Abstract
Summary
Cushing's disease (CD) is characterized by distinct syndromic features, often accompanied by obesity and depression. However, considering its gradual onset of symptoms, it is usually associated with diagnostic delays. In rare instances, CD may lead to severe infections due to the observed immunosuppression in affected individuals. We present a rare case of an undiagnosed CD in a 20-year-old male with a medical history of depression and obesity, complicated by severe COVID-19 infection. He presented to the Emergency Room with respiratory distress, hypertensive crisis, and fever, ultimately receiving the diagnosis of SARS-CoV-2 pneumonia. The patient required mechanical ventilation and intensive care unit (ICU) admission due to severe acute respiratory distress syndrome (ARDS). During ICU care, he received remdesivir and dexamethasone, subsequently developing severe hyperglycemia and worsened hypertension, requiring insulin and multiple antihypertensive agents to manage metabolic disruption. Upon physical examination, classic signs of hypercortisolism were noted. Subsequent laboratory tests and pituitary magnetic resonance imaging confirmed the diagnosis of CD. The patient underwent surgical resection with significant improvements in body composition and metabolic parameters postoperatively. After surgery, remission of hypercortisolism was evident, accompanied by notable improvements in mood and overall health. This case underscores the importance of recognizing hypercortisolism in the context of metabolic, physical, and mood changes. Timely diagnosis of CD is crucial to mitigate complications such as severe opportunistic infections and their outcomes.
Learning points
Despite some hallmark features such as proximal myopathy, easy bruising, purple striae, and facial plethora, Cushing’s disease (CD) is a challenging diagnosis due to its nonspecific signs and symptoms and gradual onset.
The case emphasizes the importance of recognizing subtle signs of CD, such as social isolation, depressive symptoms, and changes in body composition, which may be confounded by external factors like the COVID-19 pandemic.
Patients with CD are prone to severe infections due to chronic hypercortisolism-induced immunosuppression.
CD diagnostic delays are common, leading to worsening of metabolic and immune dysfunction over time. Heightened clinical suspicion and early intervention are essential to prevent diagnostic delays and optimize patient outcomes.
Keywords: Cushing's Disease, Covid-19, Immunosuppression, ARDS
Background
Cushing’s disease (CD) stands as the predominant cause of endogenous hypercortisolism, stemming from an exaggerated pathological response to ACTH stimulation caused by a pituitary tumor (1, 2). While proximal myopathy, purple striae, easy bruising, and facial plethora serve as hallmark features of hypercortisolism, CD often manifests with additional clinical manifestations such as obesity and depression, which frequently evade recognition by both patients and clinicians (1). Furthermore, the gradual development of signs and symptoms over months to years complicates the definitive diagnosis, contributing to diagnostic delays in the majority of cases. Immunosuppression commonly accompanies CD, with chronic hypercortisolism often resulting in alterations in leukocyte function, thereby heightening vulnerability to opportunistic and severe bacterial, fungal, and viral infections (3, 4, 5, 6).
The COVID-19 pandemic, due to the global spread of the SARS-CoV-2 virus between January 2020 and June 2021, presented a significant health challenge. According to previous evidence, approximately 5% of patients developed ARDS related to SARS-CoV-2 infection, leading to severe illness and increased mortality (7). Severe COVID-19 disease was more prevalent among the elderly, obese individuals, those with type 2 diabetes, and immunosuppressed patients (8). Here, we present a rare case of a previously undiagnosed young man with CD, whose diagnosis was proposed following admission with ARDS due to SARS-CoV-2 infection.
Case presentation
The authors report the case of a 20-year-old male with a previous medical history of depression and obesity with 1 year of evolution. The patient's mood fluctuations and weight changes prompted a pattern of social isolation, lasting several months before seeking outpatient assistance from Psychiatry and Nutrition clinics. Additionally, he exhibited abdominal striae, assumed in the context of weight gain, prompting a dermatology consultation that led to topical treatment with glucocorticoids.
The patient was admitted to the emergency room displaying clinical signs of respiratory distress, hypertensive crisis, fever (39°C), and cough within the preceding 3 days. Initial evaluation revealed a high respiratory rate, abnormal breath sounds with bilateral crackles, and peripheral tissue desaturation. Laboratory tests indicated leukocytosis with lymphopenia, elevated C-reactive protein levels, and a positive SARS-CoV-2 test result (Table 1). Thoracic radiographic imaging revealed bilateral airspace opacities consistent with ground-glass opacities suggestive of pneumonia.
Table 1.
Laboratory data at the admission to the emergency department.
| Laboratory test | Result | Reference range |
|---|---|---|
| Hemoglobin (g/dL) | 13.1 | 12.0–15.0 |
| Mean corpuscular volume (fL) | 89.4 | 80.0–96.1 |
| White blood cells (×109/L) | 17.2 | 4.0–10.0 |
| Platelets (109/L) | 162 | 150–400 |
| Sodium (mmol/L) | 142 | 136–145 |
| Potassium (mmol/L) | 4.2 | 3.5–5.1 |
| Phosphate (mg/dL) | 2.8 | 2.5–4.5 |
| Magnesium (mg/dL) | 1.9 | 1.7–2.2 |
| Calcium (mg/dL) | 9.1 | 8.6–10.0 |
| Albumin (g/L) | 3.5 | 3.5–5.2 |
| Creatinine (mg/dL) | 0.7 | 0.5–0.9 |
| GFR (mL/min/1.73 m2) | 89 | >60 |
| Glucose (mg/dL) | 215 | 74–110 |
| C-reactive-protein (mg/dL) | 12.4 | <0.1 |
| Hemoglobin A1c (%) | 7.9 | <5.7% |
| Triglycerides (mg/dL) | 211 | <150 |
| Total cholesterol (mg/dL) | 217 | <200 |
| HDL cholesterol (mg/dL) | 43 | >45 |
| LDL cholesterol (mg/dL) | 132 | <116 |
GFR, glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein.
Due to rapid respiratory deterioration despite initial oxygen supplementation, the patient underwent endotracheal intubation, mechanical ventilation, and transfer to the intensive care unit (ICU) with the diagnosis of ARDS secondary to SARS-CoV-2 infection. In the ICU, the patient received medical treatment with antiviral therapy (remdesivir) and systemic glucocorticoids (dexamethasone, 6 mg) according to international recommendations. However, severe hyperglycemia and hypertension ensued, requiring insulin infusion therapy and the administration of three antihypertensive agents to manage the metabolic disturbance. Dexamethasone was discontinued after 2 days to mitigate these complications. Subsequently, the patient showed clinical improvement, leading to successful extubation and transfer to the internal medicine department.
Investigation
Despite clinical improvement, additional features suggestive of hypercortisolism were noted, including moon facies, facial plethora, abdominal striae, proximal myopathy, alopecia, and ecchymoses. Given the high specificity of these findings, a comprehensive biochemical analysis was performed stemming from a high clinical suspicion of an endogenous hypercortisolism status before the introduction of oral glucocorticoids (Table 2).
Table 2.
Laboratory data after clinical stabilization (before the introduction of oral glucocorticoids).
| Laboratory test | Result | Reference range |
|---|---|---|
| 1st 24-h urinary cortisol (μg/24 h) | 416.0 | 8.0–63.0 |
| 2nd 24-h urinary cortisol (μg/24 h) | 389.2 | 8.0–63.0 |
| Morning (08:00 h)/late night (23:00 h) cortisol (μg/dL) | 19.7/29.9 | 6.2–19.4 |
| Morning (08:00 h) ACTH (pg/mL) | 134.0 | 7.2–63.3 |
| Prolactin (ng/mL) | 37.2 | 4.8–23.3 |
| Follicle-stimulating hormone (U/L) | 3.9 | 1.5–12.4 |
| Luteinizing hormone (U/L) | 5.8 | 1.7–8.6 |
| Thyroid-stimulating hormone (μUI/mL) | 2.220 | 0.270–4.200 |
| Free thyroxine (T4) (pmol/L) | 17.6 | 12.0–22.0 |
| Insulin-like growth factor-1 (IGF-1) | 145 | 115–340 |
| Total testosterone (ng/dL) | 789 | 249–836 |
The 24-h urinary-free cortisol measurements suggested a condition of hypercortisolism, with a urinary level higher than five times the upper limit of the normal range. Moreover, the biochemical profile showed cortisol rhythm reversal and elevated serum ACTH levels, indicating ACTH-dependent Cushing’s syndrome.
Magnetic resonance imaging of the pituitary gland revealed a 10.1 mm space-occupying lesion with hypointensity on T1-weighted images and lack of gadolinium enhancement within the right hemi-hypophysis, causing deviation of the pituitary stalk to the left (Fig. 1). The 8 mg dexamethasone suppression test confirmed the pituitary origin of the ACTH excess, suggesting the diagnosis of CD.
Figure 1.
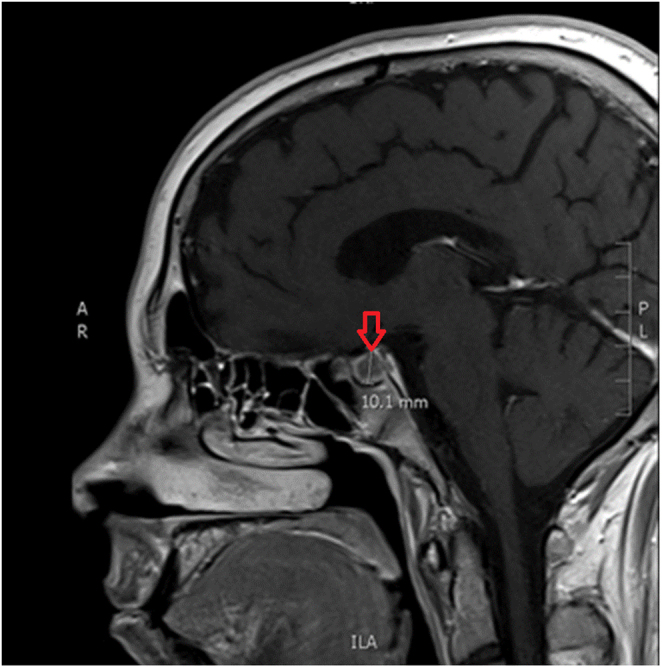
Magnetic resonance imaging revealing a 10.1 mm pituitary lesion (red row).
Treatment
The patient was promptly referred for surgical resection following current treatment guidelines, considering his age and the observed severe metabolic complications. Elective transsphenoidal surgery was performed and the pathological exam revealed the resection of a pituitary 10.1 mm corticotroph adenoma with a Ki67 of 2%. Analytical re-evaluation 1 week postoperatively confirmed remission of hypercortisolism, with 08:00 h cortisol levels of 1.8 μg/dL, and the patient requiring exogenous glucocorticoid treatment. The patient recovered full function of the hypothalamic-pituitary-adrenal axis after 9 months.
Outcome and follow-up
Notable improvements in body composition and metabolic parameters, including weight loss, blood pressure normalization, dyslipidemia improvement, and mood disorder amelioration, were observed 1 year after surgery. During the initial 2-year follow-up, the patient has remained in remission from hypercortisolism.
Discussion
We present a rare case of a 20-year-old man with undiagnosed CD, whose initial clinical presentation involved severe COVID-19 infection. Previous meta-analyses and reviews have indicated that severe ARDS secondary to SARS-CoV-2 infection is more frequently observed among patients with comorbidities such as advanced age, obesity, diabetes, and pre-existing respiratory conditions like asthma and chronic obstructive pulmonary disease (9). Given the patient's young age and lack of significant medical history, the development of ARDS following SARS-CoV-2 infection was deemed unusual, prompting suspicion of an underlying secondary cause.
There is reliable evidence stating that the severity of SARS-CoV-2 infection is associated with an inadequately regulated immune response characterized by a pro-inflammatory pattern, potentially leading to a prominent cytokine burden. Consequently, glucocorticoid therapy has been recommended to attenuate this inflammatory cascade in severe COVID-19 cases (6, 10). As a result, some authors speculate that individuals with Cushing's disease may represent an exquisite clinical subset of patients with a paradoxically reduced susceptibility to severe ARDS related to COVID-19 infection (11). However, it is important to note that persistent hypercortisolism, as seen in CD, can result in immune dysfunction, with elevated levels of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNFα), contributing to a chronic inflammatory state and facilitating conditions like ARDS (5, 11). Moreover, individuals with endogenous hypercortisolism commonly present with comorbidities that increase the risk of severe pulmonary and systemic disease in the setting of SARS-CoV-2 infection, including obesity, type 2 diabetes, hypertension, coagulopathy, and myopathy.
In this case, the authors attribute the acute clinical presentation and severe respiratory insufficiency primarily to the immunosuppression and immune dysfunction associated with endogenous hypercortisolism. The empirical use of glucocorticoids exacerbated the underlying metabolic conditions related to cortisol excess, notably hyperglycemia and hypertension. This highlights the importance of carefully evaluating patients who may not benefit from exogenous glucocorticoid treatment during SARS-CoV-2 infection, even in the context of ICU admission and severe respiratory insufficiency, to tailor treatment strategies accordingly.
CD is a rare pituitary disorder that typically affects women and adults with a median age of 40 years. Despite being the most common cause of endogenous hypercortisolism, CD diagnosis is often delayed due to its nonspecific signs and symptoms, such as depression, obesity, hypertension, dyslipidemia, and other metabolic complications (1, 2, 4).
In this particular case, social isolation emerged as one of the initial symptoms, which could have been confounded by the COVID-19 pandemic’s demands for social distancing. Additionally, changes in body composition and weight were overlooked by both the family and the general practitioner, possibly attributed to increased sedentary behavior during the pandemic. However, certain physical features such as purple striae, abdominal obesity, proximal myopathy, and cushingoid facies should have raised suspicion of CD earlier in the diagnostic process. Moreover, the use of topical glucocorticoids may have accentuated these clinical features. The delayed diagnosis resulted in worsened body dysmorphia and metabolic and immune dysfunction over time.
In conclusion, this case report underscores the importance of considering hypercortisolism in the context of metabolic and body composition changes, as well as mood disorders. It also emphasizes the critical need for early CD diagnosis to mitigate the risk of complications, including severe opportunistic infections.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
Author contribution statement
Conceptualization: MLGL; Data collection: MLGL, CL, and JSD. Data analysis: MLGL, CA, and JPC. Writing—original draft: MLGL and JPC. Writing (review and editing): MLGL, JPC, and CA. All authors read and approved the final manuscript.
References
- 1.Nieman LK Biller BMK Findling JW Newell-Price J Savage MO Stewart PM & Montori VM. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2008. 93 1526–1540. ( 10.1210/jc.2008-0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet. Diabetes and Endocrinology 2021. 9 847–875. ( 10.1016/S2213-8587(2100235-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savas M Mehta S Agrawal N van Rossum EFC & Feelders RA. Approach to the patient: diagnosis of Cushing syndrome. Journal of Clinical Endocrinology and Metabolism 2022. 107 3162–3174. ( 10.1210/clinem/dgac492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadelha M Gatto F Wildemberg LE & Fleseriu M. Cushing’s syndrome. Lancet 2023. 402 2237–2252. ( 10.1016/S0140-6736(2301961-X) [DOI] [PubMed] [Google Scholar]
- 5.Hasenmajer V Sbardella E Sciarra F Minnetti M Isidori AM & Venneri MA. The immune system in Cushing’s syndrome. Trends in Endocrinology and Metabolism 2020. 31 655–669. ( 10.1016/j.tem.2020.04.004) [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. 395 1054–1062. ( 10.1016/S0140-6736(2030566-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyes LF, Murthy S, Garcia-Gallo E, Irvine M, Merson L, Martin-Loeches I, Rello J, Taccone FS, Fowler RA, Docherty AB, et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Research 2022. 8 0, 05, 5, 2–2021. ( 10.1183/23120541.00552-2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensterle M, Herman R, Janež A, Mahmeed WA, Al-Rasadi K, Al-Alawi K, Banach M, Banerjee Y, Ceriello A, Cesur M, et al. The relationship between COVID-19 and hypothalamic-pituitary-adrenal axis: a large spectrum from glucocorticoid insufficiency to excess-the CAPISCO international expert panel. International Journal of Molecular Sciences 2022. 23. ( 10.3390/ijms23137326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal L, Garg Y, Yang P, Tabaie A, Wong AI, Mohammed A, Chinthala L, Kadaria D, Sodhi A, Holder AL, et al. eARDS: a multi-center validation of an interpretable machine learning algorithm of early onset acute respiratory distress syndrome (ARDS) among critically ill adults with COVID-19. PLoS One 2021. 16 e0257056. ( 10.1371/journal.pone.0257056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlińska A Świątkowska-Stodulska R & Sworczak K. Old problem, new concerns: hypercortisolemia in the time of COVID-19. Frontiers in Endocrinology 2021. 12 711612. ( 10.3389/fendo.2021.711612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Houten P Peng C Jaeger M van Herwaarden AE Netea MG van de Ven AC & Netea-Maier RT. Concomitant systemic inflammation and cellular immunosuppression in patients with Cushing’s syndrome. Clinical and Translational Medicine 2023. 13 e1314. ( 10.1002/ctm2.1314) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a