Abstract
Inflammatory dermatologic diseases have long been viewed as a “skin limited” disease process. Current literature on inflammatory dermatologic diseases investigates their relationship and influence on thromboembolic states and thromboembolic complications and the understanding of their pathophysiology and molecular mechanisms.
Studies specifically discuss known inflammatory skin diseases including alopecia areata, vitiligo, psoriasis, hidradenitis suppurativa, atopic dermatitis, chronic spontaneous urticaria, and autoimmune bullous diseases, and their effects on systemic inflammation, associated cardiovascular comorbidities, and thromboembolic or hypercoagulable states. The limited current literature shows potential for links between inflammatory skin diseases and hypercoagulable states. Biomarkers such as F1 + 2, D-dimer, eosinophilic cationic protein, and PAI-1 are currently being studied to outline the mechanisms connecting inflammatory skin disease to the coagulation system. Further study and larger amounts of data are needed to draw definitive conclusions, especially when interpreting biomarkers alone such as PAI-1.
The mechanisms, rates of systemic inflammation, and clinical outcomes of traditionally “skin limited” inflammatory diseases remain chronically understudied in dermatology. Many organ systems have well established connections between inflammatory disease and hypercoagulable states, but there are significant gaps in the literature regarding skin diseases. There is a significant need for comprehensive investigation of molecular mechanisms behind inflammatory dermatologic disease and hypercoagulability, how hypercoagulability effects clinical outcomes, and proper intervention to optimize patient outcomes.
Keywords: Coagulation; Hypercoagulable; Prothrombin; D-Dimer; Bullous Pemphigoid, Pemphigus; Dermatitis Herpetiformis; Alopecia Areata; Psoriasis; Vitiligo; Hidradenitis Suppurativa; Atopic dermatitis; Chronic spontaneous Urticaria
Background
The immune system, inflammatory processes, coagulation and fibrinolysis systems of the human body are intricately linked [1–7]. Thus, aberrancies in one of these systems can have significant downstream effects on the others, generating a complex feedback loop to cause a pathologic state. For instance, autoimmune disease affecting the skin can involve dysregulation of pro-inflammatory cytokines which, in turn, lead to expression of initiators of coagulation [8]. Moreover, coagulation activation with generation of thrombin is closely linked to post-translational activation cleavages of inflammatory cytokines like IL-1α [9] and interleukin-8 [10], resulting in a pro-inflammatory environment. In addition, fibrinopeptide B from cleavage of fibrinogen by thrombin is known to be chemotactic for both neutrophils and monocytes to attract inflammatory responses to areas of coagulation activation [11]. Similarly, activation of the fibrinolysis system generates the terminal protease plasmin [12], which has been shown to cleave a pro-form of interleukin-8 secreted by human dermal fibroblasts to generate an active form an interleukin-8 [13] that is potent for neutrophil signaling and activation.
Systemic inflammation has been associated with increased risk of thromboembolic events. Even traditionally “skin-limited” inflammatory diseases have increasingly been reported to be associated with hypercoagulability. This area remains largely under-explored in dermatology. This review aims to summarize current knowledge of common dermatologic diseases and association with cardiovascular comorbidities and thromboembolic states, what is known about the underlying pathophysiologic and molecular mechanisms, and the knowledge gaps in need of future investigation.
Alopecia areata
Alopecia Areata (AA) is an autoimmune disease that leads to non-scarring hair loss via infiltrate of lymphocyte cell-mediated inflammation of the hair bulb (Fig. 1A) [14]. Proinflammatory cytokines play an important role in the development of metabolic and cardiovascular complications [15]. AA has largely been considered a local disease limited to the hair follicles, however emerging evidence has supported involvement of systemic inflammation and an association with cardiovascular disease. In a study assessing the association of immune and cardiovascular biomarkers in patients with moderate to severe AA, patients with AA demonstrated elevation in general systemic immune markers (MMP12, Th1, Th2, and Th17 related) as well as atherosclerosis-associated (MMP9/CCL2) and atherosclerosis-related (IL1RN/CD40/CXCL9) biomarkers compared to controls, further pointing to an increased association between systemic involvement and risk for cardiovascular disease [16]. Interestingly, both MMP-12 (aka macrophage metalloelastase) and MMP-9 are known to cleave plasminogen to angiostatin [17, 18], where angiostatin is an antifibrinolytic protein that inhibits fibrinolysis by competing for lysine binding sites on the surface of fibrin and thus may generate an antifibrinolytic/prothrombotic state [5]. Further studies have demonstrated a significant increased level of plasma cardiac troponin in patients with AA in the absence of clinical heart disease, which may suggest higher levels of cardiac remodeling and future risk for cardiovascular disease [19, 20]. Other plasma serum markers have been explored, with higher levels of D-dimer reported in AA patients compared to controls (398.45 ± 300 vs. 244.4 ± 129.92pg/ml, p < 0.001), although plasma fibrinogen and C-reactive protein (CRP) did not differ between groups and is consistent with previous studies [19, 21].
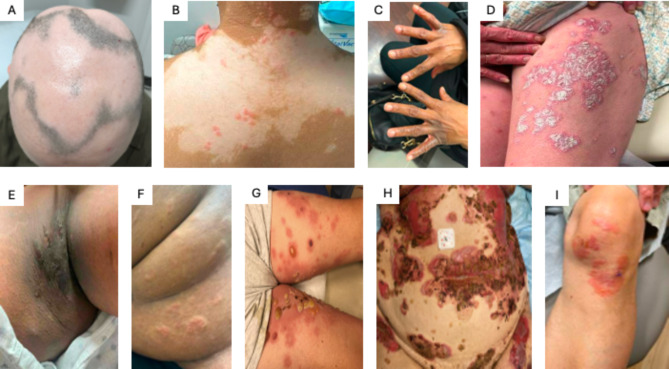
Fig. 1.
Skin Manifestations of Inflammatory Dermatologic Disease. (A) Alopecia, (B) Vitiligo, (C) Atopic Dermatitis, (D) Psoriasis, (E) Hidradenitis Suppuritiva, (F) Chronic Spontaneous Urticaria, (G) Bullous Pemphigoid, (H) Pemphigus Vulgaris, (I) Dermatitis Herpetiformis
Due to the low specificity and positive predictive value of D-dimer, other markers of venous thromboembolism in patients with AA have also been studied. Increases in soluble fibrin monomer complex (SFMC) and F1 + 2 were reported in a small case control study of 51 patients with AA, which may be associated with increased risk of VTE [22]. In addition, patients with AA have been reported to have a higher prevalence of endothelial dysfunction compared to healthy controls (42% vs. 12%; p = 0.03) [23]. This is critical, as the endothelium plays a major role in regulation of coagulation through its endothelial protein C receptor [24], thrombomodulin [25], heparan-rich surface glycocalyx [26], and also in fibrinolysis through its pre-stored tPA within Waibel-Palade bodies that gets released in response to ischemia [27, 28] and PAI-1 generation in response to inflammation [29]. Further studies have demonstrated a dose-response relationship with vascular stiffness and AA severity (beta = 0.033; 95% CI 0.009–0.057, p = 0.04), which is consistent with alterations in vascular integrity potentially increasing the risk for thrombosis based on the mechanisms described above [30].
Multiple cohort and population-based studies have demonstrated association of AA with metabolic syndrome and cardiovascular disease [22, 30–34]. In one of the only studies to explore the genomic relationship between AA and myocardial infarction (MI), O’Hagan et al. identified an association between the lysophospholipase activity pathway (gene ratio = 0.05, p = 0.006) and MHC protein binding (gene ratio = 0.04, p = 0.008), which has been linked to systemic inflammation and may further lead to increased risk of MI [34]. However, in Korea, a 1:5 case control study of 3,770 patients reported no significant differences in incidence of cardiac disease (heart failure, angina pectoris, acute MI, and chronic MI) in patients with AA compared to controls and concluded that AA was not related to an increased risk of MI [35]. This was further confirmed in a larger population-based study of 228,886 patients with AA that were matched with healthy controls and for 12 years, and further found no difference in MI incidence rates at the beginning of the study (HR 0.35; 95% CI 0.27–0.46) [32]. After 8–10 years, however, patients with AA showed an exponential increase of MI rate and experienced an increased risk of MI compared to the controls (HR 4.5; 95% CI 3.65–5.58) [32]. In a similar study assessing VTE in 30,418 patients with AA, no significant increase was observed over a 13-year follow-up period (HR 0.97; 95% CI 0.65–1.46) [36]. Furthermore, Huang et al. reported a protective effect for developing stroke (OR 0.39; 95% CI 0.18–0.97) in patients with AA, however, did not take time into consideration and only compared controls to data that was within 3 years of AA patient’s diagnosis [37].
While some results are conflicting, studies have reported an association between AA and markers for cardiovascular risk, particularly increased risk of myocardial dysfunction and infarction dependent on time, and further signify the importance of monitoring cardiovascular health in this population.
Vitiligo
Several pathogeneses of vitiligo have been proposed including genetic, autoimmune, and oxidative induced melanocyte destruction (Fig. 1B) [38]. Segmental vitiligo, the most common form, has previously been associated with increased risk of systemic disease, specifically metabolic syndrome [39, 40]. Although patients with metabolic syndrome have an increased risk of cerebral and cardiovascular events, there has not been a clear association between hypercoagulability and vitiligo. In a small case-control study, patients with vitiligo presented with a higher mean carotid intima media thickness (p < 0.001; OR 4.8, CI 1.8–12.8) and were more likely to have an atherosclerotic plaque (p = 0.006; OR 4.2, CI 1.4–12.7), which may indicate an increased cardiovascular risk [41]. Furthermore, the authors observed higher levels of serum markers for oxidative stress and a positive correlation between carotid intima media thickness and malondialdehyde, which may contribute to the development of atherosclerosis and increased cardiovascular risk.
In contrast, multiple studies have reported no association between cardiovascular risk and vitiligo, with some suggesting patients with vitiligo have a decreased risk [36, 42, 43]. In a study assessing metabolic syndrome, patients with vitiligo had significantly lower abdominal circumference and triglycerides (p < 0.001), while no other differences were observed compared to controls [42]. Furthermore, a large population-based study of 20,851 patients revealed those with vitiligo had decreased risk of peripheral vascular disease (HR, 0.98; 95% CI, 0.86–1.10) and pulmonary embolism (HR, 0.55; 95% CI, 0.36–0.86) in addition to similar risk of MI and cerebrovascular accident compared to the controls [43]. These findings were similar to Schneeweiss et al., where no increased risk of VTE was reported in vitiligo patients compared to controls (HR, 0.90; 95% CI, 0.49–1.65 vs. HR, 0.94; 95% CI, 0.84–1.05) [36].
Ultimately, given the conflicting results of the limited available studies evaluating vitiligo, cardiovascular risk and changes in coagulation/thrombosis, further studies are needed to understand whether vitiligo leads to a hypercoagulable state.
Atopic dermatitis
The etiology behind atopic dermatitis (AD) is considered multifactorial and thought to include a combination of genetic predisposition, immune dysregulation, and environmental factors leading to epidermal barrier dysfunction (Fig. 1C) [44]. Mild AD is described as ICD-10 code (L209) for AD and the prescription of topical corticosteroids or calcineurin inhibitors or ICD-10 code L209 with more than 3 claims during the same year and moderate to severe AD is defined as ICD-10 code L209 and the prescription of systemic oral medications for AD or phototherapies with more than 12 claims during the same year [45]. While primarily a dermatologic concern, a potential association between AD and hypercoagulable states has garnered increasing attention in recent years [46–52]. In a cohort study by Merola et al. examining thrombotic events in 2,061,222 patients with immune-mediated inflammatory diseases, adults with AD were found to exhibit a slightly increased risk of VTE compared to non-AD matched individuals (aHR 1.09; 95% CI 1.06–1.12) [46]. After excluding patients with underlying VTE risk factors in their study, AD was no longer significantly associated with increased VTE risk. This potential VTE risk extended to several other thrombosis-related complications in a study by Chen et al., where a cohort of 1,066 AD adults demonstrated significantly increased risk of DVT alone (HR 1.26; 95% CI 1.14–1.40) and PE alone (HR 1.30; 95% CI 1.08–1.57) in addition to overall VTE (1.28; 95% CI 1.17–1.40) [47]. Similarly, a cohort study by Warren et al.so reported an increased risk of overall VTE (aHR 1.17; 95% CI 1.12–1.22) and DVT alone (aHR 1.30; 95% CI 1.23–1.37) in AD patients; however, there was no significant increased risk for PE in AD patients (aHR 0.94; 95% CI 0.87–1.02) [48].
Investigations into MI and stroke risk in AD patients have revealed conflicting evidence, particularly for those diagnosed with moderate-to-severe AD. A retrospective cohort study by Woo et al. revealed that patients with AD ranging from mild-to-severe demonstrated a significantly increased risk of MI compared to non-AD controls (aHR 1.111; 95% CI 1.050–1.176) [45]. However, after adjusting for potential confounders, only those with moderate-to-severe AD continued to exhibit a significantly increased risk of MI (aHR 1.163; 95% CI 1.080–1.251) [45]. In another study by Silverberg et al., AD individuals were at significantly higher odds of having an MI (aOR 2.59; 95% CI 1.35–4.96) as well as stroke (aOR 1.61; 95% CI 1.27–2.05) [53]. In contrast, several studies have noted no significant increase in risk for MI and stroke in AD patients [49, 50]. A cross-sectional analysis of 259,119 participants by Drucker et al. not only found no increased risk of MI or stroke in AD patients, but additionally found a paradoxical reduction in odds of both MI (aOR 0.87; 95% CI 0.75-1.00) and stroke (aOR 0.79; 95% CI 0.66–0.95) in AD patients [49].
Despite these conflicting findings, several studies have examined coagulation properties within individuals with AD and note key differences that may potentially be contributing to a hypercoagulable state [51, 52]. In a study by Nastalek et al. examining properties of fibrin clots in AD individuals, patients with AD exhibited hypercoagulable alterations in fibrin clot characteristics when compared to matched controls [51]. These included a lower clot permeability, increased fiber thickness, accelerated clot formation, and higher maximum D-dimer levels released from clots, underscoring an increased clot mass. Another study by Tamagawa-Mineoka et al. revealed significantly elevated levels of β-Thromboglobulin and Platelet Factor 4 in patients with AD, which are both platelet chemokines released following platelet activation [52].
Overall, studies have found a no clear difference in risk for VTE and PE with conflicting evidence on MI, which highlights the need for further research investigating the complex interplay between AD, thrombotic events, and molecular mechanisms of hypercoagulability in AD.
Psoriasis
Psoriasis is a T-cell mediated inflammatory skin disease, which most frequently presents as well-demarcated salmon-colored plaques with overlying silvery scales (Fig. 1D) [54]. The acknowledgment of psoriasis as a systemic inflammatory disorder has drawn significant attention to cardiovascular health, and there are several plausible pathogenic mechanisms that may predispose patients with psoriasis to hypercoagulable states and subsequent thromboembolic events. Monocytes, for example, are known to play a significant role in the prothrombotic state of other non-dermatologic autoimmune inflammatory diseases [55], and in an in vivo murine model of psoriasis it was found that mice with psoriasis developed spontaneous aortic inflammatory changes that were monocyte-dependent and were hypercoagulable with rapid carotid artery thrombotic occlusion in response to a photochemical arterial injury relative to controls [56]. Further, in both human patients [57] and animal models [56] of psoriasis it is known that multiple cytokines are elevated, including MCP-1, which induces pro-coagulant tissue factor expression in aortic smooth muscle cells [58] and circulating blood monocytes [59]. In addition, monocytes have been recognized as being highly adherent to human dermal microvascular endothelium in an in vitro model of chronic psoriatic skin inflammation [60], and blood monocytes have also been found to be present histologically in the papillary dermal perivasculature in psoriasis patient biopsies [60]. In addition to leukocyte involvement in the potential pro-thrombotic mechanisms in psoriasis, platelets may also play a role, where a randomized controlled trial established increased platelet activation in psoriasis patients compared to controls that induced endothelial cell proinflammatory responses and upregulation of COX-1, which was significantly improved by treatment with low-dose aspirin [61]. Other studies have found a similar role for platelet activation in psoriasis, measured as plasma levels of b-thromboglobulin and platelet factor 4 via enzyme-linked immunoassay over 25 and 9 ng/mL respectively, where platelet activation was implicated in disease severity suggestive of a thromboinflammatory state as they normalize with symptoms following treatment [52].
Beyond the myriad of potential molecular mechanisms of hypercoagulability in psoriasis, several clinical studies have found increased risks of thromboembolic events in this patient population. Xu et al. reported a 20% increase in the association of psoriasis with MI (RR 1.22; 95% CI 1.05–1.42) and stroke (RR 1.21; 95% CI 1.04–1.4) [62]. A meta-analysis evaluating the association between psoriasis and risk of venous thromboembolism (VTE), deep venous thrombosis (DVT) and pulmonary embolism (PE) demonstrated an overall 1.46-fold increased risk compared to controls from 4 articles included (pHR 1.46; 95% CI 1.29–1.66) [63]. A larger, more recent meta-analysis published in 2022 that included 13 articles assessing the association of psoriasis with VTE and peripheral vascular disease demonstrated a significant increased risk for VTE (pooled HR, 1.26; 95% CI, 1.08–1.48) and peripheral vascular disease (pooled HR, 1.27; 95% CI, 1.16–1.40) among patients with psoriasis when compared to controls [64]. In an epidemiological study from Harvard using a United States database, patients with psoriasis were found to have an increased risk of developing ischemic heart disease (HR 1.90; 95% CI 1.18–3.05) and MI (HR 2.24; 95% CI 1.27–3.95) when adjusting for cardiovascular (CV) risk factors [65]. In addition, after adjusting for known CV risk factors in a large nationwide population-based study in Taiwan, patients with psoriasis had a 2 time greater risk for MI when compared to controls (HR 2.10; 95% CI 1.27–3.43) [66]. However, there is at least one large study with conflicting data, where a study of 48,523 patients with psoriasis in the United Kingdom showed that patients with psoriasis had more cardiovascular disease (CVD) risk factors and an elevated risk of major CV events, including MI, acute coronary syndrome, unstable angina, and stroke (HR 1.10; 95% CI 1.04–1.17) [67], but after controlling for CV risk factors on multivariate analysis, psoriasis was not an independent risk factor for major CV events [67]. This study differs in that it considers inflammatory arthritis an additional controlled risk factor as they reported that the risk of a major CV event was found to be 36% higher in those with psoriasis and an inflammatory arthritis compared to those who did not [67].
Examining the impact of treatment of psoriasis on CVD may also be illustrative. A meta-analysis of five clinical trials demonstrated that those receiving tumor necrosis factor (TNF)-inhibitor therapy had a significant decrease in risk of CV adverse events ((RR, 0.58; 95% CI 0.43–0.77; P < 0.001), a decrease in MI compared to those receiving methotrexate (MTX) (RR 0.65; 95% CI 0.48–0.89; p = 0.007) or topical or photo treatment (RR 0.73; 95% CI 0.59–0.90; P < 0.001), as well as a trend in reduced mortality rate compared to other therapies (RR, 0.90; 95% CI 0.54–1.50; P = 0.68) [68]. In another large observational study comparing MTX to TNF-inhibitor therapy in psoriasis patients, there was a significant decreased risk of cardiovascular events in the TNF-inhibitor group (HR 0.55; 95% CI 0.45–0.67) [69]. In a retrospective cohort study, risk of MI was decreased by 50% (HR 0.50; 95% CI 0.32–0.79) with approximately four years of a TNF-inhibitor, and reduced by 46% (HR 0.54; 95% CI 0.38–0.77) compared with other oral, topical, or phototherapy [70]. These protective effects were even more pronounced in patients older than 60 [70]. It is also noteworthy that treatment of psoriasis with biologic agents is known to significantly reduce MCP-1 levels, where (as above) MCP-1 is a known pro-coagulant cytokine that is elevated in patients with psoriasis [57].
While some conflicting reports of the association between psoriasis and CVD/thromboembolic disease exist, the studies described above support further investigation into a potential increased risk among patients with psoriasis. As a result, the American Academy of Dermatology and the National Psoriasis Foundation released evidence-based guidelines pertaining to the management and treatment of psoriasis with special attention to comorbidities and outline the role of a dermatologist in educating and screening patients with psoriasis for cardiovascular-associated disease, but did not specifically prioritize one treatment such as TNF-inhibitory therapy above other therapies [71]. Further studies are needed to establish the exact mechanisms of hypercoagulability in psoriasis and to establish the impact of various treatments on risk reduction.
Hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a non-infectious auto-inflammatory process causing recurrent painful nodules and scarring sinus tracts (Fig. 1E). While the etiology of HS is unclear, its pathogenesis is generally thought to involve a sequence of follicular hyperkeratosis followed by involuting pilosebaceous units, with subsequent inflammation resulting in expansion and tunnel formation [72]. HS is known to be highly comorbid with conditions such as metabolic syndrome, smoking, and diabetes mellitus type II, leading to poor cardiovascular health [72]. Meta-analyses of cardiovascular outcomes in HS reveal significantly increased risks of coronary artery disease (RR 1.38, 95% CI, 1.21–1.58;) and stroke (OR 1.74, 95% CI 1.45–2.09; p < 0.00001); while a population-based cohort study found increased risk of MI (aHR 1.33; 95% CI 1.04–1.68; P = 0.021) in HS [73–75].
The burden of systemic inflammation coupled with the above identified comorbidities in HS likely produce a synergistic effect, and would plausibly create a hypercoagulable state, but the literature is quite limited in this regard with significant knowledge gaps. Biomarkers indicative of systemic inflammation such as ESR [76] and CRP [77] are elevated in patients with HS, and we know from the JUPITER trial of 17,802 relatively healthy patients found to have incidental mild elevations in their CRP that they have an increased mortality from thromboembolic-related events that can be mitigated with statin therapy [78]. In a homogenous European study of 462 HS patients, there were no significant differences in levels of platelets, mean platelet volume, INR, or aPTT found [79], however these are not functional coagulation studies like more modern viscoelastic assays and are well-known to be insensitive in detecting hypercoagulable states.
While the literature suggests an increased risk of MI, the literature currently does not support the association of HS with increased venous thromboembolic events compared to healthy control populations [36].
Chronic spontaneous urticaria
Chronic spontaneous urticaria (CSU), a condition characterized by recurrent episodes of pruritic, swollen welts or hives persisting for more than six weeks, presents a multifaceted challenge in clinical management (Fig. 1F) [80]. Beyond its evident impact on skin health in patients, emerging research suggests a potential association between chronic urticaria and a hypercoagulable state. Patients with CSU have been found to have significant elevations in both D-dimer and activated Factor VII that fall dramatically after disease remission is achieved [81]. CSU patients have also been found to have an increased maximum thrombin activity, total thrombin generation, and velocity of the propagation phase of thrombin generation when compared to matched controls [82], and in a study by Takeda et al. demonstrated an increased coagulation potential using aPTT clot waveform analysis as well as having significantly increased levels of fibrinogen, D-dimer, fibrin and fibrinogen degradation products, and positive rates of soluble fibrin monomer complexes [83]. In addition, Asero and colleagues reported increases in both F1 + 2 and D-dimer levels in the plasma of CSU patients compared to controls [84]. Taken together, these findings are highly suggestive of CSU being a hypercoagulable state. However, despite these reports of increased molecular and functional signals of hypercoagulability, a study by Egeberg et al. reported no increased risk of CV events in CSU patients [85]. In their study of 1,215 CSU patients and 66,203 matched controls, they observed no significantly increased risk of MI (aHR 1.18; 95% CI 0.79–1.76), ischemic stroke (aHR 1.03; 95% CI 0.70–1.52), CV death (aHR 0.67; 95% CI 0.39–1.17), or major adverse CV events (MACE; a composite of MI, ischemic stroke, and CV death), (aHR 1.09; 95% CI 0.83–1.42). However, as is true for the other diseases discussed previously, CV events are not always related to hypercoagulability and caution should be taken in interpreting this isolated report as evidence against a prothrombotic state in CSU. Further research is required to better understand the relationship between CSU and coagulation.
Autoimmune bullous disease
Bullous pemphigoid
Autoimmune bullous disease (AIBD) is primarily driven by the activation of the adaptive immune system and is associated with perturbations in the coagulation and fibrinolytic systems (Fig. 1G) [8]. Bullous pemphigoid (BP), the most common subepidermal autoimmune bullous disease, has previously been associated with hypercoagulable states [86]. BP is caused by abnormal humoral and cellular immune response to the hemidesmosomal proteins, BP 180 and BP230 [87]. BP can be associated with significant morbidity [87]. The pathophysiology of BP is thought to involve imbalances between autoreactive T helper (Th) cells and T regulatory (Treg) cells in addition to activation of the toll-like-receptor (TLR) system independent of T-cells leading to autoantibody production via B cell stimulation [88–90]. Separate from these pathophysiologic mechanisms, a Th2 mediated cellular response due to inflammation caused by humoral hyperactivation leads to neutrophils and eosinophils releasing inflammatory cytokines [91].
There have been numerous studies showing evidence of a pathophysiologic link between BP and the coagulation system. Prothrombin fragment 1 + 2 (F1 + 2), an activation peptide involved in the process of converting prothrombin to thrombin, and D-dimer, a fibrin degradation product, have consistently been found to be elevated in patients with BP when compared to healthy controls [86, 92–95]. Of note, both F1 + 2 and D-dimer have been found to suggest a high risk of thrombosis, with one study finding both can independently predict occurrence of VTE in certain populations [96]. Levels of these markers, when compared to healthy controls, have not only been found to be elevated in skin biopsy specimens, but also in plasma and blister fluid samples of BP patients [86, 92–94]. One study by Wang et al. compared plasma levels of F1 + 2 and D-Dimer in BP patients to Herpes Zoster patients, finding BP patients had relatively higher levels of these thrombotic markers [95]. Additionally, this study found positive correlations between these two products and anti-BP180 IgG and eosinophil counts, markers of disease severity in BP [86, 95]. Remission of disease in BP patients also has been found to mirror decreases in F1 + 2 and D-Dimer [92, 95].
Eosinophils, which play an important role in the pathophysiology of BP, may also contribute to a hypercoagulable state. Eosinophilic cationic protein levels have been found to be markedly elevated in BP blister fluid and to correlate with levels of F1 + 2 and D-Dimer [97]. Tissue factor, an initiator of the extrinsic pathway of coagulation, is primarily distributed intravascularly through eosinophils [98]. Thus, while also contributing to the local inflammatory milieu, eosinophils may promote an intravascular procoagulant state in BP. One study performed by Marzano et al. found blood and tissue eosinophil levels paralleled plasma and blister fluid levels of F1 + 2 and D-Dimer in BP patients [93]. Additionally, this same study found BP patients exhibited tissue factor reactivity immunohistochemically as opposed to no reactivity in normal skin samples [93]. Furthermore, tissue factor was found to originate from eosinophils using colocalization studies [93]. Additional studies have also found tissue factor reactivity in BP patients, but not healthy controls [86, 92, 94, 99]. Finally, mean platelet volume, which may have utility in predicting coagulation events, has been found to be both related to eosinophilia and elevated in BP patients [100, 101].
A case for alterations in the fibrinolytic system as the molecular basis for hypercoagulability in BP can also be made but requires additional study. In states of health and homeostasis, fibrinolysis (the process of breaking down blood clots) happens endogenously in a continuous manner to prevent small clots throughout the body from becoming larger pathologic thromboemboli [102]. Of particular interest in the fibrinolysis pathway is plasminogen activator inhibitor-1 (PAI-1), an acute phase response protein [103] that is the primary endogenous inhibitor of plasminogen activators, as elevations in PAI-1 are known to result in resistance to endogenous fibrinolysis [103–109]. While elevations in PAI-1 are known to predispose to thromboembolic events and poor outcomes in non-dermatologic disease states [108, 109], there are very few studies to date evaluating the role of PAI-1 in the thrombotic risk present in BP patients and other dermatologic conditions [110]. The limited investigations that have been done came from Marzano et al., who found that both PAI-1 antigen levels and PAI-1 activity are significantly higher in BP patients when compared to healthy controls [110]. However, PAI-1 is known to be conformationally labile and rapidly becomes latent/inactive [111], and also gets cleaved to an inactive form by inflammatory proteases like neutrophil elastase [112] such that antigen and anti-plasminogen activator activity can be discordant, and to-date there have been no published functional studies evaluating whether or not BP patients are resistant to tPA-mediated fibrinolysis to imply a functionally prothrombotic state.
In the clinical literature there are numerous studies reflecting an increased risk of thrombotic events in patients with BP [113–119]. One of the largest of these was a multicenter cohort study performed by Cugno et al. looking at 432 patients with BP [120]. These patients were found to have a 15-fold increase of venous thromboembolism risk during the acute phase of disease [120]. Furthermore, this risk was found to be proportional to disease severity while also being heightened by concomitant risk factors [120], which has been corroborated ex-vivo in unpublished work by our group where we have found that disease severity in BP correlates with functional hypercoagulability on thromboelastography and increased clot resistance to tPA-mediated lysis secondary to increased PAI-1 activity [121]. Another cohort study involving 2,654 patients with BP compared to 26,814 comparator patients found that after propensity score-matching for 60 venous thromboembolism risk factors and severity markers, BP patients had a 2-fold greater risk of VTE [117]. A nationwide retrospective cohort study with meta analysis performed in Taiwan similarly analyzed 12,162 patients affected by BP or Pemphigus Vulgaris (PV) for risk of venous thromboembolism along with 12,162 age- and sex-matched controls; with a significantly increased incidence of venous thromboembolism discovered in those with BP and PV (HR. 1.87; 95% CI: 1.55–2.26) [114]. On the other hand, one study which specifically evaluated the causal relationship between BP and stroke using a bidirectional two-sample Mendelian randomization design found no causal impact of bullous pemphigoid on the risk of stroke [122].
The literature supports a link between BP and the coagulation system; however, further investigations are needed to determine the relationship between a hypercoagulable state in BP and biomarkers such as F1 + 2, D-dimer, eosinophilic cationic protein, and PAI-1 to define a pathophysiologic mechanism [114].
Pemphigus vulgaris
Pemphigus Vulgaris (PV) is an autoimmune blistering disease caused by autoantibodies targeting the desmosomal adhesion proteins, desmoglein 1 and desmoglein 3 (Fig. 1H) [123]. Although closely related to BP, there is less data supporting a link between PV and hypercoagulability. Specifically, fewer laboratory studies looking at coagulation parameters in this disease have been performed. One such study looked at plasma levels of F1 + 2 and D-dimer in PV patients, reporting normal levels of both with active disease and disease remission [94]. Similarly, there are very few studies which have investigated the relationship between PV and alterations of the fibrinolysis system, with the main conclusions drawn from the few available suggesting the plasminogen activator-plasmin system may play a role in the pathogenesis of PV [124–126].
On the other hand, more clinical evidence showing presence of a hypercoagulable state in PV is available [113, 127–131]. One population-based cohort study used data from the Danish National Patient registry to analyze 3,322 patients with autoimmune blistering disease, both pemphigus (601) and BP (2,410), while also taking into account baseline comorbidity burden and medication use, to determine cardiovascular outcomes [113]. These patients with AIBD were compared to a cohort of 33,195 age and sex matched controls. Incidence rates were found to be higher in the AIBD group when compared to controls for atherosclerotic cardiovascular disease (HR: 1.24; 95% CI: 1.09–1.40), venous thromboembolism (HR: 1.87; 95% CI: 1.50–2.34), and cardiovascular death (HR: 2.01; 95% CI: 1.76–2.29) [113]. Furthermore, in a large-scale population-based longitudinal study performed in Israel, 1,985 patients with pemphigus were compared to 9,874 age, sex, and ethnicity matched controls to determine risk of pulmonary embolism [131]. In this study, pemphigus was found to be an independent risk factor for incident pulmonary embolism after controlling for several confounding factors (adjusted RR: 1.98; 95% CI: 1.29–3.04) [131]. Finally, Rokni et al. performed a systematic review and meta-analysis looking at the association between autoimmune bullous disease and cardiovascular risk, with a focus on pemphigus specifically [132]. They found significant associations between pemphigus and diabetes mellitus (OR: 1.81; 95% CI: 1.26–2.60), hypertension (OR: 1.393; 1.09–1.78), and dyslipidemia (OR: 2.18; 95% CI: 1.16–4.07), all conditions predisposing one to thrombotic events [132].
Overall, limited lab data is available investigating a relationship between PV and a hypercoagulable state, and the biomarkers under study are like those of BP. Clinically, PV shows a potential relationship to thrombosis-related outcomes, but further study is needed.
Dermatitis herpetiformis
Dermatitis Herpetiformis, also known as Duhring’s disease, is an autoimmune blistering disease linked to gluten sensitivity and celiac disease (Fig. 1I). In contrast to PV, there is less clinical data and more basic science research supporting coagulation aberrations in dermatitis herpetiformis. Görög et al. found when analyzing clot lysis times, both treated and untreated dermatitis herpetiformis patients had significantly prolonged clot lysis times when compared to healthy controls [133]. Data regarding levels of F1 + 2 in dermatitis herpetiformis is conflicting. One study by Wankiewicz et al. found elevated levels of this marker in dermatitis herpetiformis patients when compared to controls, however, another study by Marzano et al. found normal levels of F1 + 2 and D-dimer in dermatitis herpetiformis patients when compared to healthy controls [86, 134]. Additionally, one study found normal levels of tissue factor expression in dermatitis herpetiformis when compared to controls [99]. PAI-1 antigen levels and plasmin-antiplasmin (PAP) complex levels have been found to be higher in dermatitis herpetiformis patients, while plasminogen levels were found to be decreased in dermatitis herpetiformis patients [135]. While the authors concluded this makes interpretation of coagulation status in dermatitis herpetiformis challenging (as PAI-1 is antifibrinolytic while high PAP and low plasminogen suggests activation of fibrinolysis), PAI-1 is a known acute phase response protein [103] and PAP levels are known to be elevated in other hypercoagulable inflammatory states that may reflect the bodies’ endogenous fibrinolysis system trying to overcome a pathologic hypercoagulable state [136], which is corroborated by the common finding of elevated D-dimer in other non-dermatologic systemic inflammatory conditions [137, 138]. In contrast to the translational literature above, however, one cohort study from Finland studied 368 dermatitis herpetiformis patients compared with matched controls and found no difference in risk of cardiovascular disease between these patients and controls. Moreover, this same study even found a relatively decreased risk of stroke in dermatitis herpetiformis patients [139].
The literature is inconclusive about if there is a relationship between dermatitis herpetiformis and coagulation abnormalities due to limited and conflicting data. Further study of a pathophysiologic link and clinical data is needed to begin investigating a potential relationship, as current data using PAI-1 antigen levels can be challenging to interpret alone.
Conclusion
Coagulation system aberrancies can be severe and life-threatening, particularly when they are undetected and comorbid to systemic disease. Hypercoagulable states have been found to occur in diseases affecting many organ systems, and our study shows the skin is no outlier. Our literature review highlights the most relevant basic science and clinical studies investigating the potential for cardiovascular and thromboembolic risks in autoimmune alopecia areata, vitiligo, psoriasis, hidradenitis suppurativa, atopic dermatitis, chronic spontaneous urticaria, and autoimmune bullous diseases. The literature described potential for associated venous disease in atopic dermatitis, associated arterial disease in hidradenitis suppurativa, and both in alopecia areata, psoriasis, and autoimmune bullous diseases. It is also clear that significant gaps remain in the literature in recognizing the pathophysiologic mechanisms behind these hypercoagulable states, as well as in determining how these hypercoagulable states affect clinical outcomes. Clinically, it is also important to investigate if a history of VTE or other related events warrants potential long term anticoagulation treatment in patients with the dermatologic conditions we describe in this paper. Future studies addressing these gaps are imperative to improve our overall disease management in inflammatory dermatologic disorders.
Acknowledgements
The authors would like to thank the Nebraska Research Initiative/Faculty Collaboration Initiative for providing the funding related to this work.
Abbreviations
- (AA)
Alopecia areata
- (AD)
Atopic dermatitis
- (AIBD)
Autoimmune bullous disease
- (BP)
Bullous pemphigoid
- (CSU)
Chronic spontaneous urticaria
- (CV)
Cardiovascular
- (CVD)
Cardiovascular disease
- (DVT)
Deep venous thrombosis
- (HS)
Hidradenitis suppurativa
- (MI)
Myocardial infarction
- (MTX)
Methotrexate
- (PAI-1)
Plasminogen activator inhibitor-1
- (PAP)
Plasmin-antiplasmin
- (PE)
Pulmonary embolism
- (PV)
Pemphigus vulgaris
- (TNF)
Tumor necrosis factor
- (VTE)
Venous thromboembolism
Author contributions
All listed authors contributed to both the writing and critical revision of the manuscript.
Funding
Nebraska Research Initiative Award (CDB, EXW), NIH NIAID R01AI165666 (EXW).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
N/A.
Consent for publication
N/A.
Competing interests
CDB has patents issued or pending related to coagulation/fibrinolysis diagnostics. CDB has previously received grant support from the Genentech, Inc., Werfen, Hikari Dx, and consulting fees from Atheneum Partners. CDB is an Associate Editor of the Thrombosis Journal. All other authors have nothing to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Divya Sharma, Sierra Thomas and Trace B. Moody contributed equally to this work.
References
- 1.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–96. [DOI] [PubMed] [Google Scholar]
- 2.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–7. [DOI] [PubMed] [Google Scholar]
- 3.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–30. [DOI] [PubMed] [Google Scholar]
- 4.Medcalf RL. Fibrinolysis, inflammation, and regulation of the plasminogen activating system. J Thromb Haemost. 2007;5(Suppl 1):132–42. [DOI] [PubMed] [Google Scholar]
- 5.Barrett CD, Moore HB, Banerjee A, Silliman CC, Moore EE, Yaffe MB. Human neutrophil elastase mediates fibrinolysis shutdown through competitive degradation of plasminogen and generation of angiostatin. J Trauma Acute Care Surg. 2017;83(6):1053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett CD, Hsu AT, Ellson CD, Kong BYM, Greenwood YW. Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin Exp Immunol. 2018;194(1):103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett CD, Moore PK, Moore EE, Moore HB, Chandler JG, Siddiqui H et al. Plasminogen degradation by Neutrophil Elastase in Pleural Infection, not high plasminogen activator Inhibitor-1 (PAI-1), is the cause of Intrapleural Lytic failure. Chest. 2024. [DOI] [PubMed]
- 8.Cugno M, Borghi A, Garcovich S, Marzano AV. Coagulation and skin autoimmunity. Front Immunol. 2019;10:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burzynski LC, Humphry M, Pyrillou K, Wiggins KA, Chan JNE, Figg N, et al. The Coagulation and Immune systems are directly linked through the activation of Interleukin-1alpha by Thrombin. Immunity. 2019;50(4):1033–42. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert CA, Luscinskas FW, Kiely JM, Luis EA, Darbonne WC, Bennett GL, et al. Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol. 1990;145(9):3033–40. [PubMed] [Google Scholar]
- 11.Richardson DL, Pepper DS, Kay AB. Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol. 1976;32(4):507–13. [DOI] [PubMed] [Google Scholar]
- 12.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129(3):307–21. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa H, Hatakeyama S, Ikesue A, Miyai H. Generation of interleukin-8 by plasmin from AVLPR-interleukin-8, the human fibroblast-derived neutrophil chemotactic factor. FEBS Lett. 1991;282(2):412–4. [DOI] [PubMed] [Google Scholar]
- 14.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia Areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1–12. [DOI] [PubMed] [Google Scholar]
- 15.Esposito K, Giugliano D. The metabolic syndrome and inflammation: association or causation? Nutr Metab Cardiovasc Dis. 2004;14(5):228–32. [DOI] [PubMed] [Google Scholar]
- 16.Glickman JW, Dubin C, Renert-Yuval Y, Dahabreh D, Kimmel GW, Auyeung K, et al. Cross-sectional study of blood biomarkers of patients with moderate to severe alopecia areata reveals systemic immune and cardiovascular biomarker dysregulation. J Am Acad Dermatol. 2021;84(2):370–80. [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88(6):801–10. [DOI] [PubMed] [Google Scholar]
- 18.Scapini P, Nesi L, Morini M, Tanghetti E, Belleri M, Noonan D, et al. Generation of biologically active angiostatin kringle 1–3 by activated human neutrophils. J Immunol. 2002;168(11):5798–804. [DOI] [PubMed] [Google Scholar]
- 19.Wang EH, Santos L, Li XY, Tran A, Kim SSY, Woo K, et al. Alopecia Areata is Associated with increased expression of Heart Disease Biomarker Cardiac Troponin I. Acta Derm Venereol. 2018;98(8):776–82. [DOI] [PubMed] [Google Scholar]
- 20.Marie E-SM, El-Sayed R, Attia GAK, Gomaa FM. Evaluation of serum cardiac troponin I and N-terminal pro-B-type natriuretic peptide levels in patients with Alopecia Areata. Clin Exp Dermatol. 2021;46(1):153–6. [DOI] [PubMed] [Google Scholar]
- 21.Shakoei S, Ghiasi M, Ziaee K. Coagulation status in patients with Alopecia Areata: a cross-sectional study. Ital J Dermatol Venerol. 2021;156(5):588–92. [DOI] [PubMed] [Google Scholar]
- 22.Waśkiel-Burnat A, Rakowska A, Zaremba M, Maciejewska M, Blicharz L, Starace M, et al. Markers of venous thromboembolism risk in patients with Alopecia Areata: is there anything to worry about? Dermatol Ther. 2023;13(8):1847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waśkiel-Burnat A, Kotowska M, Dorobek W, Smyk JM, Gąsecka A, Niemczyk A, et al. Patients with Alopecia Areata are at risk of endothelial dysfunction: results of a case-control study. Clin Exp Dermatol. 2022;47(8):1517–22. [DOI] [PubMed] [Google Scholar]
- 24.Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med. 2004;32(5 Suppl):S298–301. [DOI] [PubMed] [Google Scholar]
- 25.Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304(12):H1585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J, Chen Y. The impact of vascular endothelial glycocalyx on the pathogenesis and treatment of disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2023;34(8):465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85(12):3510–7. [PubMed] [Google Scholar]
- 28.Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogen CM, de Priester W, Westmuckett A, Lupu F. An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol. 1997;139(1):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett CD, Moore HB, Moore EE, Chandler J, Sauaia A. Combination of aspirin and rosuvastatin for reduction of venous thromboembolism in severely injured patients: a double-blind, placebo-controlled, pragmatic randomized phase II clinical trial (the STAT trial). Blood Coagul Fibrinolysis. 2023;34(8):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najafi MT, Abedini R, Ghandi N, Seraji S, Sadeghi Y. Is the severity of Alopecia Areata associated with arterial stiffness? J Res Med Sci. 2023;28:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conic RRZ, Chu S, Tamashunas NL, Damiani G, Bergfeld W. Prevalence of cardiac and metabolic diseases among patients with Alopecia Areata. J Eur Acad Dermatol Venereol. 2021;35(2):e128–9. [DOI] [PubMed] [Google Scholar]
- 32.Shin J-W, Kang T, Lee JS, Kang MJ, Huh C-H, Kim M-S, et al. Time-Dependent risk of Acute myocardial infarction in patients with Alopecia Areata in Korea. JAMA Dermatol. 2020;156(7):763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J-H, Lin H-C, Kao S, Tsai M-C, Chung S-D. Alopecia Areata increases the risk of stroke: a 3-year Follow-Up study. Sci Rep. 2015;5:11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Hagan R, Caldas SA, Correa da Rosa JM, Guttman-Yassky E, Ungar B. Alopecia Areata is associated with increased genetic risk of myocardial infarction: a mendelian randomization study. J Eur Acad Dermatol Venereol. 2023;37(11):e1341–3. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Kim YC, Choi JW. Alopecia Areata is not a risk factor for heart diseases: a 10-year retrospective cohort study. PLoS ONE. 2021;16(5):e0250216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss MC, Kim SC, Wyss R, Jin Y, Chin K, Merola JF, et al. Incidence of venous thromboembolism in patients with dermatologist-diagnosed chronic inflammatory skin diseases. JAMA Dermatol. 2021;157(7):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang KP, Joyce CJ, Topaz M, Guo Y, Mostaghimi A. Cardiovascular risk in patients with alopecia areata (AA): a propensity-matched retrospective analysis. J Am Acad Dermatol. 2016;75(1):151–4. [DOI] [PubMed] [Google Scholar]
- 38.Bergqvist C, Ezzedine K, Vitiligo. Rev Dermatology. 2020;236(6):571–92. [DOI] [PubMed] [Google Scholar]
- 39.Tanacan E, Atakan N. Higher incidence of metabolic syndrome components in vitiligo patients: a prospective cross-sectional study. Bras Dermatol. 2020;95(2):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ataş H, Gönül M. Increased risk of metabolic syndrome in patients with Vitiligo. Balkan Med J. 2017;34(3):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azzazi Y, Mostafa WZ, Sayed KS, Alhelf M, Safwat M, Mahrous A, et al. Support for increased cardiovascular risk in non-segmental vitiligo among egyptians: a hospital-based, case-control study. Pigment Cell Melanoma Res. 2021;34(3):598–604. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Martín M, de Paz NM, Mehtani P, Ferrer PC, Eliche MP, Martín BR, et al. Patients with vitiligo present fewer cardiovascular risk factors: results from a case-control study. J Eur Acad Dermatol Venereol. 2013;27(1):124–5. [DOI] [PubMed] [Google Scholar]
- 43.Kridin K, Kafri N, Cohen AD. Is vitiligo associated with an increased risk of cardiovascular outcomes? Perceptions from a population-based study. Australas J Dermatol. 2024. [DOI] [PubMed]
- 44.van den Bogaard EH, Elias PM, Goleva E, Berdyshev E, Smits JPH, Danby SG, et al. Targeting skin barrier function in atopic dermatitis. J Allergy Clin Immunol Pract. 2023;11(5):1335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woo YR, Cho M, Do Han K, Cho SH, Lee JH. Atopic dermatitis and the risk of myocardial infarction and all-cause mortality: a Nationwide Population-based Cohort Study. Allergy Asthma Immunol Res. 2023;15(5):636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merola JF, Ertmer B, Liang H, Yue X, Ofori S, Krueger W. Venous thromboembolism risk is lower in patients with atopic dermatitis than other immune-mediated inflammatory diseases: a retrospective, observational, comparative cohort study using US claims data. J Am Acad Dermatol. 2023. [DOI] [PubMed]
- 47.Chen T-L, Huang W-T, Loh C-H, Huang H-K, Chi C-C. Risk of Venous Thromboembolism Among Adults With Atopic Dermatitis. Archives of dermatology (1960). 2023;159(7):720-7. [DOI] [PMC free article] [PubMed]
- 48.Warren RB, Basey V, Lynam A, Curtis C, Ardern-Jones MR. The risk of venous thromboembolism in atopic dermatitis: a matched cohort analysis in UK primary care. British journal of dermatology (1951). 2023;189(4):427 – 36. [DOI] [PubMed]
- 49.Drucker AM, Qureshi AA, Dummer TJB, Parker L, Li WQ. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian Partnership for Tomorrow Project. British journal of dermatology (1951). 2017;177(4):1043-51. [DOI] [PubMed]
- 50.Drucker AM, Li W-Q, Cho E, Li T, Sun Q, Camargo CA, et al. Atopic dermatitis is not independently associated with non-fatal myocardial infarction or stroke among US women. Allergy (Copenhagen). 2016;71(10):1496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nastałek M, Wojas-Pelc A, Undas A. Plasma fibrin clot properties in atopic dermatitis: links between thrombosis and atopy. J Thromb Thrombolysis. 2010;30(2):121–6. [DOI] [PubMed] [Google Scholar]
- 52.Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of β-Thromboglobulin and platelet factor 4. Allergology Int. 2008;57(4):391–6. [DOI] [PubMed] [Google Scholar]
- 53.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy (Copenhagen). 2015;70(10):1300–8. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis Lancet. 2021;397(10281):1301–15. [DOI] [PubMed] [Google Scholar]
- 55.Cuadrado MJ, Lopez-Pedrera C, Khamashta MA, Camps MT, Tinahones F, Torres A, et al. Thrombosis in primary antiphospholipid syndrome: a pivotal role for monocyte tissue factor expression. Arthritis Rheum. 1997;40(5):834–41. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Gao H, Loyd CM, Fu W, Diaconu D, Liu S, et al. Chronic skin-specific inflammation promotes vascular inflammation and thrombosis. J Invest Dermatol. 2012;132(8):2067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lembo S, Capasso R, Balato A, Cirillo T, Flora F, Zappia V, et al. MCP-1 in psoriatic patients: effect of biological therapy. J Dermatolog Treat. 2014;25(1):83–6. [DOI] [PubMed] [Google Scholar]
- 58.Schecter AD, Rollins BJ, Zhang YJ, Charo IF, Fallon JT, Rossikhina M, et al. Tissue factor is induced by monocyte chemoattractant protein-1 in human aortic smooth muscle and THP-1 cells. J Biol Chem. 1997;272(45):28568–73. [DOI] [PubMed] [Google Scholar]
- 59.Ernofsson M, Siegbahn A. Platelet-derived growth factor-BB and monocyte chemotactic protein-1 induce human peripheral blood monocytes to express tissue factor. Thromb Res. 1996;83(4):307–20. [DOI] [PubMed] [Google Scholar]
- 60.Golden JB, Groft SG, Squeri MV, Debanne SM, Ward NL, McCormick TS, et al. Chronic psoriatic skin inflammation leads to increased monocyte adhesion and aggregation. J Immunol. 2015;195(5):2006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garshick MS, Tawil M, Barrett TJ, Salud-Gnilo CM, Eppler M, Lee A, et al. Activated platelets induce endothelial cell inflammatory response in Psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40(5):1340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167(6):1345–50. [DOI] [PubMed] [Google Scholar]
- 63.Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: a systematic review and meta-analysis. QJM. 2014;107(10):793–7. [DOI] [PubMed] [Google Scholar]
- 64.Chen T-L, Lee L-L, Huang H-K, Wang J-H, Chen L-Y, Tsai H-R, et al. Association of Psoriasis With Incident venous thromboembolism and peripheral vascular disease: a systematic review and Meta-analysis. JAMA Dermatol. 2022;158(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lai YC, Yew YW. Psoriasis as an independent risk factor for Cardiovascular Disease: an epidemiologic analysis using a National Database. J Cutan Med Surg. 2016;20(4):327–33. [DOI] [PubMed] [Google Scholar]
- 66.Lin H-W, Wang K-H, Lin H-C, Lin H-C. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol. 2011;64(3):495–501. [DOI] [PubMed] [Google Scholar]
- 67.Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM, et al. Psoriasis and the risk of Major Cardiovascular events: Cohort Study using the Clinical Practice Research Datalink. J Invest Dermatol. 2015;135(9):2189–97. [DOI] [PubMed] [Google Scholar]
- 68.Yang Z-S, Lin N-N, Li L, Li Y. The effect of TNF inhibitors on Cardiovascular events in Psoriasis and Psoriatic Arthritis: an updated Meta-analysis. Clin Rev Allergy Immunol. 2016;51(2):240–7. [DOI] [PubMed] [Google Scholar]
- 69.Wu JJ, Poon K-YT, Channual JC, Shen AY-J. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–50. [DOI] [PubMed] [Google Scholar]
- 70.Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76(1):81–90. [DOI] [PubMed] [Google Scholar]
- 71.Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–113. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen TV, Damiani G, Orenstein LAV, Hamzavi I, Jemec GB. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35(1):50–61. [DOI] [PubMed] [Google Scholar]
- 73.Worapongsatitaya P, Chaikijurajai T, Ponvilawan B, Ungprasert P. Hidradenitis Suppurativa and Risk of Coronary Artery Disease: a systematic review and Meta-analysis. Indian J Dermatology. 2023;68(4):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phan K, Ng WHS, Lai B, Garg A, Smith SD. Hidradenitis suppurativa and association with stroke: systematic review and meta-analysis. J Dermatolog Treat. 2022;33(4):2309–16. [DOI] [PubMed] [Google Scholar]
- 75.Kridin K, Valido K, Cohen JM, Cohen AD. Hidradenitis suppurativa and the risk of myocardial infarction, cerebrovascular accident, and peripheral vascular disease: a population-based study. Arch Dermatol Res. 2023;315(3):429–35. [DOI] [PubMed] [Google Scholar]
- 76.Gibson RS, Porter ML, Kimball AB. Erythrocyte sedimentation rate, rather than C-reactive protein, may be the preferred biomarker for hidradenitis suppurativa. JAAD Int. 2022;8:47–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarac Ozturk G, Ergun T, Peker Eyuboglu I, Akkiprik M. Serum high-sensitivity C-reactive protein, tumor necrosis factor-alpha, interleukin (IL)-1beta, IL-17A and IL-23 levels in patients with hidradenitis suppurativa. Cytokine. 2021;144:155585. [DOI] [PubMed] [Google Scholar]
- 78.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. [DOI] [PubMed] [Google Scholar]
- 79.Miller IM, Johansen ME, Mogensen UB, Zarchi K, Ellervik C, Jemec GB. Coagulation status in Hidradenitis Suppurativa: a Danish Population- and hospital-based cross-sectional study. Dermatology. 2015;231(2):119–26. [DOI] [PubMed] [Google Scholar]
- 80.Kaplan A, Lebwohl M, Gimenez-Arnau AM, Hide M, Armstrong AW, Maurer M. Chronic spontaneous urticaria: focus on pathophysiology to unlock treatment advances. Allergy. 2023;78(2):389–401. [DOI] [PubMed] [Google Scholar]
- 81.Farres MN, Refaat M, Melek NA, Ahmed EE, Shamseldine MG, Arafa NA. Activation of coagulation in chronic urticaria in relation to disease severity and activity. Allergol Immunopathol (Madr). 2015;43(2):162–7. [DOI] [PubMed] [Google Scholar]
- 82.Sakurai Y, Morioke S, Takeda T, Takahagi S, Hide M, Shima M. Increased thrombin generation potential in patients with chronic spontaneous urticaria. Allergology Int. 2015;64(1):96–8. [DOI] [PubMed] [Google Scholar]
- 83.Takeda T, Sakurai Y, Takahagi S, Kato J, Yoshida K, Yoshioka A, et al. Increase of coagulation potential in chronic spontaneous urticaria. Allergy (Copenhagen). 2011;66(3):428–33. [DOI] [PubMed] [Google Scholar]
- 84.Asero RMD, Tedeschi AMD, Coppola RP, Griffini SP, Paparella PMD, Riboldi PMD, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119(3):705–10. [DOI] [PubMed] [Google Scholar]
- 85.Egeberg A, Kofoed K, Gislason GH, Vestergaard C, Thyssen JP. Cardiovascular Risk is not increased in patients with chronic Urticaria: a Retrospective Population-based Cohort Study. Acta dermato-venereologica. 2017;97(2):261–2. [DOI] [PubMed] [Google Scholar]
- 86.Marzano AV, Tedeschi A, Berti E, Fanoni D, Crosti C, Cugno M. Activation of coagulation in bullous pemphigoid and other eosinophil-related inflammatory skin diseases. Clin Exp Immunol. 2011;165(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernard P, Antonicelli F, Bullous Pemphigoid. A review of its diagnosis, associations and Treatment. Am J Clin Dermatol. 2017;18(4):513–28. [DOI] [PubMed] [Google Scholar]
- 88.Arakawa M, Dainichi T, Ishii N, Hamada T, Karashima T, Nakama T, et al. Lesional Th17 cells and regulatory T cells in bullous pemphigoid. Exp Dermatol. 2011;20(12):1022–4. [DOI] [PubMed] [Google Scholar]
- 89.Hashimoto T, Takahashi H, Sakaguchi S. Regulatory T-cell deficiency and autoimmune skin disease: beyond the scurfy mouse and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. J Allergy Clin Immunol. 2018;142(6):1754–6. [DOI] [PubMed] [Google Scholar]
- 90.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol. 2009;21(6):626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L, Chen Z, Wang L, Luo X. Bullous pemphigoid: the role of type 2 inflammation in its pathogenesis and the prospect of targeted therapy. Front Immunol. 2023;14:1115083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cugno M, Tedeschi A, Borghi A, Bucciarelli P, Asero R, Venegoni L, et al. Activation of blood coagulation in two prototypic autoimmune skin diseases: a possible link with thrombotic risk. PLoS ONE. 2015;10(6):e0129456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marzano AV, Tedeschi A, Fanoni D, Bonanni E, Venegoni L, Berti E, et al. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol. 2009;160(2):266–72. [DOI] [PubMed] [Google Scholar]
- 94.Marzano AV, Tedeschi A, Spinelli D, Fanoni D, Crosti C, Cugno M. Coagulation activation in autoimmune bullous diseases. Clin Exp Immunol. 2009;158(1):31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S, Lu M, Zhao Z, Peng X, Li L, Cheng C, et al. Plasma levels of D-dimer and fibrin degradation products correlate with bullous pemphigoid severity: a cross-sectional study. Sci Rep. 2021;11(1):17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and thrombosis study. J Clin Oncol. 2009;27(25):4124–9. [DOI] [PubMed] [Google Scholar]
- 97.Tedeschi A, Marzano AV, Lorini M, Balice Y, Cugno M. Eosinophil cationic protein levels parallel coagulation activation in the blister fluid of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2015;29(4):813–7. [DOI] [PubMed] [Google Scholar]
- 98.Grover SP, Mackman N, Arteriosclerosis. Thromb Vascular Biology. 2018;38(4):709–25. [DOI] [PubMed] [Google Scholar]
- 99.Zebrowska A, Wagrowska-Danilewicz M, Danilewicz M, Wieczfinska J, Pniewska E, Zebrowski M, et al. Tissue factor in Dermatitis Herpetiformis and Bullous Pemphigoid: link between Immune and Coagulation System in Subepidermal Autoimmune Bullous diseases. Mediators Inflamm. 2015;2015:870428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(1):148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rifaioglu EN, Sen BB, Ekiz O, Dogramaci AC. Mean platelet volume and eosinophilia relationship in patients with bullous pemphigoid. Platelets. 2014;25(4):264–7. [DOI] [PubMed] [Google Scholar]
- 102.Fearnley GR, Fibrinolysis. Ann R Coll Surg Engl. 1967;41(1):51–4. [PMC free article] [PubMed] [Google Scholar]
- 103.Renckens R, Roelofs JJ, de Waard V, Florquin S, Lijnen HR, Carmeliet P, et al. The role of plasminogen activator inhibitor type 1 in the inflammatory response to local tissue injury. J Thromb Haemost. 2005;3(5):1018–25. [DOI] [PubMed] [Google Scholar]
- 104.Morrow GB, Whyte CS, Mutch NJ. A Serpin with a finger in many PAIs: PAI-1’s central function in Thromboinflammation and Cardiovascular Disease. Front Cardiovasc Med. 2021;8:653655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Condron M, Rowell S, Dewey E, Anderson T, Lealiiee L, Farrell D, et al. The procoagulant molecule plasminogen activator inhibitor-1 is associated with injury severity and shock in patients with and without traumatic brain injury. J Trauma Acute Care Surg. 2018;85(5):888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tjarnlund-Wolf A, Brogren H, Lo EH, Wang X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke. 2012;43(10):2833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Griemert EV, Schwarzmaier SM, Hummel R, Golz C, Yang D, Neuhaus W, et al. Plasminogen activator inhibitor-1 augments damage by impairing fibrinolysis after traumatic brain injury. Ann Neurol. 2019;85(5):667–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frischmuth T, Hindberg K, Aukrust P, Ueland T, Braekkan SK, Hansen JB, et al. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J Thromb Haemost. 2022;20(7):1618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tofler GH, Massaro J, O’Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, et al. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res. 2016;140:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marzano AV, Tedeschi A, Polloni I, Crosti C, Cugno M. Prothrombotic state and impaired fibrinolysis in bullous pemphigoid, the most frequent autoimmune blistering disease. Clin Exp Immunol. 2013;171(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berkenpas MB, Lawrence DA, Ginsburg D. Molecular evolution of plasminogen activator inhibitor-1 functional stability. EMBO J. 1995;14(13):2969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu K, Urano T, Ihara H, Takada Y, Fujie M, Shikimori M, et al. The cleavage and inactivation of plasminogen activator inhibitor type 1 by neutrophil elastase: the evaluation of its physiologic relevance in fibrinolysis. Blood. 1995;86(3):1056–61. [PubMed] [Google Scholar]
- 113.Bonnesen K, Poulsen CFB, Schmidt SAJ, Sorensen HT, Schmidt M. Autoimmune blistering disorders and cardiovascular risks: a population-based cohort study. J Am Acad Dermatol. 2024. [DOI] [PubMed]
- 114.Chen TL, Huang WT, Loh CH, Huang HK, Chi CC. Risk of incident venous thromboembolism among patients with Bullous Pemphigoid or Pemphigus Vulgaris: a Nationwide Cohort Study with Meta-Analysis. J Am Heart Assoc. 2023;12(17):e029740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kilic Sayar S, Sun GP, Kucukoglu R. Comorbidities of bullous pemphigoid: a single-center retrospective case-control study from Turkey. Dermatol Ther. 2021;34(5):e15031. [DOI] [PubMed] [Google Scholar]
- 116.Milani-Nejad N, Zhang M, Kaffenberger J. The association between bullous pemphigoid and neurological disorders: a systematic review. Eur J Dermatol. 2017;27(5):472–81. [DOI] [PubMed] [Google Scholar]
- 117.Schneeweiss MC, Merola JF, Wyss R, Silverberg JI, Mostaghimi A. Venous thromboembolism in patients with Bullous Pemphigoid. JAMA Dermatol. 2023;159(7):750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Titou H, Kerrouch H, Frikh R, Hjira N. The association between bullous pemphigoid and comorbidities: a case-control study in Moroccan patients. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31(1):7–11. [PubMed] [Google Scholar]
- 119.Yang YW, Chen YH, Xirasagar S, Lin HC. Increased risk of stroke in patients with bullous pemphigoid: a population-based follow-up study. Stroke. 2011;42(2):319–23. [DOI] [PubMed] [Google Scholar]
- 120.Cugno M, Marzano AV, Bucciarelli P, Balice Y, Cianchini G, Quaglino P, et al. Increased risk of venous thromboembolism in patients with bullous pemphigoid. The INVENTEP (INcidence of VENous ThromboEmbolism in Bullous Pemphigoid) study. Thromb Haemost. 2016;115(1):193–9. [DOI] [PubMed] [Google Scholar]
- 121.Sharma D, Barrett CD, Moore HB, Jackson JH, Sandberg TM, Gawargi FI et al. Resistance to tPA-induced fibrinolysis and activation of coagulation is present in autoimmune bullous diseases of the skin. J Thromb Haemost. 2024. [DOI] [PMC free article] [PubMed]
- 122.Shen S, Chu M, Miao H, Li L, Fang H, Li X, et al. Assessment of relationships between bullous pemphigoid and neurological diseases: a bidirectional two-sample mendelian randomization study. Exp Dermatol. 2024;33(1):e14869. [DOI] [PubMed] [Google Scholar]
- 123.Schmidt E, Kasperkiewicz M, Joly P, Pemphigus. Lancet. 2019;394(10201):882–94. [DOI] [PubMed] [Google Scholar]
- 124.Schaefer BM, Jaeger CJ, Kramer MD. Plasminogen activator system in pemphigus vulgaris. Br J Dermatol. 1996;135(5):726–32. [DOI] [PubMed] [Google Scholar]
- 125.Lombardi ML, de Angelis E, Rossano F, Ruocco V. Imbalance between plasminogen activator and its inhibitors in thiol-induced acantholysis. Dermatology. 1993;186(2):118–22. [DOI] [PubMed] [Google Scholar]
- 126.Hunziker T, Vassalli JD. Plasmin induces acantholysis in skin organ cultures. Arch Dermatol Res. 1987;279(5):341–6. [DOI] [PubMed] [Google Scholar]
- 127.Sho Y, Sakai T, Matsuda-Hirose H, Yamate T, Hatano Y. Thromboembolism and bleeding in patients with autoimmune blistering disease. Clin Exp Dermatol. 2022;47(12):2255–60. [DOI] [PubMed] [Google Scholar]
- 128.Shaheen MS, Silverberg JI. Association of inflammatory skin diseases with venous thromboembolism in US adults. Arch Dermatol Res. 2021;313(4):281–9. [DOI] [PubMed] [Google Scholar]
- 129.Leshem YA, Atzmony L, Dudkiewicz I, Hodak E, Mimouni D. Venous thromboembolism in patients with pemphigus: a cohort study. J Am Acad Dermatol. 2017;77(2):256–60. [DOI] [PubMed] [Google Scholar]
- 130.Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kridin K, Kridin M, Amber KT, Shalom G, Comaneshter D, Batat E, et al. The risk of pulmonary embolism in patients with Pemphigus: a Population-based large-scale longitudinal study. Front Immunol. 2019;10:1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rokni AM, Ayasse M, Ahmed A, Guggina L, Kantor RW, Silverberg JI. Association of autoimmune blistering disease, and specifically, pemphigus vulgaris, with cardiovascular disease and its risk factors: a systematic review and meta-analysis. Arch Dermatol Res. 2023;315(2):207–13. [DOI] [PubMed] [Google Scholar]
- 133.Gorog A, Nemeth K, Szabo L, Mayer B, Sillo P, Kolev K, et al. Decreased fibrinolytic potential and morphological changes of fibrin structure in dermatitis herpetiformis. J Dermatol Sci. 2016;84(1):17–23. [DOI] [PubMed] [Google Scholar]
- 134.Wankiewicz A, Iwan-Zietek I, Gwiezdzinski Z, Kotschy M. Levels of F(1 + 2) prothrombin fragments and thrombin - antithrombin III (TAT) complexes in patients with dermatitis herpetiformis. Med Sci Monit. 2002;8(8):BR324–7. [PubMed] [Google Scholar]
- 135.Wankiewicz A, Iwan-Zietek I, Kotschy M, Gwiezdzinski Z. Selected parameters of fibrinolysis system in patients with dermatitis herpetiformis. Med Sci Monit. 2002;8(3):CR189–92. [PubMed] [Google Scholar]
- 136.Wada K, Takahashi H, Tatewaki W, Takizawa S, Shibata A. Plasmin-a2-plasmin inhibitor complex in plasma of patients with thromboembolic diseases. Thromb Res. 1989;56(6):661–5. [DOI] [PubMed] [Google Scholar]
- 137.Borowiec A, Dabrowski R, Kowalik I, Rusinowicz T, Hadzik-Blaszczyk M, Krupa R, et al. Elevated levels of d-dimer are associated with inflammation and disease activity rather than risk of venous thromboembolism in patients with granulomatosis with polyangiitis in long term observation. Adv Med Sci. 2020;65(1):97–101. [DOI] [PubMed] [Google Scholar]
- 138.Mori S, Soejima H, Hokamaki J, Tsujita K. Clinical disease activity is a major determinant of plasma D-dimer elevation in outpatients with rheumatoid arthritis: a hospital-based cross-sectional study. Mod Rheumatol. 2024;34(2):313–21. [DOI] [PubMed] [Google Scholar]
- 139.Nilsson N, Leivo J, Collin P, Koskinen I, Kaukinen K, Huhtala H, et al. Risk of vascular diseases in patients with dermatitis herpetiformis and coeliac disease: a long-term cohort study. Ann Med. 2023;55(1):2227423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.