Hyland E, Reich BJ, Scotese C. 2023. Cretaceous climates: mapping paleo-Köppen climatic zones using a Bayesian statistical analysis of lithologic, paleontologic, and geochemical proxies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 613, 111373. ( 10.1016/j.palaeo.2022.111373) [DOI] [Google Scholar]
- 168. Uyeda JC, Harmon LJ. 2014. A novel Bayesian method for inferring and interpreting the dynamics of adaptive landscapes from phylogenetic comparative data. Syst. Biol. 63, 902–918. ( 10.1093/sysbio/syu057) [DOI] [PubMed] [Google Scholar]
- 169. Cooper RB, Allen BJ, Silvestro D. 2024. DeepDiveR—a software for deep learning estimation of palaeodiversity from fossil occurrences. bioRxiv. ( 10.1101/2024.09.03.610960) [DOI]
- 170. Chiarenza AA, Farnsworth A, Mannion PD, Lunt DJ, Valdes PJ, Morgan JV, Allison PA. 2020. Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction. Proc. Natl Acad. Sci. USA 117, 17084–17093. ( 10.1073/pnas.2006087117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Schindelz DE. 1980. Microstratigraphic sampling and the limits of paleontologic resolution. Paleobiology 6, 408–426. ( 10.1017/S0094837300003596) [DOI] [Google Scholar]
- 172. Signor PW. 1982. Species richness in the Phanerozoic: compensating for sampling bias. Geology 10, 625. () [DOI] [Google Scholar]
- 173. Foote M, Sepkoski JJ. 1999. Absolute measures of the completeness of the fossil record. Nature 398, 415–417. ( 10.1038/18872) [DOI] [PubMed] [Google Scholar]
- 174. Alroy J. 2001. Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc. Natl Acad. Sci. USA 98, 6261–6266. ( 10.1073/pnas.111144698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Raup DM. 1972. Taxonomic diversity during the Phanerozoic. Science 177, 1065–1071. ( 10.1126/science.177.4054.1065) [DOI] [PubMed] [Google Scholar]
- 176. McGowan AJ, Smith AB. 2008. Are global Phanerozoic marine diversity curves truly global? A study of the relationship between regional rock records and global Phanerozoic marine diversity. Paleobiology 34, 80–103. ( 10.1666/07019.1) [DOI] [Google Scholar]
- 177. Kalmar A, Currie DJ. 2010. The completeness of the continental fossil record and its impact on patterns of diversification. Paleobiology 36, 51–60. ( 10.1666/0094-8373-36.1.51) [DOI] [Google Scholar]
- 178. Alroy J. 2010. Geographical, environmental and intrinsic biotic controls on Phanerozoic marine diversification. Palaeontology 53, 1211–1235. ( 10.1111/j.1475-4983.2010.01011.x) [DOI] [Google Scholar]
- 179. Alroy J. 2010. Fair sampling of taxonomic richness and unbiased estimation of origination and extinction rates. Paleontol. Soc. Pap. 16, 55–80. ( 10.1017/S1089332600001819) [DOI] [Google Scholar]
- 180. Dunhill AM. 2012. Problems with using rock outcrop area as a paleontological sampling proxy: rock outcrop and exposure area compared with coastal proximity, topography, land use, and lithology. Paleobiology 38, 126–143. ( 10.1666/10062.1) [DOI] [Google Scholar]
- 181. Barrett PM, McGowan AJ, Page V. 2009. Dinosaur diversity and the rock record. Proc. R. Soc. B 276, 2667–2674. ( 10.1098/rspb.2009.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Holland S, Loughney KM. 2021. The stratigraphic paleobiology of nonmarine systems. Cambridge, UK: Cambridge University Press. ( 10.1017/9781108881869) [DOI] [Google Scholar]
- 183. Jones LA, et al. 2023. Palaeoverse: a community‐driven R package to support palaeobiological analysis. Methods Ecol. Evol. 14, 2205–2215. ( 10.1111/2041-210X.14099) [DOI] [Google Scholar]
- 184. Holland SM. 1995. The stratigraphic distribution of fossils. Paleobiology 21, 92–109. ( 10.1017/S0094837300013099) [DOI] [Google Scholar]
- 185. Holland SM. 2022. The structure of the nonmarine fossil record: predictions from a coupled stratigraphic–paleoecological model of a coastal basin. Paleobiology 48, 372–396. ( 10.1017/pab.2022.5) [DOI] [Google Scholar]
- 186. Griffin CT, Wynd BM, Munyikwa D, Broderick TJ, Zondo M, Tolan S, Langer MC, Nesbitt SJ, Taruvinga HR. 2022. Africa’s oldest dinosaurs reveal early suppression of dinosaur distribution. Nature 609, 313–319. ( 10.1038/s41586-022-05133-x) [DOI] [PubMed] [Google Scholar]
- 187. Dunne EM, Farnsworth A, Benson RBJ, Godoy PL, Greene SE, Valdes PJ, Lunt DJ, Butler RJ. 2023. Climatic controls on the ecological ascendancy of dinosaurs. Curr. Biol. 33, 206–214.( 10.1016/j.cub.2022.11.064) [DOI] [PubMed] [Google Scholar]
- 188. Nanglu K, Cullen TM. 2023. Across space and time: a review of sampling, preservational, analytical, and anthropogenic biases in fossil data across macroecological scales. Earth Sci. Rev. 244, 104537. ( 10.1016/j.earscirev.2023.104537) [DOI] [Google Scholar]
- 189. Mitchell JS, Roopnarine PD, Angielczyk KD. 2012. Late Cretaceous restructuring of terrestrial communities facilitated the end-Cretaceous mass extinction in North America. Proc. Natl Acad. Sci. USA 109, 18857–18861. ( 10.1073/pnas.1202196109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Cullen TM, Evans DC. 2016. Palaeoenvironmental drivers of vertebrate community composition in the Belly River Group (Campanian) of Alberta, Canada, with implications for dinosaur biogeography. BMC Ecol. 16, 52. ( 10.1186/s12898-016-0106-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Whiteside JH, Lindström S, Irmis RB, Glasspool IJ, Schaller MF, Dunlavey M, Nesbitt SJ, Smith ND, Turner AH. 2015. Extreme ecosystem instability suppressed tropical dinosaur dominance for 30 million years. Proc. Natl Acad. Sci. USA 112, 7909–7913. ( 10.1073/pnas.1505252112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chiarenza AA. 2024. Data from: The macroecology of Mesozoic dinosaurs. Figshare. ( 10.6084/m9.figshare.c.7506350) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.
Supplementary material is available online [192].