Abstract
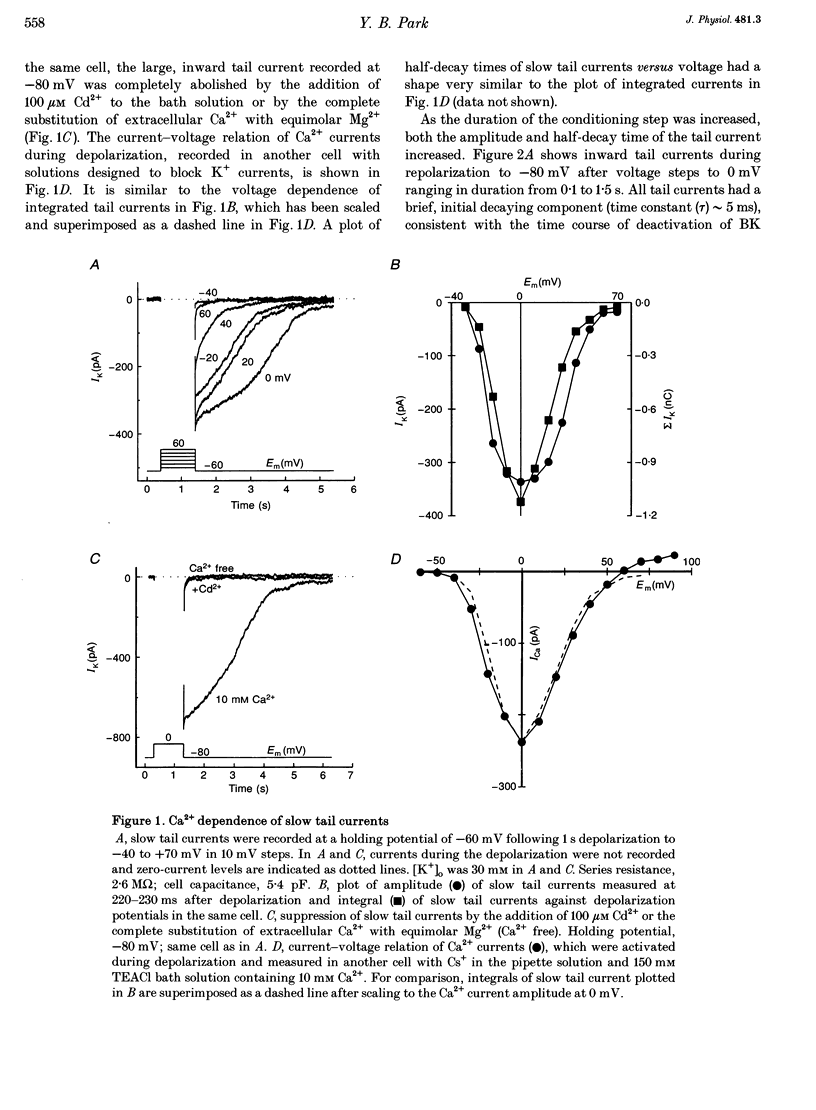
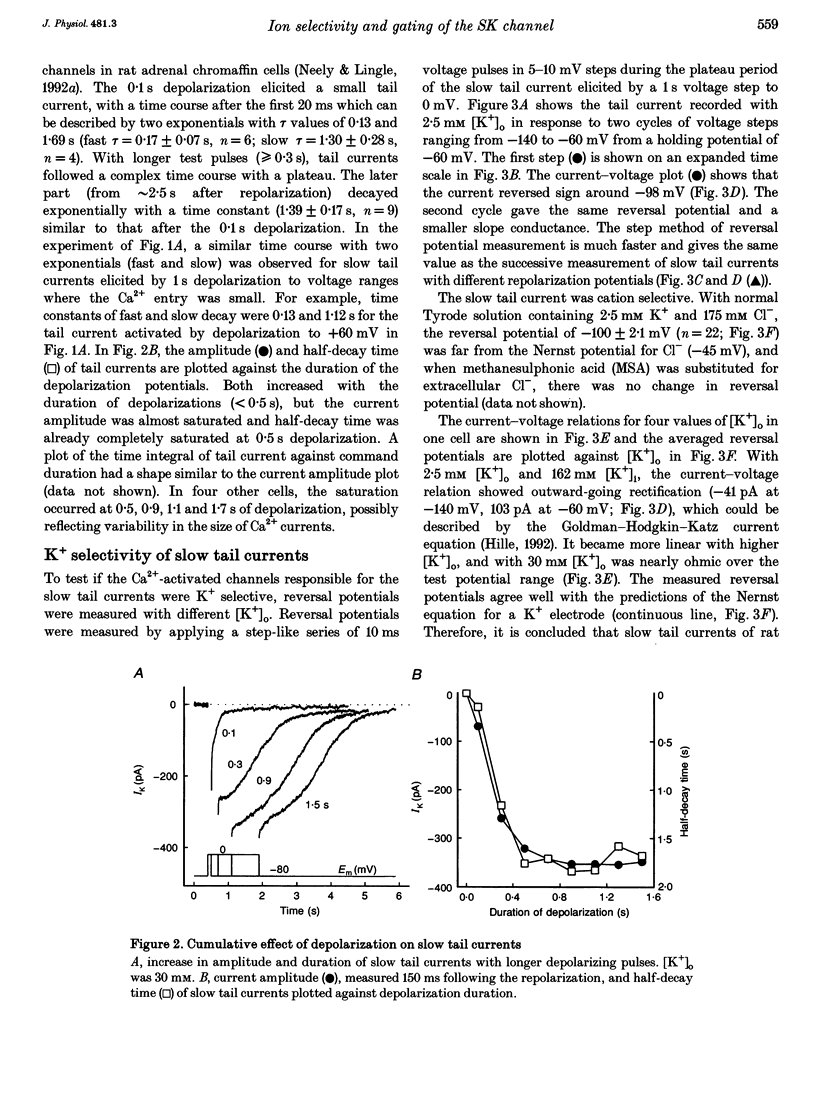
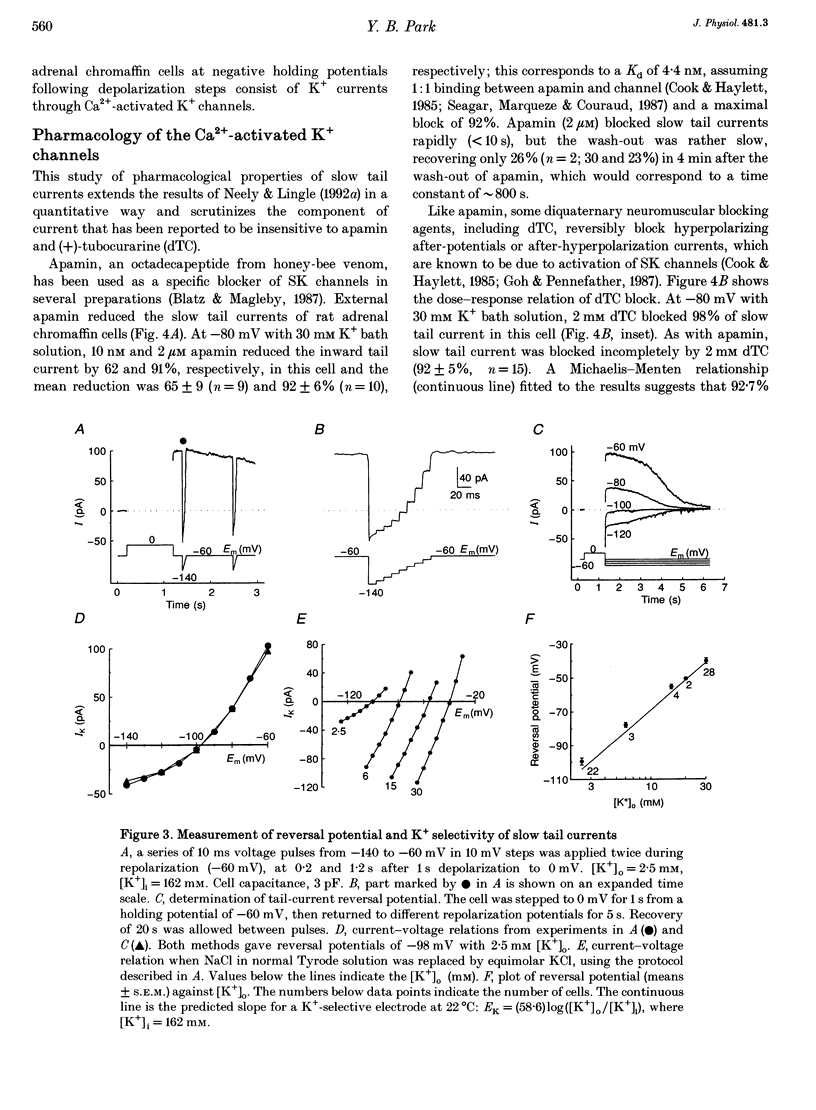
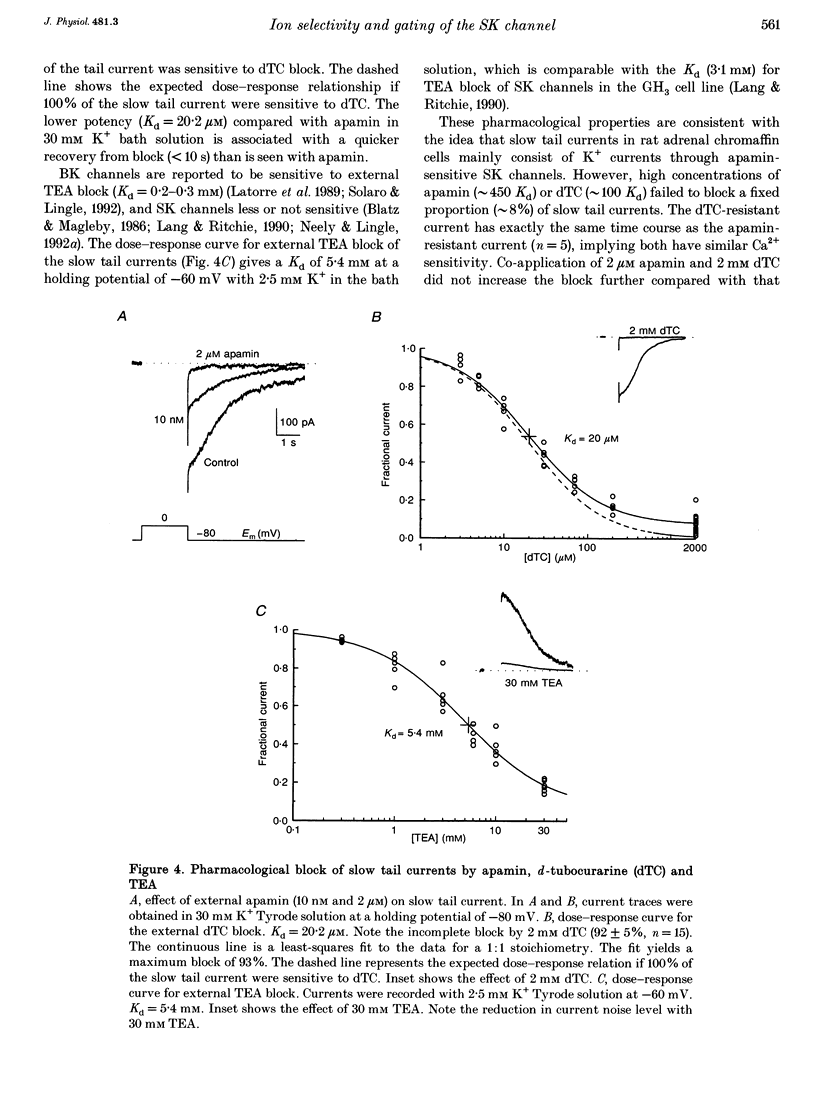
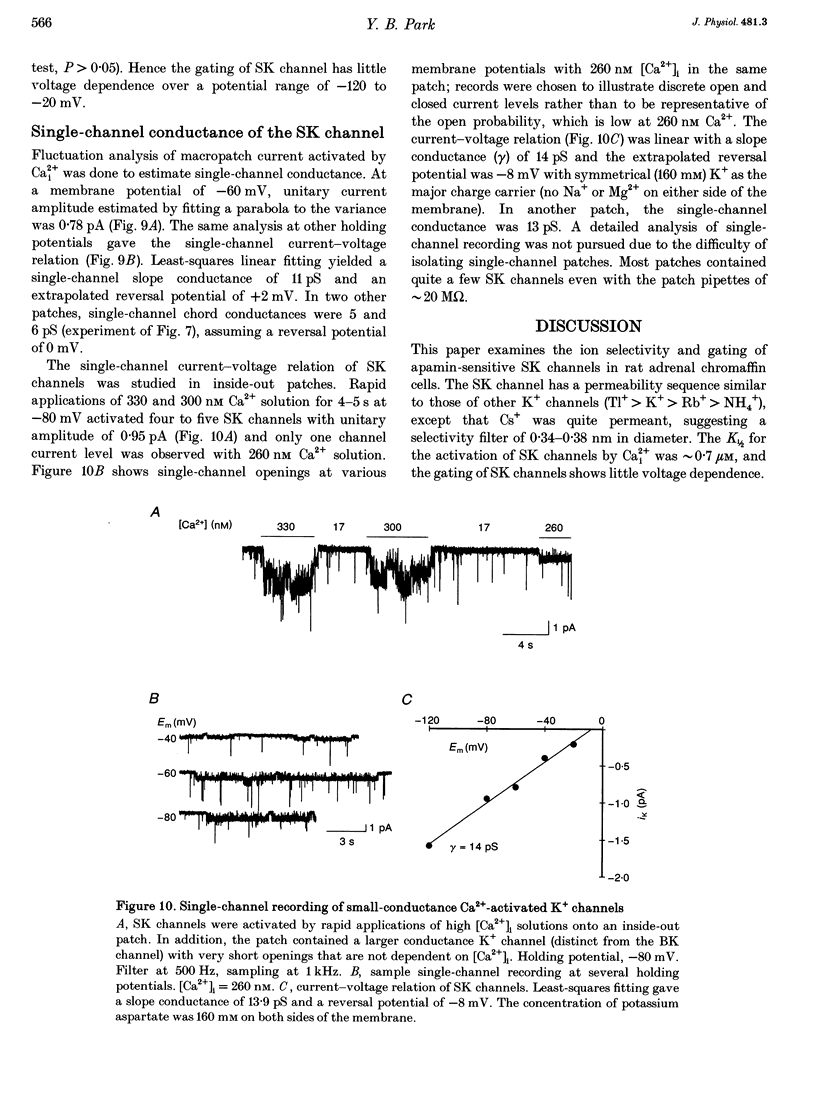
1. The ion selectivity and gating of apamin-sensitive, small conductance Ca(2+)-activated K+ (SK) channels were studied in cultured rat adrenal chromaffin cells using patch clamp techniques. 2. The amplitude of slow tail currents showed a bell-shaped dependence on depolarization potentials. Slow tail currents were abolished in a Ca(2+)-free external solution or by adding 100 microM Cd2+ to the external solution. Reversal potentials followed the predictions of the Nernst equation for a K+ electrode. 3. Slow tail currents were largely blocked by external application of apamin (dissociation constant, Kd, 4.4 nM), (+)-tubocurarine (Kd, 20 microM), and tetraethylammonium (Kd, 5.4 mM). 4. The relative permeability (PX/PK, where X may be any one of the ions listed) of SK channels was: Tl+ (1.87) > K+ (1.0) > Rb+ (0.81) > Cs+ (0.16) > NH4+ (0.11). Na+, Li+ and methylamine were not measurably permeant (PX/PK < 0.005). Open SK channels seem to have an effective pore diameter of 0.34-0.38 nm. The relative conductance (gX/gK) was: Tl+ (1.29) > K+ (1.0) > Rb+ (0.85) > Cs+ (0.45) approximately NH4+ (0.44). 5. With mixtures of Tl+ and K+, SK channels showed anomalous mole-fraction behaviour. 6. Ca2+ dependence of SK channel gating was studied using inside-out macropatches. The [Ca2+] required for half-maximal activation and the Hill coefficient were 0.69 microM and 1.7, respectively, and independent of membrane potentials. 7. Single-channel conductance was 13-14 pS (160 mM K+).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Armstrong C. M. Survival of K+ permeability and gating currents in squid axons perfused with K+-free media. J Gen Physiol. 1980 Jan;75(1):61–78. doi: 10.1085/jgp.75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo A. R., García A. G., Neher E. Small-conductance Ca(2+)-activated K+ channels in bovine chromaffin cells. Pflugers Arch. 1993 Apr;423(1-2):97–103. doi: 10.1007/BF00374966. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gater P. R., Haylett D. G., Jenkinson D. H. Neuromuscular blocking agents inhibit receptor-mediated increases in the potassium permeability of intestinal smooth muscle. Br J Pharmacol. 1985 Dec;86(4):861–868. doi: 10.1111/j.1476-5381.1985.tb11108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. Pharmacological and physiological properties of the after-hyperpolarization current of bullfrog ganglion neurones. J Physiol. 1987 Dec;394:315–330. doi: 10.1113/jphysiol.1987.sp016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Lewis R. S., Cahalan M. D. Ca(2+)-activated K+ channels in human leukemic T cells. J Gen Physiol. 1992 Jan;99(1):63–84. doi: 10.1085/jgp.99.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S., Nguyen A. N., Cahalan M. D. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol. 1993 Oct;102(4):601–630. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Tsien R. Y., Lester H. A. Activation of a potassium current by rapid photochemically generated step increases of intracellular calcium in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1987 May;84(10):3496–3500. doi: 10.1073/pnas.84.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. Conduction properties of the cloned Shaker K+ channel. Biophys J. 1993 Nov;65(5):2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2(+)-activated K+ channels in a pituitary cell line. J Physiol. 1990 Jun;425:117–132. doi: 10.1113/jphysiol.1990.sp018095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- MacKinnon R., Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990 Oct 12;250(4978):276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Effects of muscarine on single rat adrenal chromaffin cells. J Physiol. 1992;453:133–166. doi: 10.1113/jphysiol.1992.sp019221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely A., Lingle C. J. Two components of calcium-activated potassium current in rat adrenal chromaffin cells. J Physiol. 1992;453:97–131. doi: 10.1113/jphysiol.1992.sp019220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Blatz A. L., Magleby K. L. Recording from calcium-activated potassium channels. Methods Enzymol. 1992;207:194–207. doi: 10.1016/0076-6879(92)07014-f. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K. Two distinct calcium-activated potassium currents in a rat anterior pituitary cell line. J Physiol. 1987 Apr;385:591–609. doi: 10.1113/jphysiol.1987.sp016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagar M. J., Marqueze B., Couraud F. Solubilization of the apamin receptor associated with a calcium-activated potassium channel from rat brain. J Neurosci. 1987 Feb;7(2):565–570. doi: 10.1523/JNEUROSCI.07-02-00565.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. S., DeCoursey T. E. Selectivity and gating of the type L potassium channel in mouse lymphocytes. J Gen Physiol. 1991 Jun;97(6):1227–1250. doi: 10.1085/jgp.97.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro C. R., Lingle C. J. Trypsin-sensitive, rapid inactivation of a calcium-activated potassium channel. Science. 1992 Sep 18;257(5077):1694–1698. doi: 10.1126/science.1529355. [DOI] [PubMed] [Google Scholar]
- Taylor P. S. Selectivity and patch measurements of A-current channels in Helix aspersa neurones. J Physiol. 1987 Jul;388:437–447. doi: 10.1113/jphysiol.1987.sp016623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Uceda G., Artalejo A. R., López M. G., Abad F., Neher E., García A. G. Ca(2+)-activated K+ channels modulate muscarinic secretion in cat chromaffin cells. J Physiol. 1992 Aug;454:213–230. doi: 10.1113/jphysiol.1992.sp019261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom Y., Sugimori M., Llinás R. Ionic currents and firing patterns of mammalian vagal motoneurons in vitro. Neuroscience. 1985 Dec;16(4):719–737. doi: 10.1016/0306-4522(85)90090-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]


