Abstract
Coronary artery disease has a high mortality rate and is a striking public health concern, affecting a substantial portion of the global population. On the early onset of myocardial ischemia, thrombolytic therapy and coronary revascularization could promptly restore the bloodstream and nutrient supply to the ischemic tissue, efficiently preserving less severely injured myocardium. However, the abrupt re-establishment of blood flow triggers the significant discharge of previously accumulated oxidative substances and inflammatory cytokines, leading to further harm referred to as ischemia/reperfusion (I/R) injury. Diabetes significantly raises the vulnerability of the heart to I/R injury due to disrupted glucose and lipid processing, impaired insulin sensitivity and metabolic signaling, and increased inflammatory responses. Numerous studies have indicated that adipokines are crucial in the etiology and pathogenesis of obesity, diabetes, hyperlipidemia, hypertension, and coronary artery disease. Adipokines such as adiponectin, adipsin, visfatin, chemerin, omentin, and apelin, which possess protective properties against inflammatory activity and insulin resistance, have been shown to confer myocardial protection in conditions such as atherosclerosis, myocardial hypertrophy, myocardial I/R injury, and diabetic complications. On the other hand, adipokines such as leptin and resistin, known for their pro-inflammatory characteristics, have been linked to elevated cardiac lipid deposition, insulin resistance, and fibrosis. Meteorin-like (metrnl) exhibits opposite effects in various pathological conditions. However, the data on adipokines in myocardial I/R, especially in diabetes, is still incomplete and controversial. This review focuses on recent research regarding the categorization and function of adipokines in the heart muscle, and the identification of different signaling pathways involved in myocardial I/R injury under diabetic conditions, aiming to facilitate the exploration of therapeutic strategies against myocardial I/R injury in diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02357-w.
Keywords: Diabetes, Myocardial ischemia/reperfusion (I/R) injury, Adipokines
Introduction
As claimed by the most recent American Heart Association report, mortality rates stemming from cardiac and cardiovascular disease (CVD) have declined by 60% since 1950 as a result of precise diagnostic techniques and proactive medical and surgical interventions in the United States [1]. Coronary artery bypass grafting off or on-pump and direct percutaneous coronary intervention are widely recognized as the most efficacious therapeutic modalities for myocardial preservation in instances of cardiac injury [2, 3]. It is essential to understand that the sudden reintroduction of oxygen and nutrients can disturb the function and electrical activity of the heart muscle, leading to damage and exacerbating myocardial necrosis, a condition referred to as myocardial ischemia/reperfusion (I/R) injury [4]. In light of recent advancements in research on the molecular mechanisms of myocardial I/R injury, significant attention has been paid to elucidating the role of mitochondrial, lipid and glucose metabolism, oxidative products, calcium regulation, and cell signaling [5, 6]. However, there remains a lack of effective treatments for this condition in medical settings.
Conversely, there has been a slow increase in mortality rates from the end of the 2010s to 2020, which is due to the worsening of risk hazards such as diabetes, hypertension, obesity, aging population, and other related factors [1]. What is more, the global prevalence of diabetes reached 529 million individuals in 2021, with projections indicating a significant increase to 1.31 billion by the year 2050 [7]. Recent research indicates that diabetes can heighten the heart's responsiveness to I/R damage, reduce the heart's ability to respond to protective measures, disrupt energy metabolism, worsen the oxidative response and inflammatory activity in the heart, and consequently raise the likelihood of cardiomyocyte death through various mechanisms like apoptosis, necroptosis, ferroptosis, and pyroptosis [8, 9]. It is crucial to comprehend the pathogenic process that controls the advancement and worsening of myocardial I/R injury under hyperglycemia and to investigate reliable cardiac biomarkers for predicting risk.
Previously considered solely as a non-active energy receiver, white adipose tissue (WAT) has recently been acknowledged as an essential endocrine component that generates multiple peptide hormones with autocrine, paracrine, or endocrine effects on diverse physiological processes [10, 11]. These secretions comprise a varied assortment of small chemical molecules, such as cytokines and chemokines, that engage with adipose cells, immune cells, and non-regenerative cells (osteoblasts, neurocytes, retinal cells, pancreatic β cells, and cardiomyocytes) [12]. Certain agents are generated by cells other than adipocytes. In contrast, others are secreted by adipocytes and categorized as adipokines that include but are not limited to adiponectin, leptin, resistin, apelin, adipsin, visfatin, omentin, chemerin and meteorin-like (metrnl) [13]. Recent research has demonstrated that adipokines have a multifaceted impact on the insulin sensitivity, atherosclerosis, inflammation, and myocardial signaling pathogenesis. They display seemingly contradictory effects on the heart's functionality, particularly after oxidative products and I/R injury [14–16]. In a comprehensive analysis of preclinical animal studies, it was observed that adiponectin, possessing insulin-sensitizing and anti-inflammatory attributes, effectively suppressed apoptosis in cardiac muscle cells exposed to reperfusion injury by stimulating diverse molecular pathway cascades [17]. For instance, a study proposed that administering adiponectin as a supplement could potentially strengthen the responsiveness of the diabetic heart to ischemic post-conditioning through the initiation of diverse cellular signaling pathways, including Janus-activated kinase (JAK) /signal transducers and activators of transcription 3 (STAT3) and AMP-activated protein kinase (AMPK) [18]. Besides, intracerebroventricular administration of leptin significantly mitigated cardiac malfunction following I/R injury, as proved by enhanced ventricular systolic function, overall cardiac function, and mitochondrial metabolism [19]. Therefore, evidence suggests that adipokines are vital in developing myocardial I/R injury in subjects with or without diabetes, but its molecular mechanism remains largely unclear.
Previous research has mainly concentrated on individual adipokines or their receptors in relation to myocardial I/R injury or diabetes, highlighting the significant impact of adipokines on cardiovascular disease through glucose and lipid metabolism disorders [20–22]. By contrast, this review mainly examined the regulation and effects of different adipokines and associated signaling pathways and discussed their potential impacts on myocardial I/R injuries. Furthermore, a network analysis was conducted on various adipokines and their corresponding receptors using STRING tools and Cytoscape software. The aim was to delineate potential interactions among adipokines in the context of myocardial I/R injury in individuals with diabetes. This comprehensive research was undertaken to facilitate the development of innovative therapeutics and preventive strategies.
Increased vulnerability to myocardial I/R injury in diabetes
Diabetes, a metabolic disorder, is marked by high blood sugar levels resulting from insufficient insulin secretion or activity. It affects a substantial portion of the worldwide populace and is associated with various complications, particularly cardiovascular disease [23]. Of these complications, myocardial I/R injury is a prominent issue. Tissue damage arises as a consequence of the bloodstream supply briefly halted and then restored. Diabetic individuals are more sensitive to myocardial I/R injury, and the mechanisms driving this are discussed in the following sections.
Clinical perspective
Numerous studies have consistently shown that individuals with diabetes exhibit a heightened sensitivity to myocardial I/R injury when compared to non-diabetic counterparts [24, 25]. This increased risk is attributed to various underlying pathophysiological mechanisms, including hyperglycemia/hyperlipidemia, insulin resistance, compromised coronary microcirculation, endothelial dysfunction, elevated oxidative products, and dysregulated inflammatory activities [26]. Hyperglycemia is associated with an imbalanced generation of reactive oxygen species (ROS) and antioxidant defense malfunction, resulting in oxidative stress and exacerbating myocardial damage during periods of ischemia and reperfusion [27]. Studies from the same laboratory illustrated that elevated glucose levels increased myocardial infarct area during I/R in Mus musculus and enhanced ROS generation in vivo and in vitro [28, 29]. Insulin resistance, a prevalent characteristic of diabetes, hinders the uptake and utilization of glucose in cardiomyocytes, resulting in disrupted energy metabolism, mitochondrial dysfunction, and heightened susceptibility to myocardial injury [30]. Meanwhile, insulin resistance exacerbates inflammation and endothelial dysfunction, exacerbating myocardial dysfunction and augmenting susceptibility to I/R injury [31]. Diabetes-related impairment of angiogenesis impedes the development of new blood vessels in the ischemic myocardium, leading to reduced oxygen and nutrient delivery to the affected region, thereby exacerbating myocardial injury during reperfusion [32]. Additionally, endothelial dysfunction and microvascular abnormalities induced by diabetes contribute to impaired coronary flow reserve, thereby restricting the myocardium's capacity to withstand ischemic insults [33, 34]. There is compelling evidence indicating that healthy platelets possess cardioprotective properties; however, studies have shown that platelets obtained from sufferers with poorly managed type 2 diabetes mellitus (T2DM) exhibit diminished beneficial properties compared to platelets from healthy individuals [35]. Activated platelets migrate into the damaged cardiac muscle and provoke I/R injury by forming micro-emboli-small clots, enhancing platelet-leukocyte aggregation, and releasing vasoconstrictor and pro-inflammatory mediators [36]. Lastly, diabetic patients frequently present with comorbid conditions such as hypertension and dyslipidemia, which further increase their vulnerability to myocardial I/R injury [37]. Effective management of diabetes and its related cardiovascular complications is essential in minimizing the risk of myocardial I/R injury. Maintaining tight control over blood sugar levels (lifestyle changes, oral hypoglycemic medications, or insulin therapy) has been demonstrated to decrease ROS generation, inflammation, and endothelial dysfunction, ultimately reducing the area of cardiac damage during I/R [38]. Nevertheless, it is essential to acknowledge that strict glucose control may have constraints, as evidenced by previous studies demonstrating an elevated risk of cardiac attack in patients undergoing specific anti-hyperglycemic drug therapies [39], and severe hyperglycemia was more common in the strict-glucose-control group [40]. One possible explanation for this phenomenon might be the crucial character of myocardial glucose uptake and metabolism in sustaining myocardial energetic during periods of stress [41, 42].
Underlying molecular mechanisms of increased vulnerability to myocardial I/R injury in diabetes
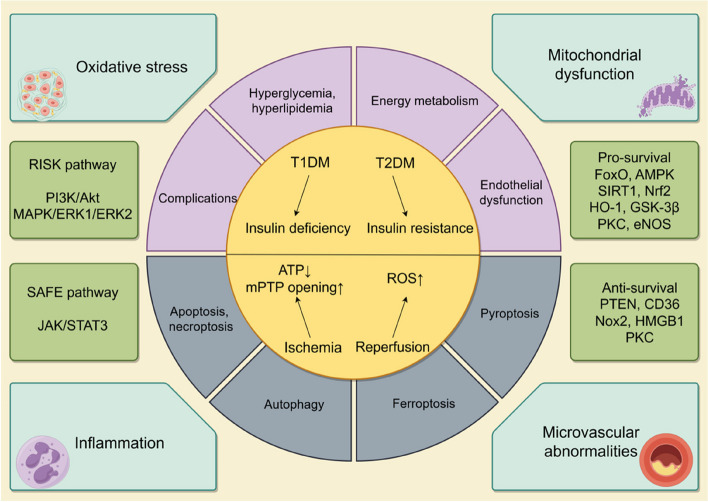
The increased vulnerability to myocardial I/R injury in diabetes to myocardial I/R injury in individuals with diabetes is a multifaceted phenomenon that is impacted by a variety of pathophysiological mechanisms. In other words, it is imperative to comprehend these underlying molecular mechanisms in order to formulate precise therapeutic approaches to mitigate myocardial damage and to enhance clinical outcomes in diabetic populations. The fundamental mechanisms of diabetes aggravating myocardial I/R injury are summarized in the Fig. 1.
Fig. 1.
Mechanism of diabetes mellitus aggravating myocardial ischemia/reperfusion (I/R) injury. Diabetes mellitus is categorized predominantly into type 1 diabetes mellitus (T1DM), distinguished by insufficient insulin production, and type 2 diabetes mellitus (T2DM), distinguished by reduced sensitivity to insulin. Diabetic condition primarily leads to hyperglycemia, hyperlipidemia, disrupted energy metabolism, endothelial dysfunction, and a range of associated complications. Under situation of myocardial ischemia, decreased ATP levels, increased opening of the mitochondrial permeability transition pore (mPTP), and the subsequent burst of ROS during myocardial reperfusion contribute to diverse types of cell deaths (including pyroptosis, necrosis, autophagy, ferroptosis, and apoptosis). Diabetes exacerbates myocardial I/R injury primarily through mechanisms involving inflammatory response, oxidative stress, disrupted mitochondrial and microvascular function and the key signal transduction pathways indicated in this action are the RISK and SAFE pathways. Current research indicates that the predominant pro-survival signaling involved are FoxO, AMPK, SIRT1, Nrf2, HO-1, GSK-3β and eNOS and the anti-survival signaling are primarily PTEN, CD36, HMGB1 and Nox2, while PKC exhibits dual function. FoxO: the class O of Forkhead box 1; AMPK: AMP-activated protein kinase; SIRT1:sirtuin 1; Nrf2: nuclear factor E2-related factor 2; HO-1: heme oxygenase-1; GSK-3β: glycogen synthase kinase-3β; eNOS: endothelial nitric oxide synthase; PTEN: Phosphatase and Tensin Homolog; CD36:cluster of differentiation 36; HMGB1: high mobility group box1protein; Nox2: Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2;PKC:protein kinase C; ERK 1: extracellular signal-regulated kinase 1; ERK2: extracellular signal-regulated kinase 2; JAK: janus-activated kinase; STAT3: signal transducers and activators of transcription 3; PI3K:phosphoinositide 3-kinase; Akt: protein kinase B; MAPK: mitogen-activated protein kinase
Research indicates that the diabetic heart may resist cardioprotective interventions. This could be attributed to disruptions in various signaling pathways and abnormal cardiomyocyte death, as observed in animal studies and in in vitro experiments, although clinical confirmation is currently insufficient [43]. The Reperfusion Injury Signaling Kinase (RISK) pathway, which encompasses the phosphoinositide 3-kinase (PI3K) /protein kinase B (Akt) and extracellular signal-regulated kinase 1 (ERK1) /extracellular signal-regulated kinase 2 (ERK2), mitogen-activated protein kinases (MAPK) signaling pathway, and the Survivor Activating Factor Enhancement (SAFE) pathway, which includes the JAK/STAT3 signaling cascade, play crucial roles in myocardial protection [44]. Impairments in above-mentioned signaling transducers diminish the responsiveness of diabetic myocardium to therapeutic interventions. Many studies have explored the cardiac protective effect of RISK and SAFE during the reperfusion period after the lethal ischemic insult. In this regard, previous findings indicated that activation of these two pathways following the onset of diabetes effectively reduced the size of infarct area caused by I/R and cell death triggered by high glucose and hypoxia/reoxygenation [45]. Further investigation demonstrated that the specific suppression of Phosphatase and Tensin Homolog (PTEN) using the inhibitor bisperoxovanadium sustained the cardioprotective benefits of post-ischemic-conditioning in streptozotocin-induced diabetic Rattus norvegicus by reactivating the PI3K/Akt and Janus-activated kinase 2 (JAK2)/STAT3 pathways [46], suggesting that the heart may regulate the phosphorylation of these kinases through the activation of PTEN under diabetic conditions. However, whether these two pathways may interact or be totally independent during myocardial I/R remains unclear. It appears that STAT5, as opposed to STAT3, may be a significant factor in the process of cardioprotection in the context of human physiology [47, 48]. Additionally, both the class O of Forkhead box (FoxO) transcription factors and the cluster of differentiation 36 (CD36)/AMPK signaling are involved in interventions combating against myocardial I/R injury via inhibiting excessive apoptotic cells, autophagy and ferroptosis as have been described in some recent studies [25, 26]. Recently, ferroptosis has emerged as an unique form of iron-dependent necrosis that differs from apoptosis, autophagy, and other established mechanisms of cell death. To date, several studies have validated the presence of myocardial cell ferroptosis in diabetic animal models, evidenced by the facts that administration of the ferroptosis agonist Erastin exacerbated myocardial I/R injury and post-ischemic cell death while the ferroptosis inhibitor Ferrostatin-1 or the utilization of the antioxidant N-acetylcysteine has been shown to attenuate myocardial I/R injury under high glucose condition [49–51]. AMPK, protein kinase C (PKC), ERK1/2, PI3K, and Akt defend against ferroptosis in myocardial tissue [52], while phosphoenolpyruvate carboxykinase-α/β (PCKα/β) inhibition also yield cardioprotective effects under high glucose [53]. In addition to the signaling mentioned above pathways and programmed cell death mechanisms, a variety of intracellular signaling molecules and cell death processes can also trigger myocardial I/R injury when hyperglycemia is present, such as necroptosis induced by necrosis-related proteins (Caspase 3, Bax, p-RIP3, p-RIP1, and p-MLKL) through the JAK2/STAT3 pathway [54], pyroptosis induced by the NLRP3 inflammasome via AMPK-Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2 signaling [55], autophagy regulated by high mobility group box1protein (HMGB1) [56], sirtuin 1 (SIRT1) /nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway activated by exaggerated generation of ROS and poor mitochondrial function [57], endothelial and vascular dysfunction with the suppression of endothelial nitric oxide synthase (eNOS) [58], inhibition of ROS-induced apoptotic rate by activating the AMPK/Akt/GSK-3β (glycogen synthase kinase-3β signaling) and Nrf2-governed antioxidant enzymes activity [59].
Impacts of adipokines on diabetes complicated with myocardial I/R injury.
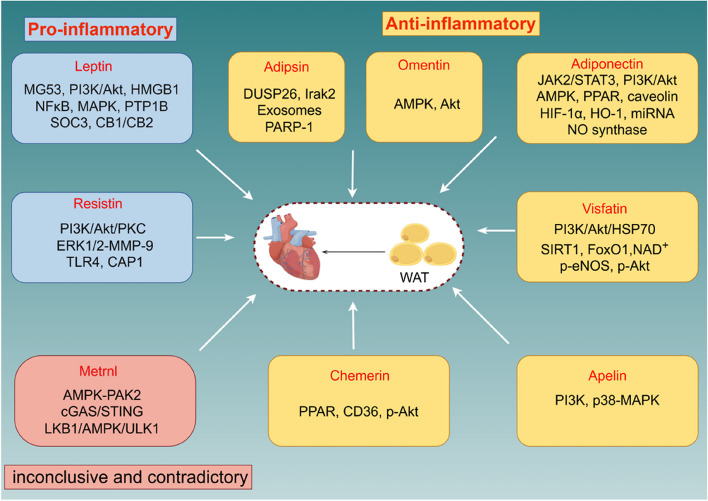
Various hormone-like molecules (adiponectin, leptin, resistin, apelin, visfatin, adipsin, omentin, chemerin, and metrnl) are classified as adipokines that are produced by WAT and play a multifaceted role in numerous diseases, including diabetes, atherosclerosis, CVD, and immune disorders [60, 61]. In obese pre-diabetic patients, abdominal fat tissue is a significant source of inflammatory and oxidative stress metabolites. Previous study found that high levels of these molecules are linked to low SIRT1 expression in adipose tissue, potentially impacting heart function both locally and systemically [62]. Furthermore, epicardial fatty tissue has been pinpointed as a leading source of CVD and metabolic disorders after an acute coronary event and coronary inflammation in T2DM given that it is closely adjacent to the coronary arteries [63, 64]. Besides, women have unique atypical risk factors that are associated with the prognosis of CVDs, such as pre-menopausal breast fat accumulation [65]. Adipokines have been implicated as playing both protective and deleterious effects of I/R injury by influencing various molecular pathways (Fig. 2). Myocardial I/R injury and diabetes are both associated with several mechanistic aspects of adipokines that have yet to be clarified.
Fig. 2.
The function and main molecular mechanism of various adipokines on myocardial ischemia/reperfusion (I/R) injury. Adipokines are mainly divided into two categories: anti-inflammatory (including adiponectin, adipsin, visfatin, chemerin, omentin and apelin, related signaling pathways are marked in each corresponding yellow box) and pro-inflammatory (leptin and resistin, related signaling pathways are marked in each corresponding blue box), while metrnl (related signaling pathway are marked in red box) exhibit opposite effects in various pathological condition. WAT: white adipose tissue; JAK2: janus-activated kinase 2; STAT3: signal transducers and activators of transcription 3; PI3K:phosphoinositide 3-kinase; Akt: protein kinase B; AMPK: AMP-activated protein kinase; PPAR: peroxisome proliferator-activated receptor; HIF-1α: hypoxia-inducible factor-1α; HO-1: heme oxygenase-1; miRNA: microRNA; NO synthase: nitric oxide synthase; HSP70: heat shock protein 70; SIRT1: sirtuin 1; FoxO1: the class O of Forkhead box 1; NAD.+: nicotinamide adenine dinucleotide; p-eNOS: phosphorylation of endothelial nitric oxide synthase; p-Akt: phosphorylation of protein kinase B; p38/MAPK: p38 mitogen-activated protein kinase; CD36: cluster of differentiation 36; PAK2: serine/threonine protein kinase 2; cGAS-STING: Cyclic GMP-AMP Synthase-Stimulator of interferon genes; LKB1: Liver Kinase B1; ULK1: UNC-51LikeAutophagyAckingKinase1; PKC: protein kinase C; ERK 1: extracellular signal-regulated kinase 1; ERK2: extracellular signal-regulated kinase 2; MMP-9: matrix metalloproteinase 9; TLR4: toll-like receptor 4; CAP1: adenylyl cyclase-associated protein 1; MG53: mitsugumin 53; HMGB1: high mobility group box1protein; NFκB: Nuclear Factor kappa B; MAPK: mitogen-activated protein kinase; PTP1B: protein tyrosine phosphatase 1B; SOC3: suppressor of cytokine signaling 3; CB1: Cannabinoid Receptor 1; CB2: Cannabinoid Receptor 2
Adiponectin
An independent research study employing various experimental methodologies initially characterized adiponectin, a protein uniquely generated by adipose cell and regulated by the ADIPOQ gene, known by various aliases including GBP-28, AdipoM1, AdipoQ, and Acrp30 in humans [66–69]. It typically exhibits high levels in the bloodstream, ranging from 3-30μg/mL, and comprises only 0.01% of pure protein in plasma, in comparison to else hormones and cytokines [70]. Meanwhile, adiponectin contains 247 amino acids including signal sequence, unstable domain, collagen-like domain, globular domain and it subsists in trimers (the fundamental unit), consists of hexamers with low molecular weights and isoforms with high molecular weights [71]. Until now, there are three central adiponectin receptors, including adiponectin receptor 1 (AdipoR1), adiponectin receptor 2 (AdipoR2) and T-cadherin (CDH13). AdipoR1 demonstrates a strong binding affinity towards globular adiponectin, whereas its interaction with full-length adiponectin is relatively weak. On the other hand, adiponectin with globular or full-length exhibits a moderate adaptability for AdipoR2 [72]. In terms of CDH13, it has been regarded as a receptor for hexameric and high molecular weights of isoforms of adiponectin [73].
Plenty of clinical trials have implied that individuals with elevated adiponectin levels have less chance of diabetes and are less susceptible to CVD [74, 75]. Meanwhile, animal experiments have indicated that insulin resistance is improved noticeably and post-ischemic myocardial infarction reduced significantly with exogenous adiponectin or adiponectin receptors agonist [76, 77]. For instance, previous studies have reported that cardiomyocytes from adiponectin knockout Mus musculus sustained severer I/R injury which could not be reverted or ameliorated by peroxisome proliferator-activated receptor (PPAR)-γ or PPAR-γ agonist [78–80]. Furthermore, cardiac adiponectin could act as an adjuster via AdipoR2 to prevent diabetic myocardial I/R injury through PI3K/Akt and JAK2/STAT3 [36, 81]. Furthermore, Cao et al. illustrated that ischemic postconditioning contributed to a significant loss in post-ischemic myocardial infarction and ROS accumulation in normal Rattus norvegicus, a phenomenon closely linked to increased expression of adiponectin and phosphorylated protein kinase B (p-Akt). The above conducive outcomes were canceled in diabetic Rattus norvegicus and the expressions of adiponectin were restrained [82]. Moreover, Li et al. proposed that the protective consequences of ischemic postconditioning are compromised in diabetes as a result of impaired adiponectin/AdipoR1/caveolin-3 signaling [83]. It is noted that caveolin has been generally identified as a tremendous latent spot in multifarious biological processes, including adiponectin signalosome formation and cardiac protection [84]. Wang et al. have shown that the knockout of caveolin-3 significantly blunts adiponectin's anti-apoptotic effect and exacerbates myocardial I/R injury. AdipoR1 co-localizes with caveolin-3 to form a complex, and the latter activates adiponectin cardioprotection signaling pathways, which are regulated by AMPK or not [85]. Recently, a study further proved that nitration of caveolin-3 at amino acid residue Tyr73 leading to signal complex dissociation is indicated in the progress of cardiac insulin and adiponectin sensitivity in the prediabetic heart, thereby exacerbating the progression of ischemic heart failure [86]. Additionally, researchers revealed that hypoadiponectinemia could decrease autophagic flux and increase myocardial I/R injury under diabetes while AdipoRon, an orally molecule that binds adiponectin receptors actively [87] restores autophagosome formation via significant phosphorylation of AMPK-Beclin-1 (Ser93/Thr119)-PtdIns3K (Ser164) and partly involves with AMPK-independent signaling [88]. Similarly, researches demonstrated that a dynamic reductive AdipoR1 expression in the heart and adiponectin concentration are contributive for the increased I/R injury responsiveness while sustained insulin therapy ameliorates myocardial reperfusion injury via increasing AMPK phosphorylation in diabetic Rattus norvegicus [89, 90]. Though some researchers have put forward that adiponectin preserves the hearts from I/R injury by deterrent of inducible nitric oxide synthase (iNOS) or endothelial NO synthase (eNOS), AMPK and Akt [91, 92], The deficiency of AMPK demonstrates limited impact on the antioxidative and antinitrative protection provided by adiponectin [93]. It is well established that both hypoxia-inducible factor-1α (HIF-1α) and HO-1 serve as crucial transcriptional regulators in hypoxic cells and act as a primary role in sustaining homeostasis in cell, further investigations have found that up-regulation of HIF-1α or HO-1 could increase adiponectin expression in diabetic mouse hearts and ultimately mitigate I/R injury [94, 95]. In microRNA (miRNA) profile aspect, findings suggested that hypoadiponectinemia in diabetic Mus musculus remarkably increased miRNA-449b expression and downregulated Nrf-1 and Ucp3 levels leading to excessive ROS generation and worse myocardial I/R injury [96]. Inhibition of miR-200c-3p and activation of the AdipoR2/STAT3 signaling induced by propofol post-conditioning alleviates diabetic myocardial I/R injury [97]. Last but not the least, the ablation of CDH13 abolished adiponectin’s cardioprotective effects and increased infarct size similarly via disrupting the stimulation of a capital adiponectin signaling pathway concentrated on AMPK phosphorylation in myocardial I/R models [98]. Subsequently, the adiponectin performs its diverse functions primarily via the intricate binding mechanisms it exhibits with the AdipoR1/R2 or CDH13 receptors, and aforementioned changes of the pathophysiological mechanisms, including oxidative stress, miRNA, transcription factors, apoptosis, autophagy, and cellular signaling mentioned above, crucially regulate cardiac metabolism, affecting myocardial I/R injury under diabetes.
Leptin
The discovery of leptin, the first adipokine synthesized and secreted by WAT encoded by the ob gene, by American scholar Jeffrey M. Friedman in 1994 marked a significant turning point in the understanding of WAT [99]. This discovery transformed the perception of WAT from a passive energy storage reservoir to a dynamic endocrine organ with active regulatory functions in behavior and metabolism. It weights 16 kDa and consists of 167 amino acids, exhibits a tertiary structure similar to that of cytokines with long chains of helices [100]. The predominant subtype of leptin receptor (LEPR), has been confirmed as the extended form that located in the hypothalamic arcuate nucleus, namely LEPRb [101]. Initially, LEPRb was deemed to be the functional receptor due to its 300 cytoplasmic residues that is longer in humans than in Mus musculus. This domain contains multiple motifs necessary for interacting with other proteins and initiating signaling pathway activation, such as the JAK-STAT3/5 and AMPK-acetyl-coenzyme A carboxylase axis [102, 103].
Study has shown that leptin acts a crucial part as the primary sensory factor for energy storage inside the human as WAT communicates with the energy metabolism to the brain through the secretion of leptin, which in turn acts on hypothalamic neurons involved in regulating appetite to suppress hunger and increase energy expenditure [104, 105]. A murine model deficient in leptin was first established in 1959, utilizing the ob/ob (leptin-deficient, caused by a single autosomal recessive mutation on the obese gene located on chromosome 6) and db/db (LEPR-deficient, caused by a single autosomal mutation on the leptin receptor gene) Mus musculus. These models have been extensively utilized in the past 20 years for the advancement of myocardial I/R injury models in T2DM [106]. Upon administering recombinant leptin to ob/ob Mus musculus, significant reductions were observed in their overall fat mass, resulting in a notable decline in food intake. Furthermore, the treatment effectively alleviated hyperglycemic and hyperinsulinemic conditions, exhibiting promising therapeutic effects in managing these metabolic abnormalities [107]. Moreover, a substantial difference is noted between Mus musculus of ob/ob and db/db genotypes and Mus musculus of wild-type genotypes in the context of post-ischemic myocardial injuries, which is attributable to multiple signal transduction pathways associated with autophagy, apoptosis, impaired insulin sensitivity, and other factors [108]. For instance, inhibition of mitsugumin 53 (MG53) E3 ligase activity mediated with MG53S255 phosphorylation [109], activation of PI3K/Akt pathway [110], down-regulation of histone 3 lysine 9 acetylation through histone deacetylase [111] and suppression of HMGB1-RAGE (receptor for advanced glycation end products) axis [112] are all reported to be associated with attenuation of myocardial ischemia reperfusion injury. However, leptin itself yields inconsistent consequences on myocardial I/R injury. Prior research revealed that the expression of leptin in both the serum and heart markedly decreased during the initial stage following myocardial I/R injury, followed by a gradual increase during the reperfusion phase [113]. In murine models, pre-administration with leptin resulted in a decline in cardiac and serum inflammation, improvement in myocardial reperfusion damage, and potentially involves the increase of PI3K-Akt-Nuclear Factor kappa B expression as a protective mechanism [114, 115]. During reperfusion, the manipulation of leptin caused a substantial reduction in infarction risk and a postponement in the opening of the mitochondrial permeability transition pore (MPTP), potentially mediated by the PI3K/Akt and p44/42 mitogen-activated protein kinase signaling transducer [116]. When compared to the aforementioned leptin-cardioprotective effects, respective clinical investigations have exhibited a significant correlation between diabetes and cardiovascular complications [22, 117]. To a certain extent, leptin is a inflammatory activator related to endothelial dysfunction, neointimal hyperplasia, thrombogenesis, cardiac hypertrophic and pro-remodeling [118–120]. Fortunately, later research found that leptin resistance is recognized as a remarkable risk indicator for CVD rather to leptin deficiency [20]. Excessive leptin and impaired leptin signaling shift cardiac substrate energy metabolism (glucose replaced by free fatty acid), and then trigger massive accumulation of lipid which induces lipid toxicity, poor mitochondrial functions, and increased generation of ROS in I/R injury under diabetic condition [121, 122]. Besides, insulin diminishes the storage of leptin and promotes its secretion directly in a physiological context, while leptin impedes the secretion of insulin, decreases the production and accumulation of fat, enhances the sensitivity of insulin receptors, and ultimately establishes equilibrium between fat homeostasis and energy homeostasis. Disruption of this equilibrium may lead to metabolic disturbances, insulin and leptin resistance coexist in diabetes, obesity and CVD due to both of them share the same signal transduction pathways such as protein tyrosine phosphatase 1B (PTP1B) and suppressor of cytokine signaling 3 (SOC3) [123, 124]. What’s more, researchers suggested that targeting cannabinoid (CB) receptors/modulating the degree or activity of endocannabinoids in tissue is beneficial to decrease insulin/leptin resistance in diabetes and mitigate the myocardial damage during I/R phase [125, 126]. Taken together, level of leptin could be identified as a potential diagnostic tool and label for diabetic myocardial I/R injury and managing appropriate leptin levels in individuals subjected to diabetes and myocardial I/R injury could be real fundamental for their metabolic well-being and holistic systemic health.
Resistin
Resistin was initially identified for its role in promoting insulin resistance which controlled by the RETN gene in humans. Discovered in Mus musculus in 2001, this molecule is part of the resistin-like molecules family, characterized by a unique cysteine repeat motif (C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-CC-X3-6-END) and exhibiting diverse expression patterns and biological functions [127]. There is ongoing argumentation with respect to the effective impact of resistin in Mus musculus and Homo sapiens. Earlier research indicated that mouse resistin is embedded in chromosome 8 and weighing 11kDa, while Homo sapiens resistin is situated in chromosome 19 and weighing 12.5kDa, share 59% identity compared to the amino acid content, 64.4% sequence identity at the messenger RNA content, but only 46.7% sequence identity at the DNA level [128]. Moreover, resistin is commonly generated by WAT in Mus musculus, whereas in Homo sapiens, macrophages are the main origination of resistin [129].
Despite the variances between humans and rodents, there is an increasing amount of demonstrations supporting that resistin acted as a mediator in the pathogenesis of inflammatory processes and the beginning of several chronic diseases, such as metabolic malfunction, CVD, and tumor [130]. On the one hand, increased contents of resistin have been described in instances of both diet-induced obese and genetically-induced obesity while treatment of anti-resistin agent has been found to against high glucose level and to mitigate insulin resistance in experimental animals with obesity in prior studies [131]. Nagaev and his co-authors contend that there exists a dearth of correlation between insulin resistance, T2DM, and resistin expression in both adipocytes and skeletal muscle. They have noted that while resistin expression is generally low in these tissues, it can still be detected in isolated adipocytes and total WAT from certain subjects [132]. Interestingly, recent study pointed out that high content of resistin is linked to escalated mortality in T2DM and expression level of greater than or equivalent to 11ng/mL indicates an elevated risk of poor outcomes [133].
It seems that fatty acid transport protein 1, CD36, AMPK, and Acetyl-CoA carboxylase are related to insulin response and resistin function under hyperglycemia [134]. Despite that, the existing data on the effects of resistin on the myocardium have been inconclusive. Researchers demonstrated that resistin can distinctly reduce apoptotic rate and post-ischemic myocardial infarction area via PI3K/Akt/PKC or ERK1/2-matrix metalloproteinase 9 dependent pathways and thus against I/R injury [135, 136]. By contrast, studies revealed that resistin yielded non- cardioprotective effects in Langendorff-perfused rodents hearts and lacking defence in human atrial muscle subjected to reoxygenation damage [137] and resistin itself even worsens cardiac I/R injury through influencing the level of atrial natriuretic peptides during reperfusion and altering biochemical indicators of myocardial injury [138]. In addition, resistin exhibits the ability to react to two distinct receptors, toll-like receptor 4 (TLR4) and adenylyl cyclase-associated protein 1 (CAP1), thereby facilitating the initiation of inflammatory processes [139]. Currently, there is a lack of understanding regarding the potential cross-talk failure between diabetes and myocardial I/R injury. This discrepancy is connected with the significant disparities observed in the genetic and proteomic configuration of the resistin molecule between rodents and humans, and a scarcity of proof concerning the resistin receptor and its downstream signaling transducer pathways. Further assessment of the roles of resistin in patients with diabetes and myocardial I/R injury could improve the comprehension of the underlying pathways involved in the physiological and pathological development of the disease and potentially lead to improved treatment strategies for affected individuals.
Apelin
In 1998, apelin was initially obtained from stomach extracts in bovine and characterized as a ligand for the human G protein-coupled receptor (also referred to apelin receptor (APJ)) [140]. It distributes in both human and mouse WAT, which is regulated by insulin and obesity [141]. Furthermore, apelin and APJ exhibit expression in different tissues, including but not limited to the cardiac tissue, lung, kidney, and tumor tissues, in addition to adipocytes [142]. Apelin peptides are produced by the enzymatic cleavage of a 77-amino-acid precursor molecule called pre-pro-apelin at the C-terminal end. These peptides exhibit diverse lengths in the circulating system, undergoing sequential cleavage to generate shorter, less well-defined forms, with the predominant isoforms being apelin-12, apelin-36, apelin-17, and apelin-13 [143]. Besides, these subtypes have the ability to bind APJ, however, conformational changes of receptors may impact protein function and effects [144]. And the preeminent physiologically active form of pyroglutamylated apelin-13 is the dominant apelin isoform inside the circulatory system and Homo sapiens plasma, with the capacity to potentially ameliorate vascular disease by inhibiting inflammation, suppressing apoptosis, reducing oxidative stress, and promoting autophagy [145, 146].
Apelin signaling system is tie up with a range of physiological responses, which is known to contribute to multiple pathological conditions, notably cardiovascular disorders and diabetes. In a study by Kartal and his co-workers, apelin-13 was administrated in diabetic Rattus norvegicus before blood flow caseation of the coronary artery and the erythrocyte deformability was significantly increased [147]. Moreover, they found that apelin-13 inhibits cardiac cell death and excessive inflammation activity from I/R injury in diabetic rodents [148]. A latest study showed that apelin exhibits a protective outcome against I/R injury through suppressing apoptotic rate and ROS production via the activation of PI3K and p38 mitogen-activated protein kinase signaling in diabetic hearts [149]. Given its protective activities, targeting the apelin for the treatment of CVD could be a therapeutic tool and more studies should be conducted to further explore new potential mechanisms on diabetic heart combined with I/R injury.
Visfatin
Visfatin, (also known as NAMPT – nicotinamide phosphoribosyltransferase or pre-B cell colony-enhancing factor (PBEF)) was isolated from abdominal WAT by a Japanese research team in 2005, which is notably abundant in visceral fat tissue in Homo sapiens and Mus musculus, with its plasma expression extent rising accompanied by the progression of obesity and diabetes [150, 151]. Prior researches demonstrated that increased content of visfatin in obese and diabetic subjects may help compensate for prolonged high blood sugar by mimicking insulin and lowering glucose and lipid levels [152, 153]. A cross-sectional multicentric study revealed that visfatin in diabetic patients who received drug treatment (such as angiotensin-converting-enzyme inhibitor, calcium channel blockers or statins) couldn’t be used as a biomarker of subclinical atherosclerosis [154]. However, the function of visfatin in the physiological and pathological processes of diabetes is controversial. Prolonged visfatin treatment leading to a diabetic phenotype in Mus musculus [155] while another study reported that serum visfatin is linked to T2DM regardless of insulin resistance and obesity [156], indicating that the dual impact of visfatin on diabetes may be influenced by its concentration and requires further comprehensive clinical investigations. Furthermore, Sadoshima and colleagues conducted a series of studies examining the relation between serum levels of visfatin and I/R injury which indicated that prevention of visfatin downregulation can effectively inhibit apoptosis and stimulate autophagic flux in cardiac myocytes in response to prolonged ischemia and I/R by activating SIRT1 with upregulation of nicotinamide adenine dinucleotide (NAD+) and ATP contents [157–159]. Finally, Xin et al. demonstrated that treatment of visfatin reduces the inflammation and apoptosis levels of myocardial cells after myocardial I/R through activation of PI3K/AKT/ heat shock protein 70 signaling axis [160] and latest study revealed that a circular RNA associated with ferroptosis mediates the visfatin-SIRT1-FoxO1-Fth1 signaling via regulating myocardial cell ferroptosis and preserving cardiac function during reperfusion injury [161]. Li et al. suggested that activating the AMPK/NAMPT signaling improves the effectiveness of sevoflurane post-conditioning in reducing myocardial I/R injury, but this conclusion was later retracted due to inaccurate and incomplete data [162]. Off note, 1-(3,6-Dibromo-carbazol-9-yl)-3-phenylamino-propan-2-ol, known for its ability to dimerize and aggregate visfatin, has been reported to decrease the infarction area in diabetic hearts throughout cardiac ischemia and reperfusion damage recently, together with molecular signaling modification for p-AKT, phosphorylated eNOS and SIRT1 [163]. Straightforwardly, more in-depth investigations are vital to examine the latent efficacy of visfatin in diabetic myocardial I/R injury.
Adipsin, omentin, chemerin, and metrnl
Adipokines discussed previously strongly correlate with diabetes and myocardial I/R injury. Moreover, other adipokines, including adipsin, omentin, chemerin and metrnl, have explicitly been linked to diabetes and myocardial I/R injury, respectively, providing valuable insights into the complex relationship between these two conditions. Additional research on these adipokines is necessary to support future in-depth clinical studies in this field.
Adipsin, known as complement factor D (CFD), was the first adipokine discovered by Spiegelman's research team in 1987 and subsequently determined to be a serine protease homolog produced and released by adipose cells, and is present in the circulatory system [164]. Activated adipsin has minimal proteolytic activity on most substrates, but can cleave complement factor B as it bind to energized complement factor C3, its function is homologous to that of C1s in the classical pathway [165]. In comparison to Mus musculus, human adipsin messenger RNA is also observed in monocytes and macrophages [166]. Spiegelman's lab and others later found that diabetic individuals subjected to β cell failure are lacking adipsin expression [167] and adipsin/C3a preserves β cells via lowering the phosphatase DUSP26 in diabetic Mus musculus, potentially leading to beneficial effects that are linked to a decreased risk of developing T2DM in humans [168]. Meanwhile, adipsin mitigates mitochondrial damage and enhances β-oxidation of fatty acid in diabetic cardiomyopathy through its interaction with Irak2 and impediment of Irak2 mitochondrial translocation [169]. Furthermore, exosomes originating from pericardial WAT mitigate post-myocardial infarction through adipsin-mediated regulation of iron homeostasis, while adipsin sourced from epicardial WAT contributes to cardiomyocyte apoptosis after myocardial infarction via mediation of PARP-1 activity [170, 171]. Based on the findings of a cytokine array analysis, adipsin emerges as a novel biomarker with potential utility in forecasting re-hospitalization and mortality among individuals with coronary disease [172]. Despite the promising future perspectives of the aforementioned adipsin and adipsin compounds, there are currently no robust clinical treatments available that can effectively repair myocardial reperfusion injury in diabetic individuals.
Omentin, metrnl, and chemerin are new adipokines discovered around 2005, which are mainly secreted by adipocytes to regulate the metabolism of adipocytes and exhibit either pro-inflammatory or anti-inflammatory properties in different clinical scenarios. Yang and colleagues discovered omentin (regulated by the ITLN1 gene) by analyzing 10,437 expressed sequence markers from a human omental fat cDNA library which consists of 313 amino acids and includes a secretory signal sequence as well as a fibrinogen-related domain [173]. Omentin exhibits high level in omental adipose tissue, specifically in stromal vascular cells rather than adipocytes. Its molecular mechanisms contribute to protective effects on glucose homeostasis by mitigating inflammatory processes, improving insulin sensitivity, enhancing endothelial function, and facilitating vasodilation in obesity and diabetic subjects [174]. It confers cardioprotective benefits by mitigating the progression of atherosclerosis and heart failure [175]. Furthermore, the systemic treatment of human omentin in rodents resulted in a decline in myocardial infarction risk and apoptotic rate following I/R, concomitant with increased levels of AMPK and Akt in heart [176]. The precise role of omentin remains uncertain at present; however, this molecule may serve as a noteworthy connection between diabetes and cardiovascular disease. Lastly, metformin and statins could elevate omentin-1 levels in patients [177], and thus, both of these medicines may be useful in the treatment of myocardial I/R injury under diabetic conditions.
In 2009, Surace et al. identified and documented the presence of the metrnl (311 amino acid sequence encoded by 936 base pair sequence) gene on human chromosome 17 using bioinformatics analysis [178, 179], it has become increasingly recognized as a high potential area of focus for investigation in a particular field of diabetes and CVD on recent years [180]. It is highly expressed in the skeletal muscle, subcutaneous fatty tissue, epididymal WAT depots and heart. Metrnl mitigates myocardial I/R injury-induced cardiomyocyte cell death by reducing over endoplasmic reticulum activity through the activation of AMPK-serine/threonine protein kinase pathway in cells [181]. Additionally, it improves diabetic cardiomyopathy by deactivating Cyclic GMP-AMP Synthase-Stimulator of interferon genes signaling in a manner dependent on Liver Kinase B1/AMPK/ UNC-51LikeAutophagyAckingKinase1-mediated autophagy [182]. Unfortunately, the relation between serum content of metrnl and the danger of heart disease in diabetic individuals remains inconclusive and contradictory, as evidenced by various controlled clinical trials or meta-analyses [183, 184], suggesting that metrnl content could be affected by various elements. The limited understanding of its receptor or direct interacting proteins hinders further investigation of metrnl in myocardial damage, both in the presence and absence of diabetes.
Regulated by the gene retinoic acid receptor responder protein 2 (RARRES2), chemerin is mainly generated by adipocytes to regulate the metabolism of adipocytes and exhibits proinflammatory and antiinflammatory properties through interaction with its main receptor, such as the chemokine-like receptor 1 (CMKLR1), G protein-coupled receptor 1 (GPR1) and C–C chemokine receptor-like 2 (CCRL2) [185]. It is produced in an unactive precursor form known as prochemerin, the latter is released and subjected to proteolytic cleavage by diverse extracellular proteases, resulting in the generation of distinct isoforms exhibiting varying degrees of biological activity. Down-regulation of chemerin is beneficial to reduce reperfusion injury in response to intestinal, kidney, lung and brain damage, with the primary mechanisms being associated with NLRP3 inflammasome-mediated pyroptosis [186–189]. Proof based on human and animal studies revealed that dysregulation of chemerin may serve as a risk indicator for hyperglycemia, vascular inflammation, angiogenesis, atherosclerosis, chronic heart failure and blood pressure modulation [190]. Elevated levels of chemerin have been linked to insulin resistance, disrupted blood glucose metabolism, and elevated blood glucose levels in Mus musculus [191]. Contrary to this, a recent study suggested that the addition of chemerin reversed cardiac dysfunction induced by lipid overload by increasing the messenger RNA levels of PPAR-γ and PPAR-dependent genes (such as CD36, Fabp4, and Fasn) and restoring the decrease in insulin-triggered Akt phosphorylation in Mus musculus treated with high-fat diet [192]. Additionally, a mendelian randomization study has identified potential associations between elevated genetically predicted levels of chemerin and a heightened risk of coronary disease [193]. The expanding number of research on chemerin's involvement in the pathological and physiological changes of CVD and diabetes has sparked interest in the potential use of chemerin and its associated signaling proteins as targets for the advancement of therapeutic medicines for the settlement of these conditions.
Potential interplay among various adipokines and receptors
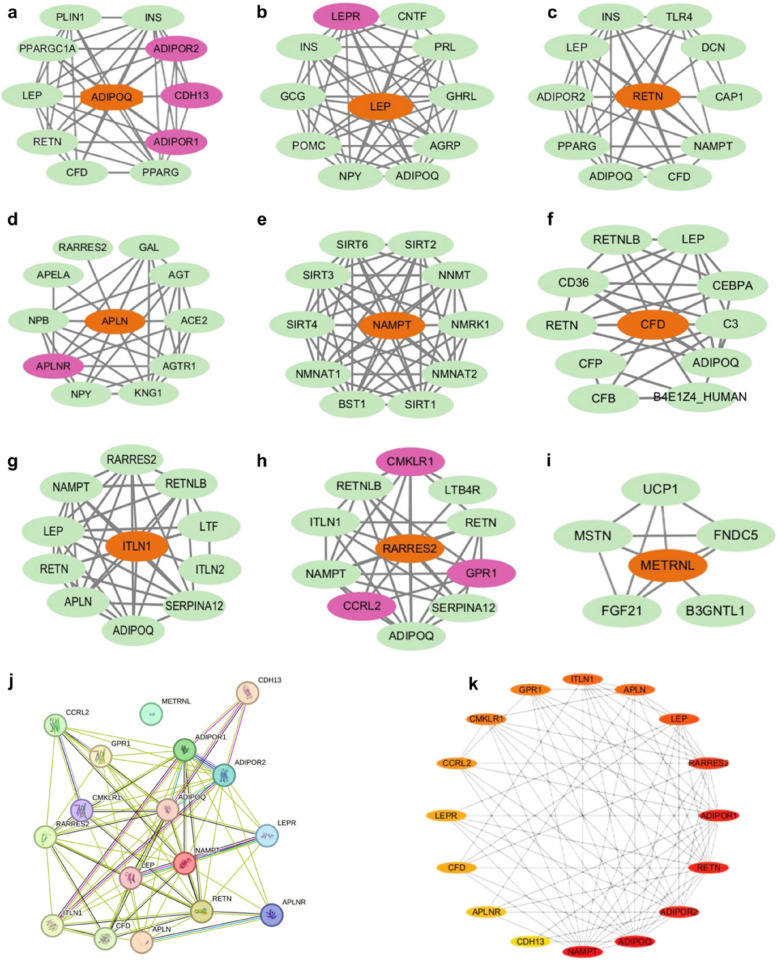
WAT is not merely a non-functional tissue but a complex and dynamic tissue that secretes adipokines in response to physiological and pathological stimuli. Due to its intricate molecular signaling pathways, WAT is crucial in maintaining body homeostasis and exerts protective or damaging effects, thus motivating and continually expanding field of research. Prior studies have primarily focused on individual adipokines or their receptors in relation to myocardial I/R injury in diabetes, rather than considering in a broader perspective. Hence, a network analysis was conducted on the aforementioned adipokines and receptors utilizing the STRING tools (http://cn.string-db.org) and Cytoscape software, aiming to outline potential interactions among adipokines in subjects who suffered myocardial I/R injury under hyperglycemia. Given the general constraint of displaying no more than a maximum of 10 interactors, it is observed that metrnl has the fewest predicted functional partners compared to the other proteins (Fig. 3a-i). Moreover, resistin exhibits the highest anticipated number of edges, with adiponectin and leptin following closely behind (Table 1). Subsequently, a more in-depth examination of the correlation between adipokines and their respective receptors was conducted, leading to the isolation of metrnl from other proteins, as the analysis was confined exclusively to the query proteins (Fig. 3j). Furthermore, a modular network was created applying the Cytohubba algorithm to reveal the core adipokine targets. The algorithm successfully identified significant network targets within the Protein–Protein Interaction (PPI) networks, with chemerin being the first target, followed by adiponectin, resistin, and visfatin.When considered collectively, adiponectin and resistin merit further investigation due to their significant potential and interconnected nature (Fig. 3k). However, this does not imply that metrnl is of lesser importance, as the PPI network is constructed using curated databases, experimentally determined data, protein homology, and other relevant factors.
Fig. 3.
The Protein–Protein Interaction (PPI) networks of potential targets of various adipokines. a. Networks of adiponectin (ADIPOQ); b. Networks of leptin (LEP); c. Networks of resistin (RETN); d. Networks of apelin (APLN); e. Networks of visfatin (NAMPT); f. Networks of adipsin (CFD); g. Networks of omentin (ITLN1); h. Networks of chemerin (RARRES2); i. Networks of metrnl (METRNL) (a-i: the orange oval represents adipokines, the green oval denotes non-self receptor proteins, and the purple oval signifies self receptor proteins); j. Networks of various adipokines and receptors; k. Ranking of adipokines and receptors (The darker node indicates a higher ranking); (all PPI enrichment p-value < 0.05)
Table 1.
The results of the Protein–Protein Interaction (PPI) networks
| Protein | Gene | Nodes | Edges | Average node degree | Average local clustering coefficient | Expected number of edges |
|---|---|---|---|---|---|---|
| Adiponectin | ADIPOQ | 11 | 44 | 8 | 0.881 | 14 |
| Leptin | LEP | 11 | 50 | 0.09 | 0.923 | 14 |
| Resistin | RETN | 11 | 39 | 7.09 | 0.862 | 15 |
| Apelin | APLN | 11 | 36 | 6.55 | 0.907 | 12 |
| Visfatin | NAMPT | 11 | 52 | 9.45 | 0.958 | 11 |
| Adipsin | CFD | 11 | 33 | 6 | 0.859 | 12 |
| Omentin | ITLN1 | 11 | 41 | 7.45 | 0.872 | 11 |
| Chemerin | RARRES2 | 11 | 37 | 6.73 | 0.796 | 11 |
| Meteorin-like protein | METRNL | 6 | 11 | 3.67 | 0.933 | 5 |
Limitation and considerations
Based on empirical evidence, among the adipokines discovered thus far, adiponectin, leptin, resistin, and apelin emerge as the most promising candidates for clinical application, particularly in the realms of myocardial protection and diabetes management. Yet, numerous potential avenues for further investigation persist, as the existing data exhibit several inconsistencies.
Elevated levels of adiponectin, known for its against inflammatory and cardioprotective effects, are linked to a declined risk of cardio damage in individuals with diabetes. Animal studies provide evidence supporting adiponectin as a cardioprotective protein in cardiovascular health [194], on the contrary, a two-year investigation found that heightened serum concentrations of adiponectin were linked to an increased likelihood of cardiovascular events leading to elevated mortality rates [195]. Besides, high-molecular-weight adiponectin is concerned with higher mortality in elder subjects compared with healthy middle-aged populations [196]. Furthermore, the expressed level of adiponectin receptors in human are influenced by gender, with males exhibiting significantly higher levels compared to females. Conversely, females demonstrate higher serum adiponectin levels than males [197]. Despite these conflicting associations, commonly referred to as the "adiponectin paradox" and previously elucidated factors such as renal dysfunction, adiponectin resistance, weight change, and hydrolysis of CDH13 [198, 199], adiponectin still remain a major puzzle in the field. Consequently, it is imperative to deepen the understanding of adiponectin's precise function and assess its potential to strike a balanced approach that minimizes diabetic myocardial I/R injury and mortality by explicitly targeting the cardiac adiponectin signaling pathway. Unlike other adipokines, adiponectin in blood does not seem to be affected by atorvastatin treatment in patients [200]. Lastly, adiponectin levels were independently associated with restenosis, but both HOMA-IR (Homeostatic model assessment of insulin resistance) and adiponectin were independently associated with de novo ischemic heart disease and the incidence of new percutaneous coronary interventions in patients with normal glucose tolerance [201].
The ob/ob and db/db rodent genotypes serve as representations of diabetes as a monogenic disorder, contrasting with the polygenic and multifactorial nature of human T2DM [202]. For instance, hyperglycemia progresses gradually and deteriorates over time in humans, while blood glucose levels exhibit transient and limited severity in ob/ob Mus musculus, with not all db/db Mus musculus experiencing the development of hyperglycemia [203, 204]. Consequently, although these models are valuable for investigation purposes, the findings may have limited applicability, especially in diabetic myocardial I/R injury. Leptin’s effects are currently incongruous and lacks comprehensive understanding, it is widely agreed upon that both elevated expression of leptin and leptin deficiency may have potential impacts for CVD. In-depth research endeavors are imperative in order to definitively ascertain whether these effects are being fine-tuned by distinct molecular signaling pathways or particular receptor isoforms. Certain hypoglycemic agents, including metformin and sodium-glucose cotransporter 2 (SGLT2) inhibitors, have been documented to enhance cardiac outcomes. Research indicates that metformin therapy, by reducing pericoronary fat levels, contributes to improved cardiovascular outcomes through the diminution of inflammatory markers, SGLT2, and leptin levels in individuals with pre-diabetes [205]. Furthermore, another study suggests that SGLT2 inhibitors may mitigate the inflammatory profile in patients with diabetes [206]. Take together, there appears to be a potential association between leptin and SGLT2.
Due to the insufficient availability of reliable and comprehensive data on resistin, it cannot be considered as a reliable independent predictor of either diabetes or CVD. However, a clinical trial showed that levels of several adipokines significantly changed in individuals suffering coronary artery bypass graft surgery with cardiopulmonary bypass, with concentrations of adiponectin and adipsin diminished, but levels of leptin and resistin significantly augmented within 24 h following the commencement of the operation [207]. Combination of several kinds of adipokines may act as a functional biomarker or risk predictor in I/R injury. Adiponectin-resistin (AR) index (fasting serum total adiponectin and resistin levels) and insulin resistance-AR (IRAR) index (integration of the AR index into an existing insulin resistance index) have been used to screen individuals with elevated risk of potential progress of T2DM and metabolic syndrome before [208], recent study found that both of them applies on cardiovascular risk in diabetic patients as well [209]. The indices pertaining to AR and IRAR underwent a marked and significant upsurge in diabetic group compared with control group and further analysis demonstrated that these indices calculated level of cardiovascular risk through area under the curve [101]. Besides, adipose-derived stem cells (ADSCs) are regarded as potential instruments for the replacement, repair, and regeneration of necrotic or impaired cells [210]. He et al. utilized ADSCs in a murine model of I/R injury, employing both resistin-treated and vehicle-treated ADSCs. Their findings indicated that ADSCs treated with resistin significantly enhanced myocardial ejection fraction and reduced myocyte apoptosis [134]. ADSCs have been the subject of numerous Phase I and II clinical trials, including the use of a transendocardial delivery system for administering stromal vascular fraction to the akinetic myocardial scar region [211], as well as intra-articular injections of allogeneic ADSCs for the treatment of knee osteoarthritis [212]. Nonetheless, the intravenous administration of ADSCs has demonstrated limited retention and survival rates of myocardial stem cells [213].
Apelin has been suggested as a novel biomarker for prognostication in myocardial ischemic patients with ST-segment elevation, with studies indicating that elevated plasma levels of apelin upon admission are in connection to a significant risk of mortality at the 6-month follow-up, thus augmenting the prognostic value provided by brain natriuretic peptide [214]. Certain researchers have posited that the endogenous release of the peptide may serve to mitigate the extent of an infarction [215] and the apelin/APJ system functions to mitigate imbalanced oxidative reaction between oxygen and lipid in mitochondrial by facilitating the formation of nitric oxide during myocardial reperfusion damage as well [216]. Furthermore, the clinical utility of apelin as a therapeutic agent is restricted by its brief half-life and the requirement for parenteral delivery. Various studies have been undertaken to investigate potential small molecule apelin agonists, yet only a few have been progressed to further evaluation [217]. Given that Apelin has been shown to interplay with caveolin in cardiomyocytes [218], it is possible that apelin may interact with adiponectin via caveolin in the context of myocardial I/R injury in diabetes, although further study is needed to test this hypothesis.
Conclusion
Taken together, adipokines should be regarded as prospective therapeutic targets for CVD, necessitating further research on optimizing adipokine levels to mitigate the systemic impact of adipokines on myocardial I/R injury in subjects with diabetes. It is advisable to begin monitoring the dynamic changes of blood adipokines in diabetic patients, given that the current investigation of various adipokines lacks a comprehensive analysis of preclinical and clinical data. Meanwhile, much deeper research is necessary to investigate potential molecular mechanisms underlying the co-occurrence of diabetes and myocardial I/R injury, mainly focusing on the interaction between oxidative response, lipid imbalance, and programmed cell death pathways. Developing small-molecule adipokine compounds, including agonists and inhibitors or synthetic adipokine analogs, is recommended to facilitate future clinical studies in this area.
Supplementary Information
Acknowledgement
The authors acknowledged VanScholar Editors Co. Ltd, Vancouver, Canada, for the assistance with the English editing.
Abbreviations
- CVD
Cardiovascular disease
- I/R
Ischemia/reperfusion
- WAT
White adipose tissue
- Metrnl
Meteorin-like
- JAK
Janus-activated kinase
- STAT3
Signal transducers and activators of transcription 3
- AMPK
AMP-activated protein kinase
- ROS
Reactive oxygen species
- RISK
Reperfusion Injury Signaling Kinase
- PI3K
Phosphoinositide 3-kinase
- Akt
Protein kinase B
- ERK1
Extracellular signal-regulated kinase 1
- ERK2
Extracellular signal-regulated kinase 2
- MAPK
Mitogen-activated protein kinases
- SAFE
Survivor Activating Factor Enhancement
- PTEN
Phosphatase and Tensin Homolog
- JAK2
Janus-activated kinase 2
- FoxO
The class O of Forkhead box
- CD36
Cluster of differentiation 36
- PKC
Protein kinase C
- HMGB1
High mobility group box1protein
- SIRT1
Sirtuin 1
- Nrf2
Nuclear factor E2-related factor 2
- HO-1
Heme oxygenase-1
- PPAR
Peroxisome proliferator-activated receptor
- p-Akt
Phosphorylated protein kinase B
- Enos
Endothelial nitric oxide synthase
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- MG53
Mitsugumin 53
- mPTP
Mitochondrial permeability transition pore
- AdipoR1
Adiponectin receptor 1
- AdipoR2
Adiponectin receptor 1
- CDH13
T-cadherin
- PPAR
Peroxisome proliferator-activated receptor
- p-Akt
Phosphorylated protein kinase B
- iNOS
Inducible nitric oxide synthase
- HIF-1α
Hypoxia-inducible factor-1α
- miRNA
MicroRNA
- LEPR
Leptin receptor
- PTP1B
Protein tyrosine phosphatase 1B
- SOC3
Suppressor of cytokine signaling 3
- CB
Cannabinoid
- TLR4
Toll-like receptor 4
- CAP1
Adenylyl cyclase-associated protein 1
- APJ
Apelin receptor
- PBEF
Pre-B cell colony-enhancing factor
- NAD+
Nicotinamide adenine dinucleotide
- CFD
Complement factor D
- RARRES2
Retinoic acid receptor responder protein 2
- CMKLR1
Chemokine-like receptor 1
- GPR1
G protein-coupled receptor 1
- CCRL2
C chemokine receptor-like 2
- PPI
Protein-Protein Interaction
- AR
Adiponectin-resistin
- IRAR
Insulin resistance-AR
- SGLT2
Sodium-glucose cotransporter 2
- ADSCs
Adipose-derived stem cells
Authors’ contributions
Ronghui Han: Literature search, data collection, chart design, and manuscript writing. Hemeng Huang: Literature search, data collection, and manuscript writing. Jianyu Zhu: Literature search, data collection and chart design. Xiaogao Jin: Literature search. Yongyan Wang: Chart design and data collection. Zhengyuan Xia: Supervision, manuscript revision and decision to submit. Youhua Xu: Manuscript revision and decision to submit. All authors read and approved the final manuscript. Hemeng Huang: Literature search, data collection, and manuscript writing. Jianyu Zhu: Literature search, data collection and chart design. Xiaogao Jin: Literature search. Yongyan Wang: Chart design and data collection. Zhengyuan Xia: Supervision, manuscript revision and decision to submit. Youhua Xu: Manuscript revision and decision to submit. All authors read and approved the final manuscript.
Funding
The project was funded by the National Natural Science Foundation of China (No.82270306) and the Science and Technology Development Fund of Macau (0055/2019/AMJ), National Key R&D Program of China (2019YFE0110500), and Research project of Guangdong Provincial Bureau of Traditional Chinese Medicine (20223016).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Youhua Xu, Email: yhxu@must.edu.mo.
Zhengyuan Xia, Email: zyxia@hku.hk.
References
- 1.Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2024;149(8):e347–913. [DOI] [PubMed] [Google Scholar]
- 2.Chang AJ, Liang Y, Hamilton SA, Ambrosy AP. Medical Decision-Making and Revascularization in Ischemic Cardiomyopathy. Med Clin North Am. 2024;108(3):553–66. [DOI] [PubMed] [Google Scholar]
- 3.Di Gioia G, Soto Flores N, Franco D, Colaiori I, Sonck J, Gigante C, et al. Coronary Artery Bypass Grafting or Fractional Flow Reserve-Guided Percutaneous Coronary Intervention in Diabetic Patients With Multivessel Disease. Circ Cardiovasc Interv. 2020;13(10): e009157. [DOI] [PubMed] [Google Scholar]
- 4.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 5.Heusch G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med. 2024;5(1):10–31. [DOI] [PubMed] [Google Scholar]
- 6.Francisco J, Del Re DP. Inflammation in Myocardial Ischemia/Reperfusion Injury: Underlying Mechanisms and Therapeutic Potential. Antioxidants (Basel). 2023;12(11):1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234. [DOI] [PMC free article] [PubMed]
- 8.Jiang Y, Cai Y, Han R, Xu Y, Xia Z, Xia W. Salvianolic acids and its potential for cardio-protection against myocardial ischemic reperfusion injury in diabetes. Front Endocrinol (Lausanne). 2024;12(14):1322474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babes EE, Bustea C, Behl T, Abdel-Daim MM, Nechifor AC, Stoicescu M, et al. Acute coronary syndromes in diabetic patients, outcome, revascularization, and antithrombotic therapy. Biomed Pharmacother. 2022;148: 112772. [DOI] [PubMed] [Google Scholar]
- 10.Pan J, Yin J, Gan L, Xue J. Two-sided roles of adipose tissue: Rethinking the obesity paradox in various human diseases from a new perspective. Obes Rev. 2023;24(1): e13521. [DOI] [PubMed] [Google Scholar]
- 11.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. 2019;16(2):83–99. [DOI] [PubMed] [Google Scholar]
- 12.Kaminska B, Kurowicka B, Kiezun M, Dobrzyn K, Kisielewska K, Gudelska M, et al. The Role of Adipokines in the Control of Pituitary Functions. Animals (Basel). 2024;14(2):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie L, Wang H, Hu J, Liu Z, Hu F. The role of novel adipokines and adipose-derived extracellular vesicles (ADEVs): Connections and interactions in liver diseases. Biochem Pharmacol. 2024;222: 116104. [DOI] [PubMed] [Google Scholar]
- 14.Polkinghorne MD, West HW, Antoniades C. Adipose Tissue in Cardiovascular Disease: From Basic Science to Clinical Translation. Annu Rev Physiol. 2024;12(86):175–98. [DOI] [PubMed] [Google Scholar]
- 15.Akoumianakis I, Antoniades C. The interplay between adipose tissue and the cardiovascular system: is fat always bad? Cardiovasc Res. 2017;113(9):999–1008. [DOI] [PubMed] [Google Scholar]
- 16.Semerena E, Nencioni A, Masternak K. Extracellular nicotinamide phosphoribosyltransferase: role in disease pathophysiology and as a biomarker. Front Immunol. 2023;17(14):1268756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue H, Zhang Q, Chang S, Zhao X, Wang M, Li W. Adiponectin protects against myocardial ischemia-reperfusion injury: a systematic review and meta-analysis of preclinical animal studies. Lipids Health Dis. 2024;23(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Yao S, Xia Z, Irwin MG. Adiponectin: mechanisms and new therapeutic approaches for restoring diabetic heart sensitivity to ischemic post-conditioning. Front Med. 2013;7(3):301–5. [DOI] [PubMed] [Google Scholar]
- 19.Omoto ACM, do Carmo JM, Nelson B, Aitken N, Dai X, Moak S, et al. Central Nervous System Actions of Leptin Improve Cardiac Function After Ischemia-Reperfusion: Roles of Sympathetic Innervation and Sex Differences. J Am Heart Assoc. 2022;11(21):e027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CC, Yellon DM. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Ther. 2011;129(2):206–19. [DOI] [PubMed] [Google Scholar]
- 21.Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin. 2018;39(7):1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsis A, Kadoglou NPE, Lambadiari V, Alexiou S, Theodoropoulos KC, Avraamides P, et al. Prognostic role of inflammatory cytokines and novel adipokines in acute myocardial infarction: An updated and comprehensive review. Cytokine. 2022;153: 155848. [DOI] [PubMed] [Google Scholar]
- 23.Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. [DOI] [PMC free article] [PubMed]
- 24.Vuori MA, Reinikainen J, Söderberg S, Bergdahl E, Jousilahti P, Tunstall-Pedoe H, et al. Diabetes status-related differences in risk factors and mediators of heart failure in the general population: results from the MORGAM/BiomarCaRE consortium. Cardiovasc Diabetol. 2021;20(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14(6):491–509. [DOI] [PubMed] [Google Scholar]
- 27.Ansley DM, Wang B. Oxidative stress and myocardial injury in the diabetic heart. J Pathol. 2013;229(2):232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Xia W, Chen J, Han K, Jiang Y, Zhang A, et al. Propofol and salvianolic acid A synergistically attenuated cardiac ischemia-reperfusion injury in diabetic mice via modulating the CD36/AMPK pathway. Burns Trauma. 2024;12:tkad055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han R, Huang H, Han H, Chen H, Zeng F, Xie X, et al. Propofol postconditioning ameliorates hypoxia/reoxygenation induced H9c2 cell apoptosis and autophagy via upregulating forkhead transcription factors under hyperglycemia. Mil Med Res. 2021;8(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai Y, Wu J, Yang Z, Wang X, Zhang D, Ma J. Mitochondrial quality control in cardiac ischemia/reperfusion injury: new insights into mechanisms and implications. Cell Biol Toxicol. 2023;39(1):33–51. [DOI] [PubMed] [Google Scholar]
- 31.Munkhjargal U, Fukuda D, Maeda J, Hara T, Okamoto S, Bavuu O, et al. LCZ696, an Angiotensin Receptor-Neprilysin Inhibitor, Ameliorates Endothelial Dysfunction in Diabetic C57BL/6 Mice. J Atheroscler Thromb. 2024;31(9):1333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bao XL, Dai Y, Lu L, Wang XQ, Ding FH, Shen WF, et al. Vasostatin-2 associates with coronary collateral vessel formation in diabetic patients and promotes angiogenesis via angiotensin-converting enzyme 2. Eur Heart J. 2023;44(19):1732–44. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zou J, Lin A, Chi J, Hao H, Chen H, et al. Oxidative stress, endothelial dysfunction, and N-acetylcysteine in type-2 diabetes mellitus. Antioxid Redox Signal. 2024;40(16–18):968–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo G, Savoia C. New Insights into Endothelial Dysfunction in Cardiometabolic Diseases: Potential Mechanisms and Clinical Implications. Int J Mol Sci. 2024;25(5):2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo I, Penna C, Musso T, Popara J, Alloatti G, Cavalot F, et al. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc Diabetol. 2017;16(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler M, Wang X, Peter K. Platelets in cardiac ischaemia/reperfusion injury: a promising therapeutic target. Cardiovasc Res. 2019;115(7):1178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manrique-Acevedo C, Hirsch IB, Eckel RH. Prevention of Cardiovascular Disease in Type 1 Diabetes. N Engl J Med. 2024;390(13):1207–17. [DOI] [PubMed] [Google Scholar]
- 38.Yang T, Zhang D. Research progress on the effects of novel hypoglycemic drugs in diabetes combined with myocardial ischemia/reperfusion injury. Ageing Res Rev. 2023;86: 101884. [DOI] [PubMed] [Google Scholar]
- 39.Asleh R, Sheikh-Ahmad M, Briasoulis A, Kushwaha SS. The influence of anti-hyperglycemic drug therapy on cardiovascular and heart failure outcomes in patients with type 2 diabetes mellitus. Heart Fail Rev. 2018;23(3):445–59. [DOI] [PubMed] [Google Scholar]
- 40.ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. [DOI] [PubMed]
- 41.Patterson B, Fields AV, Shannon RP. New insights into myocardial glucose metabolism: surviving under stress. Curr Opin Clin Nutr Metab Care. 2009;12(4):424–30. [DOI] [PubMed] [Google Scholar]
- 42.Tian H, Zhao X, Zhang Y, Xia Z. Abnormalities of glucose and lipid metabolism in myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2023;163:114827. [DOI] [PubMed] [Google Scholar]
- 43.Penna C, Andreadou I, Aragno M, Beauloye C, Bertrand L, Lazou A, et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br J Pharmacol. 2020;177(23):5312–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yellon DM, Beikoghli Kalkhoran S, Davidson SM. The RISK pathway leading to mitochondria and cardioprotection: how everything started. Basic Res Cardiol. 2023;118(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T, Mao X, Li H, Qiao S, Xu A, Wang J, et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med. 2013;63:291–303. [DOI] [PubMed] [Google Scholar]
- 46.Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan L, et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: role of the PI3K/Akt and JAK2/STAT3 pathways. Clin Sci (Lond). 2016;130(5):377–92. [DOI] [PubMed] [Google Scholar]
- 47.Gedik N, Kottenberg E, Thielmann M, Frey UH, Jakob H, Peters J, et al. Potential humoral mediators of remote ischemic preconditioning in patients undergoing surgical coronary revascularization. Sci Rep. 2017;7(1):12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res. 2012;110(1):111–5. [DOI] [PubMed] [Google Scholar]
- 49.Huang Q, Tian H, Tian L, Zhao Xs, Li L, Zhang YX, et al. Inhibiting Rev-erbα-mediated ferroptosis alleviates susceptibility to myocardial ischemia-reperfusion injury in type 2 diabetes. Free Radic Biol Med. 2023;209(Pt 1):135–50. [DOI] [PubMed] [Google Scholar]
- 50.Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol. 2020;39(2):210–25. [DOI] [PubMed] [Google Scholar]
- 51.Zhou D, Yang Y, Chen J, Zhou J, He J, Liu D, et al. N-acetylcysteine Protects Against Myocardial Ischemia-Reperfusion Injury Through Anti-ferroptosis in Type 1 Diabetic Mice. Cardiovasc Toxicol. 2024;24(5):481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryabov VV, Maslov LN, Vyshlov EV, Mukhomedzyanov AV, Kilin M, Gusakova SV, et al. Ferroptosis, a Regulated Form of Cell Death, as a Target for the Development of Novel Drugs Preventing Ischemia/Reperfusion of Cardiac Injury, Cardiomyopathy and Stress-Induced Cardiac Injury. Int J Mol Sci. 2024;25(2):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brennan S, Chen S, Makwana S, Martin CA, Sims MW, Alonazi ASA, et al. A novel form of glycolytic metabolism-dependent cardioprotection revealed by PKCα and β inhibition. J Physiol. 2019;597(17):4481–501. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F, Cao X, Zhao C, Chen L, Chen X. Empagliflozin activates JAK2/STAT3 signaling and protects cardiomyocytes from hypoxia/reoxygenation injury under high glucose conditions. J Thromb Thrombolysis. 2023;55(1):116–25. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z, et al. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med. 2020;24(12):6670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen C, Lu C, He D, Na N, Wu Y, Luo Z, et al. Inhibition of HMGB1 alleviates myocardial ischemia/reperfusion injury in diabetic mice via suppressing autophagy. Microvasc Res. 2021;138:104204. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Cai X, Zhang Q, Li X, Li S, Ma J, et al. Hydrogen sulfide restores sevoflurane postconditioning mediated cardioprotection in diabetic rats: Role of SIRT1/Nrf2 signaling-modulated mitochondrial dysfunction and oxidative stress. J Cell Physiol. 2021;236(7):5052–68. [DOI] [PubMed] [Google Scholar]
- 58.Yang K, Velagapudi S, Akhmedov A, Kraler S, Lapikova-Bryhinska T, Schmiady MO, et al. Chronic SIRT1 supplementation in diabetic mice improves endothelial function by suppressing oxidative stress. Cardiovasc Res. 2023;119(12):2190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan J, Guan Y, Mu F, Guo C, Zhang E, Yin Y, et al. Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci Rep. 2017;27(7):41491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gualillo O, González-Juanatey JR, Lago F. The emerging role of adipokines as mediators of cardiovascular function: physiologic and clinical perspectives. Trends Cardiovasc Med. 2007;17(8):275–83. [DOI] [PubMed] [Google Scholar]
- 61.Sato S. Adipo-oncology: adipocyte-derived factors govern engraftment, survival, and progression of metastatic cancers. Cell Commun Signal. 2024;22(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sardu C, Pieretti G, D’Onofrio N, Ciccarelli F, Paolisso P, Passavanti MB, et al. Inflammatory Cytokines and SIRT1 Levels in Subcutaneous Abdominal Fat: Relationship With Cardiac Performance in Overweight Pre-diabetics Patients. Front Physiol. 2018;21(9):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krauz K, Kempiński M, Jańczak P, Momot K, Zarębiński M, Poprawa I, et al. The Role of Epicardial Adipose Tissue in Acute Coronary Syndromes, Post-Infarct Remodeling and Cardiac Regeneration. Int J Mol Sci. 2024;25(7):3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Dai L, Dong Y, Ma C, Cheng P, Jiang C, et al. Coronary inflammation based on pericoronary adipose tissue attenuation in type 2 diabetic mellitus: effect of diabetes management. Cardiovasc Diabetol. 2024;23(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sardu C, Gatta G, Pieretti G, Viola L, Sacra C, Di Grezia G, et al. Pre-Menopausal Breast Fat Density Might Predict MACE During 10 Years of Follow-Up: The BRECARD Study. JACC Cardiovasc Imaging. 2021;14(2):426–38. [DOI] [PubMed] [Google Scholar]
- 66.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–9. [DOI] [PubMed] [Google Scholar]
- 67.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–703. [DOI] [PubMed] [Google Scholar]
- 68.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;221(2):286–9. [DOI] [PubMed] [Google Scholar]
- 69.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120(4):803–12. [DOI] [PubMed] [Google Scholar]
- 70.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16(5):141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: A potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151–8. [DOI] [PubMed] [Google Scholar]
- 73.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101(28):10308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siasos G, Tousoulis D, Kollia C, Oikonomou E, Siasou Z, Stefanadis C, et al. Adiponectin and cardiovascular disease: mechanisms and new therapeutic approaches. Curr Med Chem. 2012;19(8):1193–209. [DOI] [PubMed] [Google Scholar]
- 75.Lim S, Quon MJ, Koh KK. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis. 2014;233(2):721–8. [DOI] [PubMed] [Google Scholar]
- 76.Barr LA, Shimizu Y, Lambert JP, Nicholson CK, Calvert JW. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide. 2015;30(46):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han X, Wu Y, Liu X, Ma L, Li T, Sun Q, et al. Adiponectin improves coronary no-reflow injury by protecting the endothelium in rats with type 2 diabetes mellitus. Biosci Rep. 2017;37(4):BSR20170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tao L, Wang Y, Gao E, Zhang H, Yuan Y, Lau WB, et al. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res. 2010;106(2):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298(3):E663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rinaldi B, Di Filippo C, Capuano A, Donniacuo M, Sodano L, Ferraraccio F, et al. Adiponectin elevation by telmisartan ameliorates ischaemic myocardium in Zucker diabetic fatty rats with metabolic syndrome. Diabetes Obes Metab. 2012;14(4):320–8. [DOI] [PubMed] [Google Scholar]
- 81.Wang T, Qiao S, Lei S, Liu Y, Ng KF, Xu A, et al. N-acetylcysteine and allopurinol synergistically enhance cardiac adiponectin content and reduce myocardial reperfusion injury in diabetic rats. PLoS ONE. 2011;6(8):e23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao C, Liu HM, Li W, Wu Y, Leng Y, Xue R, et al. Role of adiponectin in diabetes myocardial ischemia-reperfusion injury and ischemic postconditioning. Acta Cir Bras. 2020;35(1):e202000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li H, Yao W, Liu Z, Xu A, Huang Y, Ma XL, et al. Hyperglycemia Abrogates Ischemic Postconditioning Cardioprotection by Impairing AdipoR1/Caveolin-3/STAT3 Signaling in Diabetic Rats. Diabetes. 2016;65(4):942–55. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Wang X, Jasmin JF, Lau WB, Li R, Yuan Y, et al. Essential role of caveolin-3 in adiponectin signalsome formation and adiponectin cardioprotection. Arterioscler Thromb Vasc Biol. 2012;32(4):934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sciarretta S, Frati G. The Importance of Restoring the Adiponectin Signaling Pathway to Reduce Myocardial Reperfusion Injury in Diabetes. Diabetes. 2016;65(4):826–8. [DOI] [PubMed] [Google Scholar]
- 86.Meng Z, Zhang Z, Zhao J, Liu C, Yao P, Zhang L, et al. Nitrative Modification of Caveolin-3: A Novel Mechanism of Cardiac Insulin Resistance and a Potential Therapeutic Target Against Ischemic Heart Failure in Prediabetic Animals. Circulation. 2023;147(15):1162–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Zhao J, Li R, Lau WB, Yuan YX, Liang B, et al. AdipoRon, the first orally active adiponectin receptor activator, attenuates postischemic myocardial apoptosis through both AMPK-mediated and AMPK-independent signalings. Am J Physiol Endocrinol Metab. 2015;309(3):E275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Liang B, Lau WB, Du Y, Guo R, Yan Z, et al. Restoring diabetes-induced autophagic flux arrest in ischemic/reperfused heart by ADIPOR (adiponectin receptor) activation involves both AMPK-dependent and AMPK-independent signaling. Autophagy. 2017;13(11):1855–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma Y, Liu Y, Liu S, Qu Y, Wang R, Xia C, et al. Dynamic alteration of adiponectin/adiponectin receptor expression and its impact on myocardial ischemia/reperfusion in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2011;301(3):E447–55. [DOI] [PubMed] [Google Scholar]
- 90.Pei H, Qu Y, Lu X, Yu Q, Lian K, Liu P, et al. Cardiac-derived adiponectin induced by long-term insulin treatment ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic mice via AMPK signaling. Basic Res Cardiol. 2013;108(1):322. [DOI] [PubMed] [Google Scholar]
- 91.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115(11):1408–16. [DOI] [PubMed] [Google Scholar]
- 92.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjöquist PO, et al. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMP-activated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78(1):116–22. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119(6):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA 3rd. Hypoxia inducible factor-1 upregulates adiponectin in diabetic mouse hearts and attenuates post-ischemic injury. J Cardiovasc Pharmacol. 2008;51(2):178–87. [DOI] [PubMed] [Google Scholar]
- 95.L’Abbate A, Neglia D, Vecoli C, Novelli M, Ottaviano V, Baldi S, et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293(6):H3532–41. [DOI] [PubMed] [Google Scholar]
- 96.Meng Z, Liang B, Wu Y, Liu C, Wang H, Du Y, et al. Hypoadiponectinemia-induced upregulation of microRNA449b downregulating Nrf-1 aggravates cardiac ischemia-reperfusion injury in diabetic mice. J Mol Cell Cardiol. 2023;182:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang L, Ding L, Yu S, Huang X, Ren Q. Propofol postconditioning alleviates diabetic myocardial ischemia-reperfusion injury via the miR-200c-3p/AdipoR2/STAT3 signaling pathway. Mol Med Rep. 2022;25(4):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, et al. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120(12):4342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. [DOI] [PubMed] [Google Scholar]
- 100.Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64(1):1–4. [DOI] [PubMed] [Google Scholar]
- 101.Misch M, Puthanveetil P. The Head-to-Toe Hormone: Leptin as an Extensive Modulator of Physiologic Systems. Int J Mol Sci. 2022;23(10):5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjørbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272(51):32686–95. [DOI] [PubMed] [Google Scholar]
- 103.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(Pt 1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–32. [DOI] [PubMed] [Google Scholar]
- 105.Fischer AW, Cannon B, Nedergaard J. Leptin: Is It Thermogenic? Endocr Rev. 2020;41(2):232–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stachura A, Khanna I, Krysiak P, Paskal W, Włodarski P. Wound Healing Impairment in Type 2 Diabetes Model of Leptin-Deficient Mice-A Mechanistic Systematic Review. Int J Mol Sci. 2022;23(15):8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes. 1996;104(4):293–300. [DOI] [PubMed] [Google Scholar]
- 108.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290(1):H146–53. [DOI] [PubMed] [Google Scholar]
- 109.Lv F, Wang Y, Shan D, Guo S, Chen G, Jin L, et al. Blocking MG53S255 Phosphorylation Protects Diabetic Heart From Ischemic Injury. Circ Res. 2022;131(12):962–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun D, Li S, Wu H, Zhang M, Zhang X, Wei L, et al. Oncostatin M (OSM) protects against cardiac ischaemia/reperfusion injury in diabetic mice by regulating apoptosis, mitochondrial biogenesis and insulin sensitivity. J Cell Mol Med. 2015;19(6):1296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang G, Cheng Z, Hildebrand A, Wang C, Cimini M, Roy R, et al. Diabetes impairs cardioprotective function of endothelial progenitor cell-derived extracellular vesicles via H3K9Ac inhibition. Theranostics. 2022;12(9):4415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He D, Liu D, Luo X, Chen C, Lu C, Na N, et al. HMGB1-RAGE axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte autophagy and apoptosis in diabetic mice. Biol Chem. 2023;405(3):167–76. [DOI] [PubMed] [Google Scholar]
- 113.Xue H, Yan G, Lin J, Hao X. Preliminary investigation of the changes and mechanism of Leptin after myocardial ischemia/reperfusion injury. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(11):680–3. [PubMed] [Google Scholar]
- 114.Xu T, Liu S, Wang X. Amelioration of myocardial ischemia/reperfusion injury by leptin pretreatment and ischemic preconditioning in mouse. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(2):105–8. [PubMed] [Google Scholar]
- 115.Xu S, Tao D. Leptin Alleviates Inflammatory Response in Myocardial Ischemia Reperfusion Injury. Dis Markers. 2022;9(2022):8707061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol. 2006;149(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7(1):22–9. [DOI] [PubMed] [Google Scholar]
- 118.Knudson JD, Payne GA, Borbouse L, Tune JD. Leptin and mechanisms of endothelial dysfunction and cardiovascular disease. Curr Hypertens Rep. 2008;10(6):434–9. [DOI] [PubMed] [Google Scholar]
- 119.Karmazyn M, Gan XT. Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin. Int J Mol Sci. 2024;25(2):1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Konstantinides S, Schäfer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108(10):1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Senesi P, Luzi L, Terruzzi I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int J Mol Sci. 2020;21(21):8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vilariño-García T, Polonio-González ML, Pérez-Pérez A, Ribalta J, Arrieta F, Aguilar M, et al. Role of Leptin in Obesity, Cardiovascular Disease, and Type 2 Diabetes. Int J Mol Sci. 2024;25(4):2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pedroso JAB, Silva IBD, Zampieri TT, Totola LT, Moreira TS, Taniguti APT, et al. SOCS3 Ablation in Leptin Receptor-Expressing Cells Causes Autonomic and Cardiac Dysfunctions in Middle-Aged Mice despite Improving Energy and Glucose Metabolism. Int J Mol Sci. 2022;23(12):6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhavana, Kohal R, Kumari P, Das Gupta G, Kumar Verma S. Druggable targets of protein tyrosine phosphatase Family, viz PTP1B, SHP2, Cdc25, and LMW-PTP: Current scenario on medicinal Attributes, and SAR insights. Bioorg Chem. 2024;144:107121. [DOI] [PubMed]
- 125.Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153(2):252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pacher P, Steffens S. The emerging role of the endocannabinoid system in cardiovascular disease. Semin Immunopathol. 2009;31(1):63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98(2):502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305(1):27–34. [DOI] [PubMed] [Google Scholar]
- 129.Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300(2):472–6. [DOI] [PubMed] [Google Scholar]
- 130.Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165(3):622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. [DOI] [PubMed] [Google Scholar]
- 132.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285(2):561–4. [DOI] [PubMed] [Google Scholar]
- 133.Kapłon-Cieślicka A, Tymińska A, Rosiak M, Ozierański K, Peller M, Eyileten C, et al. Resistin is a prognostic factor for death in type 2 diabetes. Diabetes Metab Res Rev. 2019;35(2): e3098. [DOI] [PubMed] [Google Scholar]
- 134.Saeedi Borujeni MJ, Esfandiary E, Taheripak G, Codoñer-Franch P, Alonso-Iglesias E, Mirzaei H. Molecular aspects of diabetes mellitus: Resistin, microRNA, and exosome. J Cell Biochem. 2018;119(2):1257–72. [DOI] [PubMed] [Google Scholar]
- 135.Gao J, Chang C, Chen Z, Wang H, Xu X, C Hamdy R, et al. Resistin, an adipocytokine, offers protection against acute myocardial infarction. J Mol Cell Cardiol. 2007;43(5):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He Y, Guo Y, Xia Y, Guo Y, Wang R, Zhang F, et al. Resistin promotes cardiac homing of mesenchymal stem cells and functional recovery after myocardial ischemia-reperfusion via the ERK1/2-MMP-9 pathway. Am J Physiol Heart Circ Physiol. 2019;316(1):H233–44. [DOI] [PubMed] [Google Scholar]
- 137.Smith CC, Lim SY, Wynne AM, Sivaraman V, Davidson SM, Mocanu MM, et al. Failure of the adipocytokine, resistin, to protect the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2011;16(1):63–71. [DOI] [PubMed] [Google Scholar]
- 138.Rothwell SE, Richards AM, Pemberton CJ. Resistin worsens cardiac ischaemia-reperfusion injury. Biochem Biophys Res Commun. 2006;349(1):400–7. [DOI] [PubMed] [Google Scholar]
- 139.Li C, Sun XN, Zhao S, Scherer PE. Crosstalk Between Adipose Tissue and the Heart: An Update. J Transl Int Med. 2022;10(3):219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–6. [DOI] [PubMed] [Google Scholar]
- 141.Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–71. [DOI] [PubMed] [Google Scholar]
- 142.Huang Z, Luo X, Liu M, Chen L. Function and regulation of apelin/APJ system in digestive physiology and pathology. J Cell Physiol. 2019;234(6):7796–810. [DOI] [PubMed] [Google Scholar]
- 143.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, et al. Molecular and functional characteristics of APJ Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275(28):21061–7. [DOI] [PubMed] [Google Scholar]
- 144.Wen R, Huang R, Xu K, Cheng Y, Yi X. Beneficial effects of Apelin-13 on metabolic diseases and exercise. Front Endocrinol (Lausanne). 2023;28(14):1285788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yamaleyeva LM, Shaltout HA, Varagic J. Apelin-13 in blood pressure regulation and cardiovascular disease. Curr Opin Nephrol Hypertens. 2016;25(5):396–403. [DOI] [PubMed] [Google Scholar]
- 146.Zeng G, Tang S, Jiang W, Yu J, Nie GY, Tang CK. Apelin-13: A Protective Role in Vascular Diseases. Curr Probl Cardiol. 2024;49(1 Pt B).102088 [DOI] [PubMed] [Google Scholar]
- 147.Kartal H, Comu FM, Kucuk A, Polat Y, Dursun AD, Arslan M. Effect of apelin-13 on erythrocyte deformability during ischaemia-reperfusion injury of heart in diabetic rats. Bratisl Lek Listy. 2017;118(3):133–6. [DOI] [PubMed] [Google Scholar]
- 148.Gunes I, Kartal H, Dursun AD, Sungu N, Polat YS, Erkent FD, et al. Effects of apelin-13 on myocardial ischemia reperfusion injury in streptozotocine induced diabetic rats. Bratisl Lek Listy. 2018;119(6):348–54. [DOI] [PubMed] [Google Scholar]
- 149.An S, Wang X, Shi H, Zhang X, Meng H, Li W, et al. Apelin protects against ischemia-reperfusion injury in diabetic myocardium via inhibiting apoptosis and oxidative stress through PI3K and p38-MAPK signaling pathways. Aging (Albany NY). 2020;12(24):25120–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang Y, Chang D, Lin K, Shin S, Lee Y. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27(6):515–27. [DOI] [PubMed] [Google Scholar]
- 151.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–30. [DOI] [PubMed] [Google Scholar]
- 152.Unlütürk U, Harmanci A, Yildiz BO, Bayraktar M. Dynamics of Nampt/visfatin and high molecular weight adiponectin in response to oral glucose load in obese and lean women. Clin Endocrinol (Oxf). 2010;72(4):469–74. [DOI] [PubMed] [Google Scholar]
- 153.Katsareli EA, Dedoussis GV. Biomarkers in the field of obesity and its related comorbidities. Expert Opin Ther Targets. 2014;18(4):385–401. [DOI] [PubMed] [Google Scholar]
- 154.Kärberg K, Forbes A, Lember M. Visfatin and Subclinical Atherosclerosis in Type 2 Diabetes: Impact of Cardiovascular Drugs. Medicina (Kaunas). 2023;59(7):1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kieswich J, Sayers SR, Silvestre MF, Harwood SM, Yaqoob MM, Caton PW. Monomeric eNAMPT in the development of experimental diabetes in mice: a potential target for type 2 diabetes treatment. Diabetologia. 2016;59(11):2477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Esteghamati A, Alamdari A, Zandieh A, Elahi S, Khalilzadeh O, Nakhjavani M, et al. Serum visfatin is associated with type 2 diabetes mellitus independent of insulin resistance and obesity. Diabetes Res Clin Pract. 2011;91(2):154–8. [DOI] [PubMed] [Google Scholar]
- 157.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105(5):481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE. 2014;9(6): e98972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hsu CP, Yamamoto T, Oka S, Sadoshima J. The function of nicotinamide phosphoribosyltransferase in the heart. DNA Repair (Amst). 2014;23:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xin B, Li P, Liu XL, Zhang XF. Visfatin relieves myocardial ischemia-reperfusion injury through activation of PI3K/Akt/HSP70 signaling axis. Eur Rev Med Pharmacol Sci. 2020;24(20):10779–89. [DOI] [PubMed] [Google Scholar]
- 161.Ju J, Li X, Zhao X, Li F, Wang S, Wang K, et al. Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J Biomed Sci. 2023;30(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li T, Yu S, Zhou C, Wang K, Wan YC. MicroRNA-206 inhibition and activation of the AMPK/Nampt signalling pathway enhance sevoflurane post-conditioning-induced amelioration of myocardial ischaemia/reperfusion injury. J Drug Target. 2020;28(1):80–91. [DOI] [PubMed] [Google Scholar]
- 163.Tur J, Badole SL, Manickam R, Chapalamadugu KC, Xuan W, Guida W, et al. Cardioprotective Effects of 1-(3,6-Dibromo-carbazol-9-yl)-3-Phenylamino-Propan-2-Ol in Diabetic Hearts via Nicotinamide Phosphoribosyltransferase Activation. J Pharmacol Exp Ther. 2022;382(2):233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237(4813):405–8. [DOI] [PubMed] [Google Scholar]
- 165.Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, et al. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244(4911):1483–7. [DOI] [PubMed] [Google Scholar]
- 166.White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, et al. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267(13):9210–3. [PubMed] [Google Scholar]
- 167.Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gómez-Banoy N, Guseh JS, Li G, Rubio-Navarro A, Chen T, Poirier B, et al. Adipsin preserves beta cells in diabetic mice and associates with protection from type 2 diabetes in humans. Nat Med. 2019;25(11):1739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jiang M, Man W, Zhang X, Zhang X, Duan Y, Lin J, et al. Adipsin inhibits Irak2 mitochondrial translocation and improves fatty acid β-oxidation to alleviate diabetic cardiomyopathy. Mil Med Res. 2023;10(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Man W, Song X, Xiong Z, Gu J, Lin J, Gu X, et al. Exosomes derived from pericardial adipose tissues attenuate cardiac remodeling following myocardial infarction by Adipsin-regulated iron homeostasis. Front Cardiovasc Med. 2022;12(9):1003282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hao S, Zhang J, Pei Y, Guo L, Liang Z. Complement factor D derived from epicardial adipose tissue participates in cardiomyocyte apoptosis after myocardial infarction by mediating PARP-1 activity. Cell Signal. 2023;101: 110518. [DOI] [PubMed] [Google Scholar]
- 172.Ohtsuki T, Satoh K, Shimizu T, Ikeda S, Kikuchi N, Satoh T, et al. Identification of Adipsin as a Novel Prognostic Biomarker in Patients With Coronary Artery Disease. J Am Heart Assoc. 2019;8(23): e013716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–61. [DOI] [PubMed] [Google Scholar]
- 174.Sena CM. Omentin: A Key Player in Glucose Homeostasis, Atheroprotection, and Anti-Inflammatory Potential for Cardiovascular Health in Obesity and Diabetes. Biomedicines. 2024;12(2):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vasamsetti SB, Natarajan N, Sadaf S, Florentin J, Dutta P. Regulation of cardiovascular health and disease by visceral adipose tissue-derived metabolic hormones. J Physiol. 2023;601(11):2099–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kataoka Y, Shibata R, Ohashi K, Kambara T, Enomoto T, Uemura Y, et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63(24):2722–33. [DOI] [PubMed] [Google Scholar]
- 177.Lin S, Li X, Zhang J, Zhang Y. Omentin-1: Protective impact on ischemic stroke via ameliorating atherosclerosis. Clin Chim Acta. 2021;517:31–40. [DOI] [PubMed] [Google Scholar]
- 178.Surace C, Piazzolla S, Sirleto P, Digilio MC, Roberti MC, Lombardo A, et al. Mild ring 17 syndrome shares common phenotypic features irrespective of the chromosomal breakpoints location. Clin Genet. 2009;76(3):256–62. [DOI] [PubMed] [Google Scholar]
- 179.Li Z, Gao Z, Sun T, Zhang S, Yang S, Zheng M, et al. Meteorin-like/Metrnl, a novel secreted protein implicated in inflammation, immunology, and metabolism: A comprehensive review of preclinical and clinical studies. Front Immunol. 2023;24(14):1098570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miao Z, Hu W, Li Z, Miao C. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41(12):1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) Attenuates Myocardial Ischemia/Reperfusion Injury-Induced Cardiomyocytes Apoptosis by Alleviating Endoplasmic Reticulum Stress via Activation of AMPK-PAK2 Signaling in H9C2 Cells. Med Sci Monit. 2020;28(26): e924564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lu Q, Ding Y, Liu Y, Wang Z, Wu Y, Niu K, et al. Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J Adv Res. 2023;51:161–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu Z, Ji H, Yao M, Wang L, Wang Y, Zhou P, et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J Cell Mol Med. 2019;23(1):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ferns GA, Fekri K, Shahini Shams Abadi M, Banitalebi Dehkordi M, Arjmand MH. A meta-analysis of the relationship between serums metrnl-like protein subfatin and risk of type 2 diabetes mellitus and coronary artery disease. Arch Physiol Biochem. 2023;129(5):1084–90. [DOI] [PubMed] [Google Scholar]
- 185.Lavis P, Bondue B, Cardozo AK. The Dual Role of Chemerin in Lung Diseases. Cells. 2024;13(2):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu L, Zhang J, Lu K, Zhang Y, Xu X, Deng J, et al. ChemR23 signaling ameliorates brain injury via inhibiting NLRP3 inflammasome-mediated neuronal pyroptosis in ischemic stroke. J Transl Med. 2024;22(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peng X, Wang W, Wang W, Qi J. Alpha-NETA, as a CMKLR1 Small Molecule Antagonist, Protects against Renal Ischemia Reperfusion Injury in Mice. Protein Pept Lett. 2022;29(11):962–70. [DOI] [PubMed] [Google Scholar]
- 188.Zhu Q, He G, Li H. Effect of Intestinal Ischemia-reperfusion Injury on the Expression of Chemerin in Mice] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37(4):440–5. [DOI] [PubMed]
- 189.Zou R, Wang M, Chen Y, Fan X, Yang B, Du J, et al. Hydrogen-Rich Saline Attenuates Acute Lung Injury Induced by Limb Ischemia/Reperfusion via Down-Regulating Chemerin and NLRP3 in Rats. Shock. 2019;52(1):134–41. [DOI] [PubMed] [Google Scholar]
- 190.Macvanin MT, Rizzo M, Radovanovic J, Sonmez A, Paneni F, Isenovic ER. Role of Chemerin in Cardiovascular Diseases. Biomedicines. 2022;10(11):2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ernst MC, Issa M, Goralski KB, Sinal CJ. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology. 2010;151(5):1998–2007. [DOI] [PubMed] [Google Scholar]
- 192.Liu R, Han Y, Huang C, Hou M, Cheng R, Wang S, et al. Adipocyte-derived chemerin rescues lipid overload-induced cardiac dysfunction. Science. 2023;26(4):106495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen D, Zhang Y, Yidilisi A, Xu Y, Dong Q, Jiang J. Causal Associations Between Circulating Adipokines and Cardiovascular Disease: A Mendelian Randomization Study. J Clin Endocrinol Metab. 2022;107(6):e2572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yi W, Sun Y, Gao E, Wei X, Lau WB, Zheng Q, et al. Reduced cardioprotective action of adiponectin in high-fat diet-induced type II diabetic mice and its underlying mechanisms. Antioxid Redox Signal. 2011;15(7):1779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Teoh H, Strauss MH, Szmitko PE, Verma S. Adiponectin and myocardial infarction: A paradox or a paradigm? Eur Heart J. 2006;27(19):2266–8. [DOI] [PubMed] [Google Scholar]
- 196.Kizer JR, Benkeser D, Arnold AM, Mukamal KJ, Ix JH, Zieman SJ, et al. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126(25):2951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fasshauer M, Paschke R, Stumvoll M. Adiponectin, obesity, and cardiovascular disease. Biochimie. 2004;86(11):779–84. [DOI] [PubMed] [Google Scholar]
- 198.Sattar N, Nelson SM. Adiponectin, diabetes, and coronary heart disease in older persons: unraveling the paradox. J Clin Endocrinol Metab. 2008;93(9):3299–301. [DOI] [PubMed] [Google Scholar]
- 199.Kalkman HO. An Explanation for the Adiponectin Paradox. Pharmaceuticals (Basel). 2021;14(12):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X, Zhang W, Zhao M, Jia G, Sun R. Effect of atorvastatin treatment on circulating adiponectin: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2019;18(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sasso FC, Pafundi PC, Marfella R, Calabrò P, Piscione F, Furbatto F, et al. Adiponectin and insulin resistance are related to restenosis and overall new PCI in subjects with normal glucose tolerance: the prospective AIRE Study. Cardiovasc Diabetol. 2019;18(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ktorza A, Bernard C, Parent V, Penicaud L, Froguel P, Lathrop M, et al. Are animal models of diabetes relevant to the study of the genetics of non-insulin-dependent diabetes in humans? Diabetes Metab. 1997;23(Suppl 2):38–46. [PubMed] [Google Scholar]
- 203.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10(2):131–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shafrir E, Ziv E, Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Ann N Y Acad Sci. 1999;18(892):223–46. [DOI] [PubMed] [Google Scholar]
- 205.Sardu C, D’Onofrio N, Torella M, Portoghese M, Mureddu S, Loreni F, et al. Metformin Therapy Effects on the Expression of Sodium-Glucose Cotransporter 2, Leptin, and SIRT6 Levels in Pericoronary Fat Excised from Pre-Diabetic Patients with Acute Myocardial Infarction. Biomedicines. 2021;9(8):904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sardu C, Massetti M, Testa N, Martino LD, Castellano G, Turriziani F, et al. Effects of Sodium-Glucose Transporter 2 Inhibitors (SGLT2-I) in Patients With Ischemic Heart Disease (IHD) Treated by Coronary Artery Bypass Grafting via MiECC: Inflammatory Burden, and Clinical Outcomes at 5 Years of Follow-Up. Front Pharmacol. 2021;15(12): 777083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Laurikka A, Vuolteenaho K, Toikkanen V, Rinne T, Leppänen T, Tarkka M, et al. Adipocytokine resistin correlates with oxidative stress and myocardial injury in patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2014;46(4):729–36. [DOI] [PubMed] [Google Scholar]
- 208.Lau CH, Muniandy S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol. 2011;21(10):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Habib SS, Al-Khlaiwi T, Butt MA, Habib SM, Al-Khliwi H, Al-Regaiey K. Novel Adiponectin-Resistin Indices and Ratios Predict Increased Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus. J Saudi Heart Assoc. 2023;35(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang T, Li T, Niu X, Hu L, Cheng J, Guo D, et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct. 2023;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Comella K, Parcero J, Bansal H, Perez J, Lopez J, Agrawal A, et al. Effects of the intramyocardial implantation of stromal vascular fraction in patients with chronic ischemic cardiomyopathy. J Transl Med. 2016;14(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen CF, Hu CC, Wu CT, Wu HH, Chang CS, Hung YP, et al. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res Ther. 2021;12(1):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bobi J, Solanes N, Fernández-Jiménez R, Galán-Arriola C, Dantas AP, Fernández-Friera L, et al. Intracoronary Administration of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells Improves Myocardial Perfusion But Not Left Ventricle Function, in a Translational Model of Acute Myocardial Infarction. J Am Heart Assoc. 2017;6(5):e005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sans-Roselló J, Casals G, Rossello X, González de la Presa B, Vila M, Duran-Cambra A, et al. Prognostic value of plasma apelin concentrations at admission in patients with ST-segment elevation acute myocardial infarction. Clin Biochem. 2017;50(6):279–84. [DOI] [PubMed] [Google Scholar]
- 215.Rastaldo R, Cappello S, Folino A, Losano G. Effect of apelin-apelin receptor system in postischaemic myocardial protection: a pharmacological postconditioning tool? Antioxid Redox Signal. 2011;14(5):909–22. [DOI] [PubMed] [Google Scholar]
- 216.Chen Z, Wu D, Li L, Chen L. Apelin/APJ System: A Novel Therapeutic Target for Myocardial Ischemia/Reperfusion Injury. DNA Cell Biol. 2016;35(12):766–75. [DOI] [PubMed] [Google Scholar]
- 217.Chapman FA, Maguire JJ, Newby DE, Davenport AP, Dhaun N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc Res. 2023;119(17):2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wu D, Xie F, Xiao L, Feng F, Huang S, He L, et al. Caveolin-1-Autophagy Pathway Mediated Cardiomyocyte Hypertrophy Induced by Apelin-13. DNA Cell Biol. 2017;36(8):611–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.