Abstract
Summary
Diabetic ketoacidosis (DKA) is a complication of diabetes mellitus (DM) that can theoretically occur in people of any age. While DKA can typically be the first presentation of type 1 DM in younger people, a first presentation is rare in older adults. Pancreatic cancer often manifests with new DM or hyperglycaemia, but very rarely as DKA. We report a case of an 89-year-old woman who was incidentally diagnosed with DKA during workup for an unwitnessed fall. Her DKA was promptly managed, and she was subsequently diagnosed with metastatic pancreatic cancer. Given the advanced stage of her malignancy, the multidisciplinary team consensus was for a palliative approach. She passed away on day 10 of the admission. To our knowledge, this is the first report of a first DKA presentation as a manifestation of pancreatic cancer in an adult aged over 70 years. To date, there is no effective screening test for pancreatic cancer in the general population. However, new-onset DM in the appropriate context might indicate the need for further evaluation. While it is possible that unresectable tumours are identified, earlier diagnosis of DM with pancreatic cancer may facilitate more timely management, including earlier advanced care planning.
Learning points
A higher clinical suspicion for pancreatic cancer is required for older adults presenting with diabetic ketoacidosis without a previously diagnosed diabetes mellitus.
A bi-directional relationship exists between diabetes and pancreatic cancer.
Pancreatic cancer generally has a very poor prognosis due to its advanced stage at diagnosis and the lack of an effective screening test.
New-onset diabetes in the appropriate context (such as weight loss) can indicate the need for further evaluation for underlying pancreatic cancer.
Keywords: Diabetes, Rare diseases/syndromes, Gastrointestinal tract
Background
Diabetic ketoacidosis (DKA) is a life-threatening complication of diabetes mellitus (DM). While it is more common in younger individuals, DKA has been observed in all age groups (1). In children and young adults, DKA usually occurs in the context of inadequate insulin therapy (either omitted doses or insufficient regimen) and new-onset DM. In contrast, in older adults, DKA is relatively uncommon and associated with higher mortality rates. In this group, DKA is usually precipitated by infection and is rarely found at the first presentation of newly detected DM.
Pancreatic adenocarcinoma is an uncommon cancer with an annual incidence of 12.9 cases per 100 000 person-years (2). It has one of the poorest cancer-related prognoses, with an overall 5-year survival rate of 5% (3). While new DM and hyperglycaemia are frequently seen in pancreatic cancer, their ability to identify a high-risk population for cancer screening is controversial (4). Here, we present a unique case of an elderly woman with the first presentation of DM as DKA, who was subsequently diagnosed with metastatic pancreatic cancer. As DKA is a rare presentation of DM in older adults, a high clinical suspicion for less common underlying conditions, including pancreatic cancer, is required in this setting.
Case presentation
An 89-year-old, pre-morbidly independent woman presented to the hospital following an unwitnessed fall at home. She denied any medical history, including previous falls, or taking regular medications. She had full recollection of the event; she fell with a head strike after her legs gave way. There was no syncope, presyncope, nor localising infective symptoms. However, she reported non-specific abdominal pain and constipation for the 2 weeks prior to admission. On further questioning, she reported functional decline and weight loss in the preceding months. Her weight was 45 kg on admission, down from 60 kg a year ago.
Initial examination showed normal vital signs. She was afebrile and fully orientated. She appeared hypovolaemic clinically. There was mild lower abdominal tenderness but no signs of peritonism. There was no evidence of traumatic injury nor abnormalities on other examinations.
Investigation
Initial workup unexpectedly revealed severe DKA with pH 7.12, serum glucose 27.0 mmol/L, beta-hydroxybutyrate ketones 7.0 mmol/L, and bicarbonate 6 mmol/L. She did not have biochemical evidence of concurrent hyperosmolar-hyperglycaemic state (serum osmolality 308 mmol/kg). Additional investigations, including full blood count, renal function, ECG, and cranial CT, were unremarkable.
Given the unusual first DKA presentation in an elderly person, further investigations for underlying precipitants were performed and summarised in Table 1. Her liver function tests showed severe cholestatic-pattern derangement. Lipase was within normal limits. C-peptide was inappropriately low (0.13 nmol/L) despite the presence of hyperglycaemia (paired serum glucose 17.2 mmol/L). Glycated haemoglobin (HbA1c) was markedly elevated at 14.8% (138 mmol/mol), and islet-autoantibodies were negative for autoimmune DM.
Table 1.
Laboratory results.
| Laboratory test | Result | Reference |
|---|---|---|
| Liver function tests and lipase | ||
| ALT (U/L) | 519 | 5–35 |
| AST (U/L) | 479 | <31 |
| ALP (U/L) | 2412 | 30–100 |
| GGT (U/L) | 2735 | <37 |
| Bilirubin (μmol/L) | 79 | <21 |
| Lipase (U/L) | 17 | 8–78 |
| Diabetes-related biochemistry and IA-Ab | ||
| HbA1c (%) | 14.8 | <6.5 |
| Glucose (mmol/L) | 17.2 | 3.3–7.7 |
| C-peptide (nmol/L) | 0.13 | 0.26–1.73 |
| Anti-GAD Ab (U/mL) | <5.0 | <5.0 |
| Anti-IA2 Ab (U/mL) | <7.5 | <7.5 |
| Anti-insulin Ab (U/mL) | <0.4 | <0.4 |
| Anti-ZnT8 Ab (RU/mL) | <10 | <10 |
| Tumour markers | ||
| CA 19-9 (U/mL) | >121 000 | <37 |
| CEA (μg/L) | 3557 | <5.0 |
Anti-GAD Ab, anti-glutamic-acid-decarboxylase antibody; Anti-IA2 Ab, anti-islet-antigen-2 antibody; Anti-ZnT8 Ab, anti-zinc-transporter-8 antibody; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; CA 19-9, cancer antigen 19-9; CEA, carcinoembryonic antigen; GGT, gamma glutamyl transferase; HbA1c, glycated haemoglobin; IA-Ab, islet auto-antibodies.
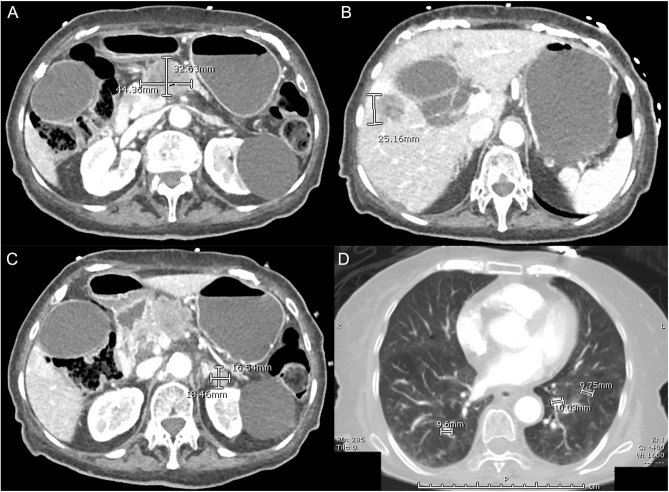
Our patient proceeded to further imaging. CT of her chest, abdomen and pelvis (Fig. 1) demonstrated a 4.4 × 3.3-cm necrotic pancreatic body mass with multiple intrahepatic, adrenal and pulmonary metastatic lesions. Tumour markers revealed marked CA 19-9 elevation (>121 000 U/mL), consistent with pancreatic adenocarcinoma.
Figure 1.
CT images demonstrating (A) pancreatic mass, (B) liver metastasis, (C) adrenal metastasis and (D) pulmonary metastases.
Treatment
Our patient was commenced on intravenous insulin infusion after adequate fluid and potassium replacement. She achieved DKA resolution within 12 h of commencement, requiring a total of 48 units of insulin neutral (Actrapid®) prior to transition to subcutaneous insulin.
Given the advanced stage of her metastatic pancreatic cancer and poor functional status, our patient was not suitable for surgical intervention or systemic therapies. The multidisciplinary team consensus was for a palliative approach. Tissue biopsy was offered but not performed due to its invasive nature. Biliary stenting was initially considered; however, due to the lack of significant symptoms of biliary obstruction, this was not pursued. She was managed with the best supportive care.
Outcome and follow-up
The patient deteriorated over the next few days with reduced consciousness and oral intake. Symptoms were adequately managed with subcutaneous analgesics and antiemetics. She passed away on day 10 of the admission in the presence of her family.
Discussion
This case highlights severe pancreatic endocrine dysfunction, which can occur with concomitant pancreatic exocrine malignancy. There are very few case reports of DKA as the initial presentation of pancreatic adenocarcinoma; however, this phenomenon has not been previously reported in an elderly person. In the six other case reports, including four with metastatic disease, patient ages ranged from 36 to 70 years, and their HbA1c levels were between 9.7% and 14.1% (82.5 and 130.6 mmol/mol) at the time of diagnosis. Our case demonstrates that the first DKA presentation in older adults should prompt comprehensive investigations to exclude sinister underlying conditions, including pancreatic cancer.
Pancreatic adenocarcinoma is a cancer of older adults and is typically diagnosed in the seventh and eighth decades of life (3). Most cases are detected at advanced stages when surgical interventions offer no survival benefit. Importantly, 85–90% of patients have no known familial or predisposing genetic syndrome. Major non-familial risk factors for pancreatic cancer include cigarette smoking, heavy alcohol consumption, chronic pancreatitis, obesity and DM (5). Despite the poor prognosis, there are currently no accurate and validated biomarkers for early detection of pancreatic cancer (2).
The literature suggests a bi-directional relationship between DM and pancreatic cancer. DM and insulin resistance are independent risk factors for pancreatic cancer. A prospective study in Finland showed that clinical DM and hyperinsulinaemia were associated with a two-fold increased risk of pancreatic cancer after adjustment for confounders (6). The precise mechanism remains unclear. The reduction in plasma adiponectin, an adipocyte-derived hormone with insulin-sensitising and anti-inflammatory properties, in the setting of DM could play a role in tumorigenesis (7). In addition, an in vitro study suggested that chronic hyperinsulinaemia may directly induce pancreatic intra-epithelial neoplasia (8).
On the other hand, DM can be precipitated by pancreatic cancer, possibly from islet cell damage. An American multi-centre prospective study revealed that DM was more prevalent in pancreatic cancer and more likely to be newly diagnosed in this setting (4). It was hypothesised that paraneoplastic adrenomedullin (AM) secretion could contribute to the development of pancreatic cancer-related DM. AM is a multifunctional hormone, expressed in many tissues including the pancreas. In vitro, its expression was upregulated in pancreatic cancer cell lines, with an inhibitory effect on beta-cell insulin secretion. In the same study, AM overexpression in mice with orthotopically implanted pancreatic cancer led to glucose intolerance (9). The observations of DKA in both locally invasive and metastatic pancreatic cancer could further support the role of paraneoplastic AM secretion, in addition to tumour mass effect, in the pathogenesis of DM.
The role of new-onset DM in the early diagnosis of pancreatic cancer is controversial. The United States Preventive Services Task Force did not recommend screening for pancreatic cancer in asymptomatic older adults with new-onset diabetes (2). On the other hand, the National Institute for Health and Care Excellence recently revised their guideline in 2022 and recommended urgent imaging to exclude pancreatic cancer for new-onset diabetes in people aged over 60 with weight loss (10). As demonstrated in our case, considering additional clinical factors, including i) first DKA presentation and ii) DM diagnosis in an elderly person, may enhance risk stratification for pancreatic cancer in adults with new-onset DM. While it is possible that unresectable tumours are identified, earlier diagnosis of DM with pancreatic cancer is likely to facilitate more timely management, including earlier advanced care planning.
In conclusion, we report a unique case of an octogenarian with a first DKA presentation secondary to undiagnosed metastatic pancreatic cancer. A high clinical suspicion for rare underlying conditions such as pancreatic cancer is required for older adults with new-onset DKA. To date, there is no effective screening test for pancreatic cancer in the general population; however, new-onset DM in the appropriate context might indicate the need for further investigation.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for the publication of their clinical details and/or clinical images was obtained from the relative of the patient.
Author contribution statement
ML: manuscript writing and literature search; SF and RB: manuscript revision; and RB was the primary physician of the patient.
References
- 1.Benoit SR Zhang Y Geiss LS Gregg EW & Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality - United States, 2000–2014. MMWR. Morbidity and Mortality Weekly Report 2018. 67 362–365. ( 10.15585/mmwr.mm6712a3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry SJ, Doubeni CA, Epling JW, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA 2019. 322 438–444. ( 10.1001/jama.2019.10232) [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 P ancreatic C ancer C ollaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterology and Hepatology 2019. 4 934–947. ( 10.1016/S2468-1253(1930347-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pannala R Leirness JB Bamlet WR Basu A Petersen GM & Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008. 134 981–987. ( 10.1053/j.gastro.2008.01.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capasso M, Franceschi M, Rodriguez-Castro KI, Crafa P, Cambie G, Miraglia C, Barchi A, Nouvenne A, Leandro G, Meschi T, et al. Epidemiology and risk factors of pancreatic cancer. Acta Biologica et Medica 2018. 89 141–146. ( 10.23750/abm.v89i9-S.7923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stolzenberg-Solomon RZ Graubard BI Chari S Limburg P Taylor PR Virtamo J & Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005. 294 2872–2878. ( 10.1001/jama.294.22.2872) [DOI] [PubMed] [Google Scholar]
- 7.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, Buring JE, Sesso HD, Lee IM, Gaziano JM, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. Journal of the National Cancer Institute 2013. 105 95–103. ( 10.1093/jnci/djs474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang AMY, Chu KH, Daly BF, Ruiter T, Dou Y, Yang JCC, de Winter TJJ, Chhuor J, Wang S, Flibotte S, et al. Effects of hyperinsulinemia on pancreatic cancer development and the immune microenvironment revealed through single-cell transcriptomics. Cancer and Metabolism 2022. 10 5. ( 10.1186/s40170-022-00282-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal G, Ramachandran V, Javeed N, Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ, et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 2012. 143 1510–1517.e1. ( 10.1053/j.gastro.2012.08.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Type 1 Diabetes in Adults: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE) 2022. (NICE Guideline, No. 17.). Available at: https://www.nice.org.uk/guidance/ng17/chapter/Recommendations. [Google Scholar]



 This work is licensed under a
This work is licensed under a